Abstract
Although there are many examples (eg, immune deviation) in which enhanced cellular responses correspond with lower humoral responses, here we demonstrate for the first time 2 models in which cytotoxic T-lymphocyte (CTL) activity is associated with an enhanced antibody response. First, C57BL/6 mice generate a stronger antibody response to ovalbumin DNA immunization than congenic bm1 mice. The latter differ from C57BL/6 mice in that the H-2Kb molecule is mutated so that the immunodominant CTL epitope of ovalbumin is no longer presented. Second, pre-existing CTLs (induced by ovalbumin peptide-priming) increased the antibody response to a second unrelated antigen (β-galactosidase) co-immunized with ovalbumin. One possible mechanism is that CTLs may release antigen from DNA-transfected cells by killing or damaging them, and this freed antigen is then accessible to dendritic cells and B cells. Our finding of CTL-mediated antibody enhancement has important implications for tumor and viral immunobiology and vaccination. (Blood. 2004;103:3073-3075)
Introduction
The dichotomy between humoral and cellular immunity has a long history.1,2 It is thought that cellular or humoral responses predispose subsequent responses to the same pathway.2,3 In one viral infection model,4-6 cytotoxic T lymphocytes (CTLs) were required for the generation of protective antibodies, suggesting a more complex interaction between the 2 pathways. Here, we established a simple in vivo model in which this relationship between cellular and humoral responses can be further examined. To our surprise, we found that generation of CTLs could actually enhance the antibody response after DNA immunization.
Materials and methods
Mice
The C57BL/6 mice and its congenic strain B6.C-H2bm1 (designated here as bm1) were bred under specific pathogen-free (spf) conditions at the institute. Perforin gene-targeted (knock-out [KO]) mice7 on a C57BL/6 background and their respective controls were housed at the Peter MacCallum Cancer Centre.
Peptides
All 4 peptides (purchased from Mimotopes, Melbourne, Australia) bind to H-2Kb. The immunodominant ovalbumin peptide (OVAp) SIINFEKL is presented on H-2Kb but not H-2Kbm1 major histocompatibility complex (MHC) class I molecules.8,9 Other peptides used were SSIEFARL from herpes simplex virus glycoprotein B (HSVp),10 GSHVEAL from insulin (INSp),11 and DAPYTNV from β-galactosidase.12 For priming, 50 μg peptide was emulsified with complete Freund adjuvant and injected subcutaneously at the base of the tail.
DNA immunization
Ovalbumin (OVA) and β-galactosidase cDNA in pCI (Promega, Madison, WI) were injected intradermally13 or by Helios gene gun (Bio-Rad, Hercules, CA; DNA loading ratio of 1 μg/injection). OVA containing HSVp that was substituted for OVAp was called OVA-hsv. For gene gun bombardment, hair around the base of the tail was plucked. The firing pressure used was 250 psi.
Immunologic assays
CTL activity was determined,13 using “stimulator” spleen cells that were irradiated (15 Gy) and incubated with 0.1 μg/mL OVAp or HSVp at 50 × 106 cells/mL for 60 minutes at 37°C. Enzyme-linked immunosorbent assay (ELISA) was performed13 with OVA or β-galactosidase protein (Sigma, St Louis, MO; 10 μg/mL in phosphate-buffered saline [PBS]) as antigen.
Results
Antibody responses to OVA DNA immunization were greater in C57BL/6 than in bm1 mice
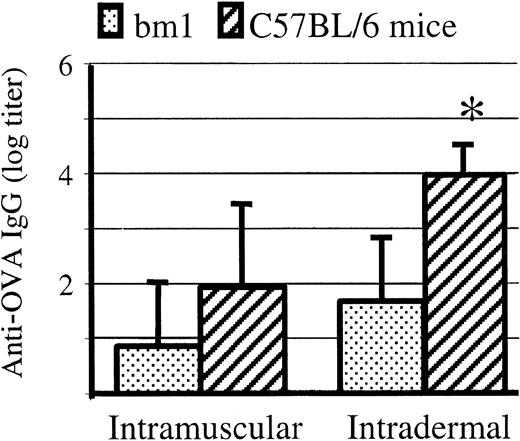
DNA immunization of C57BL/6 mice results in both CTL and antibody responses.13 The mouse strain bm1 is congenic with C57BL/6 except that its MHC has H2-Kbm1, which differs from H2-Kb by 3 amino acids and can no longer present OVAp.8 Antibody (immunoglobulin G [IgG]) levels at 4 and 6 weeks after intramuscular and intradermal DNA immunization were higher in C57BL/6 than in bm1 mice (Figure 1).
Antibody responses to intradermal DNA OVA immunization is higher in C57BL/6 than bm1 mice. Six-week-old C57BL/6 and bm1 mice were injected with DNA plasmid encoding secreted OVA. Anti-OVA IgG antibodies were measured by ELISA at 4 weeks. Antibody levels were higher in C57BL/6 than in bm1 mice following intramuscular (n = 5; P = .09) and (*) intradermal immunization (n = 8; P = .001), although significance was only shown in the latter. Mean and standard deviation are shown. Preimmune antibody in both these strains is always undetectable against OVA.
Antibody responses to intradermal DNA OVA immunization is higher in C57BL/6 than bm1 mice. Six-week-old C57BL/6 and bm1 mice were injected with DNA plasmid encoding secreted OVA. Anti-OVA IgG antibodies were measured by ELISA at 4 weeks. Antibody levels were higher in C57BL/6 than in bm1 mice following intramuscular (n = 5; P = .09) and (*) intradermal immunization (n = 8; P = .001), although significance was only shown in the latter. Mean and standard deviation are shown. Preimmune antibody in both these strains is always undetectable against OVA.
As cellular and humoral responses are supposed to be inversely related,2 we were surprised that antibody responses were higher in mice capable of a cellular response (C57BL/6) than in mice lacking a cellular response (bm1). Being genetically identical mice, except for the H-2Kb locus, the variation in antibody response would seem to be due to CTL activity in C57BL/6.
Pre-existing OVA-specific CTLs enhanced antibody responses to a second unrelated antigen co-immunized with OVA
To examine the above results further, CTLs were elicited through priming with a peptide comprising only the CTL epitope, OVAp. DNA immunization with the entire OVA molecule would then generate an antibody response. OVAp induced OVA-specific CTL in C57BL/6 mice within 2 weeks, whereas control peptide did not (data not shown). As expected, no OVA-specific IgG antibodies were generated by this regimen (data not shown).
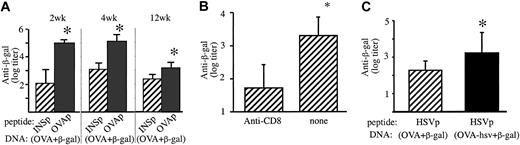
OVAp-priming of CD8+ T cells had no effect on antibody levels raised against a subsequent OVA DNA immunization (data not shown). The caveat here is that the primary OVA immunization may affect the OVA boost in contradictory ways. For example, if B cells specific for OVA can cross-present OVA on H-2Kb, they may be killed by the primed OVA-specific CTLs. This would then counteract any enhancement because of CTL-induced antigen release. This ambiguity could be abrogated with a co-immunized unrelated antigen, β-galactosidase. Two weeks after OVAp or control INSp, C57BL/6 mice were immunized by gene gun with pellets co-coated with OVA and β-galactosidase DNA. At 2 to 12 weeks IgG antibodies against β-galactosidase were significantly higher in mice primed with OVAp (Figure 2A). Moreover, if the CTLs were killed by injecting anti-CD8 antibody after OVAp priming (Figure 2B), the level of antibody was reduced (P < .01), indicating that the enhancement effect was indeed due to CTLs.
OVAp priming increased the antibody response to β-galactosidase co-immunized with OVA DNA immunization. (A) Eight-week-old male C57BL/6 mice (n = 8) were primed with either OVAp or control INSp in complete Freund adjuvant. After 2 weeks, all mice were immunized by gene gun with pellets co-coated with plasmids encoding OVA and β-galactosidase. Two and 4 weeks later, IgG antibodies against β-galactosidase were higher (P = .001) in mice primed with OVAp, compared with those primed with the control INSp. After 12 weeks, β-galactosidase antibodies remained significantly higher (P = .01) in mice primed with OVAp compared to INSp. (B) Twelve days after OVAp priming, mice (n = 8) were treated with anti-CD8 antibody (0.5 mg 53.6.7 and 0.5 mg YTS169 intraperitoneally) or not. All mice were co-immunized with OVA and β-galactosidase DNA 2 days later. Three weeks after that, ELISA titers of anti–β-galactosidase responses were determined. (C) Pre-existing HSV-specific CTLs increase the antibody response to β-galactosidase when coexpressed with OVA-hsv. Eight-week-old male C57BL/6 mice were peptide primed with HSVp. After 3 weeks, mice were DNA immunized with pellets co-coated with β-galactosidase plasmid plus either OVA or OVA-hsv (in which the CTL epitope OVAp is substituted with HSVp). HSVp-primed mice had higher β-galactosidase antibodies (P = .01) after co-immunization with OVA-hsv compared with OVA. Mean and standard deviation are shown. *Statistical significance (P < .05).
OVAp priming increased the antibody response to β-galactosidase co-immunized with OVA DNA immunization. (A) Eight-week-old male C57BL/6 mice (n = 8) were primed with either OVAp or control INSp in complete Freund adjuvant. After 2 weeks, all mice were immunized by gene gun with pellets co-coated with plasmids encoding OVA and β-galactosidase. Two and 4 weeks later, IgG antibodies against β-galactosidase were higher (P = .001) in mice primed with OVAp, compared with those primed with the control INSp. After 12 weeks, β-galactosidase antibodies remained significantly higher (P = .01) in mice primed with OVAp compared to INSp. (B) Twelve days after OVAp priming, mice (n = 8) were treated with anti-CD8 antibody (0.5 mg 53.6.7 and 0.5 mg YTS169 intraperitoneally) or not. All mice were co-immunized with OVA and β-galactosidase DNA 2 days later. Three weeks after that, ELISA titers of anti–β-galactosidase responses were determined. (C) Pre-existing HSV-specific CTLs increase the antibody response to β-galactosidase when coexpressed with OVA-hsv. Eight-week-old male C57BL/6 mice were peptide primed with HSVp. After 3 weeks, mice were DNA immunized with pellets co-coated with β-galactosidase plasmid plus either OVA or OVA-hsv (in which the CTL epitope OVAp is substituted with HSVp). HSVp-primed mice had higher β-galactosidase antibodies (P = .01) after co-immunization with OVA-hsv compared with OVA. Mean and standard deviation are shown. *Statistical significance (P < .05).
Pre-existing HSV-specific CTLs enhanced the antibody response to secondary antigen co-immunized with antigen containing the cognate CTL epitope
To ensure that this phenomenon was not peculiar to OVA-specific CTLs, we primed mice with HSVp. For the secondary gene gun immunization, β-galactosidase plasmid plus either the canonical OVA plasmid or OVA-hsv (in which OVAp was replaced by HSVp) were used. Only in the latter would the peptide-primed CTLs generated be cognate for the secondary immunization and only in the latter did we find increased antibody levels (Figure 2C).
Discussion
For both OVA and HSV systems, a boosting effect on the antibody response to antigen was coexpressed with an antigen targeted by pre-existing specific CTLs. Providing the second immunization contained the cognate CTL epitope immunization, antibody against β-galactosidase was elevated in both systems. We concluded therefore, that at least in the case of DNA immunization in which antigen is expressed endogenously, CTLs can enhance the antibody response.
There are several possible explanations as to the mechanism of this CTL-induced antibody enhancement. First, activated CD8+ T cells may provide some form of alternative “help” to B cells. One report described CD8+ T cells switching to a CD8-CD4- phenotype and secretion of Th2 cytokines; such cells could help B cells produce IgG, at least in vitro.14 CD8+ T cells have not been shown to directly provide help for B cells in vivo. We investigated this by DNA-immunizing mice lacking CD4+ T cells (transgenic for anti-CD4 antibody15 or MHC class II deficient). They did not mount antibody responses to OVA; thus, CD8+ T cells cannot substitute for CD4+ T-cell help. Therefore, we favor a second explanation. Most cells transfected by DNA are not professional antigen-presenting cells but are parenchymal or stromal cells.16 Such cells can be a reservoir of cell-associated antigen that can be released by CTL attack. This released antigen from apoptotic or damaged cells is then available for B cells and dendritic cells.17,18 This also explains why the antibody enhancement was not seen in protein immunization (bm1 and C57BL/6 mice responded equally; data not shown).
Our peptide-priming experiments in perforin-deficient mice showed a similar boosting effect as in wild-type mice. Perhaps this is not surprising because CTLs can inflict injury to cells by many other mechanisms, such as by way of Fas ligand, TRAIL (tumor necrosis factor–related apoptosis-inducing ligand), or by secreting cytotoxic cytokines such as tumor necrosis factor, lymphotoxin, and interferon-γ.
The generation of CTLs against one antigen (the CTL target antigen) enhancing the humoral response of another (secondary antigen) has major implications in antibody responses to tumor neoantigens and viral infections, because multiple antigens would be expressed in the one cell. It is tempting to speculate how our findings also impinge on the heterologous prime-boost strategies that have been shown to be so promising for genetic immunization.19-21 Their success may partly be mediated by DNA-primed CTLs that release antigen to improve the secondary immune response.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-07-2305.
Supported by the National Health and Medical Research Council (NHMRC) of Australia, the Ramaciotti Foundation, and a National Institutes of Health/Juvenile Diabetes Research Foundation Program Grant. C.M.D. was supported by a scholarship from the Melbourne University Scheme.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal