Abstract
Activating and expanding T cells using T-cell receptor (TCR) cross-linking antibodies and interleukin 2 (IL-2) results in potent cytotoxic effector cells capable of recognizing a broad range of malignant cell targets, including autologous leukemic cells. The mechanism of target cell recognition has previously been unknown. Recent studies show that ligation of NKG2D on natural killer (NK) cells directly induces cytotoxicity, whereas on T cells it costimulates TCR signaling. Here we demonstrate that NKG2D expression is up-regulated upon activation and expansion of human CD8+ T cells. Antibody blocking, redirected cytolysis, and small interfering RNA (siRNA) studies using purified CD8+ T cells demonstrate that cytotoxicity against malignant target cells occurs through NKG2D-mediated recognition and signaling and not through the TCR. Activated and expanded CD8+ T cells develop cytotoxicity after 10 to 14 days of culture, coincident with the expression of the adapter protein DAP10. T cells activated and expanded in low (30 U/mL) and high (300 U/mL) concentrations of IL-2 both up-regulated NKG2D expression equally, but only cells cultured in high-dose IL-2 expressed DAP10 and were cytotoxic. Collectively these results establish that NKG2D triggering accounts for the majority of major histocompatibility complex (MHC)–unrestricted cytotoxicity of activated and expanded CD8+ T cells, likely through DAP10-mediated signaling. (Blood. 2004;103: 3065-3072)
Introduction
Significant progress has been made in understanding the molecular mechanisms underlying natural killer (NK) cell–mediated cytotoxicity against malignant target cells. Triggering of NK cells is regulated by both activating and inhibitory cell surface immunoreceptors. In humans, such receptors are divided into at least 3 groups that include: (1) the killer immunoglobulin receptors (KIRs), (2) natural cytotoxicity receptors (NCRs), and (3) the c-type lectin receptors (for reviews, see Lanier1 and Moretta et al2 ). NKG2D is one member of the c-type lectin-activating receptor family that is evolutionarily conserved and is located within the NK gene complex on human chromosome 12p12-p13.3 NKG2D is expressed on all NK cells and is a promiscuous receptor that recognizes at least 6 counterligands. These include the major histocompatibility complex (MHC) class I-like molecules, MICA and MICB, and members of the ULBP family (ULPB1-4), named for the ability of some members to bind to the UL-16 protein of cytomegalovirus (CMV).4-9 Other potential NKG2D ligands have been described at a molecular level, but their cell surface expression and NKG2D-binding capacity have not yet been demonstrated.10 Interestingly, the ligands for NKG2D appear to have a pattern of expression that is relatively restricted to malignant tissue.6,11 Given this, NKG2D may be particularly important in the recognition of malignant cells. NKG2D has a significant role in the triggering of interleukin-2 (IL-2)–activated NK cells because ligation induces calcium flux, cytokine release, and cytotoxicity.4,5
NKG2D has also been identified on γ/δ T cells and CD8+ T-cell receptor (TCR) α/β T cells.8,12 In contrast to NK cells, cross-linking NKG2D on antigen-specific, CD8+ cytotoxic T lymphocyte (CTL) clones does not induce cytokine production or calcium flux or trigger cytotoxicity.4 NKG2D signaling does, however, augment cytotoxic and proliferative responses of T cells on antigen encounter, thus qualifying NKG2D as a T-cell costimulatory molecule.12
It has long been known that ex vivo–activated and –expanded T cells become blastlike in appearance, up-regulate cytotoxic effector molecules, and on prolonged activation with high doses of IL-2 develop MHC-unrestricted cytotoxicity against a variety of malignant cell targets including autologous leukemic blasts.13-17 Cells activated and expanded in the presence of interferon-γ (IFN-γ), followed by anti-CD3 monoclonal antibody (mAb) and IL-2 have been termed cytokine-induced killer (CIK) cells. These T cells have broad in vitro and in vivo biologic activity following both syngeneic and allogeneic bone marrow transplantation (BMT) in rodent models.18,19 Infusion of cells of this type have been shown to reduce the risk of tumor recurrence in patients with hepatocellular carcinoma following surgical resection,20 and such cells are also under exploration in the setting of autologous and allogeneic hematopoietic cell transplantation. The exact molecular structures that account for this functional activity are unknown. Here we have evaluated ex vivo–activated and –expanded CD8+ T cells, which display MHC-unrestricted, TCR-independent cytotoxicity against malignant targets and demonstrate a central role for NKG2D-mediated recognition and signaling in cytotoxic function.
Materials and methods
Cell isolation and generation of ex vivo–expanded and –activated T cells
T cells were activated and expanded as previously described.21 Briefly, mononuclear cells were isolated from healthy donors by Ficoll-Hypaque density centrifugation and washed 3 times with phosphate-buffered saline (PBS). The final product was resuspended at 2 × 106 cells/mL in complete RPMI (cRPMI) consisting of 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 50μg/mL 2-mercaptoethanol (2-ME) at 37°C, 5% CO2. On day 0, cells were activated with IFN-γ (1000 U/mL; Genentech, South San Francisco, CA) and the following day, cells were stimulated with OKT-3 (25 ng/mL; OrthoBioTech, Raritan, NJ) and recombinant IL-2 (rIL-2; 300 U/mL; Chiron, Emeryville, CA). Thereafter, cells were stimulated with rIL-2 (300 U/mL) every 3 to 5 days and fresh media was added to maintain a cell density of 1.5 to 2 × 106/mL for a total of 14 to 28 days. In select experiments cells were manipulated in the exact same manner except they received low doses of IL-2 (30 U/mL).
Cell lines
The U266 (American Type Culture Collection [ATCC], Manassas, VA) and 721-221 (kind gift from Peter Parham, Stanford University) cell lines were cultured in cRPMI. The P815 (ATCC) was cultured in complete Dulbecco modified Eagle medium (cDMEM).
Flow cytometry and cell selection
Activated and expanded T cells or tumor targets were stained with the following mAbs: CD3 (UCHT1), CD4 (SK3), CD8 (RPA-T8), CD56 (NCAM16-2), isotype control (IgG) directly conjugated to either fluorescein isothiocyanate (FITC) or phycoerythrin (PE), all from Becton Dickinson (San Jose, CA). Unconjugated antibodies against NKG2D (M585), MICA (M673), MICB (M362), ULBP1 (M295), ULBP2 (M311), and ULBP3 (M551) were all kind gifts of David Cosman (Amgen, Seattle, WA). Secondary antibody staining was accomplished with FITC, PE, or peridinin chlorophyll protein (PerCp)–conjugated rat-antimouse Ig (PharMingen, San Diego, CA) or Texas red–conjugated goat-antimouse Ig (Caltag, Burlingame, CA). About 1 × 106 cells were removed from culture, washed with fluorescence-activating cell sorting (FACS) staining solution (PBS containing 2% FCS), collected by centrifugation, and resuspended in 100 μL FACS staining solution. Cells were then stained with the mentioned antibodies or isotype controls for 15 minutes at 4°C. Excess antibody was removed and cells were evaluated by FACS analysis at the Stanford shared FACS facility (Stanford University Medical School), using a FASCscan instrument (Becton Dickinson). Data were analyzed by FlowJo software (version 2.5.1; Tree Star, San Carlos, CA).
Where stated, CD8+ T cells were purified using the following method. Freshly isolated peripheral blood lymphocytes (PBLs; from healthy donors) were enriched prior to culture using magnetic bead sorting with directly conjugated CD8 microbeads, according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). After this enrichment, cells were cultured as described until 24 to 48 hours prior to use when cells were further purified using FACS. The cells were labeled CD3 and CD8 antibodies and analyzed and sorted on a dual laser FACS (Becton Dickinson Immunocytometry Systems, Mountain View, CA), which was modified and made available through the FACS shared-user group at Stanford University.
51Cr release assay
Tumor targets were labeled with 51Cr (Dupont-NEN, Boston, MA) by incubating 1 × 106 cells in 300 μCi (11.1 MBq) 51Cr for 2 hours at 37°C, 5% CO2. The labeled cells were washed 3 times with PBS, resuspended in cRPMI, and plated in 96-well plates at a concentration of 1 × 104 cells/mL in triplicate. Effector cells were added at specified ratios (either 10:1 or 40:1) and incubated for 4 hours at 37°C, 5% CO2. For cytotoxicity assays that were performed with blocking mAbs, either isotype control or blocking antibodies against NKG2D were added to effector cells for 30 minutes prior to the addition of tumor cell targets (final concentration, 20 μg/mL). At the completion of the assay, supernatant was collected and counted using a γ counter (Cobra/AII, Packard Bioscience, Meriden, CT). The percentage specific 51Cr lysis was calculated using the following equation: % specific lysis = 100 × (test release) - (spontaneous release)/(maximal release) - (spontaneous release).
Redirected cytolysis
For redirected killing assays 1 × 106 P815 cells were labeled with 51Cr as described (see “51Cr release assay”). Excess 51Cr was removed by washing as described and the cells were resuspended in 500 μL. NKG2D, CD3, or isotype control antibodies were added (final concentration, 20 μg/mL) and incubated for 30 minutes. The P815 target cells were then used as targets in a 51Cr release assay as described.
Small interfering RNA
Sequences complementary to NKG2D (5′-CGGGGUCAGGGAGGUGGUGUU-3′) were generated by the Pan Facility (Stanford University) and were transfected into purified activated and expanded CD8+ T cells at days 14 to 21 of culture using oligofectamine according to the manufacturer's instructions (Invitrogen, San Diego, CA). Cells were used for FACS analysis and 51Cr release assays 24 and 72 hours after transfection.
Polymerase chain reaction
Activated and expanded CD8+ T cells were FACS sorted at various times during culture (days 0, 3, 7, 10, 14, and 21). Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Santa Clarita, CA) and reverse transcribed to cDNA with Superscript II RNase H- Reverse Transcriptase (Gibco BRL, Gaithersburg, MD) and oligo dT random primer hexamers (500 μg/mL). cDNA (0.5 μL) was amplified with recombinant Taq polymerase (Gibco BRL) for 35 cycles. The following primers were used: 5′-CTGGGAGATGAGTGAATTTCATA-3′ (NKG2D sense) and 5′-GACTTCACCAGTTTAAGTAAATC-3′ (NKG2D antisense) for NKG2D (417 bp), 5′-CATCTGGGTCACATCCTCTT-3′ (DAP10 sense) and 5′-CAGAAGTCAAAGGTCCAAGC-3′ (DAP10 antisense) for DAP10 (306 bp), and 5′-CCGCAAAGACCTGTACGCCA-3′ (actin sense) and 5′-TGGACTTGGGAGAGGACTGG-3′ (actin antisense) for actin (650 bp).
Western blotting
Cell lysates (50 μg) were prepared from purified cell populations of activated and expanded T cells at various time points in culture using freshly made lysis buffer (10 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 8, 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1% NP-40, 0.5% deoxycholate, 0.1% SDS [sodium dodecyl sulfate], protease inhibitor cocktail [complete protease inhibitor; Roche, Indianapolis, IN], phenylmethylsulfonyl fluoride (PMSF). Samples were separated on a 15% polyacrylamide gel and transferred to a polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 5% nonfat milk and probed with a DAP10-specific antibody and then a species-specific secondary horseradish peroxidase (HRP)–conjugated antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were developed using chemiluminescence (ECL; Amersham Piscataway, NJ).
Results
Expression and functional activity of NKG2D on ex vivo–activated and –expanded T cells
NKG2D is a recently identified cell surface molecule that plays a significant role in NK cell–mediated target recognition and cytolysis.4,22,23 NKG2D is also expressed on some T-cell subsets.4,22 Because activated and expanded T cells attain significant MHC-unrestricted cytotoxicity against a variety of malignant cell lines and fresh tumor isolates,13,14 we evaluated these cells for the expression of NKG2D by PCR. As shown in Figure 1A, NKG2D message was detected at all time points tested. To further characterize the NKG2D expression in cell subsets, FACS analysis was performed on both freshly isolated (day 0) and ex vivo–activated and –expanded cell subsets after 14 to 21 days of culture. CD4+ T cells had minimal expression of NKG2D at day 0 and there was little up-regulation of this molecule over the culture period (Figure 1B). In contrast, freshly isolated CD8+ T cells had a substantially higher density of cell surface NKG2D than CD4+ cells and this was further up-regulated after 14 to 21 days of culture (Figure 1B). We have previously reported that activated and expanded T cells that coexpress the cell surface molecule CD56 account for a substantial proportion of the cytotoxic effector cells capable of lysing transformed cells.24 Whereas these CD3+CD56+ cells make up a small proportion of freshly isolated T cells, they account for about 10% to 40% of ex vivo–expanded T cells after 14 to 21 days of culture.24 Like CD8+ T cells, freshly isolated CD3+CD56+ cells were found to have high expression of NKG2D, and this expression increased after 14 to 21 days of culture (Figure 1B). We also compared the relative expression of NKG2D on NK cells (CD3-CD16+) and found that both freshly isolated NK cells and the few NK cells that survived the culture conditions (< 5%) had a lower density of NKG2D than either CD8+ T cells or CD3+CD56+ T cells both at rest and after activation.
NKG2D expression over time in culture. (A) Cells were isolated at the stated time points and reverse transcription-polymerase chain reaction (RT-PCR) was performed using NKG2D-specific primers. (B) Cells were stained with antibodies and analyzed by FACS analysis as described in “Materials and methods.” Shown are FACS histograms at either day 0 or at day 21 of culture. Dotted histograms represent isotype control antibodies and solid lines NKG2D. Mean fluorescent intensity (MFI) of NKG2D is shown in the upper right of each histogram. Results are representative of 3 or more experiments.
NKG2D expression over time in culture. (A) Cells were isolated at the stated time points and reverse transcription-polymerase chain reaction (RT-PCR) was performed using NKG2D-specific primers. (B) Cells were stained with antibodies and analyzed by FACS analysis as described in “Materials and methods.” Shown are FACS histograms at either day 0 or at day 21 of culture. Dotted histograms represent isotype control antibodies and solid lines NKG2D. Mean fluorescent intensity (MFI) of NKG2D is shown in the upper right of each histogram. Results are representative of 3 or more experiments.
To investigate whether NKG2D plays a functional role in the recognition and cytolysis of tumor target cells we performed 51Cr release assays using purified activated and expanded CD8+ T cells isolated at days 14 to 21 of culture. As shown in Figure 2A, activated and expanded CD8+ T cells mediate cytolysis against the plasmacytoma cell line U266 with 40% to 50% specific killing at an effector-target ratio (E/T ratio) of 10:1. The addition of blocking mAbs directed against NKG2D significantly attenuated cytotoxicity, whereas an isotype control antibody had little effect on toxicity. The counterligands for NKG2D have been identified and include MICA, MICB, and ULPB 1, 2, and 3.6,8 Evaluation of the U266 cell line for these ligands revealed cell surface expression of MICA and ULBP3 (Figure 2B). Blocking MICA or ULBP3 either individually or together attenuated cytolysis by 50% to 100% (Figure 2C). Thus, NKG2D on activated and expanded CD8+ T cells plays a significant role in the recognition of malignant targets and triggering of T-cell cytotoxicity.
Activated and expanded CD8+ T cells function through NKG2D. (A) Cytolysis against the plasmacytoma cell line U266. Effectors were combined with targets (E/T = 10:1) either alone (▪) or in the presence of isotype control antibodies (▦) or NKG2D antibodies (□). Antibodies were used at a final concentration of 20 μg/mL and results are representative of 3 or more experiments. Error bars indicate standard deviations. (B) FACS histograms demonstrating the presence or absence of NKG2D ligands on the U266 cell line. Dotted lines represent isotype control and solid lines, the antibody directed against the stated NKG2D counterligand. (C) 51Cr release assay was performed as in panel A, except in the presence of antibodies directed against NKG2D, MICA, ULPB3, or isotype control antibodies. Data are expressed as a percent reduction in cytotoxicity.
Activated and expanded CD8+ T cells function through NKG2D. (A) Cytolysis against the plasmacytoma cell line U266. Effectors were combined with targets (E/T = 10:1) either alone (▪) or in the presence of isotype control antibodies (▦) or NKG2D antibodies (□). Antibodies were used at a final concentration of 20 μg/mL and results are representative of 3 or more experiments. Error bars indicate standard deviations. (B) FACS histograms demonstrating the presence or absence of NKG2D ligands on the U266 cell line. Dotted lines represent isotype control and solid lines, the antibody directed against the stated NKG2D counterligand. (C) 51Cr release assay was performed as in panel A, except in the presence of antibodies directed against NKG2D, MICA, ULPB3, or isotype control antibodies. Data are expressed as a percent reduction in cytotoxicity.
NKG2D can trigger TCR-independent cytotoxicity of activated T cells
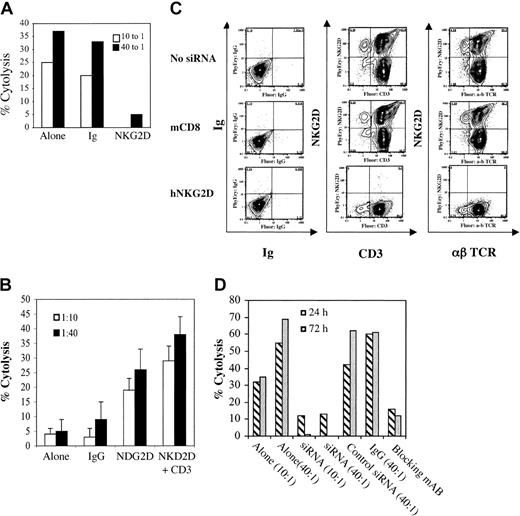
NKG2D signaling directly induces NK cell–mediated cytolysis, whereas in T cells NKG2D is thought to transmit costimulatory signals, similar to CD28.12,22 Our previous studies have indicated that ex vivo–activated and –expanded CD8+ T cells function through an MHC-unrestricted, TCR-independent mechanism.25,26 Despite this, it remains possible that the cytotoxicity against a particular malignant cell line could be explained by a small population of antigen-specific T cells and that blocking NKG2D simply attenuated TCR costimulation. To address this issue, we performed cytotoxicity assays using purified activated and expanded CD8+ T cells and the lymphoblastic B-cell line 721-221 as targets. Because these target cells do not express MHC class I,27 peptide antigen cannot be presented to CD8+ T cells and, therefore, TCR-mediated target recognition cannot occur. We found that highly purified activated and expanded CD8+ T cells mediated potent cytolysis against the 721-221 cell line, supporting previous observations of MHC-unrestricted cytotoxicity. Moreover, antibody blocking of NKG2D almost completely attenuated cytotoxicity, whereas isotype control antibodies had minimal effects on cytotoxicity (Figure 3A). To investigate the functional outcome of selectively triggering NKG2D on CD8+ ex vivo–activated and –expanded T cells, we performed reverse cytolysis assays using the Fc receptor-bearing murine cell line P815. In general, ex vivo–activated and –expanded CD8+ human T cells display little cytotoxicity against these murine target cells (Figure 3B). Similarly, when loaded with nonspecific antibodies (isotype control antibodies) the cytotoxicity was minimal. In contrast, P815 cells incubated with antibodies against NKG2D were able to directly trigger cytolysis in FACS-purified CD8+ activated and expanded T cells. To further define the role of NKG2D in malignant cell recognition and cytolysis, small interfering RNA (siRNA) was generated against NKG2D. Six different siRNAs were generated and transfected into activated and expanded cells at day 14 to 17 of culture. The sequence complementary to bp 1546 to 1567 induced almost complete suppression of NKG2D 72 hours after transfection (Figure 3C) and was used for the experiments described. This siRNA specifically suppressed NKG2D protein synthesis because a control siRNA (against murine CD8 [mCD8]) showed no inhibition of NKG2D expression relative to the nontransfected control. Also noteworthy is that this NKG2D siRNA had no influence on either CD3 or αβ TCR expression (Figure 3C). siRNA transfected cells were then used in functional assays (51Cr release). Cells transfected with mCD8 siRNA (control) or nontransfected cells mediated significant toxicity against malignant targets. In contrast, cells treated with NKG2D siRNA were incapable of mounting a cytolytic response, comparable to nontransfected cells treated with NKG2D-blocking antibody (Figure 3D). Taken together, these results show that triggering through NKG2D is both necessary and sufficient for cytolysis of targets by ex vivo–activated and –expanded CD8+ T cells.
Activated and expanded CD8+ T cells recognize targets through a NKG2D-dependent and TCR-independent pathway. (A) Cytolysis of 721-221 cell lines by activated and expanded CD8+ T cells either alone or in the presence of isotype control antibodies or antibody against NKG2D (at 20 μg/mL). (B) Redirected cytolysis against murine P815 cells either alone, with isotype control antibody, anti-NKG2D, or anti-NKG2D and anti-CD3 (at 20 μg/mL). Error bars indicate standard deviation (C) FACS analysis after NKG2D-specific siRNA. Shown is the FACS analysis of activated and expanded T cells at day 14 to 21 of culture treated with siRNA complementary to human NKG2D (hNKG2D) or controls (nontransfected cells and cells transfected with mCD8 siRNA). siRNA complementary to NKG2D induces a more than 95% inhibition of NKG2D expression at 72 hours after transfection, but has no effect on either CD3 or αβ TCR relative to controls. (D) Cytotoxicity of cells treated with siRNA. Untreated cells or cells treated with control siRNA (against mCD8) induced significant cytotoxicity against the U266 cell line, whereas cells transfected with hNKG2D siRNA or nontransfected cells treated with NKG2D-blocking antibodies had no cytotoxicity. All are representative of 3 or more experiments.
Activated and expanded CD8+ T cells recognize targets through a NKG2D-dependent and TCR-independent pathway. (A) Cytolysis of 721-221 cell lines by activated and expanded CD8+ T cells either alone or in the presence of isotype control antibodies or antibody against NKG2D (at 20 μg/mL). (B) Redirected cytolysis against murine P815 cells either alone, with isotype control antibody, anti-NKG2D, or anti-NKG2D and anti-CD3 (at 20 μg/mL). Error bars indicate standard deviation (C) FACS analysis after NKG2D-specific siRNA. Shown is the FACS analysis of activated and expanded T cells at day 14 to 21 of culture treated with siRNA complementary to human NKG2D (hNKG2D) or controls (nontransfected cells and cells transfected with mCD8 siRNA). siRNA complementary to NKG2D induces a more than 95% inhibition of NKG2D expression at 72 hours after transfection, but has no effect on either CD3 or αβ TCR relative to controls. (D) Cytotoxicity of cells treated with siRNA. Untreated cells or cells treated with control siRNA (against mCD8) induced significant cytotoxicity against the U266 cell line, whereas cells transfected with hNKG2D siRNA or nontransfected cells treated with NKG2D-blocking antibodies had no cytotoxicity. All are representative of 3 or more experiments.
Cytotoxicity and expression of DAP10 over time in culture
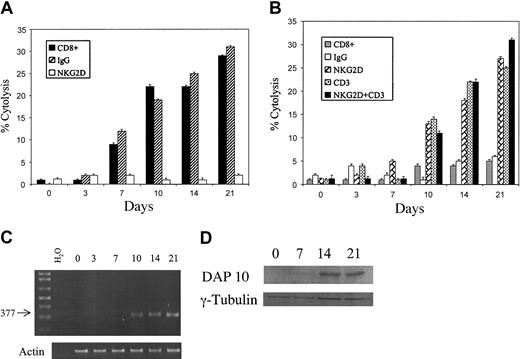
Given that ex vivo–activated and –expanded CD8+ T cells mediate cytotoxicity, much like NK cells, we performed cytotoxicity and redirected lysis studies of purified CD8+ T cells over a time course following activation with anti-CD3 mAbs and IL-2. On initial isolation CD8+ T cells were not capable of inducing significant cytotoxicity or redirected lysis (Figure 4A-B). Cytotoxicity, which was inhibited by anti-NKG2D mAbs, was first detectable after 7 days in culture and then much more readily observed by day 10 (Figure 4A). Similarly, redirected cytotoxicity was measurable by day 10 in culture (Figure 4B).
Induction of cytotoxicity and redirected cytolysis correlates with the expression of DAP10. T cells were activated and expanded as described in “Materials and methods.” Cells were pre-enriched using MACS on day 0 and then cultured until 24 to 48 hours prior to use (at various time points in culture days 0, 3, 7, 10, 14, and 21) when they were purified by FACS. Cells were rested for 24 to 48 hours in cRPMI and IL-2 (300 U/mL) and then used for either cytotoxicity assays (A) or redirected cytolysis assays (B), or freshly sorted cells were used to harvest RNA for RT-PCR (C). For cytotoxicity assays (A), targets were the U266 cell line. Either no antibody was used (▪) or isotype control antibody (▨) or anti-NKG2D antibody (□) was added. For redirected cytolysis experiments (B), CD8+ T cells at various times in culture were combined with P815 cells alone (▦) or with P815 cells that were incubated with isotype control antibodies (□), anti-NKG2D (▨), anti-CD3 (  ) or anti-NKG2D and anti-CD3 (▪). For panels A and B, antibodies were used at 20 μg/mL, E/T = 10:1, and error bars indicate standard deviation. For PCR experiments (C), DAP10-specific primers were used and the amplified product was separated on a 0.9% agarose gel. (D) For Western blotting experiments, purified CD8+ cells were isolated at various times in culture and extracts (50 μg) were separated on a 15% acrylamide gel, then transferred to PVDF membranes, and probed using either a DAP10 or γ-tubulin–specific antibody (loading control). Results are representative of 2 or more individual experiments.
) or anti-NKG2D and anti-CD3 (▪). For panels A and B, antibodies were used at 20 μg/mL, E/T = 10:1, and error bars indicate standard deviation. For PCR experiments (C), DAP10-specific primers were used and the amplified product was separated on a 0.9% agarose gel. (D) For Western blotting experiments, purified CD8+ cells were isolated at various times in culture and extracts (50 μg) were separated on a 15% acrylamide gel, then transferred to PVDF membranes, and probed using either a DAP10 or γ-tubulin–specific antibody (loading control). Results are representative of 2 or more individual experiments.
Induction of cytotoxicity and redirected cytolysis correlates with the expression of DAP10. T cells were activated and expanded as described in “Materials and methods.” Cells were pre-enriched using MACS on day 0 and then cultured until 24 to 48 hours prior to use (at various time points in culture days 0, 3, 7, 10, 14, and 21) when they were purified by FACS. Cells were rested for 24 to 48 hours in cRPMI and IL-2 (300 U/mL) and then used for either cytotoxicity assays (A) or redirected cytolysis assays (B), or freshly sorted cells were used to harvest RNA for RT-PCR (C). For cytotoxicity assays (A), targets were the U266 cell line. Either no antibody was used (▪) or isotype control antibody (▨) or anti-NKG2D antibody (□) was added. For redirected cytolysis experiments (B), CD8+ T cells at various times in culture were combined with P815 cells alone (▦) or with P815 cells that were incubated with isotype control antibodies (□), anti-NKG2D (▨), anti-CD3 (  ) or anti-NKG2D and anti-CD3 (▪). For panels A and B, antibodies were used at 20 μg/mL, E/T = 10:1, and error bars indicate standard deviation. For PCR experiments (C), DAP10-specific primers were used and the amplified product was separated on a 0.9% agarose gel. (D) For Western blotting experiments, purified CD8+ cells were isolated at various times in culture and extracts (50 μg) were separated on a 15% acrylamide gel, then transferred to PVDF membranes, and probed using either a DAP10 or γ-tubulin–specific antibody (loading control). Results are representative of 2 or more individual experiments.
) or anti-NKG2D and anti-CD3 (▪). For panels A and B, antibodies were used at 20 μg/mL, E/T = 10:1, and error bars indicate standard deviation. For PCR experiments (C), DAP10-specific primers were used and the amplified product was separated on a 0.9% agarose gel. (D) For Western blotting experiments, purified CD8+ cells were isolated at various times in culture and extracts (50 μg) were separated on a 15% acrylamide gel, then transferred to PVDF membranes, and probed using either a DAP10 or γ-tubulin–specific antibody (loading control). Results are representative of 2 or more individual experiments.
NKG2D is incapable of directly signaling and instead uses a transmembrane adapter protein to transmit surface signals into the cell. The only adapter protein known to bind to human NKG2D is DAP10.28 To investigate the expression of DAP10 over time in culture, PCR was performed for the expression of DAP10 transcripts. DAP10 was not amplified from freshly isolated purified CD8+ T cells, but by day 10 in culture it was consistently detectable (Figure 4C). To confirm that DAP10 was amplified, gel fragments were purified and sequenced. Near complete concordance with the DAP10 published sequence was observed (not shown). To confirm that DAP10 message was translated into protein, Western blotting was performed. DAP10 protein expression could consistently be detected in CD8+ selected cells at days 14 and 21 of culture (Figure 4D). Interestingly, DAP10 could not be identified in purified CD4+ cells at any time point (not shown). Thus, cytotoxic potential developed concurrently with the expression of the NKG2D adaptor molecule DAP10.
Effect of IL-2 dose on DAP10 expression
Culturing cytotoxic T-cell clones in low doses of IL-2 is one method of reducing “nonspecific” cytotoxicity. Thus, we reasoned that the dose of IL-2 might be critical for the development of cytotoxicity in cultured T cells. To formally evaluate the impact of IL-2 dose on cytotoxicity, T cells were activated and expanded in media containing either 30 U/mL or 300 U/mL IL-2 and cells were assayed for the expression of NKG2D, DAP10, and cytotoxic function. There was essentially no difference in the cell surface expression of NKG2D when cells were cultured in either 30 U/mL or 300 U/mL IL-2 (Figure 5A). In contrast, there was a marked difference in cytotoxicity because cells cultured in 30 U/mL IL-2 mediated no cytolysis, whereas cells from the same donor cultured at the higher does of IL-2 (300 U/mL) mediated significant cytolysis (Figure 5B). Evaluation of DAP10 expression by RT-PCR showed that cells cultured with low doses of IL-2 (30 U/mL) had no detectable DAP10 transcripts, whereas DAP10 could be readily identified in cells cultured with 300 U/mL IL-2 (Figure 5C). Thus, cytotoxicity of expanded T cells is likely to be regulated at the level of the expression of the adapter protein DAP10 and this is in turn regulated through IL-2 signaling.
IL-2 dose influences cytotoxicity and DAP10 expression but has no effect on NKG2D cell surface expression. Cells were isolated from donors as described in “Materials and methods” and divided into 2 separate fractions that were treated identically except that one received high doses of IL-2 (300 U/mL) and the other low-dose IL-2 (30 U/mL). (A) FACS analysis shows no difference in NKG2D expression on CD3+CD8+ cells at IL-2 doses of either 30 or 300 U/mL. Dotted histograms represent isotype control antibodies and solid lines NKG2D. (B) Cytolysis against 721-221 cells (E/T = 10:1) demonstrates no cytolysis of targets when cells were cultured with 30 U/mL IL-2, but significant cytolysis when cultured in 300 U/mL. (C) Expression of DAP10 by RT-PCR was detected in cells that were cultured in high (300 U/mL) but not low doses of IL-2 (30 U/mL). Results are representative of 3 individual experiments.
IL-2 dose influences cytotoxicity and DAP10 expression but has no effect on NKG2D cell surface expression. Cells were isolated from donors as described in “Materials and methods” and divided into 2 separate fractions that were treated identically except that one received high doses of IL-2 (300 U/mL) and the other low-dose IL-2 (30 U/mL). (A) FACS analysis shows no difference in NKG2D expression on CD3+CD8+ cells at IL-2 doses of either 30 or 300 U/mL. Dotted histograms represent isotype control antibodies and solid lines NKG2D. (B) Cytolysis against 721-221 cells (E/T = 10:1) demonstrates no cytolysis of targets when cells were cultured with 30 U/mL IL-2, but significant cytolysis when cultured in 300 U/mL. (C) Expression of DAP10 by RT-PCR was detected in cells that were cultured in high (300 U/mL) but not low doses of IL-2 (30 U/mL). Results are representative of 3 individual experiments.
Discussion
T cells that are activated and expanded with CD3 cross-linking antibodies and IL-2 attain significant cytotoxic activity against a wide variety of malignant cell lines and autologous leukemic blasts after 10 to 20 days of culture. Murine activated and expanded T cells mediate cytolysis against both syngeneic and allogeneic targets in vitro and eradicate minimal residual malignant cells after autologous BMT.18 When adoptively transferred after allogeneic BMT, such cells have a reduced propensity to induce graft-versus-host disease18,19,29 but retain graft-versus-tumor effects.18,19 Ex vivo–activated and –expanded T cells can be generated from patients with active leukemia and can mediate cytolysis against autologous malignant cells.15-17,24 Phase 1 clinical trials have established that the administration of ex vivo–activated and –expanded T cells is well tolerated with few infusion-related events.20,30-32 A randomized, controlled trial by Takayama and coworkers demonstrated that following surgical resection for hepatocellular carcinoma, patients receiving multiple infusions of autologous activated and expanded T cells had a significantly lower risk of disease recurrence compared to those randomized to no treatment.20
Previous studies have suggested that activated and expanded T cells recognize tumor targets through an MHC-unrestricted, TCR-independent mechanism because antibody masking of either the TCR, MHC class I or II, or the coreceptors CD4 and CD8 had no impact on cytotoxicity.25 Despite this MHC-unrestricted target recognition, CD3 or TCR signaling remains intact because cross-linking of these receptors can induce either cytotoxic granule content release33,34 or activation-induced cell death.21 Cell-to-cell contact is necessary for cytotoxicity because antibodies directed against CD54 or CD11c (or both) significantly attenuate cytotoxicity.25 Studies with perforin knock-out mice demonstrate that tumor cytolysis occurs through a granule release pathway (granzyme/perforin) and not through FasL.19 Although informative, these studies have failed to identify the exact receptor ligand pairs used for tumor recognition. Here we demonstrate that ex vivo–activated and –expanded CD8+ T cells use NKG2D to recognize tumor targets, and by blocking this interaction, we attenuate the majority of cytotoxicity.
NKG2D is expressed on the surface of all NK cells and some T cells.22,23 Previous studies have demonstrated that stimulation of T cells with either CD3 antibodies or CD3/CD28 antibody-coated beads increases cell surface NKG2D over 96 hours.4,22 Our results support these findings because we also found that NKG2D expression increases on T cells after ex vivo activation and expansion over the 14- to 21-day culture period. Our studies further show that NKG2D expression was significantly higher on CD8+ (versus CD4+) T cells after activation and expansion, again consistent with other investigators.4 We have previously shown that T cells that express CD56 are the major cytotoxic effector cells in these cultures,24 but interestingly, although CD3+CD56+ T cells had high levels of cell surface NKG2D compared to CD3+CD56- cells, NKG2D expression was not significantly higher than the CD3+CD8+ population overall, suggesting that cell surface NKG2D itself cannot account for the increased cytotoxicity in this subpopulation. Also interesting was the fact that when compared to CD8+ T cells or CD3+CD56+ T cells, NKG2D expression was lower on both resting and activated NK cells.
Multiple studies support the concept that NKG2D ligation results in different functional outcomes on either NK cells or T cells. More specifically, in NK cells NKG2D triggering induces activating signals,22,23 whereas in T cells the same stimulus induces costimulatory signals.4,12 Using CMV-specific CTLs, Groh et al12 demonstrated that on TCR triggering, NKG2D stimulation enhances cytokine production, T-cell proliferation, and cytolysis. Importantly, NKG2D signaling augmented cytotoxicity only when TCR triggering occurred (ie, when CTL clones were MHC matched with targets). If the CTLs and target cells were MHC mismatched, NKG2D did not directly activate cytolysis despite high levels of MICA on the target cells.12 There are, however, some populations of T cells that do possibly use NKG2D as a direct activating molecule. Intraepithelial lymphocytes (IELs) that are cultured with IL-15 up-regulate surface NKG2D and display potent NKG2D-mediated redirected cytolysis.35 In addition, certain γ/δ T clones (using the Tδ1 receptor) use both the TCR and NKG2D to bind to MICA, thus receiving an activating and a costimulatory signal through the same molecule (ie, signal 1 through TCR/MICA and signal 2 through NKG2D/MICA).11,36 Using purified activated and expanded CD8+ T cells and targets that cannot present peptide antigen (MHC class I negative), we found that NKG2D-blocking antibodies significantly attenuated cytolysis. Using siRNA complementary to NKG2D resulted in a near total inhibition of NKG2D protein on the cell surface and this in turn abolished cytotoxicity (while not affecting either CD3 or αβ TCR expression), thus directly demonstrating a TCR-independent, MHC-unrestricted, NKG2D-dependent mechanism of cytotoxicity.
In murine lymphocytes alternate splicing of NKG2D mRNA results in a long and short species (NKG2D-L and NKG2D-S) that differentially associate with the adapter proteins DAP10 and DAP12.37 NKG2D-S interacts with either DAP10 or DAP12, where as NKG2D-L preferentially associates with DAP10. On activation, both murine NK cells and T cells express NKG2D-S and NKG2D-L. In NK cells DAP10 and DAP12 could be detected, whereas in short-term activated T cells only DAP10 was detected.37 This differential expression of DAP12 possibly explains how NKG2D triggering results in different functional outcomes in T cells versus NK cells (for a review, see Long38 ). More recent studies question the importance of DAP12 in the direct activating function of NKG2D because NK cells derived from DAP12-/- mice or from mice deficient in DAP12 downstream signaling molecules (Syk or ZAP70) have near normal cytotoxicity (which is mediated through a DAP10, phosphatidylinositol 3-kinase [PI3K] pathway).39
In human NK cells DAP12 is expressed, but unlike DAP10, DAP12 has not been shown to associate with NKG2D,28 and to date, the only known adapter protein to associate with human NKG2D is DAP10.42 We found that human activated and expanded T cells expressed DAP10 at the onset of cytotoxicity (day 10 of culture). Only cells cultured in high doses of IL-2 (300 U/mL) showed expression of this adapter protein and were capable of cytotoxic function. T cells cultured in low doses of IL-2 (30 U/mL) did not express DAP10, nor were they capable of mediating cytolysis despite high levels of cell surface NKG2D. Thus, the activation state of the cells may critically influence the ability to directly signal through NKG2D. In our studies, such activation was induced by high doses of IL-2 (300 U/mL). How high-dose IL-2 (300 U/mL) might influence the activation status and expression of proteins in the NKG2D-DAP10 signaling pathway requires further study, but one possible explanation is that high doses of IL-2 may also stimulate the IL-15 receptor and this may in turn regulate the expression of DAP10. Interestingly, the addition of IL-15 to cultured T cells induces both CD56 expression and enhances MHC-unrestricted cytotoxicity.35,40,41
NKG2D interacts with at least 6 counterligands, including MICA, MICB, ULBP1, ULBP2, ULBP3, and ULBP4. MICA and MICB are nonclassical MHC molecules that are highly polymorphic but do not bind peptide.7 They are either absent or expressed at low levels on resting tissues and up-regulated in response to cellular “stress” including heat shock, viral infection, and malignant transformation.7,11 Although initially thought to be present only on epithelial malignancies, more recent studies document the expression of these NKG2D ligands on a variety of both solid and liquid tumors.11,43,44 The UL-16 binding proteins, ULBP1 to 3 also appear to be preferentially expressed on malignant tissues, but mRNA has been detected in normal tissues.6 Malignant cell lines have not yet been systematically examined for ULBP4 expression. Given the relatively restricted expression of these ligands and the effects that they have on T and NK cells, such a system is an ideal target for immunotherapy. In fact, overexpression of NKG2D ligands can induce potent rejection of tumors in murine model systems.45,46
Through these studies we have found that CD8+ T cells expanded with TCR cross-linking antibodies and high doses of IL-2 predominately use NKG2D to identify malignant cell targets and mediate cytolysis. It is not entirely clear why T cells, which have been endowed with a mechanism of exquisite target specificity (ie, the TCR), are also able to mediate NKG2D-driven MHC-unrestricted cytotoxicity. Moreover, it is not known whether such a process exists under physiologic conditions or if such findings are a result of the ex vivo expansion conditions. It may be that NKG2D is used by T cells as a direct activating molecule only under conditions of extreme circumstance, which result in high local concentrations of cytokines, but further study is clearly required. Regardless, understanding the mechanism of how expanded CD8+ T cells recognize and mediate cytotoxicity may provide important information into T-cell biology and will aid in the rational design of clinical trials using such cells as immune-based therapy for malignancies.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-06-2125.
Supported by National Institutes of Health grants K08 HL04505-03 (M.R.V.), P01 CA49605 (R.S.N.), and R01 CA8006 (R.S.N.) and a grant from the Dana Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr David Cosman for generously providing the antibodies against NKG2D, MICA, MICB, and ULBP1-3 and Drs Thai Cao and Mo Dao for the many lengthy and helpful discussions.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal