Abstract
Immunoglobulin E (IgE) bound to multivalent antigen (Ag) elicits mast cell degranulation but not survival; on the contrary, IgE in the absence of Ag (IgE(-Ag)) induces survival only but not degranulation. Although these distinct responses are mediated through the same receptor, FcϵRI, the molecular mechanism generating the divergence is largely unknown. We recently showed that the signals through FcRγ chain are essential for IgE(-Ag)–induced mast cell survival as well as IgE(+Ag)–induced degranulation. To determine whether the cellular output is regulated by the quantity of FcRγ signal, we expressed CD8/FcRγ chimeras (CD8/γ) in bone marrow–derived mast cells (BMMCs) from FcRγ-/- mice to manipulate the strength of FcRγ signals by anti-CD8 cross-linking. Cross-linking of CD8/γ induced mast cell survival and degranulation. Survival was induced by weaker stimulation than needed for degranulation in terms of anti-CD8 concentration and the valency of chimera. However, sustained extracellular signal-regulated kinase (Erk) activation seems to regulate survival even when the activation signal was strong enough to elicit degranulation. Generation of sustained Erk activation by active mitogen-activated protein kinase kinase (MEK) induced BMMC survival. These results suggest that the duration and the magnitude of FcRγ signals may determine mast cell survival and degranulation, respectively. (Blood. 2004;103:3093-3101)
Introduction
Immunoglobulin E (IgE) triggers antiparasitic immunity or allergic responses via binding to FcϵRI on the surface of mast cells and basophils.1 Rodent FcϵRI is expressed as a tetramer composed of specific α, β (FcϵRIα, FcϵRIβ), and common γ (FcRγ) homodimers shared with FcγRI or FcγRIII.2 The α chain mediates binding to IgE. The β and γ chains possess immunoreceptor tyrosine-based activation motifs (ITAMs)3 within their cytoplasmic domains. Cross-linking of FcϵRI with IgE and multivalent antigen (IgE(+Ag)) initiates an activation signal cascade via tyrosine phosphorylation of these ITAMs by Lyn. Syk is then recruited to the phospho-ITAMs of FcϵRIγ, where it is activated to phosphorylate various substrates in the downstream cascade, which leads to degranulation or cytokine production.1,4 It is recently reported that IgE in the absence of Ag (IgE(-Ag)) actively promotes mast cell survival in addition to passive sensitization.5,6 IgE(+Ag) can evoke degranulation but not survival, whereas IgE(-Ag) induces survival but not degranulation.6 Although both these responses are mediated through FcϵRI, the molecular mechanism underlying it remains to be fully elucidated.6,7 We have recently demonstrated that ITAM in the FcRγ chain is essential for IgE(-Ag)–induced mast cell survival as well as degranulation and cytokine production evoked by IgE(+Ag).50 This suggests that distinct cellular responses like mediator release and survival are triggered by a similar signaling mechanism, in an FcRγ ITAM-dependent manner. One possible hypothesis for the distinct responses mediated by the same receptor is that the strength of FcRγ signals determines the type of response similar to the situation observed during immature T- or B-cell development.8-12 Extracellular signal-regulated kinase (Erk), a pleiotropic mitogen-activated protein (MAP) kinase, is one of the possible molecules that transmit different nature of ligand-receptor interaction into appropriate cellular output.8,13 Sustained Erk activation is reported to induce cell survival in many types of cells.14 In mast cells, it has been reported that IgE(-Ag) elicits more prolonged Erk activation than IgE(+Ag).6
IgE(-Ag)–induced Erk activation is abrogated by the disruption of the lipid raft structure.6 Lyn is exclusively localized in these rafts. Although FcϵRI is virtually absent in rafts of resting cells,15 it translocates into rafts upon IgE(+Ag) stimulation.15,16 In T cells, constitutive localization of pre–T-cell receptor (pre-TCR) within the raft is suggested to confer survival signals during early thymic development.17 It is therefore possible that IgE(-Ag) may also induce the prolonged association of FcϵRI with rafts to deliver survival signals.
In this report, the correlation between FcRγ signal strength or duration and mast cell responses was investigated using FcRγ chimera with CD8, a coreceptor expressed in T cell, in bone marrow–derived mast cells (BMMCs).
Materials and methods
Mice
C57BL/6 mice were obtained from Japan SLC (Hamamatsu, Japan). The establishment and characterization of FcRγ-/- mice have been described previously.18 Lyn-/- mice were kindly provided by T. Yamamoto (University of Tokyo). All mice were maintained within a filter-air laminar flow enclosure and provided with standard laboratory food and water ad libitum. All animal experiments were performed in accordance with Helsinki protocol and Chiba University guidelines.
Antibodies
Mouse antidinitrophenol (anti-DNP) IgE (H1 DNP-ϵ-26) was kindly provided by F. Liu (La Jolla Institute, Ontario, CA). H1 DNP-ϵ-26 was centrifuged at 100 000g for 10 minutes before use to remove possible aggregated complex contaminants. 53.6.7, anti-CD8α, was purchased from eBioscience (San Diego, CA). Anti-CD8α Fab was prepared by papain digestion. Briefly, 200 μg anti-CD8 was digested with 0.02 mg/mL papain in 1 mL digestion buffer (0.02 M EDTA [ethylenediaminetetraacetic acid] and 0.02 M l-cysteine) at 37°C for 4 hours. After the reaction was stopped with 3 mM iodoacetamide, digested Fc portion or undigested whole antibody (Ab) was removed with protein A–Sepharose (30 μL × 3) and protein G–Sepharose (30 μL × 1). After dialysis against phosphate-buffered saline (PBS), the purity and titer of anti-CD8α Fab were confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) (more than 95%) and cell surface staining, respectively. Polyclonal rabbit antimouse CD8α was kindly provided by R. Zamoyska (National Institute for Medical Research [NIMR], London, England) for immunoblotting. Fluorescein isothiocyanate (FITC)–conjugated antimouse IgE monoclonal antibody (mAb) (R35-72) and phycoerythrin (PE)–conjugated antimouse c-kit mAb (2B8) were purchased from BD Pharmingen (San Diego, CA) and eBioscience, respectively. Anti–phospho-Erk, anti–phospho–Jun N-terminal kinase (anti–phospho-JNK), and anti–phospho-p38 Abs were purchased from Promega (Madison, WI). Antiphospho-Akt (Ser473) was from Cell Signaling Technology (Beverly, MA). Anti-FcRγ Ab was from UBI (Lake Placid, NY). Anti-Lyn was kindly provided by T. Yamamoto (University of Tokyo, Tokyo, Japan). Goat antirat Ig was purchased from Cappel (West Chester, PA).
Construction
To construct the CD8/γ chimera, the cytoplasmic domain of murine FcRγ (amino acids [aa's] 45 to 86) was fused to the extracellular and transmembrane domain of murine CD8α (aa's 1 to 217) by polymerase chain reaction (PCR). For mutant CD8(CS)/γ, C151(TGC) and C166(TGT) of CD8 were mutated to S(TCC) and S(TCT), respectively. For the LAT/γ chimera, the cytoplasmic domain of FcRγ (aa's 45 to 86) was fused to the truncated LAT (aa's 1 to 36). Fragments encoding these chimeras were cloned into the retroviral vector, pMX or pMX-IRES-GFP (kindly provided by T. Kitamura, University of Tokyo).19 MEK.WT and MEK.ΔSESE were kindly provided by E. Nishida (Kyoto University, Kyoto, Japan).
Retroviral infection and BMMC induction
Retrovirus transfections were performed as previously described.20 BM cells were obtained from femurs and tibias of 10- to 15-week-old FcRγ-/-, wild-type, or Lyn-/- mice, and 5 × 105 cells were suspended in RPMI supplemented with 10% fetal calf serum (FCS) and 10% supernatant of X-63–interleukin-3 (X-63–IL-3) cells as a source of IL-3 (kindly provided by H. Karasuyama, Tokyo Medical and Dental University, Tokyo, Japan).21 For retrovirus infection, 10-fold concentrated virus supernatant was added to cell cultures at days 0, 1, and 2. After 6 days, CD8+ cells were sorted by MACS (Miltenyi Biotec, Bergisch Gladbach, Germany) and cultured in the same medium. Adherent cells were removed every 3 to 5 days. After 4 weeks of culture, more than 95% of the cells were c-kit+ and CD8+.
Degranulation assays
For antigen cross-linking, mast cells were sensitized with 1 μg/mL mouse anti-DNP IgE at 37°C for 4 hours and then with the indicated amount of dinitrophenol–human serum albumin (DNP-HSA). Degranulation assays were performed as previously described.22 Briefly, 5 × 104 cells per 100 μL mast cells were treated with various amounts of anti-CD8 or IgE for 30 minutes in Tyrode buffer (130 mM NaCl, 5 mM KCl, 1.4 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], and 0.1% bovine serum albumin [BSA], pH 7.4) in 96-well plates (Corning Costar, Cambridge, MA). Supernatants and cell lysates were assayed for β-hexosaminidase activity. The percentage of specific β-hexosaminidase release was calculated as [supernatant activity/(supernatant activity + cell lysate activity)] × 100. Prostaglandin D2 (PGD2) in supernatant was measured by enzyme immunoassay (EIA) (Cayman Chemical, Ann Arbor, MI). For long-term degranulation, mast cells were cultured in RPMI containing 10% FCS. At indicated times, supernatant was harvested and histamine concentration was measured by Histamine enzyme-linked immunosorbent assay (ELISA) kit (MBL, Nagoya, Japan).
Survival assays
A total of 5 × 104 mast cells were cultured in 200 μL IL-3–free medium with various treatments on flat-bottom 96-well plates (Corning Costar). At indicated days, cells were stained with propidium iodide (PI), and live cells (PI-negative cells) were counted by FACSCalibur (BD Bioscience, San Jose, CA).
Internalization assay
CD8 chimera–expressing BMMCs were treated with anti-CD8 at 37°C for indicated periods. For secondary cross-linking, cells were incubated with anti-CD8 on ice for 30 minutes, washed twice, and treated with goat antirat Ig at 37°C. Surface expression was assessed by staining with PE-conjugated antirat Ig κ light chain (MRK-1; BD Pharmingen). Data were expressed by percentage of surface expression when mean fluorescence intensity (MFI) of 0 minutes was set at 100%.
Stimulation with immobilized IgE(+Ag)
A 24-well plate (FALCON, Lincoln Park, NJ) was coated with 30 μg/mL DNP-HSA at 37°C for 2 hours. After washing 3 times with PBS, 10 μg/mL IgE was added and incubated at 37°C for 1 hour. After washing 3 times with PBS, BMMCs were inoculated on the coated well at a concentration of 1 × 106/mL and centrifuged at 400g (1500 rpm) for 2 minutes, followed by incubation at 37°C for indicated periods.
Ca2+ mobilization
BMMCs (1 × 107/mL) were incubated with 10 μM indo-1-AM (Molecular Probes, Eugene, OR) in the presence of F127 and 0.2% FCS at 37°C for 30 minutes. Cells were washed twice and resuspended in 500 μL Hanks balanced salt solution (HBSS) containing 1% FCS and stimulated with anti-CD8. For antigen stimulation, normal BMMCs were sensitized with 1 μg/mL IgE overnight before antigen stimulation. Ca2+ mobilization was analyzed in a BD-LSR (BD Bioscience) equipped with UV lasers. Ratio metric analysis of Ca2+-bound indo-1 (FL5)/Ca2+-free indo-1 (FL4) was performed using CellQuest (BD Bioscience) and FlowJo (Tree Star, San Carlos, CA) software programs.
Western blot analysis
For phospho-Erk analysis, BMMCs were cultured in the absence of IL-3 for 2 hours followed by stimulation with indicated conditions and lysed in 1% Nonidet P-40 (NP-40) lysis buffer as previously described.23 For the precipitation of Ab-bound chimera, cell lysates were incubated with protein G–Sepharose at 4°C for 1 hour.
Results
CD8/FcRγ chimera–induced degranulation, cytokine production, and cell survival in mast cells
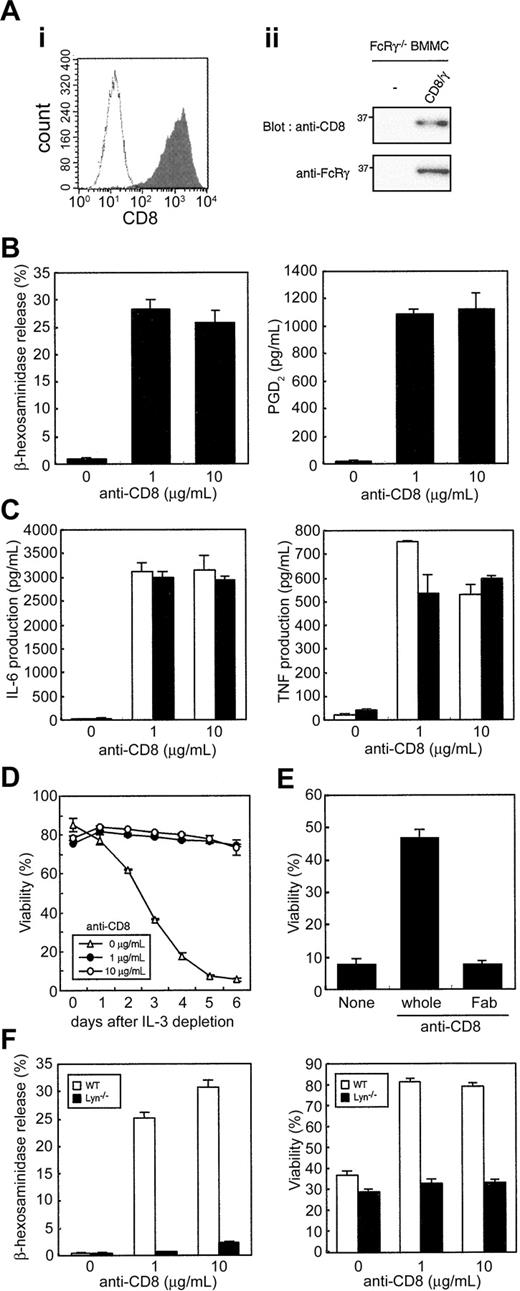
Initially, we investigated whether the strength of FcRγ signals determined the outcome of mast cell responses. For this purpose, we established CD8/FcRγ chimera (CD8/γ)–expressing BMMCs so that the strength of FcRγ signaling could be manipulated by cross-linking with various concentrations of anti-CD8. To avoid the possible involvement of endogenous FcRγ chain, FcRγ-/- BM cells were used for retrovirus-mediated gene transfer. Similar chimeras have been used in the analysis of cell lines but not in normal mast cells.24-26 When CD8-expressing cells were sorted and cultured for 4 weeks in the presence of IL-3, approximately 95% of the cells expressed CD8 and c-kit, but not FcϵRI on the cell surface, as expected (Figure 1Ai; and data not shown). The expression of the entire chimeric molecule was confirmed by immunoblotting with anti-CD8 and anti-FcRγ (Figure 1Aii).
CD8/γ-induced degranulation, cytokine production, and survival in FcRγ-deficient BMMCs. (A) Establishment of FcRγ-deficient BMMCs expressing CD8/γ. Bone marrow cells were infected with CD8/γ and cultured as described in “Materials and methods.” (i) BMMCs were stained with FITC-labeled anti-CD8 and analyzed by FACSCalibur (gray shaded histogram). The open histogram indicates uninfected control cells. (ii) Total cell lysates were blotted with polyclonal anti-CD8 (top) and anti-FcRγ (bottom). (B) Degranulation and prostaglandin synthesis upon CD8/γ cross-linking. FcRγ-deficient BMMCs expressing the CD8/γ chimera were stimulated with indicated amount of soluble anti-CD8 for 30 minutes, and β-hex release (left) and PGD2 production (right) were measured. (C) Cytokine production upon CD8/γ cross-linking. Cells were stimulated as described for panel B for 4 hours (□) and 24 hours (▪). Production of IL-6 (left) and TNF (right) was determined by ELISA. (D) Survival induction upon CD8/γ cross-linking. Cells were stimulated with the indicated amount of anti-CD8 in the absence of IL-3. Viability was assessed by propidium iodide (PI) staining. (E) Comparison of divalent versus monovalent anti-CD8 on survival induction. CD8/γ-expressing cells were left untreated or treated with 3 μg/mL whole anti-CD8 or anti-CD8 Fab in the absence of IL-3. After 4 days of treatment, viability was determined as described. (F) Degranulation and survival in Lyn-deficient BMMCs expressing CD8/γ. WT BMMCs or Lyn-deficient BMMCs expressing CD8/γ were established as described in “Materials and Methods.” β-hex release (left) and survival after IL-3 depletion for 4 days (right) were determined. Data were means ± SDs of triplicate assays.
CD8/γ-induced degranulation, cytokine production, and survival in FcRγ-deficient BMMCs. (A) Establishment of FcRγ-deficient BMMCs expressing CD8/γ. Bone marrow cells were infected with CD8/γ and cultured as described in “Materials and methods.” (i) BMMCs were stained with FITC-labeled anti-CD8 and analyzed by FACSCalibur (gray shaded histogram). The open histogram indicates uninfected control cells. (ii) Total cell lysates were blotted with polyclonal anti-CD8 (top) and anti-FcRγ (bottom). (B) Degranulation and prostaglandin synthesis upon CD8/γ cross-linking. FcRγ-deficient BMMCs expressing the CD8/γ chimera were stimulated with indicated amount of soluble anti-CD8 for 30 minutes, and β-hex release (left) and PGD2 production (right) were measured. (C) Cytokine production upon CD8/γ cross-linking. Cells were stimulated as described for panel B for 4 hours (□) and 24 hours (▪). Production of IL-6 (left) and TNF (right) was determined by ELISA. (D) Survival induction upon CD8/γ cross-linking. Cells were stimulated with the indicated amount of anti-CD8 in the absence of IL-3. Viability was assessed by propidium iodide (PI) staining. (E) Comparison of divalent versus monovalent anti-CD8 on survival induction. CD8/γ-expressing cells were left untreated or treated with 3 μg/mL whole anti-CD8 or anti-CD8 Fab in the absence of IL-3. After 4 days of treatment, viability was determined as described. (F) Degranulation and survival in Lyn-deficient BMMCs expressing CD8/γ. WT BMMCs or Lyn-deficient BMMCs expressing CD8/γ were established as described in “Materials and Methods.” β-hex release (left) and survival after IL-3 depletion for 4 days (right) were determined. Data were means ± SDs of triplicate assays.
Next, we examined whether CD8/γ engagement induced BMMC activation. Upon cross-linking CD8/γ with soluble anti-CD8, β-hexosaminidase (β-hex) secretion as an indicator for degranulation, (Figure 1B, left), PGD2 secretion as a representative of prostaglandin (PG) synthesis (Figure 1B, right), and cytokine production such as IL-6 and tumor necrosis factor (TNF) (Figure 1C) were measured. The activation levels of CD8/γ-stimulated mast cells, as assessed using these criteria, were equivalent to those exhibited by wild-type BMMCs stimulated with IgE and Ag (IgE(+Ag)) (data not shown; and Sakurai et al).50 We also investigated whether stimulation with anti-CD8 induced BMMC survival in the absence of any exogenous growth factors such as IL-3. Anti-CD8 treatment clearly enhanced survival of FcRγ-/- BMMCs expressing CD8/γ (Figure 1D). Because even low concentrations of soluble anti-CD8 mAb induced mast cell survival, we examined whether cross-linking with monovalent mAb is sufficient for survival. We compared survival induced by monovalent versus divalent anti-CD8. As shown in Figure 1E, anti-CD8 Fab did not evoke mast cell survival, suggesting that cross-linking of the chimera by a divalent Ab is required to induce survival. Degranulation and survival by CD8/γ was abrogated in both Lyn-/- BMMCs (Figure 1F) and in wild-type BMMCs expressing truncated CD8 lacking the cytoplasmic region containing the ITAM (data not shown). This suggested that phosphorylation of ITAM within the cytoplasmic domain of CD8/γ triggered survival and degranulation. These results confirm that cross-linking of chimeric FcRγ is sufficient for induction of degranulation, PG synthesis, and cytokine production in BMMCs lacking endogenous FcRγ. Furthermore, our data demonstrate for the first time that FcRγ engagement is sufficient for the induction of BMMC survival.
Distinct signal thresholds for the induction of mast cell survival and degranulation
Next, we examined the correlation between signal strength through CD8/γ and various cellular responses, particularly degranulation and cell survival. CD8/γ-expressing BMMCs were stimulated by cross-linking with various concentrations of soluble anti-CD8. Concentrations as low as 0.01 μg/mL anti-CD8 mAb induced a significant level of mast cell survival in CD8/γ-expressing BMMCs. The dose of anti-CD8 inducing half maximum response (EC50) for survival was 0.036 μg/mL (Figure 2A).
Distinct threshold for the induction of survival and degranulation. (A) Dose-response curve based on the concentration of input anti-CD8. FcRγ-deficient BMMCs expressing CD8/γ were stimulated with various amounts of anti-CD8 mAb, and β-hex release (•) and cell viability (○) were measured at 30 minutes and 4 days, respectively. Responses were expressed as percentage of each maximum response. Data were means ± SDs of triplicate assays. Similar results were obtained from 4 independent experiments. (B) Dose-response curve based on the percentage of bound anti-CD8. The same cells were treated with the indicated amounts of anti-CD8, followed by staining with PE-labeled antirat Ig κ light chain (inset). Background-subtracted mean fluorescence intensity (MFI) for each concentration is shown. The responses shown in panel A are expressed based on the percentage of bound anti-CD8.
Distinct threshold for the induction of survival and degranulation. (A) Dose-response curve based on the concentration of input anti-CD8. FcRγ-deficient BMMCs expressing CD8/γ were stimulated with various amounts of anti-CD8 mAb, and β-hex release (•) and cell viability (○) were measured at 30 minutes and 4 days, respectively. Responses were expressed as percentage of each maximum response. Data were means ± SDs of triplicate assays. Similar results were obtained from 4 independent experiments. (B) Dose-response curve based on the percentage of bound anti-CD8. The same cells were treated with the indicated amounts of anti-CD8, followed by staining with PE-labeled antirat Ig κ light chain (inset). Background-subtracted mean fluorescence intensity (MFI) for each concentration is shown. The responses shown in panel A are expressed based on the percentage of bound anti-CD8.
The concentration of anti-CD8 mAb required for degranulation as measured by β-hex release was much higher (EC50 = 0.26 μg/mL) than that needed for cell survival. Degranulation was only induced by concentrations higher than 0.1 μg/mL anti-CD8. This was confirmed by analyzing histamine release, another known indicator of degranulation. The high stimulation threshold required for degranulation was not simply due to the short stimulation period because anti-CD8–induced histamine release after 24 hours of stimulation also shows similar dose responses (data not shown). Figure 2B (inset) shows the correlation between the input concentration of anti-CD8 and surface-bound anti-CD8 as assessed by mean fluorescence intensity (MFI) using antirat Ig staining. When each cellular response was plotted as the percentage of the engaged CD8/γ, the difference in the signaling thresholds required for mast cell survival and degranulation became even more prominent (Figure 2B).
Sustained Erk activation correlates with mast cell survival
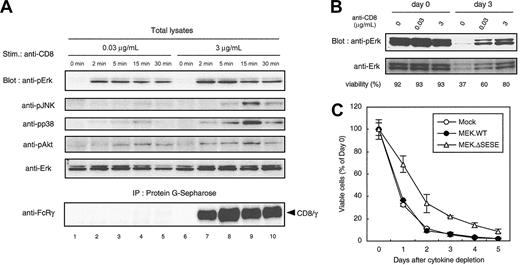
To investigate differences in cellular signaling events associated with survival and degranulation, active forms of MAP kinases (MAPKs) were analyzed using antiphospho-MAPK mAbs. CD8/γ-expressing cells were stimulated with either 0.03 μg/mL anti-CD8 mAb, which increased survival but did not induce degranulation, or with 3 μg/mL anti-CD8 that induced both survival and degranulation. The amount of anti-CD8 mAb bound to CD8/γ reached a plateau within 2 minutes after the treatment with 3 μg/mL anti-CD8 (Figure 3A, lower panel, lanes 7-10). In contrast, CD8/γ could hardly be detected by 0.03 μg/mL anti-CD8 (Figure 3A, lower panel, lanes 2-5). Nevertheless, comparable levels of sustained Erk activation were observed with both 0.03 μg/mL and 3 μg/mL mAbs. Activation of prosurvival kinase Akt, assessed by phoshphorylation at Ser473, was also induced by both concentrations of anti-CD8. In contrast, the phosphorylation levels of JNK and p38 upon stimulation with 0.03 μg/mL anti-CD8 mAb were much lower compared with the levels when 3 μg/mL was used (Figure 3A). Erk activation was sustained for days in survival-inducing condition (Figure 3B).
Sustained Erk activation in survival-inducing condition. (A) Phosphorylation of MAPKs upon anti-CD8 stimulation. FcRγ-deficient BMMCs expressing CD8/γ were incubated without IL-3 for 2 hours at 37°C followed by treatment with 0.03 μg/mL or 3 μg/mL anti-CD8 for the indicated periods. Total cell lysates were blotted with Abs for phosho-Erk (anti-pErk), phospho-JNK (anti-pJNK), phosho-p38 (anti-pp38), phosho-Akt (anti-pAkt), and Erk (Erk). Lysates were also precipitated with protein G–Sepharose, and the amount of anti-CD8–bound CD8/γ was determined by immunoblot with anti–FcRγ (bottom panel). (B) Erk activation is sustained for days. A total of 2 × 105 CD8/γ-bearing BMMCs were cultured in the presence of the indicated amounts of anti-CD8. Cells were harvested at day 0 (immediately after IL-3 depletion) and day 3. Total lysates were blotted with anti-pErk and anti-Erk Abs. Viability of each cell is shown below. (C) Effect of active MEK on BMMC survival. Mature BMMCs from normal B6 mice were infected with pMX-IRES-GFP vector alone (Mock; ○), wild-type MEK (MEK.WT; •), or constitutive active MEK (MEK.ΔSESE; ▵) with 100 ng/mL stem cell factor (SCF) and 10 ng/mL IL-3. Forty-eight hours after infection, cells were cultured in cytokine-free medium for 5 days. Viable GFP-positive cells were determined by flow cytometry and expressed as percentage of day 0. Data were means ± SDs of triplicate assays. Similar results were obtained from 5 independent experiments.
Sustained Erk activation in survival-inducing condition. (A) Phosphorylation of MAPKs upon anti-CD8 stimulation. FcRγ-deficient BMMCs expressing CD8/γ were incubated without IL-3 for 2 hours at 37°C followed by treatment with 0.03 μg/mL or 3 μg/mL anti-CD8 for the indicated periods. Total cell lysates were blotted with Abs for phosho-Erk (anti-pErk), phospho-JNK (anti-pJNK), phosho-p38 (anti-pp38), phosho-Akt (anti-pAkt), and Erk (Erk). Lysates were also precipitated with protein G–Sepharose, and the amount of anti-CD8–bound CD8/γ was determined by immunoblot with anti–FcRγ (bottom panel). (B) Erk activation is sustained for days. A total of 2 × 105 CD8/γ-bearing BMMCs were cultured in the presence of the indicated amounts of anti-CD8. Cells were harvested at day 0 (immediately after IL-3 depletion) and day 3. Total lysates were blotted with anti-pErk and anti-Erk Abs. Viability of each cell is shown below. (C) Effect of active MEK on BMMC survival. Mature BMMCs from normal B6 mice were infected with pMX-IRES-GFP vector alone (Mock; ○), wild-type MEK (MEK.WT; •), or constitutive active MEK (MEK.ΔSESE; ▵) with 100 ng/mL stem cell factor (SCF) and 10 ng/mL IL-3. Forty-eight hours after infection, cells were cultured in cytokine-free medium for 5 days. Viable GFP-positive cells were determined by flow cytometry and expressed as percentage of day 0. Data were means ± SDs of triplicate assays. Similar results were obtained from 5 independent experiments.
Our data demonstrating sustained Erk activation is associated with survival are consistent with a previous report.6 To test whether sustained Erk activation is sufficient for the survival, the active form of MAPK kinase (MEK), a direct activator of Erk, was introduced into BMMCs. Wild-type MEK (MEK.WT) showed no enhancement of survival over the vector alone (Mock). In contrast, the active form of MEK (MEK.ΔSESE) significantly, albeit moderately, enhanced survival (Figure 3C). This suggests that constitutive activation of Erk is capable of inducing survival in normal BMMCs. To confirm the correlation between sustained Erk activation and survival, BMMC survival was analyzed during transient Erk activation by further cross-linking of anti-CD8 mAb with a secondary Ab. Erk phosphorylation was induced more strongly by secondary cross-linking than anti-CD8 alone. The level reached a peak at 2 minutes and rapidly decreased, whereas anti-CD8 alone induced a sustained level of Erk phosphorylation (Figure 4A). Secondary cross-linking also induced rapid internalization of CD8/γ from the surface (Figure 4B), with similar kinetics to Erk phosphorylation. In terms of survival, viability of BMMCs cross-linked with anti-CD8 plus second Ab was significantly lower compared with those treated with anti-CD8 alone (Figure 4C). These results suggest that sustained but not transient Erk activation correlates with cell survival.
Prolonged but not weak signals induced survival. (A) Kinetics of Erk phosphorylation after secondary cross-linking of CD8/γ. (i) FcRγ-deficient BMMCs expressing CD8/γ were cultured in the absence of IL-3 for 2 hours and stimulated with 3 μg/mL anti-CD8 or anti-CD8 plus goat antirat Ig for the indicated times. Total cell lysates were blotted with anti-phospho-Erk (upper panels) or anti-Erk (lower panels) to verify equal loading of proteins. (ii) The intensity of each band was quantified using LAS-1000 (Fuji, Tokyo, Japan) and expressed as arbitrary units. (B) Down-regulation of surface CD8/γ by secondary cross-linking. Cells were treated with anti-CD8 only or anti-CD8 plus goat antirat Ig Abs as described for panel A. At the indicated times, CD8/γ surface expression was assessed as described in “Materials and methods.” (C) Survival of CD8/γ-bearing BMMCs by secondary cross-linking. Cells were treated with anti-CD8 only or anti-CD8 plus anti–rat Ig Abs. Cell viability was determined similarly to Figure 1D. (D) Kinetics of Erk phosphorylation after stimulation with immobilized IgE(+Ag) in normal BMMCs. IL-3–starved FcRγ+/+ BMMCs were stimulated either with 30 ng/mL DNP-HSA after one hour of sensitization (IgE(+Ag)), with plate-coated IgE(+Ag) as indicated in “Materials and methods” (immobilized IgE(+Ag)), or with 10 μg/mL soluble IgE (IgE(-Ag)). Erk phoshphorylation was determined similarly to panel A. (E-F) Degranulation and survival of normal BMMCs. BMMCs were stimulated with plate-coated Ag, plate-coated IgE(+Ag), or IgE similarly to panel D. After 4 days, histamine concentration in culture supernatant (E) and cell viability (F) were determined by ELISA and FACSCalibur, respectively. Data were means ± SDs of triplicate assays.
Prolonged but not weak signals induced survival. (A) Kinetics of Erk phosphorylation after secondary cross-linking of CD8/γ. (i) FcRγ-deficient BMMCs expressing CD8/γ were cultured in the absence of IL-3 for 2 hours and stimulated with 3 μg/mL anti-CD8 or anti-CD8 plus goat antirat Ig for the indicated times. Total cell lysates were blotted with anti-phospho-Erk (upper panels) or anti-Erk (lower panels) to verify equal loading of proteins. (ii) The intensity of each band was quantified using LAS-1000 (Fuji, Tokyo, Japan) and expressed as arbitrary units. (B) Down-regulation of surface CD8/γ by secondary cross-linking. Cells were treated with anti-CD8 only or anti-CD8 plus goat antirat Ig Abs as described for panel A. At the indicated times, CD8/γ surface expression was assessed as described in “Materials and methods.” (C) Survival of CD8/γ-bearing BMMCs by secondary cross-linking. Cells were treated with anti-CD8 only or anti-CD8 plus anti–rat Ig Abs. Cell viability was determined similarly to Figure 1D. (D) Kinetics of Erk phosphorylation after stimulation with immobilized IgE(+Ag) in normal BMMCs. IL-3–starved FcRγ+/+ BMMCs were stimulated either with 30 ng/mL DNP-HSA after one hour of sensitization (IgE(+Ag)), with plate-coated IgE(+Ag) as indicated in “Materials and methods” (immobilized IgE(+Ag)), or with 10 μg/mL soluble IgE (IgE(-Ag)). Erk phoshphorylation was determined similarly to panel A. (E-F) Degranulation and survival of normal BMMCs. BMMCs were stimulated with plate-coated Ag, plate-coated IgE(+Ag), or IgE similarly to panel D. After 4 days, histamine concentration in culture supernatant (E) and cell viability (F) were determined by ELISA and FACSCalibur, respectively. Data were means ± SDs of triplicate assays.
Although strong cross-linking with IgE(+Ag) does not significantly induce survival,5 high anti-CD8 concentrations could still induce substantial BMMC survival (Figure 2A). These results raise the possibility that failure to induce BMMC survival by IgE(+Ag) was due to the limited duration of signaling rather than excessive signaling. To test this, we utilized an approach that enabled signal duration to be manipulated. IgE(+Ag) induces FcϵRI internalization, which may terminate prolonged activation,27 whereas IgE(+immobilized Ag) does not cause receptor internalization.28,29 This implies that immobilized Ag can transduce both strong and prolonged signals simultaneously. Indeed, the plate-coated DNP-HSA plus anti-DNP IgE induced strong and sustained Erk activation (Figure 4D). Furthermore, Figure 4E shows that IgE(+immobilized Ag) but not IgE(-Ag) elicited significant amounts of histamine release, indicating that a strong signal sufficient for degranulation was delivered. Nevertheless, survival was also promoted upon stimulation with IgE(+immobilized Ag) in a manner similar to IgE(-Ag) (Figure 4F). These data suggest that even strong FcRγ signals can induce mast cell survival as long as Erk activation is sufficiently sustained.
Because Ca2+ mobilization is one of the key events associated with FcϵRI triggering via IgE(+Ag), we analyzed the relationship between Ca2+ mobilization and mast cell survival. Any immediate increases in intracellular Ca2+ concentrations were examined by stimulation of indo-1–labeled CD8/γ-expressing mast cells with graded concentrations of anti-CD8 mAb. Survival and degranulation were also measured. Immediate Ca2+ mobilization was principally correlated with anti-CD8 mAb concentration. Upon stimulation with 0.01 μg/mL mAb, Ca2+ mobilization was hardly detected (Figure 5A), but substantial cell survival was induced (Figure 2). Similarly, when FcϵRI on normal BMMCs was stimulated, IgE(-Ag) did not induce Ca2+ mobilization in our assay, whereas IgE(+Ag) did (Figure 5B). These results suggest that the transient increase in [Ca2+]i seems to be highly correlated with degranulation rather than survival signals.
Increased [Ca2+]i correlated with mast cell degranulation rather than survival. (A) FcRγ-deficient BMMCs expressing CD8/γ were loaded with indo-1 and stimulated with graded amounts of anti-CD8. The increase of [Ca2+]i was determined and expressed as described in “Materials and methods.” Bars on the right side represent cell survival (white bar) and β-hex release (black bar) at the same mAb concentrations. (B) Normal BMMCs were also stimulated with 10 μg/mL anti-DNP IgE (top graph) or a graded amount of DNP-HSA after sensitization with 1 μg/mL anti-DNP IgE for 16 hours at 37°C.
Increased [Ca2+]i correlated with mast cell degranulation rather than survival. (A) FcRγ-deficient BMMCs expressing CD8/γ were loaded with indo-1 and stimulated with graded amounts of anti-CD8. The increase of [Ca2+]i was determined and expressed as described in “Materials and methods.” Bars on the right side represent cell survival (white bar) and β-hex release (black bar) at the same mAb concentrations. (B) Normal BMMCs were also stimulated with 10 μg/mL anti-DNP IgE (top graph) or a graded amount of DNP-HSA after sensitization with 1 μg/mL anti-DNP IgE for 16 hours at 37°C.
FcRγ localization to lipid rafts is not sufficient to promote survival
A recent report suggested that disruption of lipid rafts abrogates IgE(-Ag)–mediated signal transduction.6 To investigate whether constitutive localization of FcRγ chain to lipid rafts triggers downstream survival signals, the intracellular domain of the FcRγ chain was fused to the N-terminal 36 amino acids of linker of activated T cells (LAT) containing palmitoylation sites that target proteins to lipid rafts (Figure 6A). Density gradient centrifugation of FcRγ-/- BMMCs expressing chimeric LAT/γ showed that LAT/γ was predominantly localized in the lipid raft fraction as expected (Figure 6B, middle). In addition, the chimera LAT(CA)/γ, which contains the mutation C27/30A in the N-terminal of LAT that abolishes raft localization,30,31 was also constructed (Figure 6B). The expression of each chimera, LAT/γ and LAT(CA)/γ, was comparable with that of CD8/γ and higher than endogenous FcRγ in WT BMMCs as determined by an anti-FcRγ blot (data not shown). In addition, FcRγ-/- BMMCs infected with pMX-IRES-GFP vector only (Mock), LAT/γ, and LAT(CA)/γ all developed normally in terms of c-kit expression (data not shown). The survival advantage of chimera-expressing population was compared by culturing bulk BMMCs infected with vector only, LAT/γ, and LAT(CA)/γ in the absence of IL-3 and examining the percentage of viable cells at indicated days by chasing the bicistronic expression of GFP. As shown in Figure 6C, there was no difference in the survival rates of GFP-positive (infected) and GFP-negative (uninfected internal control) populations in each line. Taken together, this suggests that raft localization of FcRγ is not sufficient to augment survival and that further cross-linking within the raft may be required to trigger survival signals.
Constitutive raft localization is not sufficient for BMMC survival. (A) Schematic representation of LAT/γ and LAT(CA)/γ chimera. (B) Subcellular localization of LAT/γ. FcRγ-deficient BMMCs expressing pMX-IRES-GFP vector only (Mock), LAT/γ, or LAT(CA)/γ were subjected to density gradient centrifugation as previously described. Each fraction was blotted with anti-FcRγ (left) and anti-Lyn (right) as a control. (C) Cell survival after IL-3 depletion. pMX-IRES-GFP (Mock)–, LAT/γ-, and LAT(CA)/γ-infected bulk BMMCs were cultured in the absence of IL-3, and the percentage of viable cells at the indicated days was examined. The percentages of bicistronic GFP-positive population were around 30% in each line. Data were expressed as the percentage of viable cell numbers at day 0 for the GFP-negative (uninfected internal control) and GFP-positive (infected) gated population.
Constitutive raft localization is not sufficient for BMMC survival. (A) Schematic representation of LAT/γ and LAT(CA)/γ chimera. (B) Subcellular localization of LAT/γ. FcRγ-deficient BMMCs expressing pMX-IRES-GFP vector only (Mock), LAT/γ, or LAT(CA)/γ were subjected to density gradient centrifugation as previously described. Each fraction was blotted with anti-FcRγ (left) and anti-Lyn (right) as a control. (C) Cell survival after IL-3 depletion. pMX-IRES-GFP (Mock)–, LAT/γ-, and LAT(CA)/γ-infected bulk BMMCs were cultured in the absence of IL-3, and the percentage of viable cells at the indicated days was examined. The percentages of bicistronic GFP-positive population were around 30% in each line. Data were expressed as the percentage of viable cell numbers at day 0 for the GFP-negative (uninfected internal control) and GFP-positive (infected) gated population.
Degranulation required higher valency of cross-linking than cell survival
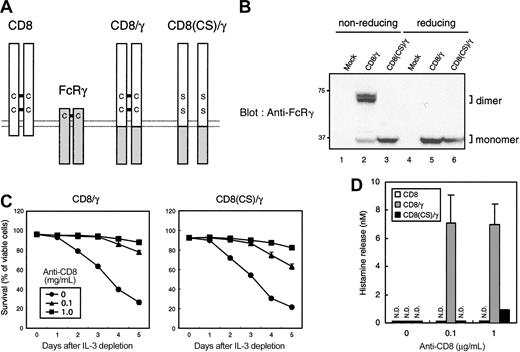
We adopted another mutagenesis approach to investigate the effect of valency of the chimera on the induction of mast cell survival and degranulation. Because CD8α dimerizes via a disulfide linkage between extracellular domains,32 each CD8/γ chimera is presumably expressed as a homodimer on the cell surface (Figure 7A). Indeed, most of CD8/γ was detected as a dimer in nonreducing SDS-PAGE and a monomer in reducing conditions (Figure 7B, lanes 2 and 5). To compare the signaling capacity of monomeric versus dimeric CD8/γ, 2 cysteine residues involved in the interchain disulfide linkage were mutated to serine (CD8(CS)/γ). CD8(CS)/γ was expressed as a monomer even under a nonreducing condition (Figure 7B, lane 3). This was also confirmed by an anti-CD8 blot (data not shown). Surface staining with anti-CD8 mAb showed no difference in the fluorescence intensity between CD8/γ- and CD8(CS)/γ-expressing cells (data not shown), indicating that this mutation impaired only valency of the chimera without changing surface expression or anti-CD8 affinity.
Monomeric CD8/γ failed to induce degranulation but not survival upon anti-CD8 treatment. (A) Schematic representation of CD8/γ and CD8(CS)/γ. (B) Lack of dimer formation in CD8(CS)/γ. The 2B4 T-cell hybridomas were infected with vector alone (Mock), CD8/γ, or CD8(CS)/γ. Cells were lysed and analyzed by SDS-PAGE in the presence (reducing) and absence (nonreducing) of 2.5 μM 2-mercaptoethanol in the sample buffer. The membrane was blotted with anti-FcRγ. (C) Cell survival after IL-3 depletion. BMMCs expressing truncated CD8 (CD8), CD8/γ, and CD8(CS)/γ were sorted and cultured in the presence of different concentrations of anti-CD8 after IL-3 depletion. Percentage of viable cells at the indicated days is shown. (D) Histamine release upon anti-CD8 stimulation. Each cell line was stimulated using the same conditions as for panel C for 30 minutes. Histamine release into the culture supernatant was determined by ELISA. N.D. indicates not detected. Data were means ± SDs of triplicate assays.
Monomeric CD8/γ failed to induce degranulation but not survival upon anti-CD8 treatment. (A) Schematic representation of CD8/γ and CD8(CS)/γ. (B) Lack of dimer formation in CD8(CS)/γ. The 2B4 T-cell hybridomas were infected with vector alone (Mock), CD8/γ, or CD8(CS)/γ. Cells were lysed and analyzed by SDS-PAGE in the presence (reducing) and absence (nonreducing) of 2.5 μM 2-mercaptoethanol in the sample buffer. The membrane was blotted with anti-FcRγ. (C) Cell survival after IL-3 depletion. BMMCs expressing truncated CD8 (CD8), CD8/γ, and CD8(CS)/γ were sorted and cultured in the presence of different concentrations of anti-CD8 after IL-3 depletion. Percentage of viable cells at the indicated days is shown. (D) Histamine release upon anti-CD8 stimulation. Each cell line was stimulated using the same conditions as for panel C for 30 minutes. Histamine release into the culture supernatant was determined by ELISA. N.D. indicates not detected. Data were means ± SDs of triplicate assays.
We then compared the mast cell function upon anti-CD8 stimulation of CD8/γ- and CD8(CS)/γ-expressing cells. Mast cell survival in CD8(CS)/γ-expressing BMMCs was comparable to CD8/γ-expressing BMMCs when anti-CD8 was added at concentrations of 0.1 μg/mL and 1 μg/mL. In contrast, CD8/γ-expressing BMMCs, but not CD8(CS)/γ-expressing BMMCs, induced substantial levels of degranulation (Figure 7D) in response to anti-CD8 stimulation. Again, this result suggests that the signaling threshold for mast cell survival is lower than that required for degranulation.
Discussion
We have recently demonstrated that BMMCs from FcRγ-/- mice reconstituted with ITAM-mutated FcRγ fail to induce survival, cytokine production, and degranulation.50 This indicates that FcRγ plays a central role in both IgE(-Ag)– and IgE(+Ag)–dependent responses. How these distinct cellular responses are regulated through the same receptor subunit in the same cellular context is not clear. We hypothesized that the strength of the FcRγ signals determined the outcome of the response. To verify it we established CD8/FcRγ-expressing BMMCs to stimulate FcRγ directly in a qualitative manner. Cross-linking of FcRγ at a low valency was sufficient to induce mast cell survival, whereas strong cross-linking was required for degranulation. Cross-linking was required for survival because anti-CD8 Fab could not induce survival. This is consistent with the model that cell surface aggregation of FcϵRI is required for IgE(-Ag)–induced survival although the nature of IgE cross-linking in the absence of Ag is not yet clear.33 Requirements of Lyn (Figure 1F) and the cytoplasmic region of FcRγ (Figure 7D) for the CD8/γ chimera to function suggested that phosphorylation of the ITAMs was important. Notably, IgE(+Ag)–mediated degranulation is not impaired in Lyn-/- BMMCs.34 The differential requirement of Lyn in degranulation through FcϵRI and CD8/γ may be explained by the distinct contribution of FcϵRIβ, which is phosphorylated by Lyn and binds SH2 domain–containing 5′ inositol phosphatase (SHIP) in vitro.35 These results demonstrate that the FcRγ signal is necessary and sufficient for the induction of various mast cell responses and also suggest that the quantity of the signals can control the types of responses induced. Notably, the types of responses induced cannot be attributed to different sensitivities of the assay system utilized because EC50 values were essentially constant regardless of the sensitivity of the assay. Our data also confirm that survival signals were triggered by FcϵRI subunits rather than other IgE-binding molecules such as galectin-3, consistent with the previous report.5
Mast cell survival induced by IgE(-Ag) is associated with simultaneous FcϵRI up-regulation,36 but whether this up-regulation is required for mast cell survival has not been investigated. FcϵRI up-regulation is FcRγ-ITAM independent50 but FcϵRIα dependent.37,38 Consistently, we showed surface expression of CD8/γ was not significantly up-regulated upon anti-CD8 treatment despite the fact that mast cell survival was enhanced (Figure 4B). Thus, mast cell survival can occur in the absence of receptor up-regulation.
The involvement of lipid rafts in the IgE(+Ag) response via FcϵRI is well documented.16 Kalesnikoff et al reported that disruption of raft structure also abrogated IgE(-Ag)–induced Erk activation.6 In T cells, constitutive localization of pre-TCR within rafts is thought to trigger survival signals in early thymocyte development.17 It is possible that IgE(-Ag)–induced FcϵRI association with lipid raft confers survival signals without aggregation. However, we found forced expression of FcRγ within lipid rafts did not promote BMMC survival by itself, suggesting that a certain type of receptor cross-linking is required to promote mast cell survival after the recruitment of FcRγ to the lipid raft.39
At first sight it seems contradictory that we found high concentrations of anti-CD8 mAb still induced mast cell survival as well as degranulation because previous work shows that massive cross-linking of FcϵRI by IgE(+Ag) does not trigger survival signals.5 This may reflect the difference in systems between the present CD8/γ system and the physiologic IgE-FcϵRI system. The strength and duration of FcϵRI signals are mutually exclusive in physiological conditions because IgE plus multivalent Ag induces strong activation signals as well as receptor internalization simultaneously, and the latter leads to rapid desensitization to Ag.27 However, our in vitro studies using CD8 chimeras indicated that sustained FcRγ signals regulated mast cell survival even when the activation signal was strong enough to elicit degranulation (Figure 4). Although the difference of the dose-response curve between survival and degranulation was significant, it was smaller than expected. This indicates that distinct responses can be induced by slight alterations of the FcRγ signal. Under physiological conditions, the full signaling capacity of FcϵRI can be achieved by cooperation between the β and γ chains. Although the role of β chain as an amplifier of FcϵRI signal is well characterized,40-42 it also associates with SHIP in vitro,35 which is known to function as a negative regulator. The presence of the β chain may confer the FcϵRI complex with the additional ability to draw a strict boundary between survival and degranulation. Indeed, because substantial degranulation was observed upon IgE(-Ag) stimulation in SHIP-deficient BMMCs, β chain–associated SHIP may play a role in avoiding unnecessary (if any harmful) degranulation upon IgE(-Ag) stimulation.33
We and others have shown that antigen receptor signal strength or duration or both determines the fate of immature T and B cells.9,43 Recently, Gonzalez-Espinosa et al reported that strength of FcϵRI signals determines the cytokine profile produced by mast cells.44 Thus, it could be that a general property of Ag receptors via downstream signal transduction machinery is to convert quantitative or durational input into qualitative output. Consistently, Torigoe et al showed that various FcϵRI ligand avidity strengths could elicit distinct responses by recruiting different downstream signaling molecules,45 just like agonistic or partial agonistic peptides during T-cell receptor (TCR) signaling.46 Currently, intracellular molecular mechanisms that convert “strength” and “duration” into distinct responses are not known. The “kinetic proofreading model” is widely accepted for several Ag receptors including FcϵRI.45 In this model, downstream signals depend not only on the concentration but also on the lifetime of the “activated” receptor. However, it is difficult to explain the distinct outcome by IgE(-Ag) and IgE(+Ag) stimulation using this model.47 Our observation that even lower Ag concentrations were unable to induce survival (data not shown) suggests that “a sustained signal” cannot be simply proofread by weak transient signals. The precise molecular mechanism that senses “sustained” activation is unknown. One possible explanation is that such signals are translated into sustained Erk signals that can induce cell survival in many types of cells.13,14 Two antiapoptotic molecules belonging to the bcl-2 family, bcl-xL and A1, are involved in mast cell survival.6,48 Activation of prosurvival kinase Akt was also observed in survival-inducing condition (Figure 3A), suggesting the Akt-induced bcl-xL activation pathway may contribute to the survival. The correlation between sustained Erk activation and the expression of bcl-2 family proteins needs to be clarified. Although forced prolonged Erk activation could bypass partial survival (Figure 3C), whether such sustained signals directly induce survival or not is still unclear. Recent reports have shown that weak FcϵRI signals favor the production of certain cytokines or chemokines, which could be one of the candidates responsible for the survival described here.6,44,47
The involvement of Ca2+ signals in degranulation is well documented.1 Consistently, our data show that Ca2+ mobilization correlates well with degranulation. On the other hand, survival appeared to be independent of transient Ca2+ mobilization. However, we cannot exclude the possibility that undetectable Ca2+ levels were triggered in IgE(-Ag)–stimulated mast cells, because Huber et al reported that anti-DNP IgE could induce slight Ca2+ influx even in a wild-type BMMC.33 Although the precise molecular mechanism is still unclear, our system would provide an efficient tool for analyzing downstream effector molecules that direct these divergent responses. The conclusion that prolonged cross-linking of FcRγ is sufficient to induce mast cell survival may support the implication that the high-affinity Fc receptor for IgG associated with FcRγ can also regulate the survival of FcγR-expressing cells such as phagocytes.49 Furthermore, the present study could lead to the development of therapeutic strategies that specifically inhibit certain allergic responses mediated by FcϵRI on mast cells.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-08-2944.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs K. Horikawa and T. Yamamoto for Lyn-deficient mice and reagents; Drs S. Hiraoka, D. Sakurai, and H. Arase for discussion; Ms. M. Sakuma, R. Shiina, and M. Unno for technical help; and H. Yamaguchi and Y. Kurihara for secretarial help.



![Figure 5. Increased [Ca2+]i correlated with mast cell degranulation rather than survival. (A) FcRγ-deficient BMMCs expressing CD8/γ were loaded with indo-1 and stimulated with graded amounts of anti-CD8. The increase of [Ca2+]i was determined and expressed as described in “Materials and methods.” Bars on the right side represent cell survival (white bar) and β-hex release (black bar) at the same mAb concentrations. (B) Normal BMMCs were also stimulated with 10 μg/mL anti-DNP IgE (top graph) or a graded amount of DNP-HSA after sensitization with 1 μg/mL anti-DNP IgE for 16 hours at 37°C.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/8/10.1182_blood-2003-08-2944/6/m_zh80080459910005.jpeg?Expires=1763575716&Signature=04Y3TXJhFZ2SL56T2x90ZoCG0Bn2vJvckCqmGy~-PwyghWovfVeF6NagPCs8OyQuAGIhXoNeCI5wo9dbqIJmcYLKeKmxPx~kJoUcz8qAQmSeyBKkofxLq4V7IPniAV9sg2WhYPGcYL~Np8YyC0LQjfWxVQCb8ZDJNfwl4DTt7RzqaUayX2UDKWwBcj2hGGY051NnWNXtey6OHXrWFDC4jCN5wugWilsC~3EruT~NVQj0UlR67yShFAY41hej0WHLwD-8mccUtpkELhRfXNyNuHEK6SZMJKpz8p6W0G-9~gcciZCXS0fapqlP1TsRmy9lh14N~P87kwGmvENjdHeSDQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal