Abstract
Whole-blood assays (WBAs) have been successfully used as a simple tool for immuno-epidemiological field studies evaluating cellular immune responses to mycobacterial and viral antigens. Rather unexpectedly, we found very poor cytokine responses to malaria antigens in WBAs in 2 immuno-epidemiological studies carried out in malaria endemic populations in Africa. We have therefore conducted a detailed comparison of cellular immune responses to live (intact) and lysed malaria-infected erythrocytes in WBAs and in peripheral blood mononuclear cell (PBMC) cultures. We observed profound inhibition of both proliferative and interferon-γ responses to malarial antigens in WBAs as compared with PBMC cultures. This inhibition was seen only for malaria antigens and could not be overcome by increasing either antigen concentration or responder cell numbers. Inhibition was mediated by intact erythrocytes and occurred early in the culture period, suggesting that failure of antigen uptake might underlie the lack of T-cell responses. In support of this hypothesis, we have shown that intact uninfected erythrocytes specifically inhibit phagocytosis of infected red blood cells by peripheral blood monocytes. We propose that specific biochemical interactions with uninfected erythrocytes inhibit the phagocytosis of malaria-infected erythrocytes and that this may impede T-cell recognition in vivo. (Blood. 2004; 103:3084-3092)
Introduction
Naturally acquired immunity to erythrocytic stages of malaria parasites is multifactorial, involving both antibody- and cell-mediated effector mechanisms and targeting a diverse array of antigens (recently reviewed in Artavanis-Tsakonas et al1 ). In general, protective antibodies recognize determinants exposed on the surface of merozoites or parasite-derived molecules that are inserted into the outer membrane of the infected red blood cell (iRBC). Cellular immune responses, on the other hand, may be induced by either surface-expressed or internal antigens, following phagocytosis of the iRBC and subsequent processing and presentation of malarial peptides to T lymphocytes. Once activated, these T lymphocytes can provide B-cell help, activate macrophages to phagocytose and kill iRBC, and possibly mediate direct killing of infected cells. However, not all such immune responses are beneficial. Many antimalarial antibodies are not associated with protection, and the activation of T cells may promote pathological levels of inflammation in the nonimmune host (reviewed in Miller et al2 ). Differentiation of protective immune responses and their antigenic targets from irrelevant or pathogenic responses is thus a major challenge for vaccine development (reviewed in Good3 ). Extensive immuno-epidemiological studies are required to unravel the relationship between in vitro cellular responses to blood stage antigens and acquired immunity.
Cellular responses to malarial antigens have classically been assayed using in vitro restimulation of separated peripheral blood mononuclear cells (PBMCs). However, PBMC separation is time consuming, expensive, and requires relatively large blood samples and thus imposes major logistic constraints on epidemiological studies, especially in children. Using whole-blood assays (WBAs) instead of PBMCs is faster, cheaper, and requires smaller blood volumes. Over the last 30 years, the WBA has been successfully used to evaluate cellular responses to mitogens,4-8 bacterial,9 mycobacterial,10-15 parasite,16,17 and viral antigens,18,19 but in 2 separate field studies we have found that cytokine responses to malaria antigens in WBAs were unexpectedly low.20,21 We therefore conducted a detailed comparison of cellular immune responses to live (intact) and lysed malaria-infected erythrocytes in WBAs and in peripheral blood mononuclear cell (PBMC) cultures to determine the suitability of WBAs for the study of T-cell responses to malaria antigens. Much to our surprise, we have observed that proliferative and interferon-γ (IFN-γ) responses to blood stage malarial antigens were markedly inhibited in WBAs when compared with responses obtained from separated PBMCs. As intraerythrocytic malaria parasites are confined to the blood circulation, uptake and presentation of malaria antigens to the immune system take place predominantly in the spleen rather than the lymph nodes, and the WBAs may thus be a closer approximation of the in vivo situation than PBMC assays. We therefore considered it relevant to determine which components of whole blood interfere with T-cell activation, and we have attempted to elucidate the mechanism of this inhibition.
Patients, materials, and methods
Subjects
Seventeen healthy adult volunteers were recruited at the London School of Hygiene and Tropical Medicine (LSHTM). Age, sex, ethnic origin, and previous exposure to malaria were documented. Blood groups were determined using AB and D grouping sera (Lome Laboratories, Twyford, United Kingdom). Details of all donors are shown in Table 1. Ethical approval for this study was obtained from the ethical review committee of the London School of Hygiene and Tropical Medicine. Informed consent was obtained from all participants in the study in accordance with the Declaration of Helsinki.
Characteristics of blood donors
Donor . | Age, y . | Sex . | Blood group . | Ethnicity . | Previous malaria exposure . |
|---|---|---|---|---|---|
| 1 | 43 | M | A pos | African | Resident of urban Ghana, regular attacks in childhood, some slide confirmed |
| 2 | 31 | F | O pos | Middle Eastern | None |
| 3 | 50 | M | B neg | North African | Childhood in Sudan, several malaria attacks; living in Europe for last 10 years |
| 4 | 25 | F | O pos | European | None |
| 5 | 33 | F | O neg | European | None |
| 6 | 38 | F | B pos | European | Has traveled to Africa on prophylaxis; one unconfirmed attack, 15 years ago* |
| 7 | 24 | F | O pos | European | None |
| 8 | 31 | F | A pos | European | Has traveled to Kenya on prophylaxis |
| 9 | 33 | M | O pos | European | Lived in Cote d'lvoire for 3 years on prophylaxis, one (unconfirmed) attack 4 years ago |
| 10 | 59 | M | O pos | European | Traveled to Africa, Asia, and South America on prophylaxis |
| 11 | 46 | F | A pos | European | Traveled to Africa and Asia on prophylaxis |
| 12 | 37 | F | A pos | European | Traveled to Asia and South America without prophylaxis, no attacks |
| 13 | 30 | F | B pos | Asian | Childhood in Nigeria and Middle East, regular travel to India on prophylaxis |
| 14 | 29 | F | O pos | European | Traveled to Ghana on prophylaxis |
| 15 | 29 | M | A pos | European | Traveled to Mexico, Vietnam, and Cambodia on prophylaxis |
| 16 | 24 | F | AB pos | European | Worked in Malawi 2002, 2 confirmed malaria attacks |
| 17 | 51 | M | O pos | Asian | Childhood in highland Kenya, adult life in Europe, traveled on prophylaxis, no attacks |
Donor . | Age, y . | Sex . | Blood group . | Ethnicity . | Previous malaria exposure . |
|---|---|---|---|---|---|
| 1 | 43 | M | A pos | African | Resident of urban Ghana, regular attacks in childhood, some slide confirmed |
| 2 | 31 | F | O pos | Middle Eastern | None |
| 3 | 50 | M | B neg | North African | Childhood in Sudan, several malaria attacks; living in Europe for last 10 years |
| 4 | 25 | F | O pos | European | None |
| 5 | 33 | F | O neg | European | None |
| 6 | 38 | F | B pos | European | Has traveled to Africa on prophylaxis; one unconfirmed attack, 15 years ago* |
| 7 | 24 | F | O pos | European | None |
| 8 | 31 | F | A pos | European | Has traveled to Kenya on prophylaxis |
| 9 | 33 | M | O pos | European | Lived in Cote d'lvoire for 3 years on prophylaxis, one (unconfirmed) attack 4 years ago |
| 10 | 59 | M | O pos | European | Traveled to Africa, Asia, and South America on prophylaxis |
| 11 | 46 | F | A pos | European | Traveled to Africa and Asia on prophylaxis |
| 12 | 37 | F | A pos | European | Traveled to Asia and South America without prophylaxis, no attacks |
| 13 | 30 | F | B pos | Asian | Childhood in Nigeria and Middle East, regular travel to India on prophylaxis |
| 14 | 29 | F | O pos | European | Traveled to Ghana on prophylaxis |
| 15 | 29 | M | A pos | European | Traveled to Mexico, Vietnam, and Cambodia on prophylaxis |
| 16 | 24 | F | AB pos | European | Worked in Malawi 2002, 2 confirmed malaria attacks |
| 17 | 51 | M | O pos | Asian | Childhood in highland Kenya, adult life in Europe, traveled on prophylaxis, no attacks |
Where only travel to or work in an endemic area is mentioned, the donor has otherwise lived in malaria-free areas.
Whole-blood assay (WBA)
Venous blood was collected into sterile containers with preservative-free sodium heparin (CP Pharmaceuticals, Wrexham, United Kingdom) at 10 IU/mL of blood. The blood was diluted in RPMI 1640 containing 100 U/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine (all Gibco BRL, Paisley, United Kingdom) (RPMI-PSG) and aliquoted at 200 μL per well into sterile 96-well, round-bottomed tissue-culture plates (Nalge Nunc International, Hereford, United Kingdom). Antigens and controls were added to triplicate wells, and cultures were incubated at 37°C with 5% CO2 for periods of up to 7 days. Culture supernatants (150 μL per well) from triplicate wells were collected, pooled, and frozen at -80°C for cytokine analysis. Standard WBAs were conducted at a 1:10 dilution of whole blood; modified assays used a 1:3 dilution of whole blood.
Peripheral blood mononuclear cell (PBMC) assay
Heparinized venous blood was diluted with an equal volume of RPMI-PSG, layered onto Histopaque-1077 (Sigma, Dorset, United Kingdom), and centrifuged at 400g for 30 minutes. The PBMC layer was collected and washed twice with Hanks buffered saline solution (HBSS) (Gibco BRL). The cells were resuspended in RPMI-PSG with 10% autologous plasma at 106 cells/mL. These were plated in triplicate, at a volume of 200 μL per well, into sterile 96-well tissue-culture plates. Antigens were added and assays were completed exactly as described above for WBAs.
Preparation of platelets, granulocytes, erythrocytes, and erythrocyte lysate
Platelet-rich plasma was isolated from whole blood by centrifugation (250g, 10 minutes) as described elsewhere22 and diluted back to the original whole-blood volume in RPMI-PSG with 10% autologous plasma. Granulocytes were separated from whole blood by centrifugation over a double gradient of histopaque-1119 and histopaque-1077 (Sigma) for 30 minutes at 700g, washed 3 times, and resuspended in RPMI-PSG with 10% autologous plasma at 5 × 106 cells/mL, which represents the average concentration in whole blood. Whole blood, from which platelets were removed (as above), was centrifuged at 400g for 10 minutes and the buffy coat removed. The erythrocyte pellet was washed 3 times in cold isotonic phosphate buffered saline (PBS). Purified intact erythrocytes were resuspended at 0.5 (50%) hematocrit in RPMI-PSG with 10% autologous plasma. Erythrocyte lysate was obtained from this suspension by 2 rapid freeze-thawing cycles in liquid nitrogen and a 37°C water bath. Giemsa-stained slides of these preparations confirmed that they were free from contamination by other cell types; the erythrocyte lysate showed no intact cells and centrifugation at 700g did not result in a cell pellet, both confirming lysis of the erythrocytes.
Antigens and mitogens
Plasmodium falciparum (clone 3D7) was maintained in continuous culture at 37°C in 3% O2, 4% CO2, 93% N2 with fresh human A+ erythrocytes at 0.05 (5%) hematocrit and RPMI 1640 supplemented with 5.96 g/L HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Sigma), 2.325 g/L sodium hydrogen bicarbonate (AnalaR VWR, Poole, United Kingdom), 1 mg/L hypoxanthine (Gibco BRL), and 10% human AB serum (Sigma). Cultures were shown to be free from mycoplasma contamination by polymerase chain reaction (PCR) (Takara; BioWhittaker, Wokingham, United Kingdom). Infected red blood cells (iRBCs) at schizont stage were separated on a gradient of 60% Percoll (Sigma), 34% RPMI, and 6% 10 × PBS, washed and adjusted to required concentrations. Plasmodium falciparum schizont lysate (PfSL) was made by 2 rapid freeze-thaw cycles in liquid nitrogen and a 37°C water bath. A similar freeze-thaw preparation of uninfected red blood cells (uRBCs) was used as a control. Purified protein derivative of Mycobacterium tuberculosis (PPD) (Statens Serum Institute, Copenhagen, Denmark) and the mitogen phytohemagglutinin (PHA) (Sigma), both at 5 μg/mL final well concentration, were used as positive controls. Growth medium (GM; RPMI-PSG) was used as a negative control.
Lymphocyte proliferation assays
After removal of supernatants on day 6, 1 μCi (0.037 MBq) of [3H]-thymidine (Amersham, Amersham, United Kingdom) in 100 μL fresh RPMI-PSG was added to each well. Eighteen hours later the cells were harvested onto cellulose filters, and tritium incorporation was measured by liquid scintillation counting (Topcount; Canberra Packard, Meriden, CT).
Cytokine ELISA
IFN-γ was measured in the culture supernatants by sandwich enzyme-linked immunosorbent assay (ELISA) using commercially available antibodies and recombinant IFN-γ standard (Pharmingen BD, Oxford, United Kingdom). All samples were tested in duplicate. Samples with optical densities (ODs) above the highest point on the standard curve (2000 pg/mL) were retested at 1:10 and 1:100 dilutions. Where volumes of supernatant were insufficient for titration, values above 2000 pg/mL were based on extrapolation. The lower limit of detection (LLD) was defined as lowest point on the linear part of the standard curve. For each figure, curves of all contributing ELISA plates were reviewed, and the highest LLD was used as a conservative estimate of the overall LDD for that figure.
Phagocytosis assays
PBMCs in RPMI-PSG (106 cells/mL) were allowed to adhere in chamber slides (Nalge Nunc International) for 2 hours at 37°C with 5% CO2. Nonadherent cells were washed off with warm HBSS and discarded. RPMI-PSG with 10% autologous plasma was added, and the cells were incubated with 5 × 105/mL iRBCs or an equivalent number of 2.8 μM latex beads (Polysciences, Warrington, PA), and pre-incubated with autologous plasma in the presence or absence of uninfected erythrocytes at concentrations equivalent to a 1:10 dilution of whole blood. After 2 or 18 hours the supernatants were removed into labeled tubes. The wells were rinsed twice with warm HBSS to remove further nonadherent cells, which also were collected into the labeled tubes and pelleted by centrifugation. Erythrocytes on the chamber slides and in the pellets were lysed by osmosis with distilled water and the cell debris washed off. Nonadherent cells were transferred to glass slides by cytospin (Shandon, Pittsburgh, PA); all slides were dried, fixed with methanol, stained with Giemsa (Sigma), and visualized by light microscopy. Slides were also stained with O-diansidine HCL (Sigma) to look for erythrophagocytosis.
Results
IFN-γ and lymphoproliferative responses to parasite antigens are inhibited in WBAs
It has been repeatedly shown that cells from most adults with little or no previous malaria exposure respond to iRBCs and PfSL by IFN-γ production and lymphocyte proliferation.23-25 Both IFN-γ production and proliferation follow a classical recall antigen response pattern, peaking after 6 days of in vitro culture with the responding cells being CD45RO+ αβ TCR+ lymphocytes.23,25-28 To our surprise, whole blood assays gave positive IFN-γ responses to phytohemagglutinin (PHA) and PPD, but no IFN-γ response to either iRBCs or PfSL. Among the 17 donors for whom data are shown, cells from only 2 produced IFN-γ and then only at very low concentrations. These data are representative of more than 60 donors tested at various times (data not shown).
IFN-γ was assayed in culture supernatant by ELISA after 6 days and lymphocyte proliferation after 7 days (Figure 1). There was variation in the magnitude of the response between individual donors, but they all followed a similar pattern. As expected, in PBMC cultures, cells from all donors responded to PHA, PPD, iRBCs, and PfSL by IFN-γ production (Figure 1A) and lymphocyte proliferation (Figure 1B). Consistent with previous literature, cells from all donors responded to PHA and PPD in WBAs, although in all cases both IFN-γ and lymphoproliferative responses were somewhat reduced compared to PBMC assays.
IFN-γ production and lymphoproliferative responses to malaria antigens are inhibited in WBAs. PBMCs (106/mL) and 1:10 diluted whole-blood assays (WBAs) derived from single blood samples were incubated for 7 days with PHA (5 μg/mL), PPD (5 μg/mL), 5 × 105/mL iRBCs, 5 × 105/mL PfSL, 5 × 105/mL uninfected red blood cells (uRBCs), or growth medium only (GM). IFN-γ (A) was assayed by ELISA in 6-day supernatants, and lymphoproliferation (B) was assessed after 7 days. In each column, the filled symbols on the left represent values obtained from PBMC assays, and the open symbols on the right represent values obtained from WBAs; each point represents data from one donor. Two donors had previous exposure to malaria (donor nos. 1,  and ⋄; and 3, ▪ and □), and 3 donors were malaria nonexposed (donor nos. 7, • and ○; 4, *; and 5, ▴ and ▵).The lower limit of detection for the IFN-γ ELISA (125 pg/mL) (A) and the background level of [3H] incorporation (B) are indicated by dotted lines.
and ⋄; and 3, ▪ and □), and 3 donors were malaria nonexposed (donor nos. 7, • and ○; 4, *; and 5, ▴ and ▵).The lower limit of detection for the IFN-γ ELISA (125 pg/mL) (A) and the background level of [3H] incorporation (B) are indicated by dotted lines.
IFN-γ production and lymphoproliferative responses to malaria antigens are inhibited in WBAs. PBMCs (106/mL) and 1:10 diluted whole-blood assays (WBAs) derived from single blood samples were incubated for 7 days with PHA (5 μg/mL), PPD (5 μg/mL), 5 × 105/mL iRBCs, 5 × 105/mL PfSL, 5 × 105/mL uninfected red blood cells (uRBCs), or growth medium only (GM). IFN-γ (A) was assayed by ELISA in 6-day supernatants, and lymphoproliferation (B) was assessed after 7 days. In each column, the filled symbols on the left represent values obtained from PBMC assays, and the open symbols on the right represent values obtained from WBAs; each point represents data from one donor. Two donors had previous exposure to malaria (donor nos. 1,  and ⋄; and 3, ▪ and □), and 3 donors were malaria nonexposed (donor nos. 7, • and ○; 4, *; and 5, ▴ and ▵).The lower limit of detection for the IFN-γ ELISA (125 pg/mL) (A) and the background level of [3H] incorporation (B) are indicated by dotted lines.
and ⋄; and 3, ▪ and □), and 3 donors were malaria nonexposed (donor nos. 7, • and ○; 4, *; and 5, ▴ and ▵).The lower limit of detection for the IFN-γ ELISA (125 pg/mL) (A) and the background level of [3H] incorporation (B) are indicated by dotted lines.
By contrast, parasite antigen-induced responses were below the LLD of the assay (IFN-γ) or below background levels observed for control antigens (lymphoproliferation). The only exception was that cells from one donor with previous exposure to malaria (donor no. 3) gave a low positive IFN-γ response to iRBCs in the WBA. We concluded therefore that inhibition of cytokine production and lymphocyte proliferation in WBAs is specific for malaria antigens and is a universal finding in all donors.
Effect of parasite antigen dose
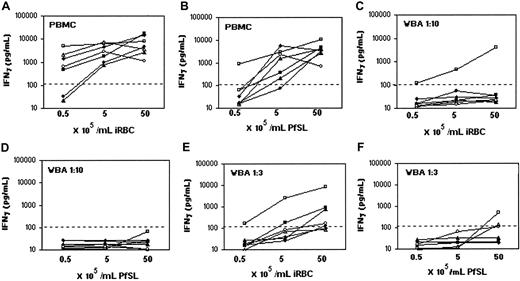
As antigens and mitogens were used at concentrations that had previously been found to be optimal for PBMC assays, we considered the possibility that the concentration of malaria antigens used was suboptimal for WBAs. We thus compared IFN-γ and proliferative responses to increasing concentrations of malaria antigens in parallel PBMC and WBA assays using cells from 7 donors with varying levels of previous malaria exposure. As in previous studies,23,25,29 the optimum concentration for IFN-γ responses to both iRBC and PfSL in PBMC assays was approximately 5 × 105 schizont-infected red blood cells per milliliter (Figure 2A and 2B, respectively). In the WBAs, cells from one previously exposed donor (donor no. 3) produced detectable levels of IFN-γ in response to iRBCs, but cells from none of the donors produced IFN-γ in response to PfSL at any of the concentrations used (Figure 2C-D). Proliferative responses as measured by [3H]-thymidine incorporation on day 7 showed an identical pattern to the IFN-γ responses (data not shown).
Effect of antigen concentration and dilution of whole blood on the IFN-γ response to malaria antigens. IFN-γ (pg/mL) was assayed in supernatants of cells from 3 malaria-exposed (donor nos. 1, ▿; 3, □; and 6, ○) and 4 malaria-nonexposed (donor nos. 2,  ; 4, ▪; 5, ▴; and 7, •) donors incubated for 6 days with 0.5, 5.0, or 50 × 105/mL iRBCs (A,C,E), or equivalent amounts of PfSL (B,D,F). PBMC assays (A-B), whole blood diluted 1:10 (C-D), and whole blood diluted 1:3 (E-F) from the same samples were compared. IFN-γ concentrations in control cultures with uninfected RBCs were always below the LDD of the assay (125 pg/mL) and are not shown. Each donor is represented by a different symbol. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL). We therefore made a direct side-by-side comparison between PBMC and WBA for 5 donors.
; 4, ▪; 5, ▴; and 7, •) donors incubated for 6 days with 0.5, 5.0, or 50 × 105/mL iRBCs (A,C,E), or equivalent amounts of PfSL (B,D,F). PBMC assays (A-B), whole blood diluted 1:10 (C-D), and whole blood diluted 1:3 (E-F) from the same samples were compared. IFN-γ concentrations in control cultures with uninfected RBCs were always below the LDD of the assay (125 pg/mL) and are not shown. Each donor is represented by a different symbol. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL). We therefore made a direct side-by-side comparison between PBMC and WBA for 5 donors.
Effect of antigen concentration and dilution of whole blood on the IFN-γ response to malaria antigens. IFN-γ (pg/mL) was assayed in supernatants of cells from 3 malaria-exposed (donor nos. 1, ▿; 3, □; and 6, ○) and 4 malaria-nonexposed (donor nos. 2,  ; 4, ▪; 5, ▴; and 7, •) donors incubated for 6 days with 0.5, 5.0, or 50 × 105/mL iRBCs (A,C,E), or equivalent amounts of PfSL (B,D,F). PBMC assays (A-B), whole blood diluted 1:10 (C-D), and whole blood diluted 1:3 (E-F) from the same samples were compared. IFN-γ concentrations in control cultures with uninfected RBCs were always below the LDD of the assay (125 pg/mL) and are not shown. Each donor is represented by a different symbol. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL). We therefore made a direct side-by-side comparison between PBMC and WBA for 5 donors.
; 4, ▪; 5, ▴; and 7, •) donors incubated for 6 days with 0.5, 5.0, or 50 × 105/mL iRBCs (A,C,E), or equivalent amounts of PfSL (B,D,F). PBMC assays (A-B), whole blood diluted 1:10 (C-D), and whole blood diluted 1:3 (E-F) from the same samples were compared. IFN-γ concentrations in control cultures with uninfected RBCs were always below the LDD of the assay (125 pg/mL) and are not shown. Each donor is represented by a different symbol. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL). We therefore made a direct side-by-side comparison between PBMC and WBA for 5 donors.
Effect of responder cell numbers
We considered that the number of lymphocytes per culture might be somewhat lower in whole blood diluted 1:10 than in the conventional PBMC assay (0.8-2.4 × 105 compared with 2 × 105 leukocytes per well, based on an average adult leukocyte count of 4-10 × 106/mL of whole blood) and that the number of responding T cells may thus have fallen below detectable levels in the WBAs. We compared the IFN-γ response to malaria antigens in whole blood diluted 1:3 with 1:10 WBA and PBMC assays (Figure 2E-F). The one donor whose cells responded in the 1:10 WBA (donor no.3) also responded in the 1:3 WBA, with a similar amount of IFN-γ being produced. Cells from 2 other donors, both malaria nonexposed (donor nos. 4 and 5), produced above background levels of IFN-γ in the 1:3 WBA, but only in response to iRBCs and only at the highest antigen concentration used. [3H]-thymidine incorporation could not be measured in 1:3 diluted whole blood assays, as erythrocyte debris blocked the harvester filters, as previously reported.10 However, taking this data together, we conclude that IFN-γ and proliferative responses to iRBCs and PfSL are consistently and profoundly suppressed in WBAs, irrespective of prior exposure to malaria, and that this is not simply due to suboptimal antigen concentration or responder cell numbers.
Uninfected erythrocytes, but not platelets or granulocytes, inhibit responses to malaria antigens
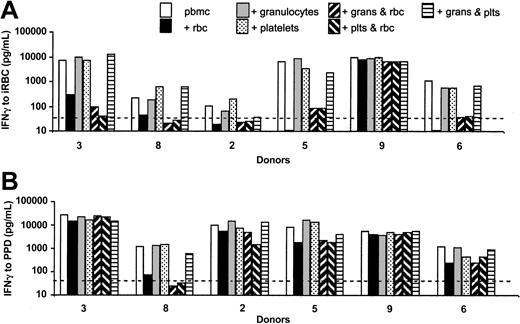
As both PBMCs and WBA 1:10 contain 10% autologous plasma, we reasoned that cellular, rather than humoral, components of whole blood must be responsible for the inhibition of T-cell responses to malaria antigens. We therefore made purified preparations of all the major cell types that are present in WBAs but not PBMCs, for instance, erythrocytes, granulocytes, and platelets, and added these—alone or in combination—to PBMC cultures (Figure 3).
Uninfected erythrocytes, but not platelets or granulocytes, inhibit IFN-γ responses to malaria antigens. IFN-γ (pg/mL) was assayed in supernatants of PBMCs cultured for 6 days with 5 × 105/mL iRBCs (A) or 5 μg/mL PPD (B) in the presence or absence of purified erythrocytes, purified granulocytes, or purified platelets (all at concentrations equivalent to 1:3 dilution of whole blood) alone or in combination. Responses to uRBCs and GM were below the LDD of the assay (not shown); the pattern of responses seen with PfSL was identical to that seen for iRBCs (data not shown). No IFN-γ or proliferation was detected in cultures of erythrocytes, granulocytes, or platelets alone (ie, without PBMCs) (not shown). Data from 6 donors are shown. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL).
Uninfected erythrocytes, but not platelets or granulocytes, inhibit IFN-γ responses to malaria antigens. IFN-γ (pg/mL) was assayed in supernatants of PBMCs cultured for 6 days with 5 × 105/mL iRBCs (A) or 5 μg/mL PPD (B) in the presence or absence of purified erythrocytes, purified granulocytes, or purified platelets (all at concentrations equivalent to 1:3 dilution of whole blood) alone or in combination. Responses to uRBCs and GM were below the LDD of the assay (not shown); the pattern of responses seen with PfSL was identical to that seen for iRBCs (data not shown). No IFN-γ or proliferation was detected in cultures of erythrocytes, granulocytes, or platelets alone (ie, without PBMCs) (not shown). Data from 6 donors are shown. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL).
The addition of platelets or granulocytes, at concentrations equivalent to 1:3 diluted whole blood, had no suppressive effect on IFN-γ responses to parasite antigens (Figure 3A) or PPD (Figure 3B). By contrast, the addition of purified erythrocytes to PBMC cultures had a strong suppressive effect on parasite responses in all except one donor (donor no. 9). In some donors, responses to PPD also were affected by the addition of erythrocytes (Figure 3B), but overall the reduction in IFN-γ production was much less marked than for iRBCs, and IFN-γ levels remained well above the LLD. Responses to PfSL followed the same pattern as responses to iRBCs (not shown), and proliferative responses to both parasite antigens and to PPD measured at day 7 followed the same pattern as the IFN-γ responses (not shown). We concluded therefore that uninfected erythrocytes, and not granulocytes or platelets, are responsible for the inhibition of the T-lymphocyte response to malaria antigens in whole blood.
Intact uninfected erythrocytes, but not erythrocyte lysates, inhibit IFN-γ responses to malaria antigens
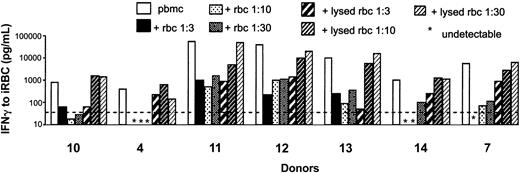
To determine whether intact erythrocytes or products that might be released by erythrocyte lysis were responsible for the observed inhibition, we added intact erythrocytes or equivalent amounts of erythrocyte lysate to PBMC cultures and assessed the effect on iRBCs, PfSL, and PPD-induced IFN-γ production by PBMCs. Intact erythrocytes at all concentrations markedly suppressed PBMC responses to iRBCs, whereas the erythrocyte lysates had much less inhibitory effect, showing suppression only at concentrations equivalent to a 1:3 dilution of whole blood (Figure 4). Responses to PfSL also were less suppressed by lysate than by intact erythrocytes and as anticipated neither intact nor lysed erythrocytes had a consistent impact on the PPD response (data not shown). We conclude that intact erythrocytes are required for the inhibition of responses to malaria antigens.
Intact uninfected erythrocytes, but not erythrocyte lysates, inhibit IFN-γ responses to malaria antigens. IFN-γ (pg/mL) was assayed in supernatants of PBMCs cultured for 6 days with 5 × 105/mL iRBCs in the presence or absence of intact or lysed uninfected erythrocytes (at decreasing concentrations equivalent to 1:3, 1:10, and 1:30 diluted whole blood, respectively). An identical pattern of responses was obtained for PBMCs stimulated with PfSL (data not shown). No IFN-γ was detected in control cultures containing only lysed or intact erythrocytes (ie, without PBMCs) (not shown). Data from 7 donors are shown. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL).
Intact uninfected erythrocytes, but not erythrocyte lysates, inhibit IFN-γ responses to malaria antigens. IFN-γ (pg/mL) was assayed in supernatants of PBMCs cultured for 6 days with 5 × 105/mL iRBCs in the presence or absence of intact or lysed uninfected erythrocytes (at decreasing concentrations equivalent to 1:3, 1:10, and 1:30 diluted whole blood, respectively). An identical pattern of responses was obtained for PBMCs stimulated with PfSL (data not shown). No IFN-γ was detected in control cultures containing only lysed or intact erythrocytes (ie, without PBMCs) (not shown). Data from 7 donors are shown. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL).
The interaction between uninfected erythrocytes and parasite antigens does not suppress IFN-γ responses to PPD
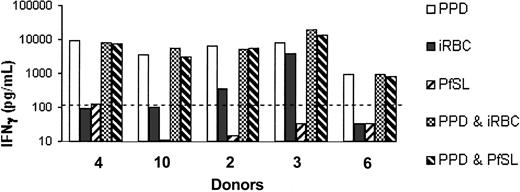
In an attempt to understand the mechanism by which uninfected red blood cells specifically inhibit responses to malaria antigens we tested whether the interaction between uninfected erythrocytes, malaria antigens, and PBMCs was able to suppress the response to an unrelated antigen, PPD (Figure 5). PBMCs were cultured with PPD or malaria antigens alone and with PPD plus iRBCs or PfSL in the presence of uninfected erythrocytes (at a concentration equivalent to 1:10 dilution of whole blood). As expected, responses to malaria antigens alone were suppressed in the presence of erythrocytes, but the PPD response was intact. Interestingly, addition of malaria antigens to the PPD cultures had no obvious inhibitory effect on the PPD response (Figure 5). This indicates that the inhibitory effects of uninfected red blood cells are antigen specific and do not affect the functioning of antigen-presenting cells or T cells per se.
The interaction between uninfected erythrocytes and parasite antigens does not suppress IFN-γ responses to PPD. IFN-γ (pg/mL) was assayed in 6-day supernatants of PBMCs cultured with uninfected red blood cells (equivalent to 1:10 dilution of whole blood) plus 5 μg/mL PPD; 5 × 105/mL iRBCs; 5 × 105/mL lysed schizonts (PfSL); PPD plus iRBCs; or PPD plus PfSL. Data from 5 donors are shown. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL).
The interaction between uninfected erythrocytes and parasite antigens does not suppress IFN-γ responses to PPD. IFN-γ (pg/mL) was assayed in 6-day supernatants of PBMCs cultured with uninfected red blood cells (equivalent to 1:10 dilution of whole blood) plus 5 μg/mL PPD; 5 × 105/mL iRBCs; 5 × 105/mL lysed schizonts (PfSL); PPD plus iRBCs; or PPD plus PfSL. Data from 5 donors are shown. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL).
Uninfected erythrocytes inhibit early stages of the response to malaria antigens
To determine the crucial time-point for inhibition of the response to malaria antigens, uninfected red cells were added to PBMC cultures at different times during incubation with iRBCs or PfSL. The addition of uninfected erythrocytes at time zero (ie, at the same time as the malaria antigens) or during the first 2 hours of incubation led to marked suppression of IFN-γ production. The addition of erythrocytes after 6 to 24 hours of culture had much less impact on the amount of IFN-γ detected at 6 days in PBMC cultures stimulated with either iRBCs (Figure 6) or PfSL (not shown).
Uninfected red cells inhibit early stages of the IFN-γ response to malaria antigens. IFN-γ (pg/mL) was assayed in supernatants of PBMCs cultured for 6 days with 5 × 105/mL iRBCs or 5 × 105/mL PfSL, PPD, or control antigens (not shown). PBMCs were cultured alone or with uninfected red blood cells (equivalent to 1:10 dilution) added to cultures immediately (time 0), after 30 minutes, or after 2, 6, 24, or 72 hours. Data from 5 donors are shown. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL).
Uninfected red cells inhibit early stages of the IFN-γ response to malaria antigens. IFN-γ (pg/mL) was assayed in supernatants of PBMCs cultured for 6 days with 5 × 105/mL iRBCs or 5 × 105/mL PfSL, PPD, or control antigens (not shown). PBMCs were cultured alone or with uninfected red blood cells (equivalent to 1:10 dilution) added to cultures immediately (time 0), after 30 minutes, or after 2, 6, 24, or 72 hours. Data from 5 donors are shown. The dotted line indicates the LLD for the IFN-γ ELISA (125 pg/mL).
Uninfected erythrocytes inhibit phagocytosis of iRBC and PfSL
Taking together the observations that suppression of parasite responses requires intact erythrocytes and occurs early in the immune response but leaves monocytes and T lymphocytes capable of responding to PPD, we reasoned that the most likely explanation was specific interference by uninfected erythrocytes with phagocytosis of parasitized erythrocytes and subsequent reduction in antigen processing and presentation. To test this hypothesis, PBMC-derived monocytes were allowed to adhere to chamber slides and subsequently cultured with iRBCs, PfSL, and GM in the presence or absence of uninfected erythrocytes. As a control, we compared the inhibitory effect of erythrocytes on phagocytosis of iRBCs with their effects on latex beads of similar size. To determine whether physical obstruction by uninfected erythrocytes was responsible for inhibition, we assessed whether phagocytosis of iRBCs or latex beads would be improved by shaking the cultures during incubation.
In the absence of erythrocytes, phagocytosis of whole schizonts, fragments of parasites, and malaria pigment were clearly observed in 50% to 80% of adherent monocytes after 2 hours (Figure 7C); similar levels of phagocytosis of latex beads were seen (Figure 7E; Table 2). After 18 hours, parasite pigment could still be found, but whole schizonts were less commonly recognizable in the adherent monocytes (not shown). By contrast, in the presence of erythrocytes, no phagocytosis of parasite material or latex beads was observed after 2 hours (Figure 7D,F) or 18 hours (not shown). We did not see any uptake of uninfected erythrocytes by monocytes, either with Giemsa or with O-diansidine HCL staining (not shown), indicating that competition between uninfected and infected erythrocytes as a result of erythrophagocytosis (which has been reported in samples from acute malaria cases30 ) is an unlikely explanation for reduced phagocytosis of iRBCs. Shaking the cultures only slightly improved uptake of both iRBCs and latex beads in the presence of erythrocytes, and only after 18 hours' incubation (Table 2).
Effects of uninfected erythrocytes on the phagocytosis of iRBCs and latex beads by adherent cells. PBMCs (106/mL) were allowed to adhere to chamber-well slides. Nonadherent cells were washed off, and the remaining adherent fraction was subsequently incubated in duplicates for 2 or 18 hours (3 donors each) with 5 × 105/mL iRBCs, PfSL (not shown), GM, or equivalent numbers of 2.8-μm latex beads, in the presence or absence of 1:10 diluted uninfected erythrocytes, Giemsa stained, and visualized by light microscopy. Representative images are shown of adherent monocytes (A) and their reduced numbers following addition of uninfected erythrocytes (B) (magnification, × 100 for both panels), phagocytosis of iRBCs by adherent monocytes in the absence (C) or presence (D) of uninfected erythrocytes, and phagocytosis of latex beads by adherent monocytes in the absence (E) or presence (F) of uninfected erythrocytes (magnification, × 1300; oil immersion for panels C-F). Arrows indicate iRBCs or latex beads.
Effects of uninfected erythrocytes on the phagocytosis of iRBCs and latex beads by adherent cells. PBMCs (106/mL) were allowed to adhere to chamber-well slides. Nonadherent cells were washed off, and the remaining adherent fraction was subsequently incubated in duplicates for 2 or 18 hours (3 donors each) with 5 × 105/mL iRBCs, PfSL (not shown), GM, or equivalent numbers of 2.8-μm latex beads, in the presence or absence of 1:10 diluted uninfected erythrocytes, Giemsa stained, and visualized by light microscopy. Representative images are shown of adherent monocytes (A) and their reduced numbers following addition of uninfected erythrocytes (B) (magnification, × 100 for both panels), phagocytosis of iRBCs by adherent monocytes in the absence (C) or presence (D) of uninfected erythrocytes, and phagocytosis of latex beads by adherent monocytes in the absence (E) or presence (F) of uninfected erythrocytes (magnification, × 1300; oil immersion for panels C-F). Arrows indicate iRBCs or latex beads.
Uninfected erythrocytes inhibit phagocytosis of iRBCs by peripheral blood monocytes
. | Percentage of monocytes containing phagocytosed iRBCs or latex beads . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | Adherent cells . | . | Detached monocytes . | . | |||
| Incubation time and donor . | Static . | Shaken . | Static . | Shaken . | |||
| 2 h incubation | |||||||
| Donor no. 9 | |||||||
| iRBCs without uRBCs | 14 | 23 | — | — | |||
| iRBCs with uRBCs | 1 | 0 | 4 | — | |||
| Beads without uRBCs | 39.5 | 15 | — | — | |||
| Beads with uRBCs | 0.5 | 2 | 36.7 | — | |||
| Donor no. 15 | |||||||
| iRBCs without uRBCs | 11 | 6 | — | — | |||
| iRBCs with uRBCs | 0 | 1 | 7 | 12.8 | |||
| Beads without uRBCs | 27.5 | 43 | — | — | |||
| Beads with uRBCs | 0 | 11 | 66 | 50 | |||
| Donor no. 16 | |||||||
| iRBCs without uRBCs | 23 | 6 | — | — | |||
| iRBCs with uRBCs | 0.5 | 5 | 4 | 5 | |||
| Beads without uRBCs | 66 | 44 | — | — | |||
| Beads with uRBCs | 1.5 | 13 | 36 | 34 | |||
| 18 h incubation | |||||||
| Donor no. 6 | |||||||
| iRBCs without uRBCs | 41 | 44.5 | — | — | |||
| iRBCs with uRBCs | 2 | 5 | 7 | NP | |||
| Beads without uRBCs | 57 | 20.5 | — | — | |||
| Beads with uRBCs | 1 | 10.5 | 80 | NP | |||
| Donor no. 17 | |||||||
| iRBCs without uRBCs | 33 | 39.5 | NP | — | |||
| iRBCs with uRBCs | 1.67 | 8 | — | NP | |||
| Beads without uRBCs | 36 | 38.5 | NP | — | |||
| Beads with uRBCs | 2.9 | 8.5 | 52 | NP | |||
| Donor no. 5 | |||||||
| iRBCs without uRBCs | 32.5 | 31 | — | — | |||
| iRBCs with uRBCs | — | 6 | 12 | NP | |||
| Beads without uRBCs | 28.9 | 47 | — | — | |||
| Beads with uRBCs | 6.3 | 13 | 52 | 50 | |||
. | Percentage of monocytes containing phagocytosed iRBCs or latex beads . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | Adherent cells . | . | Detached monocytes . | . | |||
| Incubation time and donor . | Static . | Shaken . | Static . | Shaken . | |||
| 2 h incubation | |||||||
| Donor no. 9 | |||||||
| iRBCs without uRBCs | 14 | 23 | — | — | |||
| iRBCs with uRBCs | 1 | 0 | 4 | — | |||
| Beads without uRBCs | 39.5 | 15 | — | — | |||
| Beads with uRBCs | 0.5 | 2 | 36.7 | — | |||
| Donor no. 15 | |||||||
| iRBCs without uRBCs | 11 | 6 | — | — | |||
| iRBCs with uRBCs | 0 | 1 | 7 | 12.8 | |||
| Beads without uRBCs | 27.5 | 43 | — | — | |||
| Beads with uRBCs | 0 | 11 | 66 | 50 | |||
| Donor no. 16 | |||||||
| iRBCs without uRBCs | 23 | 6 | — | — | |||
| iRBCs with uRBCs | 0.5 | 5 | 4 | 5 | |||
| Beads without uRBCs | 66 | 44 | — | — | |||
| Beads with uRBCs | 1.5 | 13 | 36 | 34 | |||
| 18 h incubation | |||||||
| Donor no. 6 | |||||||
| iRBCs without uRBCs | 41 | 44.5 | — | — | |||
| iRBCs with uRBCs | 2 | 5 | 7 | NP | |||
| Beads without uRBCs | 57 | 20.5 | — | — | |||
| Beads with uRBCs | 1 | 10.5 | 80 | NP | |||
| Donor no. 17 | |||||||
| iRBCs without uRBCs | 33 | 39.5 | NP | — | |||
| iRBCs with uRBCs | 1.67 | 8 | — | NP | |||
| Beads without uRBCs | 36 | 38.5 | NP | — | |||
| Beads with uRBCs | 2.9 | 8.5 | 52 | NP | |||
| Donor no. 5 | |||||||
| iRBCs without uRBCs | 32.5 | 31 | — | — | |||
| iRBCs with uRBCs | — | 6 | 12 | NP | |||
| Beads without uRBCs | 28.9 | 47 | — | — | |||
| Beads with uRBCs | 6.3 | 13 | 52 | 50 | |||
Phagocytosis of iRBCs and latex beads by adherent and detached monocytes after 2 or 18 hours, in static or shaken cultures, was quantified by Giemsa staining of chamber-well slides or cytospin preparations, respectively. One hundred monocytes were counted for each duplicate slide, and the mean percentage of monocytes positive for phagocytosis of iRBCs or latex beads is given.
— indicates no monocytes seen on the slide; and NP, monocytes seen, but quantification of phagocytosis not possible.
Interestingly, in the presence of uninfected erythrocytes, the number of cells that remained adherent to the slides was reduced by approximately 50%. This effect was seen in all wells containing adherent cells and uninfected erythrocytes, irrespective of whether parasite antigen or latex beads were present, and thus seems to be a direct result of the presence of erythrocytes (Figure 7A vs 7B). To determine whether detached monocytes might be able to phagocytose parasite material or latex beads, we examined stained cytospin preparations of culture supernatants. When no erythrocytes had been added to the adherent cells, no monocytes were found in cytospins. When uninfected erythrocytes had been added, detached monocytes were found in culture supernatants, but very few of the detached cells contained iRBCs; by contrast, latex beads were very effectively phagocytosed by detached cells in the presence of uninfected erythrocytes (Table 2).
In summary, therefore, in the presence of excess uninfected erythrocytes, monocyte adherence to plastic is disrupted and phagocytosis of iRBCs is inhibited in both adherent and detached monocytes. However, phagocytosis of latex beads is inhibited only in the adherent fraction.
Discussion
Whole-blood assays have revolutionized the measurement of cellular immune responses in epidemiological studies. Direct comparisons of PBMC assays and WBAs have shown good correlations for both proliferation and cytokine production, and WBAs have proven an effective simple tool for large scale studies of immune responses to whole mycobacteria and soluble antigen preparations.10-15,31 WBAs have been shown to be suitable for measuring responses to a variety of parasites,17 bacteria,9 viruses,18 all suggesting that antigen uptake, processing, presentation, and subsequent T-cell activation can occur in whole-blood assays. It was thus a considerable surprise to find, in 2 separate field studies, that cellular responses to malaria antigens in WBAs were markedly lower than expected from our prior experience with PBMC assays and were frequently undetectable.20,21 In both studies, strong cytokine responses to PHA and PPD were observed in WBAs, confirming the findings of others and demonstrating that the problem with WBA appeared to be specific to malaria antigens. Our strong suspicion from field studies that WBAs were not optimal for assessing T-cell responses to malaria antigens has been confirmed in this comparative study, where we have shown that lymphoproliferative and IFN-γ responses to blood stage malaria antigens, which are easily detected in PBMC assays, are totally inhibited in whole blood while responses to soluble antigens (PPD) and mitogens (PHA) are similar in both assays.
In vivo, malaria parasites are effectively confined to the blood. Uptake of circulating parasite material by antigen-presenting cells occurs primarily in the red pulp of the spleen, where macrophages phagocytose and destroy any abnormal (including malaria-infected) or aged erythrocytes, while allowing healthy erythrocytes to re-enter the blood circulation via the venous sinuses. Importantly, therefore, antigen uptake occurs under slow flow conditions and in the presence of uninfected erythrocytes; thus in several important respects WBAs resemble the in vivo situation more closely than the widely used PBMC assays. It is thus of real interest to determine the mechanism of inhibition of cellular responses to iRBCs and PfSL in WBAs.
As autologous plasma was present in both PBMC assays and WBAs, we realized that a cellular, rather than a humoral, component of whole blood was instrumental in the observed inhibition. We did consider whether TGF-β released from platelets (which represent a major store of preformed TGF-β) might inhibit IFN-γ responses.32 However, purified platelets were not able to suppress IFN-γ responses in PBMC cultures. We also considered the possibility that activated T cells might be undergoing apoptosis in the WBA, as specific apoptosis of malaria-reactive T cells has been reported in vivo during murine malaria infections,33,34 and high levels of T-cell apoptosis have been reported in vitro in PBMCs from malaria-infected donors.35,36 We have indeed found higher numbers of apoptotic (annexin V+, propidium iodide-) cells in WBA than in PBMC cultures analyzed by flow cytometry, but this effect was seen for all antigens and controls and thus could not explain the malaria antigen–specific suppression observed in WBAs (data not shown).
Systematic examination of the effect of different cell types on the PBMC response demonstrated that the inhibitory effects depended entirely on the presence of uninfected erythrocytes. Intact erythrocytes were far more inhibitory than erythrocyte lysates; they interfered with early events in T-cell activation and did not affect responses to soluble antigens. The simplest explanation for these observations was that uninfected erythrocytes inhibited uptake (and subsequent antigen presentation) of iRBCs and schizont lysates. This hypothesis was supported by the subsequent experiments. Uninfected erythrocytes may physically obstruct phagocytosis of iRBCs and latex beads by adherent cells, presumably through restriction of cell movement and contact, although inhibition of phagocytosis of iRBC is only partially ameliorated by prolonged shaking of the cultures. Given that in normal blood, and in WBAs, erythrocytes outnumber monocytes by approximately 14 000:1, this is perhaps not surprising and may underlie the frequently reported observation that T-cell responses in WBAs are somewhat lower than in PBMCs.5,6,10,16 Physical obstruction is likely to have a greater effect on large particles such as iRBCs and latex beads than on soluble antigens, which may explain why responses to PPD remain intact while responses to iRBCs and PfSL are inhibited. However, simple physical obstruction does not explain why detached monocytes cells are able to phagocytose latex beads but not iRBCs and would not explain why other large particulate antigens, including whole pathogens, appear to induce strong cellular immune responses in whole-blood assays.9,11,12,14,15,17 In particular, the inhibition of uptake of iRBC and schizont lysates by detached monocytes appears to be specific.
We wondered whether the phenomenon of rosetting—adherence of iRBCs to uninfected erythrocytes—might contribute to their poor uptake.37 However, the parasite clone we have used (3D7) had previously been shown not to form rosettes,38 and we did not observe rosette formation in any of our cultures (data not shown). We also excluded that the effect was an artifact caused by the use of heparin as anticoagulant. Heparin, at the concentrations present in our assays, has been reported to inhibit rosette formation,39,40 indicating that it can modify the interaction between iRBCs and uninfected erythrocytes. However, we found that uninfected erythrocytes exerted their inhibitory effect regardless of whether heparin, citrate, or EDTA (ethylenediaminetetraacetic acid) was used (not shown but provided for review). Similarly, the effect was independent of ABO and Rh blood group.41 We have not investigated the role of various reported rosetting ligands (CD36, heparan sulfate, CR1/CD35, thrombospondin, IgM, and IgG; reviewed in Craig and Scherf42 and Vogt et al43 ) but, as 3D7 does not form rosettes, we feel that they are unlikely to play a key inhibitory role in WBA.
An alternative explanation for our findings is that uninfected erythrocytes bind malaria antigens released from iRBCs, effectively acting as an antigen sink and preventing their uptake by antigen-presenting cells. Our group has previously shown that the parasite antigens recognized by cells from nonimmune donors are membrane associated25 ; these may be proteins that are physically anchored into parasite or red cell membranes or soluble glycolipid containing molecules that rapidly interpolate into red cell membranes as the infected cell lyses. While intact uninfected erythrocytes would not be phagocytosed, lysed uninfected red cells that might also adsorb malaria antigens would themselves be rapidly phagocytosed by antigen-presenting cells (APCs), explaining why only intact erythrocytes inhibit T-cell responses. While this hypothesis clearly needs further investigation, adsorption of glycolipid-associated proteins by red cell membrane “sinks” may partly explain the relatively low immunogenicity of and relative paucity of T-cell responses to glycosyl-phosphatidyl-inositols (GPI)–linked epitopes in antigens such as merozoite surface protein-1 compared to more distant regions of the same molecule.44-46 Conversely, it is possible that soluble peptide or recombinant protein malaria antigens (lacking the GPI anchor) might be adequately presented in WBAs. Whether inhibition of antigen presentation by intact erythrocytes has any more far-reaching implications, such as maintenance of peripheral tolerance to erythrocyte-associated antigens, remains to be seen.
Finally, it is intriguing that good IFN-γ responses could consistently be detected in WBAs for 1 of our 17 donors (donor no. 3) and that uninfected erythrocytes consistently had only a modest effect on PBMC responses in another donor (donor no. 9). Although both donors reported previous exposure to malaria, this was not recent and similar responses were not seen in other exposed donors. We thus considered it unlikely that malaria exposure had expanded the T-cell repertoire of these donors to such an extent that they could overcome the effects of limited antigen presentation. Rather, we think that their IFN-γ response was due to activation of natural killer (NK) cells, as analysis of NK cell IFN-γ production by flow cytometry has revealed that, in contrast to other donors in this study, these 2 donors make extremely strong NK cell responses to iRBCs.47 NK cell responses are rapid and require a high ratio of iRBCs to responder cells26 but do not require antigen processing or presentation and are thus unlikely to be affected by inhibition of iRBC phagocytosis. The notion that innate responses to malaria remain (at least partially) intact in whole blood but that adaptive (ie, T-cell) responses are suppressed is supported first by our observation that iRBCs can induce interleukin-12 (IL-12) responses in whole-blood cultures (M. Walther and E.M.R., manuscript in preparation) and second by the very strong correlation we observed between IFN-γ, IL-12, and TNF-γ responses in WBAs in endemic populations.20
In summary, we believe that the failure of T lymphocytes to respond to malaria antigens in WBAs is due to impaired uptake and presentation of complex malaria antigens by antigen-presenting cells. We suggest that this is due to both nonspecific impairment of phagocytosis of particulate antigens and specific inhibition of iRBC-APC interactions and adsorption of membrane-associated antigens by uninfected erythrocytes. The potential for such interactions to impair development of memory T-cell responses to blood stage malaria antigens in vivo, or to contribute to peripheral tolerance to self-antigens, requires investigation.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-08-2867.
Supported by the Wellcome Trust Training Fellowship 060115 (S.S.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Claire Swales for assistance with culturing and mycoplasma testing of malaria parasites and Carolynne Stanley for help with collecting the blood samples. We also thank Prof Hazel Dockrell, Dr Rosemary Weir, and other members of the mycobacterial research group at London School of Hygiene and Tropical Medicine for their expert opinions on whole blood assays and Dr Kiki Bodman-Smith for help with phagocytosis assays.

![Figure 1. IFN-γ production and lymphoproliferative responses to malaria antigens are inhibited in WBAs. PBMCs (106/mL) and 1:10 diluted whole-blood assays (WBAs) derived from single blood samples were incubated for 7 days with PHA (5 μg/mL), PPD (5 μg/mL), 5 × 105/mL iRBCs, 5 × 105/mL PfSL, 5 × 105/mL uninfected red blood cells (uRBCs), or growth medium only (GM). IFN-γ (A) was assayed by ELISA in 6-day supernatants, and lymphoproliferation (B) was assessed after 7 days. In each column, the filled symbols on the left represent values obtained from PBMC assays, and the open symbols on the right represent values obtained from WBAs; each point represents data from one donor. Two donors had previous exposure to malaria (donor nos. 1, and ⋄; and 3, ▪ and □), and 3 donors were malaria nonexposed (donor nos. 7, • and ○; 4, *; and 5, ▴ and ▵).The lower limit of detection for the IFN-γ ELISA (125 pg/mL) (A) and the background level of [3H] incorporation (B) are indicated by dotted lines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/8/10.1182_blood-2003-08-2867/6/m_zh80080459900001.jpeg?Expires=1769178227&Signature=Ryp3Tv~-waRFdRJeEIa3lzCewrpVZeS8P1IPiFVmRZTrmA8qpnIelt7rS~Z0v0vhiM08NpH2hxKrYHjhkj3wkjxgrFWdtopbRPcwLiXRW8j2uz1elERnvy-nFlDgbAirsF8iZUqjcakXKZ96HgcbkjlhvMqqKaCW0I4aVGoeKu6BeR48Kk9eYCVN4gPUb6J049I0YDNx2EMU8S~GR5KBt6RxJ5CNGKcrapFGFKhJG4DlqYp66IOgvhOZi-Zl5OMQwosDImmXUGW9yLOiuKlwdgX5~INS7OYIoBUQP~e0jADoaOGZwuXNUEcqEhuo~ZHoqPEGS6XBG4b6ezP1~e16gQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal