Abstract
Chronic myelogenous leukemia (CML) results from malignant transformation of a primitive hematopoietic cell by the BCR/ABL oncogene. The breakpoint cluster region/ABL (BCR/ABL) tyrosine kinase inhibitor imatinib mesylate (imatinib) is highly effective in inducing remissions in CML. However, the effects of imatinib on intracellular signaling in primary progenitor cells are not well described. We show that imatinib exposure resulted in a significant dose-responsive reduction in BCR/ABL kinase activity in CML CD34+ cells. However, imatinib treatment resulted in an increase in activity of p42/44 mitogen-activated protein kinase (MAPK), an important downstream effector of BCR/ABL. Increased MAPK activity was growth factor dependent. Pharmacologic inhibition of MAPK using MAPK/extracellular signal–regulated kinase kinase–1/2 (MEK-1/2) inhibitors significantly reduced CML progenitor proliferation. Combined treatment with a MEK-1/2 inhibitor and imatinib significantly increased suppression of CML progenitors compared with either inhibitor alone. In contrast, imatinib treatment resulted in a small reduction in AKT activity. Combined treatment with a phosphatidylinositol-3 (PI-3) kinase inhibitor and imatinib significantly increased suppression of CML progenitor growth compared with either inhibitor alone. We conclude that inhibition of BCR/ABL kinase activity in CML progenitors by imatinib results in a growth factor-dependent compensatory increase in MAPK activity and in only partial inhibition of PI-3 kinase activity. These mechanisms may contribute to incomplete elimination of CML progenitors by imatinib. (Blood. 2004;103:3167-3174)
Introduction
Chronic myelogenous leukemia (CML) is a clonal hematopoietic disorder resulting from malignant transformation of a primitive hematopoietic cell.1 Stem cell transformation in CML results in abnormal proliferation and expansion of malignant progenitors, precursors, and mature hematopoietic cells.1,2 Clinically, CML inevitably progresses from an initial chronic phase (CP) through an accelerated phase (AP) and a terminal blast crisis (BC) or an acute leukemic phase. Malignant cells in CML have a characteristic karyotypic abnormality, the translocation t(9;22).3 At the molecular level, this translocation results in the formation of an abnormal BCR/ABL fusion gene.4 Several studies indicate that the BCR/ABL gene is required and sufficient for development of CML.5-7 The fusion of N-terminal breakpoint cluster region (BCR) sequences to the ABL tyrosine kinase (TK) results in constitutive activation and enhancement of the TK activity of the BCR/ABL protein (p210BCR/ABL).8 Abnormal TK activity is critical for the transforming ability of p210BCR/ABL.9 Deregulated TK activity results in activation of several downstream signaling pathways, including the Ras/mitogen-activated protein kinase (Ras/MAPK), phosphatidylinositol-3 kinase/AKT (PI-3K/AKT), and signal transducer and activator of transcription (STAT) pathways, which are implicated in mitogenic signaling and enhancement of survival.1,10
Imatinib mesylate (imatinib) (STI571; Gleevec; Novartis Pharmaceuticals, Basel, Switzerland) is a potent small molecule inhibitor of the BCR/ABL TK.11 Imatinib also inhibits the c-ABL, c-KIT, and platelet-derived growth factor (PDGF) receptor TKs. Preclinical studies showed that imatinib inhibits the growth of BCR/ABL-expressing cell lines in vitro and in vivo.12 In clinical trials, imatinib treatment results in cytogenetic response in a very high proportion of CML patients and is now a frontline treatment for this disease.13-18 However, since follow-up is limited, it is not clear as yet whether responses to imatinib will be durable. Recent reports indicate that molecular remissions may be rare and that BCR/ABL transcripts, although significantly reduced, may be detected in most patients with complete cytogenetic responses (CCRs) to imatinib when sensitive reverse transcriptase–polymerase chain reaction (RT-PCR) analyses are used.19 In some patients, especially in AP and BC, relapses can be observed even after induction of CCR.16,17,20 It is therefore important to investigate whether imatinib effectively targets the malignant primitive progenitors in which the disease originates.
In previous studies, others and we have shown that in vitro exposure to imatinib results in a dose-dependent inhibition of CML-committed and primitive progenitor growth.12,21-23 However, suppression of progenitor growth was partial and occurred mainly through inhibition of abnormally increased proliferation rather than through a selective increase in apoptosis. Consistent with this, Graham et al24 reported that primitive, quiescent Philadelphia chromosome–positive (Ph+) progenitor cells from CML patients have reduced sensitivity to imatinib in vitro. We have further shown that residual BCR/ABL+ CD34+ progenitors persist in CML patients in CCR on imatinib therapy.25 Therefore, imatinib effectively suppresses, but may not eliminate, malignant progenitors in CML patients. It is not yet clear whether imatinib effectively inhibits BCR/ABL kinase activity in CML progenitor cells. In addition, the effects of imatinib on signaling pathways downstream to BCR/ABL in primary progenitor cells are not clear. Improved understanding of the mechanisms of response or lack of response of CML primary progenitors to imatinib may allow rational design of therapies to enhance targeting of malignant progenitors resistant to elimination by imatinib alone.
The Ras signaling pathway appears to play an essential role in transformation of BCR/ABL+ cell lines.26 BCR-ABL can activate multiple alternative pathways leading to Ras activation.10,27 Ras activation leads to the phosphorylation and activation of downstream signaling proteins belonging to the MAPK pathway. Of the 3 major MAPK signaling pathways, p42/44 MAPK, also known as extracellular signal–regulated kinase (ERK), is a key mediator of proliferation, differentiation, and survival signals, whereas c-Jun N-terminal kinase (JNK) and p38 MAPK are activated in response to cellular stress and inflammatory cytokines and mediate induction of apoptosis.28 The p42/44 MAPK has been reported to be phosphorylated in BCR/ABL-expressing cell lines.10 Constitutive activation of p42/44 MAPK signaling can drive oncogenic transformation in fibroblasts and growth-factor independence in hematopoietic cell lines.29,30 Several studies suggest a role for MAPK signaling in BCR/ABL-driven proliferation and survival signaling.31-34
The aim of the current study was to investigate the effects of imatinib on BCR/ABL-activated intracellular signaling pathways in CML primary progenitors, and the relationship of these signaling mechanisms to inhibition of progenitor growth. We investigated whether imatinib effectively suppressed BCR/ABL TK activity in CML CD34+ cells. We also evaluated its effects on MAPK signaling, a potentially important downstream signaling mechanism contributing to hematopoietic cell transformation by BCR/ABL. The effects of imatinib on another downstream pathway, the PI-3K signaling pathway, were also studied.
Patients, materials, and methods
Subjects
Bone marrow samples from CML patients and healthy donors, peripheral blood stem cell samples from healthy donors, and umbilical cord blood samples were obtained under guidelines approved by the Institutional Review Board of the City of Hope National Medical Center (Duarte, CA).
Selection of CD34+ progenitors
Bone marrow mononuclear cells (BMMNCs) were isolated by Ficoll-Hypaque (Sigma Diagnostics, St Louis, MO) density gradient centrifugation (specific gravity, 1.077) for 30 minutes at 400g as previously described.35 CD34+ cells were selected from BMMNCs by means of immunomagnetic column separation (Miltenyi Biotech, Auburn, CA).36
Cell culture and exposure to inhibitors
CD34+ cells were cultured in tissue-culture plates (Falcon; BD Biosciences, Bedford, MA) at 37°C in a humidified atmosphere with 5% CO2 in serum-free medium (SFM) (StemCell Technologies, Vancouver, BC, Canada) supplemented with growth factors (GFs) at concentrations similar to that found in stroma-conditioned medium from long-term bone marrow cultures (200 pg/mL granulocyte-macrophage colony-stimulating factor [GM-CSF]; 1 ng/mL G-CSF; 200 pg/mL stem cell factor [SCF]; 50 pg/mL leukemia inhibitory factor [LIF]; 200 pg/mL macrophage-inflammatory protein–1α [MIP-1α]; and 1 ng/mL interleukin 6 [IL6]).35 Cells were cultured for 48 to 96 hours prior to being harvested and assayed in progenitor, proliferation, and apoptosis assays. Where indicated, imatinib mesylate, the MAPK extracellular signal-regulated kinase–1/2 (MEK-1/2) inhibitors PD98059 or U0126 (Calbiochem, San Diego, CA), or the PI-3 kinase inhibitors LY294002 or Wortmannin (Sigma Diagnostics) were added at the indicated concentrations.
Progenitor assays
CD34+ cells, cultured for 96 hours alone or in the presence of inhibitors, were plated in methylcellulose progenitor culture for 14 to 18 days and assessed for the presence of GM colony-forming units (CFU-GMs) and erythroid burst-forming units (BFU-Es) as previously described.35
Apoptosis assay
Apoptosis was detected as previously described.21 Briefly, CD34+ cells were cultured for 48 hours alone or in the presence of inhibitors, labeled with annexin V–fluorescein isothiocyanate (annexin V–FITC) and Via-Probe 7-amino-actinomycin D (7-AAD) (BD PharMingen, San Diego, CA), and analyzed by flow cytometry. Apoptotic cells were defined as annexin V–FITC+ and 7-AAD-.
Proliferation assay
Proliferation was evaluated by means of a 5- (and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes, Eugene, OR) labeling assay, as previously described with a small modification.21 CD34+ cells labeled with CFSE were cultured for 96 hours alone or in the presence of inhibitors. At the end of the culture period, proliferation was analyzed by flow cytometry (FACScalibur; Becton Dickinson, San Jose, CA). ModFit software (Verity, Topsham, ME) was used to fit the data, determine the percentage of cells in each generation, and generate a proliferation index.
Western blot analysis
CD34+ cells (2 × 105 cells per condition) were cultured in medium containing low concentrations of GF (as described in “Cell culture and exposure to inhibitors”) in the presence or absence of inhibitors at indicated concentrations for 16 hours. In experiments testing the effects of high GF concentrations, a 25-fold higher concentration of this GF cocktail was used. Lysates were prepared in buffer containing 0.5% Nonidet P-40 (Sigma Diagnostics) and 0.5% sodium deoxycholate supplemented with phenylmethylsulfonyl fluoride (1 mM), protease inhibitors mixture, and phosphatase inhibitors (50 mM NaF, 1 mM Na3VO4) (all from Sigma Diagnostics). Proteins were resolved on 10% sodium dodecyl sulfate–polyacrylamide get electrophoresis (SDS-PAGE) gels and transferred to nitrocellulose membrane. Primary antibodies used were as follows: anti–Crk-like (anti-CRKL) rabbit polyclonal antibody (sc-319; Santa Cruz Biotechnology, Santa Cruz, CA), anti–phosphorylated p42/44 MAPK mouse monoclonal antibody (sc-7383; Santa Cruz Biotechnology), and anti-p42/44 MAPK rabbit polyclonal antibody (sc-94; Santa Cruz Biotechnology). Horseradish peroxidase– or alkaline phosphatase–conjugated secondary antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA). Antibody detection was done by means of the Superfemto kit (Pierce Biotechnology, Rockford, IL) or Western-light system (Tropix, Bedford, MA). The protein expression level was determined by densitometry with the use of ImageQuant software (Amersham Pharmacia Biotech, Piscataway, NJ). Each experiment included the CML cell line K562 as a positive control.
MAP kinase and AKT kinase assays
CD34+ cells (3 × 105 cells per condition) were cultured for 16 hours in medium containing low concentrations of GF with or without imatinib as described in the preceding section. Samples were assayed with the use of AKT kinase or p44/42 MAPK assay kits obtained from Cell Signaling Technology (Beverly, MA) following the manufacturer's instructions. AKT or p44/42 MAPK were immunoprecipitated and subjected to an in vitro kinase reaction using glycogen synthase kinase–3 (GSK-3) and ELK-1 fusion proteins as substrates, respectively. The products of the kinase reactions were resolved by electrophoresis, and Western blotting with the use of primary antibodies to phospho–GSK-3α/β or phospho–ELK-1 was performed. One third of the lysate was used for Western blotting for actin.
Statistics
Results of data obtained from multiple experiments were reported as the mean ± 1 standard error of the mean (SEM). Significance levels were determined by Student t test analysis.
Results
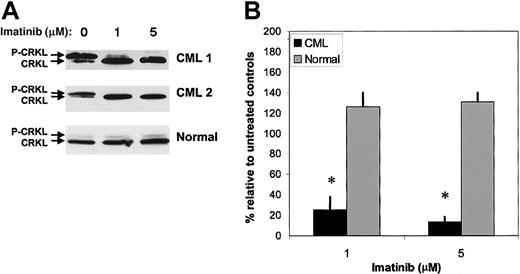
The effect of imatinib on BCR/ABL TK activity in CML CD34+ cells was investigated. The CRKL phosphorylation assay has been developed and validated as a practical method for evaluating the effect of imatinib treatment on BCR/ABL TK activity in primary CML cells.37 CML and normal CD34+ cells were cultured in low concentrations of GF, with or without addition of imatinib (1 to 5 μM). The concentrations of imatinib used correspond to levels achieved in patients being treated with imatinib.14 Phosphorylated CRKL (P-CRKL) is distinguishable from the nonphosphorylated form by its slower migration (Figure 1). CML CD34+ cells showed elevated CRKL phosphorylation (69% ± 8%; n = 6) compared with normal CD34+ cells (13% ± 2%; n = 3; P < .005) in the absence of imatinib. Imatinib exposure resulted in inhibition of CRKL phosphorylation in CML CD34+ cells in a dose-dependent manner (Figure 1; Table 1). In time-course studies, inhibition of CRKL phosphorylation was seen as early as 2 hours after imatinib exposure (data not shown). Imatinib did not have significant effects on CRKL phosphorylation in normal CD34+ cells. These results indicate that imatinib effectively inhibits BCR/ABL TK activity in CML CD34+ cells.
Inhibition of CRKL phosphorylation in CML CD34+ cells by imatinib treatment. CML (n = 6) and normal (n = 3) CD34+ cells (2 × 105) were exposed to imatinib (1 and 5 μM) for 16 hours in medium containing low concentrations of growth factors as described in “Patients, materials, and methods.” Whole-cell lysates were electrophoresed on 10% SDS-PAGE and Western blotted with an anti-CRKL antibody. P-CRKL could be distinguished from nonphosphorylated CRKL by its slower migration. (A) Representative results for 2 CML samples and a normal control sample are shown. (B) Densitometry analysis was performed, and the percentage of phosphorylated CRKL determined as described in “Results.” Means ± SEM of P-CRKL/CRKL ratios for treated cells expressed relative to controls are shown for CML (▪) and normal (▦) samples. Significant differences in paired t tests for treated CD34+ cells compared with untreated controls are indicated (*P < .005).
Inhibition of CRKL phosphorylation in CML CD34+ cells by imatinib treatment. CML (n = 6) and normal (n = 3) CD34+ cells (2 × 105) were exposed to imatinib (1 and 5 μM) for 16 hours in medium containing low concentrations of growth factors as described in “Patients, materials, and methods.” Whole-cell lysates were electrophoresed on 10% SDS-PAGE and Western blotted with an anti-CRKL antibody. P-CRKL could be distinguished from nonphosphorylated CRKL by its slower migration. (A) Representative results for 2 CML samples and a normal control sample are shown. (B) Densitometry analysis was performed, and the percentage of phosphorylated CRKL determined as described in “Results.” Means ± SEM of P-CRKL/CRKL ratios for treated cells expressed relative to controls are shown for CML (▪) and normal (▦) samples. Significant differences in paired t tests for treated CD34+ cells compared with untreated controls are indicated (*P < .005).
Effect of imatinib on CRKL and MAPK phosphorylation and CFC growth in individual CML patient samples
Sample . | Phase . | Prior imatinib at time of sampling . | Response to imatinib treatment . | P-CRKL . | P-MAPK . | CFC . |
|---|---|---|---|---|---|---|
| 1 | CP | No | Did not receive | 5 | 129 | 18 |
| 2 | AP | No | NA | 4 | 192 | 32 |
| 3 | AP | No | Did not receive | 35 | 165 | 39 |
| 4 | CP | No | NA | 0 | 50 | 46 |
| 5 | CP | No | CCR | 30 | 179 | 67 |
| 6 | CP | Yes | Initial CHR followed by loss of response | 79 | 219 | 106 |
Sample . | Phase . | Prior imatinib at time of sampling . | Response to imatinib treatment . | P-CRKL . | P-MAPK . | CFC . |
|---|---|---|---|---|---|---|
| 1 | CP | No | Did not receive | 5 | 129 | 18 |
| 2 | AP | No | NA | 4 | 192 | 32 |
| 3 | AP | No | Did not receive | 35 | 165 | 39 |
| 4 | CP | No | NA | 0 | 50 | 46 |
| 5 | CP | No | CCR | 30 | 179 | 67 |
| 6 | CP | Yes | Initial CHR followed by loss of response | 79 | 219 | 106 |
CML CD34+ cells were exposed to imatinib (1 mM) as described in “Patients, materials, and methods.” The effect of imatinib on P-CRKL, on P-MAPK, and on CFC growth in 6 individual CML patient samples is shown. Values shown are results for treated cells expressed as percentage of untreated controls.
CFC indicates colony-forming cells; NA, data not available; and CHR, complete hematologic remission.
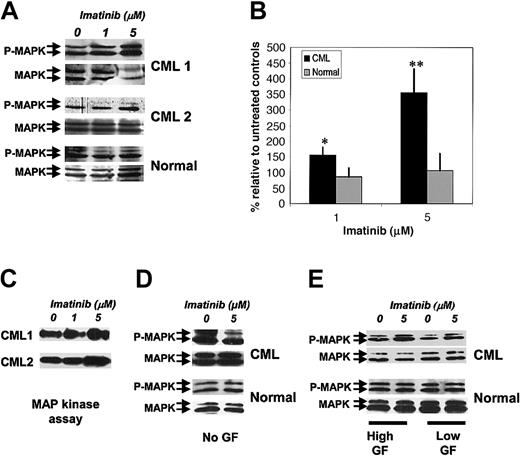
Activation of the Ras-MAPK pathway is considered to play an important role in BCR/ABL-induced transformation of hematopoietic cells.31-34 To evaluate the effect of imatinib on MAPK activity in CML CD34+ cells, Western blotting was performed with antibodies to the activated dual phosphorylated (Thr202, Tyr204) form of p42/44 MAPK (P-MAPK). Imatinib exposure failed to inhibit P-MAPK in CML CD34+ cells in the tested dose range (1-5 μM). Instead, the level of P-MAPK was increased in CML CD34+ cells in an imatinib dose–responsive manner. Total MAPK expression remained unchanged, and an increase in the P-MAPK/MAPK ratio was seen (Figure 2A-B). Time-course studies showed that increased MAPK phosphorylation was seen as early as 2 hours after imatinib exposure (data not shown). Imatinib treatment did not result in significant change in the P-MAPK/MAPK ratio in normal CD34+ cells, indicating that enhanced MAPK signaling is specific to CML progenitors. To confirm the results of Western blotting, we directly assessed MAPK activity by evaluating the ability of immunoprecipitated MAPK to phosphorylate the substrate protein ELK. These studies confirmed that imatinib treatment resulted in significantly increased MAPK activity in CML CD34+ cells (Figure 2C). We evaluated the relationship of increased MAPK activity to exogenous GFs. P-MAPK levels were either unchanged or reduced in CML CD34+ cells treated with imatinib in the absence of exogenous GF imatinib (Figure 2D), indicating that increased MAP kinase activity is GF dependent. Cells treated with imatinib in the presence of high concentrations of GF also demonstrated up-regulation of P-MAPK, indicating that this effect was not abrogated at saturating GF concentrations (Figure 2E).
Enhancement of p42/44 MAPK phosphorylation in CML CD34+ cells by imatinib treatment. (A) CML and normal CD34+ cells (2 × 105) were exposed to imatinib (1 and 5 μM) for 16 hours in medium containing low concentrations of GFs. Whole-cell lysates were prepared, electrophoresed on 10% SDS-PAGE, and Western blotted with antibodies to dual phosphorylated p42/44 MAPK (P-MAPK). Blots were stripped and reprobed with antibodies to total p42/44 MAPK (MAPK). Representative results for 2 CML samples and a normal control sample are shown. (B) Cumulative results for 6 CML and 3 normal samples are shown. P-MAPK/MAPK ratios were calculated by densitometry. Mean ± SEM of P-MAPK/MAPK ratio for treated cells expressed relative to controls is shown for CML (▪) and normal (▦) samples. Significant differences in a paired t test for treated CD34+ cells compared with untreated controls are indicated (*P < .07; **P < .02). (C) MAPK kinase assays were performed on CML CD34+ cells cultured with low concentrations of GFs in the absence and presence of imatinib. The p42/44 MAPK was immunoprecipitated, and in vitro kinase assays were performed with the use of an ELK-1 fusion protein as substrate followed by Western blot with an anti–phospho-ELK-1 antibody. Representative results for 2 CML samples are shown. (D) Results of Western blotting for P-MAPK and MAPK in CML and normal CD34+ cells cultured with or without imatinib (5 μM) in medium without addition of GFs. (E) Results of Western blotting for P-MAPK and MAPK in CML and normal CD34+ cells cultured with or without imatinib (5 μM) in medium containing high (25-fold higher) compared with low concentrations of GFs.
Enhancement of p42/44 MAPK phosphorylation in CML CD34+ cells by imatinib treatment. (A) CML and normal CD34+ cells (2 × 105) were exposed to imatinib (1 and 5 μM) for 16 hours in medium containing low concentrations of GFs. Whole-cell lysates were prepared, electrophoresed on 10% SDS-PAGE, and Western blotted with antibodies to dual phosphorylated p42/44 MAPK (P-MAPK). Blots were stripped and reprobed with antibodies to total p42/44 MAPK (MAPK). Representative results for 2 CML samples and a normal control sample are shown. (B) Cumulative results for 6 CML and 3 normal samples are shown. P-MAPK/MAPK ratios were calculated by densitometry. Mean ± SEM of P-MAPK/MAPK ratio for treated cells expressed relative to controls is shown for CML (▪) and normal (▦) samples. Significant differences in a paired t test for treated CD34+ cells compared with untreated controls are indicated (*P < .07; **P < .02). (C) MAPK kinase assays were performed on CML CD34+ cells cultured with low concentrations of GFs in the absence and presence of imatinib. The p42/44 MAPK was immunoprecipitated, and in vitro kinase assays were performed with the use of an ELK-1 fusion protein as substrate followed by Western blot with an anti–phospho-ELK-1 antibody. Representative results for 2 CML samples are shown. (D) Results of Western blotting for P-MAPK and MAPK in CML and normal CD34+ cells cultured with or without imatinib (5 μM) in medium without addition of GFs. (E) Results of Western blotting for P-MAPK and MAPK in CML and normal CD34+ cells cultured with or without imatinib (5 μM) in medium containing high (25-fold higher) compared with low concentrations of GFs.
We investigated the functional significance of these changes in intracellular signaling following imatinib treatment. Treatment of CML CD34+ cells with 1 μM imatinib resulted in significant reduction in CFC growth compared with untreated controls (49% ± 13%; n = 6; P = .013), an observation consistent with our previously described results.21 Inhibition of normal CFCs at this dose was significantly less than that of CML CFCs (14% ± 8%; n = 6; P = .048). There was significant correlation between inhibition of CML CFC growth and inhibition of BCR/ABL TK activity in CML CD34+ cells (r2 = 0.74; P < .02) (Table 1). Increased MAPK activity did not correlate well with the degree of TK suppression or with inhibition of CFC growth. Although the correlation between inhibition of CFC growth and CRKL inhibition was good overall, patient no. 4 was an exception in that CFC growth was less inhibited than would be expected on the basis of the high level of inhibition of CRKL phosphorylation. It is possible this patient may have acquired additional non–BCR/ABL-related abnormalities in growth regulation that could contribute to reduced inhibition of progenitor growth despite effective inhibition of BCR/ABL TK activity and that could alter the MAPK response to BCR/ABL TK inhibition. In sample no. 6, from a patient with clinical resistance to imatinib, treatment with imatinib failed to inhibit BCR/ABL TK activity, but up-regulation of MAPK signaling was still seen, suggesting that actions of imatinib on kinases other than BCR/ABL may be involved in MAPK up-regulation.
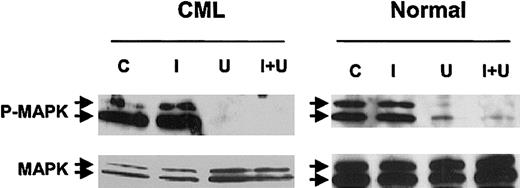
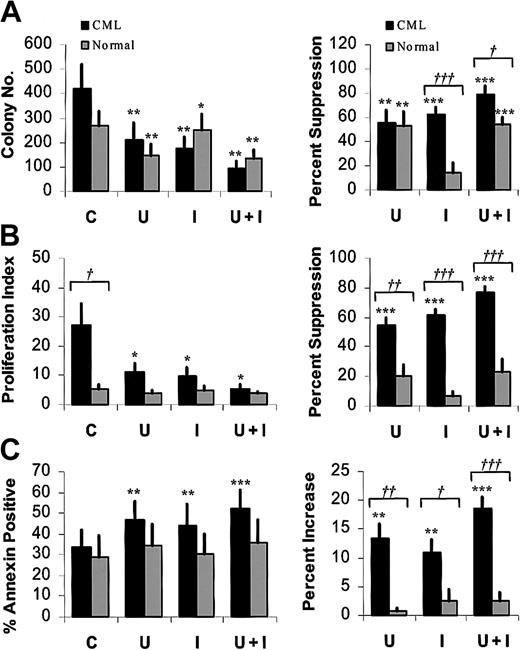
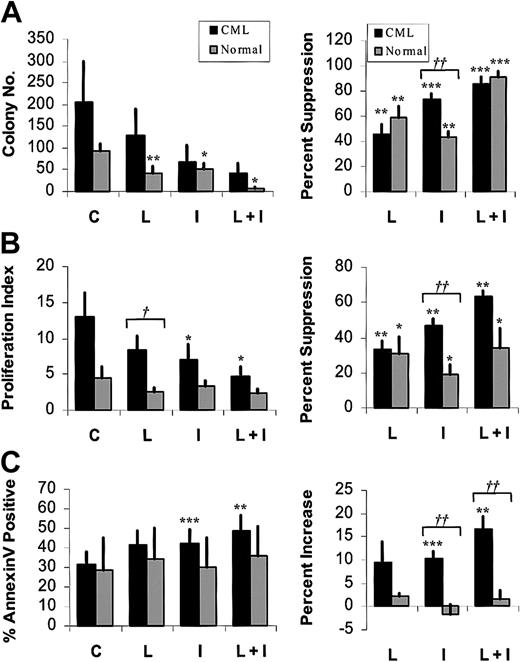
To further investigate the significance of MAPK activity in imatinib-treated CML progenitors, we studied the effects of U0126 and PD98059, pharmacologic inhibitors of the p42/44 MAPK–activating enzyme MEK-1/2. Preliminary dose-response studies indicated that U0126 or PD98059 markedly reduced P-MAPK levels in BCR/ABL-transformed TF-1 cell lines at a concentration of 25 μM, (data not shown). We confirmed that 25 μM U0126 (Figure 3) or PD98059 (not shown) inhibited MAPK phosphorylation in CML and normal CD34+ cells, in both the presence and absence of imatinib. Inhibition was more complete with U0126 than with PD98059. P-MAPK levels were higher in CML cells than in normal CD34+ cells after culture for 96 hours both with and without imatinib treatment (data not shown). U0126 treatment led to significant inhibition of both CML and normal CFC growth (Figure 4A). As expected, imatinib treatment also resulted in significant inhibition of CML CFC growth. Combined treatment with U0126 and imatinib resulted in significantly increased suppression of CML CFCs beyond that seen with either inhibitor alone (U0126 versus U0126 plus imatinib, P = .033; imatinib versus U0126 plus imatinib, P = .019), but did not result in increased suppression of normal CFCs beyond that observed with U0126 alone. Similar results were obtained with PD98059 (data not shown).
Effect of MEK-1/2 inhibitor treatment on MAPK phosphorylation in CML and normal CD34+ cells. CD34+ cells from a CML patient and a healthy control were exposed to 25 μM U0126 for 16 hours in the presence or absence of 1 μM imatinib in medium containing low concentrations of GF. Whole-cell lysates were prepared, electrophoresed on 10% SDS-PAGE, and Western blotted with antibodies to dual phosphorylated p42/44 MAPK (P-MAPK) and total p42/44 MAPK (MAPK). Results of representative experiments are shown. C indicates control cells; I, the presence of 1 μM imatinib; U, the presence of 25 μM U0126; U + I, the presence of both.
Effect of MEK-1/2 inhibitor treatment on MAPK phosphorylation in CML and normal CD34+ cells. CD34+ cells from a CML patient and a healthy control were exposed to 25 μM U0126 for 16 hours in the presence or absence of 1 μM imatinib in medium containing low concentrations of GF. Whole-cell lysates were prepared, electrophoresed on 10% SDS-PAGE, and Western blotted with antibodies to dual phosphorylated p42/44 MAPK (P-MAPK) and total p42/44 MAPK (MAPK). Results of representative experiments are shown. C indicates control cells; I, the presence of 1 μM imatinib; U, the presence of 25 μM U0126; U + I, the presence of both.
Effects of the MEK-1/2 inhibitor U0126 on imatinib-treated CML and normal CD34+ cells. CD34+ cells were cultured in the presence of low GF, either without inhibitors (C) or in the presence of 1 μM imatinib (I), 25 μM U0126 (U), or both (U + I). Data plotted are for CML (▪) and normal (▦) cells. Significant differences between imatinib-treated CD34+ cells and untreated controls (paired t tests; *P < .05, **P < .01, ***P < .001) and between CML and normal CD34+ cells (unpaired t tests; †P < .05, ††P < .01, †††P < .001) are indicated. (A) CML and normal CFC growth. CML (n = 7) and normal (n = 6) CD34+ cells were cultured as described for 96 hours and plated in methylcellulose progenitor culture for 14 to 18 days, and CFC frequency was enumerated. The left panel shows colony numbers per 2000 CD34+ cells (mean ± SEM). In the right panel, CFC data are presented as the percentage of suppression of CFCs compared with untreated controls. (B) Proliferation of CML and normal CD34+ cells in a CFSE-labeling assay. CML (n = 5) and normal (n = 4) CD34+ cells were labeled with CFSE, treated for 96 hours as indicated, and assessed for cell division by flow cytometry as described in the “Patients, materials, and methods.” ModFit software was used to fit the data and generate a proliferation index. In the left panel, the proliferation index (mean ± SEM) is plotted. In the right panel, results are presented as the percentage of suppression of proliferation compared with untreated controls. (C) Apoptosis of CML and normal CD34+ cells. CML (n = 7) and normal (n = 6) CD34+ cells were treated for 48 hours and assessed for apoptosis by flow cytometry after labeling with annexin V–FITC and 7-AAD. The left panel shows the percentage of apoptotic cells (mean ± SEM). The increase in apoptosis above that of untreated cells (percentage treated - percentage untreated) is shown in the right panel.
Effects of the MEK-1/2 inhibitor U0126 on imatinib-treated CML and normal CD34+ cells. CD34+ cells were cultured in the presence of low GF, either without inhibitors (C) or in the presence of 1 μM imatinib (I), 25 μM U0126 (U), or both (U + I). Data plotted are for CML (▪) and normal (▦) cells. Significant differences between imatinib-treated CD34+ cells and untreated controls (paired t tests; *P < .05, **P < .01, ***P < .001) and between CML and normal CD34+ cells (unpaired t tests; †P < .05, ††P < .01, †††P < .001) are indicated. (A) CML and normal CFC growth. CML (n = 7) and normal (n = 6) CD34+ cells were cultured as described for 96 hours and plated in methylcellulose progenitor culture for 14 to 18 days, and CFC frequency was enumerated. The left panel shows colony numbers per 2000 CD34+ cells (mean ± SEM). In the right panel, CFC data are presented as the percentage of suppression of CFCs compared with untreated controls. (B) Proliferation of CML and normal CD34+ cells in a CFSE-labeling assay. CML (n = 5) and normal (n = 4) CD34+ cells were labeled with CFSE, treated for 96 hours as indicated, and assessed for cell division by flow cytometry as described in the “Patients, materials, and methods.” ModFit software was used to fit the data and generate a proliferation index. In the left panel, the proliferation index (mean ± SEM) is plotted. In the right panel, results are presented as the percentage of suppression of proliferation compared with untreated controls. (C) Apoptosis of CML and normal CD34+ cells. CML (n = 7) and normal (n = 6) CD34+ cells were treated for 48 hours and assessed for apoptosis by flow cytometry after labeling with annexin V–FITC and 7-AAD. The left panel shows the percentage of apoptotic cells (mean ± SEM). The increase in apoptosis above that of untreated cells (percentage treated - percentage untreated) is shown in the right panel.
Progenitor proliferation was measured by labeling cells with CFSE and evaluating cell division by flow cytometry (Figure 4B). As previously reported,21 untreated CML CD34+ cells demonstrated significantly enhanced proliferation compared with their normal counterparts (P = .043), and a marked reduction in CML CD34+ cell proliferation was seen following imatinib treatment. Treatment with U0126 also resulted in a significant reduction in CML CD34+ cell proliferation. The combination of U0126 and imatinib resulted in significantly increased inhibition of CML CD34+ cell proliferation beyond that seen with either inhibitor alone (U0126 versus U0126 plus imatinib, P = .001; imatinib versus U0126 plus imatinib, P = .006). Inhibition of normal CD34+ cell proliferation by U0126 did not reach statistical significance (P = .076). Imatinib did not result in significant inhibition of normal CD34+ cell proliferation. The combination of imatinib with U0126 did not result in increased suppression of normal CD34+ cell proliferation beyond that observed with U0126 alone. Exposure to imatinib or MEK inhibitors alone resulted in a small but significant increase in apoptosis in CML CD34+ cells compared with controls (Figure 4C). Combined exposure to imatinib and MEK inhibitors was associated with a small increase in apoptosis beyond that seen with either inhibitor alone (U0126 versus U0126 plus imatinib, P = .02; imatinib versus U0126 plus imatinib, P = .009). Neither MEK inhibitors nor imatinib resulted in a significant increase in apoptosis in normal CD34+ cells. These results suggest that the enhanced activity of the inhibitor combination may result from both enhanced suppression of CML CD34+ cell proliferation and increased apoptosis.
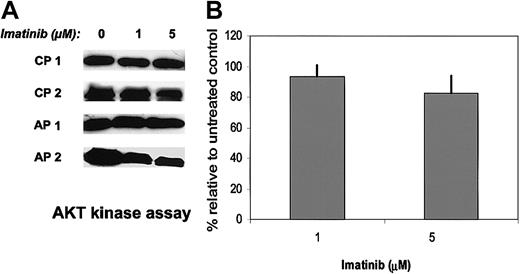
We also evaluated the effect of imatinib on PI-3K/AKT kinase signaling, another downstream effector of BCR/ABL. Klejman et al38 have reported that imatinib inhibits PI-3K/AKT kinase activity in CML BC cells and that combinations of PI-3K inhibitors and imatinib resulted in enhanced and selective inhibition of CML CFC growth. Here we observed that imatinib treatment inhibited AKT kinase activity in CD34+ cells from some CML patients but not others and that pooled results for AKT kinase inhibition by imatinib were not statistically significant (Figure 5). AKT kinase inhibition did not correlate with disease stage, prior imatinib exposure, or imatinib response. The effect of the PI-3K inhibitors LY294002 (LY) and Wortmannin on CML and normal CD34+ growth were tested. LY (2 μM) and Wortmannin (12.5 nM) concentrations were as described in the Klejman study. The effects of LY were more prominent than Wortmannin and are shown in Figure 6. Treatment of CML CD34+ cells with LY resulted in significant inhibition of CML CFC growth and suppression of proliferation. Normal CD34+ cells treated with LY also showed significant decreases in CFC growth and proliferation. As expected, treatment of CML CD34+ cells with imatinib alone resulted in significant suppression of CFC growth, inhibition of proliferation, and induction of apoptosis. Suppression of CML CFC growth following exposure to a combination of imatinib and LY was significantly increased compared with either LY or imatinib alone. (LY versus LY plus imatinib, P = .002; imatinib versus LY plus imatinib, P = .009). Treatment of CML CD34+ cells with the combination of LY and imatinib also significantly enhanced inhibition of proliferation relative to either inhibitor alone (LY versus LY plus imatinib, P = .011; imatinib versus LY plus imatinib, P = .012), but only increased apoptosis significantly when compared with imatinib alone (LY versus LY plus imatinib, P = .105; imatinib versus LY plus imatinib, P = .025). Normal CD34+ cells treated with the combination of LY and imatinib showed enhanced CFC suppression relative to either inhibitor alone and increased apoptosis relative to imatinib alone. In summary, addition of PI-3K inhibitors to imatinib resulted in significantly increased suppression of CML progenitor growth compared with imatinib alone and also resulted in similar suppression of normal progenitor growth.
Effects of imatinib on activities of AKT kinase in CML samples. AKT kinase assays were performed on CML CD34+ cells cultured with or without imatinib (1 and 5 μM) for 16 hours in medium containing low concentrations of GF. AKT was immunoprecipitated, and in vitro kinase assays were performed with the use of a GSK-3 fusion protein as substrate followed by Western blotting with an anti–phospho–GSK-3α/β antibody. The difference between imatinib treated cells and untreated cells was not significant (P = .4 for 1 μM imatinib; P = .18 for 5 μM imatinib). (A) Representative results for 2 CP and 2 AP patients. (B) Pooled results for treated cells relative to controls is shown (mean ± SEM, n = 6).
Effects of imatinib on activities of AKT kinase in CML samples. AKT kinase assays were performed on CML CD34+ cells cultured with or without imatinib (1 and 5 μM) for 16 hours in medium containing low concentrations of GF. AKT was immunoprecipitated, and in vitro kinase assays were performed with the use of a GSK-3 fusion protein as substrate followed by Western blotting with an anti–phospho–GSK-3α/β antibody. The difference between imatinib treated cells and untreated cells was not significant (P = .4 for 1 μM imatinib; P = .18 for 5 μM imatinib). (A) Representative results for 2 CP and 2 AP patients. (B) Pooled results for treated cells relative to controls is shown (mean ± SEM, n = 6).
Effects of the PI-3K inhibitor LY294002 on imatinib-treated CML and normal CD34+ cells. CD34+ cells were cultured with low GF, either without inhibitors (C), or in the presence of 1 μM imatinib (I), 2 μM LY294002 (L), or both (L + I). Data plotted are for CML (▪) and normal (▦) cells. Significant differences between treated CD34+ cells and untreated controls (paired t tests; *P < .05, **P < .01, ***P < .001) and between CML and normal CD34+ cells (unpaired t tests; †P < .05, ††P < .01, †††P < .001) are indicated. (A) Growth of CML and normal CFCs. CML (n = 5) and normal (n = 4) cells were cultured as described for 96 hours and then plated in methylcellulose progenitor culture for 14 to 18 days, and CFC frequency was enumerated. The left panel shows colony numbers per 2000 CD34+ cells (mean ± SEM). In the right panel, data are presented as the percentage of suppression of CFCs compared with untreated controls. (B) Proliferation of CML and normal CD34+ cells in a CFSE-labeling assay. CML (n = 4) and normal (n = 5) CD34+ cells were labeled with CFSE and treated for 96 hours and assessed for proliferation by flow cytometry. ModFit software was used to fit the data and generate a proliferation index. In the left panel, the proliferation index (mean ± SEM) is plotted. In the right panel, results are presented as the percentage of suppression of proliferation compared with untreated controls. (C) Apoptosis of CML and normal CD34+ cells. CML (n = 7) and normal (n = 4) CD34+ cells were treated for 48 hours and assessed for apoptosis by flow cytometry after labeling with annexinV–FITC and 7-AAD. The left panel shows the percentage of apoptotic cells (mean ± SEM). The increase in apoptosis above that of untreated cells (percentage treated - percentage untreated) is shown in the right panel.
Effects of the PI-3K inhibitor LY294002 on imatinib-treated CML and normal CD34+ cells. CD34+ cells were cultured with low GF, either without inhibitors (C), or in the presence of 1 μM imatinib (I), 2 μM LY294002 (L), or both (L + I). Data plotted are for CML (▪) and normal (▦) cells. Significant differences between treated CD34+ cells and untreated controls (paired t tests; *P < .05, **P < .01, ***P < .001) and between CML and normal CD34+ cells (unpaired t tests; †P < .05, ††P < .01, †††P < .001) are indicated. (A) Growth of CML and normal CFCs. CML (n = 5) and normal (n = 4) cells were cultured as described for 96 hours and then plated in methylcellulose progenitor culture for 14 to 18 days, and CFC frequency was enumerated. The left panel shows colony numbers per 2000 CD34+ cells (mean ± SEM). In the right panel, data are presented as the percentage of suppression of CFCs compared with untreated controls. (B) Proliferation of CML and normal CD34+ cells in a CFSE-labeling assay. CML (n = 4) and normal (n = 5) CD34+ cells were labeled with CFSE and treated for 96 hours and assessed for proliferation by flow cytometry. ModFit software was used to fit the data and generate a proliferation index. In the left panel, the proliferation index (mean ± SEM) is plotted. In the right panel, results are presented as the percentage of suppression of proliferation compared with untreated controls. (C) Apoptosis of CML and normal CD34+ cells. CML (n = 7) and normal (n = 4) CD34+ cells were treated for 48 hours and assessed for apoptosis by flow cytometry after labeling with annexinV–FITC and 7-AAD. The left panel shows the percentage of apoptotic cells (mean ± SEM). The increase in apoptosis above that of untreated cells (percentage treated - percentage untreated) is shown in the right panel.
Discussion
We have directly investigated the effect of imatinib on intracellular signaling in CML primary progenitor cells. We show that imatinib treatment results in inhibition of BCR/ABL TK activity in CML CD34+ progenitor cells. However, imatinib treatment failed to inhibit p42/44 MAPK, a potential downstream target of the BCR/ABL TK, and instead an unexpected dose-responsive and GF-dependent increase in MAPK activity was seen. Pharmacologic inhibition of MAPK activity with MEK-1/2 inhibitors in combination with imatinib resulted in additional suppression of CML progenitor growth beyond that seen with imatinib treatment alone.
Others and we have previously shown that imatinib treatment results in significant suppression of CML progenitor growth.12,21-24 Progenitor suppression results mainly from inhibition of proliferation although a small increase in apoptosis is also seen.21,22,24 However, suppression is incomplete, and residual BCR/ABL+ progenitors can persist following imatinib treatment, even in patients in CCR.25 One potential explanation is that imatinib has reduced activity in CML CD34+ progenitors compared with more differentiated cells or cell lines. In the current study, we directly evaluated the effect of imatinib on BCR/ABL TK activity. CRKL phosphorylation was used as a marker of BCR/ABL TK activity.37,39 Imatinib treatment resulted in significant dose-responsive inhibition of CRKL phosphorylation in CML CD34+ cells. The degree of reduction of CRKL phosphorylation was similar to that previously described for primary CML whole-blood or marrow cells37 and for BCR/ABL-expressing cell lines following treatment with imatinib (S.C. and R.B., unpublished observations, March 2003). The degree of inhibition of BCR/ABL TK activity in CML CD34+ cells by imatinib correlated well with inhibition of CFC growth. Progenitor suppression was incomplete, even in samples demonstrating highly effective inhibition of BCR/ABL TK activity. However low-level kinase activity always persists and may be sufficient to maintain viability of BCR/ABL-expressing progenitors.
BCR/ABL TK activity results in abnormal phosphorylation of a large number of intracellular proteins, including adaptor proteins, receptors, enzymes, and cytoskeletal proteins, that are implicated as playing a role in BCR/ABL-induced transformation.1 However, the role of specific downstream pathways in the transformation of primary CML progenitor cells is unclear. The p42/44 MAPK plays an important role in integration of signals from extracellular stimuli regulating cell proliferation, differentiation, and survival.28,40 Several studies in hematopoietic cell lines indicate that BCR/ABL activates p42/44 MAPK signaling.26,32-34 However, a recent study did not find an association between BCR/ABL expression and MAPK activation in human leukemia lines.41 Studies evaluating the effect of imatinib on MAPK signaling in CML cell lines have also given mixed results. Dan et al42 reported that exposure to imatinib resulted in dephosphorylation of p42/44 MAPK. On the other hand, Yu et al43 observed that exposure of BCR/ABL-expressing K562 cells to low concentrations of imatinib (200 nM) resulted in an increase in p42/44MAPK phosphorylation. We show here that imatinib treatment results in dose-responsive increase in MAPK phosphorylation and catalytic activity in CML CD34+ progenitor cells. Imatinib also resulted in increased, rather than reduced, Ras activation in BCR/ABL-expressing TF-1 hematopoietic cells (M.H. and R.B., unpublished observations, September 2003). Increased MAPK activity did not correlate well with the degree of TK suppression or inhibition of CML CFC growth. There was considerable interpatient variability in the relationship between imatinib effects on BCR/ABL TK activity, P-MAPK levels, and CFC growth. This may be related in part to varying degrees of BCR/ABL TK resistance to imatinib in primary samples. Additional non–BCR/ABL-related abnormalities in growth regulation could also contribute to reduced inhibition of progenitor growth despite effective inhibition of BCR/ABL TK activity and could alter the MAPK response to BCR/ABL TK inhibition. Finally, increased MAPK signaling could be seen even in imatinib-resistant cells, suggesting that inhibition of non-BCR/ABL TK mechanisms could contribute to MAPK up-regulation. CML CD34+ cells demonstrate abnormal sensitivity to c-KIT stimulation, and it is possible that some of the results obtained may have been influenced by inhibition of c-KIT, which could occur even where imatinib sensitivity was lost.44
Our studies using MEK-1/2 inhibitors provide evidence that persistent MAPK signaling in imatinib-treated cells is of functional significance. Inhibition of MAPK signaling led to suppression of CML and normal progenitor growth. A significant inhibition of CML CD34+ cell proliferation and increase in apoptosis was seen. The combination of imatinib with a MEK inhibitor resulted in significantly increased inhibition of CML, but not normal progenitor growth, compared with either inhibitor alone. Our results suggest that inhibition of MAPK signaling has an antiproliferative effect on CML progenitors, which is enhanced in combination with imatinib, and a significant but less prominent effect on cell viability. Yu et al43 observed that exposure to imatinib in combination with MEK1/2 inhibitor PD184352 resulted in a dramatic increase in mitochondrial dysfunction and apoptosis in K562 cells. Differences between our results and those of Yu et al are probably related to differences in intracellular signaling and regulation of apoptosis between primary progenitor cells and the K562 cell line, which is a propagated BC CML-derived cell line with multiple additional genetic abnormalities besides the BCR/ABL translocation,45 and several-fold–increased BCR/ABL expression compared with primary CML cells.46 As a result, K562 cells may have a greater dependence on BCR/ABL TK activity and/or MAPK activity for maintenance of viability.47
Increased MAPK activity in imatinib-treated CML progenitors may represent a compensatory response to inhibition of BCR/ABL TK activity. Cultured CML CD34+ cells had increased P-MAPK levels compared with normal CD34+ cells. In the absence of exogenous GF, treatment with imatinib either reduced or did not change MAPK activity in CML CD34+ cells. The imatinib-induced increase in MAPK activity was seen even at saturating GF concentrations. Therefore imatinib-mediated inhibition of BCR/ABL TK activity may be associated with an enhanced response of CML progenitors to GF-induced physiologic stimulation of MAPK activity. Loss of negative feedback mechanisms operating in BCR/ABL-transformed cells may contribute to increased MAPK signaling in imatinib-treated cells. For example, the docking protein p62DOK is prominently phosphorylated in CML progenitor cells,48 is a negative modulator of RAS and MAPK signaling,49 and can oppose p210BCR/ABL-induced leukemogenesis in an in vivo mouse model.49 We have observed that imatinib treatment leads to marked reduction in p62DOK phosphorylation in CML CD34+ cells (S.C. and R.B., unpublished observations, December 2002). However, p62DOK has also been reported to bind p120 Ras GTPase-activating protein (GAP) and potentiate signaling through Ras,50 and further studies will be required to determine whether reduction in p62DOK phosphorylation after imatinib treatment could play a role in altered MAPK signaling in CML progenitor cells.
In the network model of signaling pioneered by Iyengar and colleagues, signaling cascades are viewed as interacting pathways that integrate multiple inputs to shape a defined output, rather than as linear pathways that relay information.51-53 The interaction of different pathways and the dynamic modulation of activities of components within signaling pathways can create a variety of biologic outputs. In CML progenitors, BCR/ABL signaling may result in constitutive activation of several signaling cascades, which may override physiologic signaling through these pathways. Reduction of constitutive BCR/ABL-mediated activation of these pathways following imatinib treatment may result in compensatory up-regulation of GF-mediated physiologic signaling through the Ras/MAPK pathway. MAPK signaling may have different outcomes depending on amplitude and duration of signal, the activity of feedback mechanisms, and interactions with other signaling pathways. Enhanced GF-dependent MAPK signaling in imatinib-treated CML progenitors could contribute to incomplete inhibition of progenitor growth, but may not be sufficient to maintain the enhanced level of proliferation seen in untreated CML progenitors, which may require sustained activation of MAPK, PI-3K, and other signaling pathways by the BCR/ABL kinase. As discussed in the next paragraph, continued signaling through the PI-3K pathway may also contribute to maintenance of viability of imatinib-treated cells.
Inhibition of other pathways downstream of BCR/ABL may contribute to imatinib-mediated suppression of progenitor growth, including pathways downstream of Ras other than p42/44 MAPK.26 Other potential candidates include the PI-3K/AKT and Janus kinase–STAT (Jak-STAT) signaling pathways, as well as transcription factors such as c-Jun, c-myc, and nuclear factor–κB (NF-kB), which have been shown to be important for transformation of CML cell lines.1 Klejman et al38 reported that imatinib treatment inhibited PI-3K/AKT activity in CML BC cells and that combined imatinib and PI-3K treatment selectively targeted CML progenitor growth and spared normal marrow cells. In our study, imatinib treatment reduced AKT kinase activity in CD34+ cells from some CML CP and AP patients, but this effect was not consistent. Treatment with the PI-3K inhibitor LY2 significantly inhibited CML CFC growth. Addition of LY to imatinib resulted in increased suppression of CML progenitor growth and proliferation and increased apoptosis compared with imatinib alone. These results suggest that inhibition of PI-3K signaling is incomplete in imatinib-treated cells and that further inhibition of PI-3K signaling can enhance inhibition of progenitor growth. Our results indicating that LY also inhibited normal progenitor growth when used alone and in combination with imatinib are consistent with previous reports that PI-3K signaling plays an important role in GF-induced proliferation and survival of normal progenitors.54,55 Differences between these results and those of Klejman et al could be related to the different cell populations studied or the specific experimental conditions.
Our studies support the exploration of combined inhibition of BCR/ABL TK activity and MAPK signaling as a strategy to enhance therapeutic targeting of CML progenitors. PD184352, a novel, highly potent, and selective inhibitor of the MEK kinase, which is bioavailable orally, has been recently shown to suppress the growth of colon tumors in vivo without causing overt toxicity.56 Resistance to imatinib in CML is often related to evolution of point mutations in the ABL kinase domain.37,57 Although certain kinase domain mutations are completely insensitive to imatinib, several mutations retain intermediate levels of sensitivity to inhibition by imatinib.58 For mutations demonstrating residual imatinib sensitivity, combination of imatinib and additional agents could be of therapeutic benefit,59 and combined use of imatinib and MAP kinase inhibitors could be considered. Since MEK inhibitors block the growth of both normal and CML progenitors, toxicity to normal cells may be an important consideration.
In conclusion, our results indicate that suppression of primary CML progenitor growth by imatinib is associated with enhanced MAPK signaling that is GF dependent. Increased MAPK activity is of functional significance, and combined suppression of BCR/ABL TK activity and MAPK signaling can enhance inhibition of CML progenitor growth.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-04-1271.
Supported in part by grants R01 CA95684 (National Institutes of Health) (R.B.); Translational Research Grant 6468 (Leukemia and Lymphoma Society) (R.B.); RPG-99-202-01-LBC (American Cancer Society) (R.B.); and GCRC grant 5MOIRR0043 (National Institutes of Health). R.B. is a Clinical Scholar of the Leukemia and Lymphoma Society.
S.C. and M.H. contributed equally to this paper.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Helen Xu and Tinisha McDonald for their excellent technical assistance with sample preparation and progenitor selection. We would also like to thank Allen Lin, CRA; Allison Sano, RN; Debra Vasquez, RN; and the physicians and staff in the Division of Hematology/BMT for assistance with obtaining patient samples.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal