Abstract
The junctional membrane protein vascular endothelial (VE)–cadherin mediates contact inhibition of growth and inhibits apoptosis of endothelial cells. In this article we show that VE-cadherin induces expression of growth arrest–specific 1 (Gas1), an integral membrane protein up-regulated in nonproliferating cells. By comparing syngenic endothelial cell lines, we found that Gas1 mRNA was increased by 3-fold in VE-cadherin–positive cells in comparison to VE-cadherin–null cells. Ectopic expression of Gas1 in endothelial or 293 cells strongly reduced apoptosis without affecting cell growth. Addition of vascular endothelial growth factor (VEGF) also up-regulated Gas1 and this effect was augmented more so in confluent nonproliferating cells than in sparse cultures. VE-cadherin–blocking antibody partially inhibited VEGF-induced Gas1, suggesting that VE-cadherin clustering is required for an optimal response to this stimulus. Inhibition of phosphoinositole-3-OH kinase (PI3-kinase) pathway by Wortmannin prevented Gas1 synthesis and the antiapoptotic effect of VEGF, but, in cells ectopically expressing Gas1, Wortmannin was ineffective. Furthermore, inhibition of Gas1 expression by short interfering RNA (siRNA) both in vitro and in allantois organ cultures made endothelial cells refractory to the antiapoptotic effect of VEGF. Overall these data indicate that Gas1 induction by VE-cadherin and VEGF in endothelial cells requires activation of PI3-kinase. Gas1 expression positively correlates with inhibition of endothelial cell apoptosis and may contribute to the integrity of resting endothelium. (Blood. 2004;103:3005-3012)
Introduction
Endothelial cells constitute a continuous monolayer that lines and protects the vessel wall. Changes in the integrity of the endothelium may result in uncontrolled permeability to plasma proteins and circulating cells leading to inflammatory and thrombotic reactions and lipid infiltration.1 In resting conditions, endothelial cells are contact inhibited in their growth and present a remarkably long half-life and resistance to noxious stimuli.2 Evidence suggests that, upon establishment of intercellular contacts, junctional adhesive proteins transfer intracellular signals that negatively modulate cell proliferation and sensitivity to apoptotic stimuli.3
Vascular endothelial (VE)–cadherin is an endothelial-specific junctional protein that promotes homophilic, Ca++-dependent adhesion at cell-to-cell adherens junctions.4-6 Through its cytoplasmic tail, VE-cadherin binds to p120, β-catenin, and plakoglobin, which indirectly mediate the anchorage to actin microfilaments.7-9 β-Catenin and, in some conditions, plakoglobin and p120, when released and stabilized in the cytosol, can translocate to the nucleus and influence gene transcription.10,11 We have previously shown that, similarly to other members of the cadherin family, VE-cadherin is required for contact inhibition of cell growth12-14 and for protection from apoptosis induced by serum deprivation.15,16 VE-cadherin expression may therefore modulate genes important in cell cycle regulation and programmed cell death.
The nonproliferative state of several types of cells is accompanied by the up-regulation of a group of 6 genes called growth arrest–specific (Gas) genes. These proteins are structurally unrelated but may exert a coordinated action in modulating cell growth and survival.17 Through gene profile analysis, we identified Gas1 as a gene markedly up-regulated by VE-cadherin expression. Gas1 is an integral membrane protein up-regulated by cell confluency and growth inhibition.
In other cell types Gas1 is induced in response to stimuli that drive cells to G0 phase.18 Ectopic expression of Gas1 inhibits fibroblast and tumor cell proliferation in a p53-dependent way.19-21 However, Gas1 mechanism of action is largely unknown and its activities may vary in different cell systems22,23 and in the embryo.24,25 In this article we show that expression of Gas1 in endothelial cells does not significantly inhibit cell growth but strongly protects the cells from apoptosis. We also found that, besides VE-cadherin, VEGF also up-regulates Gas1 consistently with its antiapoptotic activity15,26 but independently from its mitogenic effect.
Overall, these data disclose a novel activity of Gas1 and suggest that in endothelial cells this mediator may have a more general biologic significance by promoting cell stabilization and resistance to toxic agents.
Materials and methods
Cells and culture conditions
The 293 cells (American Type Culture Collection CRL 1573, Manassas, VA) were grown in Iscove modified Dulbecco minimum essential medium (D-MEM; Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS; Life Technologies). Murine endothelial cells with homozygous null mutation of VE-cadherin gene, isolated from embryonic stem cells,15,27 were cultured in D-MEM (Life Technologies) supplemented with 10% FCS (HyClone, Logan, UT) as previously described.8,15,27 Culture medium for human umbilical vein endothelial cells (HUVECs) was M-199 (Life Technologies) supplemented with 50 μg/mL endothelial cell growth supplement (ECGS; Sigma Chemical, St Louis, MO) and 100 μg/mL heparin (Sigma Chemical).8 Disposable plastic culture was from Falcon (Becton Dickinson Labware, Franklin Lakes, NJ).
Plasmid construction and cell infection
The 293 cells were cotransfected with pVgRXR plasmid and empty cDNA or human VE-cadherin wild-type cDNA28 pIND (sphingosine 1–phosphate [SP1]) mammalian expression vector29 (Ecdysone-Inducible Expression Kit; Invitrogen, Carlsbad, CA). After 20 days of neomycin (fc 600 μg/mL) and zeocin (fc 400 μg/mL) selection, different clones were isolated and treated for 24 hours at 37°C with 1 μM of the ecdysone analog muristerone A (Invitrogen) to induce the expression of the exogenous gene. The correct VE-cadherin expression and cellular localization were tested by Western blot and immunofluorescence analysis (see “Immunofluorescence”).
Wild-type VE-cadherin or a truncated mutant, lacking the last 80 amino acids of the cytoplasmic tail30 and mouse or human Gas118,31 cDNAs, were cloned into a retroviral vector pINCO, expressing green fluorescent protein (GFP) as previously described.32
The infection efficiency was tested measuring VE-cadherin or GFP expression by fluorescence-activated cell scanner cytometry (FACS) performed by a FACStar Plus apparatus (Becton Dickinson, Mountain View, CA). To avoid clonal selection heterogeneity, cells were sorted and homogeneous cell populations (expressing GFP by > 90%) were used for the experiments. For each cDNA, control cells were infected with pINCO expressing GFP only.
Antibodies
Mouse antihuman VE-cadherin monoclonal antibody (mAb), clone BV9, was produced in our laboratory as described.33 Rabbit polyclonal antibody against Gas1 (anti-229) and rat antimouse platelet endothelial cell adhesion molecule 1 (PECAM-1; MEC 13.3) were characterized previously.34,35 Mouse anti–γ-tubulin mAb was purchased from Sigma Chemical. Rhodamine (TRITC)–conjugated and fluorescein isothiocyanate (FITC)–conjugated secondary antibodies reactive against mouse and rat immunoglobulin (IgGs) were purchased from Jackson Immunoresearch Laboratories (West Grove, PA). Antirabbit and antimouse peroxidase-conjugated secondary antibodies (for Gas1 and γ-tubulin) were from Sigma. Western blots were performed according to standard protocols, and the proteins were visualized by enhanced chemiluminescence (ECL; Amersham Biosciences, Little Chalfont, United Kingdom).34-36 The signals on film (Hyperfilm ECL; Amersham Biosciences) were scanned and quantified using the National Institutes of Health (NIH) Image 1.62 program for Macintosh computer, developed at the NIH and freely available at http://rsb.info.nih.gov/nih-image/website.
Immunofluorescence
VE-cadherin-transfected 293 cells were cultured on glass coverslips, and immunofluorescence was performed as described.8 Briefly, cells were fixed in 3% formaldehyde from paraformaldehyde (PAF) for 15 minutes and permeabilized with 0.5% Triton X-100 before staining. After incubation with anti–VE-cadherin BV9 antibody for 1 hour at 37°C, cells were labeled with appropriate TRITC-conjugated secondary antibody (Jackson Immunoresearch Laboratories). Fluorescence was detected with a fluorescence microscope (Leica DMR, Wetzlar, Germany) and images were recorded with a Hamamatsu 3CCD camera (Hamamatsu Photonics, Hamamatsu-City, Japan) before processing through Adobe Photoshop software for Macintosh (Adobe Systems, San Jose, CA).
GeneChip protocol
Total RNAs from confluent 293 cells expressing or not expressing VE-cadherin were obtained as described.37 Oligo array hybridization probes were prepared and used according to the Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix, Santa Clara, CA), and the hybridization results were analyzed with Affymetrix GeneChip Software, according to standard Affymetrix procedure.38,39
Cell growth assay
To evaluate DNA synthesis, a double staining of total nuclei and nuclei of cells in S phase was performed as described.12 Briefly, endothelial cells were cultured on fibronectin-coated (7 μg/mL; Sigma Chemical) glass coverslips in 24-well plates, and 30 μM bromodeoxyuridine (BrdU; Roche Diagnostic, Mannheim, Germany) was added to the culture medium for 4 hours at 37°C. Cells were then fixed with 3% PAF (see “Immunofluorescence”) and treated for 10 minutes with 2N HCl. BrdU was revealed by a specific mAb (Amersham Biosciences) followed by TRITC-conjugated antibody to mouse immunoglobulin (Jackson Immunoresearch Laboratories) in the presence of Hoechst 33258 (0.1 μg/mL; Sigma Chemical). Coverslips were then mounted in Mowiol 4-88 (Calbiochem-Novabiochem, San Diego, CA) and examined for immunofluorescence staining. The percentage of BrdU uptake for each type of cell was calculated using the following formula: (number BrdU-positive cells × 100)/number cell nuclei.
For growth curves, murine endothelial cells were seeded (3 × 104 cells/cm2) in 24-well plates in D-MEM with 10% FCS. For synchronization, 24 hours later cells were serum starved for 24 hours in serum-free MCDB-131 medium (Life Technologies) supplemented with 1% bovine serum albumin (BSA; Sigma Chemical). Fresh D-MEM with 10% FCS was then added and cells were counted at different days thereafter. Medium was changed only at 3 days after the beginning of the experiment.
Apoptosis
To induce apoptosis, endothelial cells were treated as described.15,40 Briefly, 4 × 104 cells were seeded in a 96-well plate in 100 μL serum-free MCDB-131 medium supplemented with 1% BSA. Cells were cultured for 72 hours either in the presence or absence of 80 ng/mL of VEGF (R&D Systems, Minneapolis, MN). For apoptosis assays, 293 cells were cultured in 3-dimensional collagen gels as reported previously.27 After 8 days, cells were treated for 30 minutes with 5 mg/mL of collagenase A (Sigma Chemical) and resuspended in phosphate-buffered saline (PBS). Apoptosis was quantified by measuring DNA fragmentation (TUNEL [terminal deoxynucleotidyl transferase {TdT}–mediated deoxyuridine triphosphate {dUTP} nick end labeling] detection method; Roche Diagnostic) or by Cell Death Detection ELISA plus (Roche Diagnostic). To block phosphoinositole-3-OH kinase (PI3-kinase) activity in endothelial cells, 0.1 μM Wortmannin (Sigma Chemical) was added 15 minutes before stimulation with VEGF.
Quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR)
Two micrograms of total RNA from different samples were reverse transcribed in a final volume of 50 μL using Taqman Reverse Transcriptions Reagents (Perkin Elmer Applied Biosystems, Foster City, CA). To avoid amplification of contaminating genomic DNA, samples were previously treated with RQ1 RNAse-free DNAse (Promega, Madison, WI). Primers and probe sequences for human or mouse Gas1 and mouse β2 microglobulin were designed using the primer design software Primer Express (Perkin Elmer Applied Biosystems). Both the human and mouse Gas1 probes were labeled with the reporter dye FAM (6-carboxy-fluorescein), whereas the mouse β2 microglobulin probe and the human glyceraldehyde phosphate dehydrogenase (GAPDH) Taqman predeveloped assay reagent (PDAR) were labeled with the reporter dye VIC. All PCR reactions were performed using an ABI Prism 7700 Sequence Detection System (Perkin Elmer Applied Biosystems). For any sample, the Gas1 expression level, normalized to the housekeeping genes β2 microglobulin (in case of murine cells) or GAPDH (in case of human cells), was determined using the comparative threshold cycle (CT) method as previously described.41
RNA interference
RNA interference (RNAi) of Gas1 expression was induced with short interfering RNA (siRNA) directed against the gene. Positive 21-nucleotide siRNA (UGG.CGC.UGC.UGC.AGC.UGC.U.dT.dT) and (A.GCA.GCU.GCA.GCA.GCG.CCA.dT.dT) targeted human Gas1 mRNA sequence in position 484-502. The scramble II Duplex, siACE-RNAi, was used as negative control. Both positive and control oligonucleotides were from Dharmacon Research (Dharmacon RNA Technologies, Lafayette, CO).
HUVECs were seeded at a density of 20 000 cells/cm2 the day before transfection and they were about 40% confluent when they were transfected with 100 nM positive or scramble oligonucleotides in Oligofectamine (Invitrogen) and Optimem (Life Technologies) without serum or BSA. Before transfection the cells were washed once with Optimem. Transfection medium was maintained on cells for 4 hours, then it was removed and substituted with complete medium. Transfection was repeated after 24 hours and cells were used for the experiments after further 24 hours. Higher concentrations or number of transfections of both positive and scrambled oligonucleotides caused cell toxicity and were excluded from the study. Gas1 expression was tested by Western blot as described above.
Allantois culture
For ex vivo experiments, pregnant female BALB/c mice (Charles River Italia, Calco, Italy) were used. Allantoises were isolated as previously described.42,43 Briefly, embryos at 8.5 dpc (days after coitus) were isolated and the allantoises were excised, washed in PBS (4°C), and then pipetted into NUNC 4-chambered culture slides (Nalgene Nunc International, Roskilde, Denmark) containing 0.4 mL D-MEM with 10% FCS and 1% penicillin-streptomycin. Explants were cultured at 37°C in 5% CO2 incubator for 18 hours and transfected with 100 nM positive or scrambled oligonucleotides as described in “RNA interference.” Transfection was repeated after 24 hours. To induce apoptosis, cultured allantoises were then exposed for an additional 24 hours to serum-free MCDB-131 medium supplemented with 1% BSA in the presence or absence of 80 ng/mL VEGF as described in “Apoptosis.” Allantois cultures were then fixed and permeabilized as shown previously in detail,42 exposed to PECAM-1 antibody, and apoptotic cells were detected by TUNEL staining. Images were processed and analyzed using ImageJ 1.30k, freely available at http://rsb.info.nih.gov/ij/download.html website.
Results
Gas1 is induced by VE-cadherin expression
To investigate VE-cadherin–induced gene expression, we transfected the 293 cell line with a eukaryotic expression vector containing human VE-cadherin cDNA under the control of an ecdysone-inducible promoter. As reported in Figure 1A by Western blot analysis, while untreated 293 cells did not express detectable VE-cadherin, they did so after treatment with ecdysone. By immunofluorescence analysis, VE-cadherin was also correctly organized at intercellular junctions (Figure 1B). Gene expression pattern induced by VE-cadherin was studied by oligo array hybridization (Affymetrix GeneChip Expression Analysis, chip Hu6800).
VE-cadherin expression induces Gas1 in 293 cells. The 293 cells were stimulated for 24 hours at 37°C with 1μM of the ecdysone analog muristerone A to express transfected VE-cadherin. Western blot (A) and immunofluorescence (B) analysis confirmed VE-cadherin expression and its correct localization at cell-cell contacts only in stimulated (Ecdysone +) 293 cells. Original magnification, × 400 (B). (C-D) Analysis of Gas1 up-regulation in ecdysone-treated 293 cells by Northern blot and quantitative RT-PCR. The RT-PCR data are means ± SD of 3 different experiments performed in triplicates and are expressed as relative amounts using “Ecdysone -” cell lysate as reference value. Cells transfected with the empty vector and treated with ecdysone did not show detectable VE-cadherin expression by Western blot or up-regulation of Gas1 mRNA using Northern blot analysis (not shown). For Northern blot, a comparable amount of total RNA was loaded as shown by ethidium bromide staining of the membrane. The position of 2 ribosomal RNAs (28S and 18S) is indicated.
VE-cadherin expression induces Gas1 in 293 cells. The 293 cells were stimulated for 24 hours at 37°C with 1μM of the ecdysone analog muristerone A to express transfected VE-cadherin. Western blot (A) and immunofluorescence (B) analysis confirmed VE-cadherin expression and its correct localization at cell-cell contacts only in stimulated (Ecdysone +) 293 cells. Original magnification, × 400 (B). (C-D) Analysis of Gas1 up-regulation in ecdysone-treated 293 cells by Northern blot and quantitative RT-PCR. The RT-PCR data are means ± SD of 3 different experiments performed in triplicates and are expressed as relative amounts using “Ecdysone -” cell lysate as reference value. Cells transfected with the empty vector and treated with ecdysone did not show detectable VE-cadherin expression by Western blot or up-regulation of Gas1 mRNA using Northern blot analysis (not shown). For Northern blot, a comparable amount of total RNA was loaded as shown by ethidium bromide staining of the membrane. The position of 2 ribosomal RNAs (28S and 18S) is indicated.
Several genes were consistently up- or down-regulated by the presence of VE-cadherin, many of them related to the control of cell cycle, cell adhesion, cytoskeleton organization, and apoptosis (F.B. et al., manuscript in preparation). Among the up-regulated genes, we were particularly intrigued by Gas1 for its role in cell growth inhibition.17,18 VE-cadherin induction of Gas1 in 293 cells was confirmed by both Northern blot and RT- PCR (Figure 1C-D).
Since VE-cadherin is specifically expressed in endothelial cells, it was important to extend the analysis to this cell type. As reported in Figure 2A, in freshly isolated sparse HUVECs the synthesis of Gas1 was increased upon exposure to serum and, more marked, by reaching confluency.
Confluency and VE-cadherin up-regulate Gas1 in endothelial cells. (A) Quantitative RT-PCR analysis of Gas1 mRNA in freshly isolated HUVECs. Confluent (5 × 104/cm2) and sparse (4 × 103/cm2) cells were cultured for 24 hours in the presence or absence of 20% FCS. At the end of this period mRNA was extracted as described in “Materials and methods.” RT-PCR data are means ± SD of 3 separate experiments performed in triplicate and are expressed as relative amounts using “sparse cells/serum -” as reference value. (B) Quantitative RT-PCR analysis of isogenic endothelial cells VE-cadherin–null or expressing the VE-cadherin wild-type (VE-cadherin positive) or truncated (tVE) cDNA. Cells were seeded at the same density and cultured for 3 days in the presence of 10% FCS and then extracted for Gas1 mRNA evaluation. RT-PCR values are means ± SD of 4 different experiments performed in triplicate and are expressed as relative amounts using “VE-cadherin null” cell lysate as reference value.
Confluency and VE-cadherin up-regulate Gas1 in endothelial cells. (A) Quantitative RT-PCR analysis of Gas1 mRNA in freshly isolated HUVECs. Confluent (5 × 104/cm2) and sparse (4 × 103/cm2) cells were cultured for 24 hours in the presence or absence of 20% FCS. At the end of this period mRNA was extracted as described in “Materials and methods.” RT-PCR data are means ± SD of 3 separate experiments performed in triplicate and are expressed as relative amounts using “sparse cells/serum -” as reference value. (B) Quantitative RT-PCR analysis of isogenic endothelial cells VE-cadherin–null or expressing the VE-cadherin wild-type (VE-cadherin positive) or truncated (tVE) cDNA. Cells were seeded at the same density and cultured for 3 days in the presence of 10% FCS and then extracted for Gas1 mRNA evaluation. RT-PCR values are means ± SD of 4 different experiments performed in triplicate and are expressed as relative amounts using “VE-cadherin null” cell lysate as reference value.
To test whether VE-cadherin could play a role in density-induced Gas1 expression, we compared VE-cadherin–null endothelial cells with the same cells expressing wild-type or a truncated form of the protein (“Materials and methods”). This mutant lacks the binding domain for β-catenin and therefore the anchorage to actin.30 As previously published,15,36 more than 90% of transfectants express VE-cadherin (wild-type or mutant form) at cell-to-cell contacts and present all endothelial markers tested retaining their endothelial characteristics. As shown in Figure 2B, confluent cells expressing wild-type VE-cadherin showed about 3-fold higher level of Gas1 mRNA than VE-cadherin–null cells or cells transfected with the truncated mutant. Sparse VE-cadherin–positive cells did not show detectable Gas1 levels (not shown).
Gas1 protects cells from apoptosis
Despite measurable levels of Gas1 mRNA (Figure 2B), we were unable to detect the protein in murine endothelial cells (Figure 3A). This is likely due to low antiserum sensitivity and low levels of Gas1 in these endothelial cell lines.
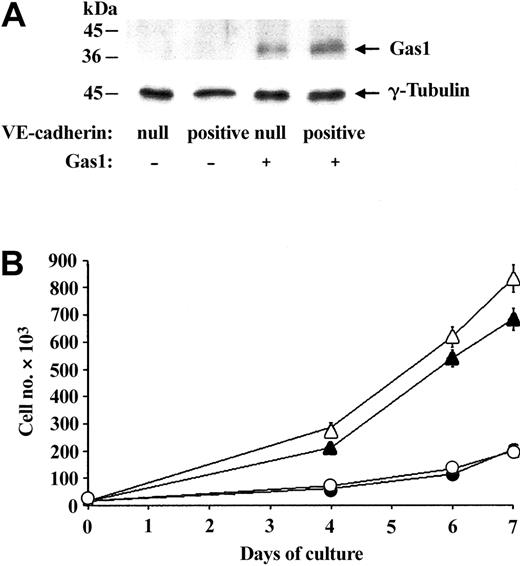
Ectopic expression of Gas1 does not change endothelial cell growth. (A) Western blot analysis of Gas1 expression. Equal amounts of extracts of murine VE-cadherin–null and VE-cadherin–positive endothelial cells transfected or not with Gas1 cDNA were studied. Cells were seeded at the same density and cultured in the presence of 10% FCS for 3 days before extraction. In cells overexpressing Gas1 the protein was detectable at the right molecular weight. Western blot of γ-tubulin was used as control. (B) Effect of overexpression of Gas1 on endothelial cell proliferation. Growth curves of VE-cadherin–null (▵ and ▴) and VE-cadherin–positive cells (○ and •), overexpressing (▵ and ○) or not overexpressing (▴ and •) Gas1 cDNA. The growth curves were performed as described in “Materials and methods” and data are means of 2 experiments performed in triplicate.
Ectopic expression of Gas1 does not change endothelial cell growth. (A) Western blot analysis of Gas1 expression. Equal amounts of extracts of murine VE-cadherin–null and VE-cadherin–positive endothelial cells transfected or not with Gas1 cDNA were studied. Cells were seeded at the same density and cultured in the presence of 10% FCS for 3 days before extraction. In cells overexpressing Gas1 the protein was detectable at the right molecular weight. Western blot of γ-tubulin was used as control. (B) Effect of overexpression of Gas1 on endothelial cell proliferation. Growth curves of VE-cadherin–null (▵ and ▴) and VE-cadherin–positive cells (○ and •), overexpressing (▵ and ○) or not overexpressing (▴ and •) Gas1 cDNA. The growth curves were performed as described in “Materials and methods” and data are means of 2 experiments performed in triplicate.
To understand the biologic significance of Gas1 up-regulation, we ectopically expressed murine Gas1 cDNA (or empty vector) in VE-cadherin–null and –positive cells using a retroviral construct. By Western blot analysis, as reported in Figure 3A, the infection resulted in increased amounts of Gas1 levels in both cell types. To test whether Gas1 could take part in inhibition of growth, we compared the proliferation rate of VE-cadherin–null or –positive cells in the presence or absence of Gas1 overexpression. As previously reported,12,44 VE-cadherin–null cells reached a significantly higher density than VE-cadherin–positive cells and, surprisingly, Gas1 overexpression did not change the growth pattern of either VE-cadherin–null and –positive cells (Figure 3B; Table 1).
Effect of overexpression of Gas1 on endothelial cell proliferation
Cells . | . | . | |
|---|---|---|---|
| VE-cadherin . | Gas1 . | %BrdU uptake . | |
| Null | − | 53.3 ± 5.1 | |
| Positive | − | 26.7 ± 3.2 | |
| Null | + | 54.4 ± 6.2 | |
| Positive | + | 28.1 ± 1.5 | |
Cells . | . | . | |
|---|---|---|---|
| VE-cadherin . | Gas1 . | %BrdU uptake . | |
| Null | − | 53.3 ± 5.1 | |
| Positive | − | 26.7 ± 3.2 | |
| Null | + | 54.4 ± 6.2 | |
| Positive | + | 28.1 ± 1.5 | |
DNA synthesis, expressed as % of cells positive to BrdU. The percentage of BrdU uptake for each cell type was performed as described in “Materials and methods” and was calculated using the following formula: number BrdU-positive cells × 100/number cell nuclei. BrdU data are means ± SD of 3 different experiments performed in triplicates.
As previously observed,15 in the absence of serum, VE-cadherin–null cells died more effectively than in VE-cadherin–positive cells (Figure 4). Gas1 expression reduced cell apoptosis significantly, in both VE-cadherin-null and -positive cells (Figure 4). This effect was particularly apparent in VE-cadherin-null cells where apoptosis was reduced to values comparable to VE-cadherin-positive cells. Codistribution of GFP and TUNEL staining confirmed these results, showing that a much lower number of endothelial cells expressing Gas1 died compared with controls (Table 2). The protective effect of Gas1 expression on apoptosis was not endothelial specific but was also observed in 293 cells (Table 2).
Gas1 reduces endothelial cell apoptosis by serum deprivation. VE–cadherin-null and VE-cadherin–positive endothelial cells transfected or not transfected with Gas1 cDNA were cultured in absence of serum for 72 hours. Cell apoptosis was quantified by measuring DNA fragmentation as oligonucleosomes OD (absorbance A405nm - A490nm)/μg proteins of cell extract. The values are means ± SD of 4 separate experiments performed in triplicate.
Gas1 reduces endothelial cell apoptosis by serum deprivation. VE–cadherin-null and VE-cadherin–positive endothelial cells transfected or not transfected with Gas1 cDNA were cultured in absence of serum for 72 hours. Cell apoptosis was quantified by measuring DNA fragmentation as oligonucleosomes OD (absorbance A405nm - A490nm)/μg proteins of cell extract. The values are means ± SD of 4 separate experiments performed in triplicate.
Ectopic expression of Gas 1 inhibits apoptosis of 293 and endothelial cells
Cells and their transfected DNA . | No. of cells analyzed . | No. of GFP-positive cells . | No. of apoptotic GFP-positive cells . | No. of apoptotic GFP-negative cells . | Relative inhibition of apoptosis, % . |
|---|---|---|---|---|---|
| 293 | |||||
| GFP | 354 | 216 | 75 | 50 | 3 |
| GFP + Gas1 | 382 | 245 | 2 | 49 | 98 |
| VE-cadherin positive | |||||
| GFP | 425 | 382 | 48 | 6 | 13 |
| GFP + Gas1 | 453 | 410 | 13 | 6 | 77 |
| VE-cadherin null | |||||
| GFP | 410 | 372 | 145 | 16 | 7 |
| GFP + Gas1 | 434 | 391 | 12 | 17 | 92 |
Cells and their transfected DNA . | No. of cells analyzed . | No. of GFP-positive cells . | No. of apoptotic GFP-positive cells . | No. of apoptotic GFP-negative cells . | Relative inhibition of apoptosis, % . |
|---|---|---|---|---|---|
| 293 | |||||
| GFP | 354 | 216 | 75 | 50 | 3 |
| GFP + Gas1 | 382 | 245 | 2 | 49 | 98 |
| VE-cadherin positive | |||||
| GFP | 425 | 382 | 48 | 6 | 13 |
| GFP + Gas1 | 453 | 410 | 13 | 6 | 77 |
| VE-cadherin null | |||||
| GFP | 410 | 372 | 145 | 16 | 7 |
| GFP + Gas1 | 434 | 391 | 12 | 17 | 92 |
293, VE-cadherin–positive, and –null endothelial cells transfected either with GFP or GFP + Gas1 vector were cultured in absence of serum as described in “Materials and methods.” Apoptosis was measured by TUNEL assay. The values are means of triplicate from a typical experiment out of 3 performed. The relative inhibition of apoptosis was calculated by the following formula: percent = (% apoptotic GFP-negative cells - % apoptotic GFP-positive cells)/% apoptotic GFP-negative cells.
Gas1 is induced by VEGF
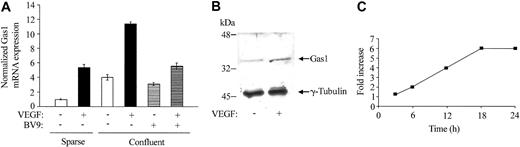
We tested whether Gas1 could be induced by other agents able to inhibit endothelial cell apoptosis. As reported in Figure 2A, serum increased Gas1 synthesis in sparse cells. The figure also shows that addition of VEGF to both sparse and confluent HUVECs induced a marked increase in Gas1 levels, as detected by RT-PCR (Figure 5A) and Western blot analysis (Figure 5B). In time course experiments, the effect of VEGF was relatively slow, becoming apparent between 6 and 12 hours and reaching maximal values around 18 hours (Figure 5C). The effect was unrelated to cell proliferation, since in confluent cells this concentration of VEGF (80 ng/mL) was poorly active in inducing BrdU uptake (< 5% of positive cells).
VEGF up-regulates Gas1 expression in HUVECs. (A) Quantitative RT-PCR analysis of Gas1 mRNA expression. Cells were seeded in sparse (4 × 103 cells/cm2) or confluent (5 × 104 cells/cm2) conditions in complete medium. Before addition of VEGF (80 ng/mL for 24 hours) cells were starved for 24 hours in serum-free medium supplemented with 1% BSA. Confluent cells were added with 50 μg/mL of anti–VE-cadherin–blocking mAb (BV9) for 1 hour before addition of VEGF. Data are means ± SD of 3 separate experiments performed in triplicate and are expressed as relative amounts using “sparse, VEGF -, mAb anti–VE-cadherin -” cell lysate as reference value. (B) Western blot analysis of Gas1 expression after VEGF treatment. (C) Time course of Gas1 mRNA expression after VEGF treatment. Confluent cells were activated with VEGF for the indicated times as described above. Data are means of 2 experiments performed in quadruplicate.
VEGF up-regulates Gas1 expression in HUVECs. (A) Quantitative RT-PCR analysis of Gas1 mRNA expression. Cells were seeded in sparse (4 × 103 cells/cm2) or confluent (5 × 104 cells/cm2) conditions in complete medium. Before addition of VEGF (80 ng/mL for 24 hours) cells were starved for 24 hours in serum-free medium supplemented with 1% BSA. Confluent cells were added with 50 μg/mL of anti–VE-cadherin–blocking mAb (BV9) for 1 hour before addition of VEGF. Data are means ± SD of 3 separate experiments performed in triplicate and are expressed as relative amounts using “sparse, VEGF -, mAb anti–VE-cadherin -” cell lysate as reference value. (B) Western blot analysis of Gas1 expression after VEGF treatment. (C) Time course of Gas1 mRNA expression after VEGF treatment. Confluent cells were activated with VEGF for the indicated times as described above. Data are means of 2 experiments performed in quadruplicate.
Confluent cells, however, produced higher levels of Gas1 in response to the growth factor compared with sparse cells. This difference was abolished adding the VE-cadherin–blocking mAb BV9, which is able to dismantle the cadherin from intercellular junctions.40 This suggests that, besides expression, clustering of this protein at cell-to-cell contacts is required for optimal VEGF response (Figure 5A).
Activation of PI3-kinase is necessary for Gas1 induction but not for its antiapoptotic activity
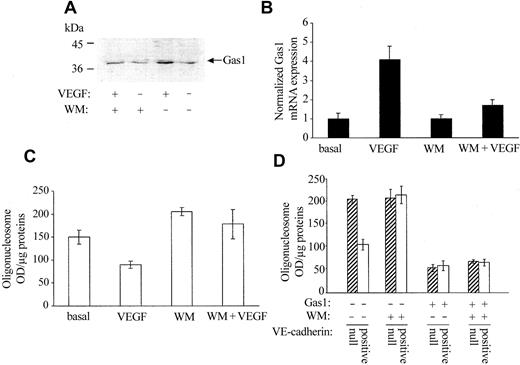
VEGF and VE-cadherin protect endothelial cells from apoptosis by activating PI3-kinase and inducing Akt phosphorylation.15 We tested whether inhibition of this pathway also affects Gas1 synthesis and/or activity. As reported in Figure 6A, Wortmannin, an inhibitor of PI3-kinase activity, inhibited Gas1 expression both in control and in VEGF-treated endothelial cells. Serum-starved HUVECs undergo apoptosis within 72 hours (Figure 6C basal). When they were treated with VEGF, Gas1 expression was increased by about 4 times (Figure 6B VEGF) and apoptosis was reduced (Figure 6C). Addition of Wortmannin further increased apoptosis in resting cells and inhibited the protective effect of VEGF (Figure 6C WM and WM+VEGF). Gas1 synthesis was inversely related to the apoptotic values and was strongly reduced by the addition of the drug (Figure 6B WM and WM+VEGF). Wortmannin was effective in increasing death of VE-cadherin–positive cells up to a value comparable to VE-cadherin–null cells (Figure 6D, compare ▨ and □). However, ectopic expression of Gas1 reduced apoptosis in both cells types in a way independent of Wortmannin. Overall, these data indicate that activation of PI3-kinase is needed for Gas1 synthesis but not for its activity.
PI3-kinase activation is required for Gas1 expression but not for its antiapoptotic activity. (A) Confluent HUVECs were treated with 0.1 μM Wortmannin (WM) 15 minutes before stimulation with 80 ng/mL VEGF for 24 hours. Gas1 synthesis was evaluated by Western blot. VEGF increases 3 times the amount of Gas1 in endothelial cell extracts (OD values: 5307 for resting cells and 14 879 for cells activated with VEGF); WM decreased both the basal and VEGF-induced Gas1 synthesis by 23% and 40%, respectively (OD values: 4126 vs 5307 for resting cells treated with WM and 8964 vs 14 879 for cells treated with VEGF and WM). Three independent experiments gave comparable results. (B) Cells were analyzed for Gas1 mRNA expression and cell apoptosis (C) by quantitative RT-PCR and DNA fragmentation, respectively. VEGF reduced apoptosis and increased Gas1 mRNA synthesis. Both effects were strongly inhibited by cell treatment with WM. The values are means ± SD of 4 separate experiments performed in duplicate. RT-PCR data are expressed as relative amounts taking “control cells” cell lysate as reference value. (D) Effect of WM on apoptosis of VE-cadherin–null and –positive cells transfected or not transfected with Gas1 cDNA. WM (0.1 μM) was added to the cells for the last 24 hours of duration of the experiment of apoptosis (Figure 4 legend). Data are means ± SD of 3 separate experiments performed in triplicate.
PI3-kinase activation is required for Gas1 expression but not for its antiapoptotic activity. (A) Confluent HUVECs were treated with 0.1 μM Wortmannin (WM) 15 minutes before stimulation with 80 ng/mL VEGF for 24 hours. Gas1 synthesis was evaluated by Western blot. VEGF increases 3 times the amount of Gas1 in endothelial cell extracts (OD values: 5307 for resting cells and 14 879 for cells activated with VEGF); WM decreased both the basal and VEGF-induced Gas1 synthesis by 23% and 40%, respectively (OD values: 4126 vs 5307 for resting cells treated with WM and 8964 vs 14 879 for cells treated with VEGF and WM). Three independent experiments gave comparable results. (B) Cells were analyzed for Gas1 mRNA expression and cell apoptosis (C) by quantitative RT-PCR and DNA fragmentation, respectively. VEGF reduced apoptosis and increased Gas1 mRNA synthesis. Both effects were strongly inhibited by cell treatment with WM. The values are means ± SD of 4 separate experiments performed in duplicate. RT-PCR data are expressed as relative amounts taking “control cells” cell lysate as reference value. (D) Effect of WM on apoptosis of VE-cadherin–null and –positive cells transfected or not transfected with Gas1 cDNA. WM (0.1 μM) was added to the cells for the last 24 hours of duration of the experiment of apoptosis (Figure 4 legend). Data are means ± SD of 3 separate experiments performed in triplicate.
Inhibition of Gas1 synthesis blocked the antiapoptotic effect of VEGF
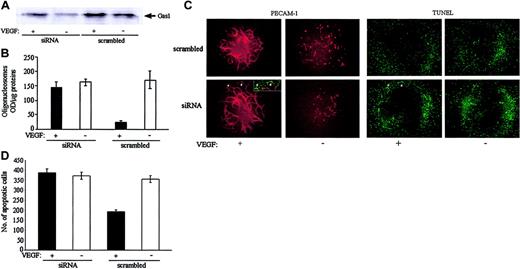
To further investigate whether Gas1 expression was required for the antiapoptotic effect of VEGF, HUVECs were targeted with Gas1-directed short interfering RNA (siRNA) or corresponding scrambled oligonucleotides. None of the transfected cells presented changes in morphology, retraction, or junctional distribution of VE-cadherin (not shown). As reported in Figure 7A, by Western blot analysis, siRNA directed to Gas1 decreased the synthesis of the protein by 70% and 50% in resting and VEGF-activated endothelial cells, respectively. In parallel, inhibition of Gas1 synthesis prevented the antiapoptotic effect of VEGF in the absence of serum.
Inhibition of Gas1 expression by siRNA prevented the antiapoptotic effect of VEGF. (A) Cells were incubated with siRNA as indicated in “Materials and methods” and then treated with VEGF (80 ng/mL for 24 hours) or control medium. Expression of Gas1 was evaluated by Western blot. Gas1 siRNA inhibited the expression of the protein by 70% in resting conditions (OD values: 2267 vs 7528 for control and siRNA treated cells, respectively) and by 50% in VEGF-treated cells (OD values: 6288 vs 12 430 for control and siRNA treated cells, respectively). (B) Cells were incubated with Gas1 siRNA and VEGF as described above. Cell apoptosis was quantified by measuring DNA fragmentation as oligonucleosomes. Data are means ± SD of a quadruplicate of a typical experiment. Two independent experiments gave comparable results. (C) Allantois explants were cultured, transfected with siRNA, and treated with VEGF (80 ng/mL for 24 hours) or control medium as described in “Materials and methods” (original magnification, × 100). VEGF is required for the formation of a well-organized vascular network as evidentiated by labeling with an anti–PECAM-1 mAb. In contrast, in absence of the growth factor, endothelial cells are unable to form vessels and many of them undergo apoptosis evaluated as DNA fragmentation (TUNEL) (C-D). Gas1 siRNA reduced the protective effect of VEGF and double-labeling PECAM-1/TUNEL demonstrates apoptotic cells in newly forming vascular structures at the periphery of the network (arrowheads and the insert). The number of apoptotic cells in allantoises (D) has been quantified as described in “Materials and methods.” Data are means ± SD of triplicate of a typical experiment. Two independent experiments gave comparable results.
Inhibition of Gas1 expression by siRNA prevented the antiapoptotic effect of VEGF. (A) Cells were incubated with siRNA as indicated in “Materials and methods” and then treated with VEGF (80 ng/mL for 24 hours) or control medium. Expression of Gas1 was evaluated by Western blot. Gas1 siRNA inhibited the expression of the protein by 70% in resting conditions (OD values: 2267 vs 7528 for control and siRNA treated cells, respectively) and by 50% in VEGF-treated cells (OD values: 6288 vs 12 430 for control and siRNA treated cells, respectively). (B) Cells were incubated with Gas1 siRNA and VEGF as described above. Cell apoptosis was quantified by measuring DNA fragmentation as oligonucleosomes. Data are means ± SD of a quadruplicate of a typical experiment. Two independent experiments gave comparable results. (C) Allantois explants were cultured, transfected with siRNA, and treated with VEGF (80 ng/mL for 24 hours) or control medium as described in “Materials and methods” (original magnification, × 100). VEGF is required for the formation of a well-organized vascular network as evidentiated by labeling with an anti–PECAM-1 mAb. In contrast, in absence of the growth factor, endothelial cells are unable to form vessels and many of them undergo apoptosis evaluated as DNA fragmentation (TUNEL) (C-D). Gas1 siRNA reduced the protective effect of VEGF and double-labeling PECAM-1/TUNEL demonstrates apoptotic cells in newly forming vascular structures at the periphery of the network (arrowheads and the insert). The number of apoptotic cells in allantoises (D) has been quantified as described in “Materials and methods.” Data are means ± SD of triplicate of a typical experiment. Two independent experiments gave comparable results.
In order to investigate the role of Gas1 in newly forming vessels, we used ex vivo analysis of vasculogenesis and angiogenesis in the allantois.42 As reported in Figure 7C, at 24 hours after isolation and culture of allantoises in the presence of VEGF, it is possible to evidence a well-organized vascular network stained by PECAM-1 antibody. In absence of VEGF, vascular structures were lost as a result of endothelial cell apoptosis (Figure 7C-D). Addition of Gas1 siRNA reduces the protective effect of VEGF (Figure 7D). As shown in the insert and indicated by arrowheads (Figure 7C), many TUNEL-positive cells were concentrated along the vessels at the periphery of the network and could be costained with PECAM-1, indicating their endothelial nature. A diffuse TUNEL staining could be observed also in the presence of VEGF, which is likely due to cells of mesodermal origin present in the allantois, which undergo apoptosis due to serum deprivation. These cells do not express VEGF receptors and cannot be protected by addition of the growth factor.
Discussion
In normal vessels, endothelial cells remain for several years in a resting state characterized by contact inhibition of cell growth and resistance to apoptotic stimuli. Junctional proteins may contribute to this quiescent condition by transferring intracellular signals and influencing gene expression.5 In the present article we found that confluency induces Gas1 expression in the endothelium. This effect was dependent upon expression of a functional VE-cadherin, since cells expressing wild-type VE-cadherin presented higher levels of Gas1 compared with VE-cadherin–null cells or cells expressing a truncated mutant of the protein.30
Gas1 is a membrane-associated protein down-regulated during G0-to-S phase transition and up-regulated by cell quiescence.17,18 Little is known on the signaling pathway(s) that regulate Gas1 expression. Ectopic expression of Gas1 in fibroblasts or tumor cell lines exerts a growth-suppressing effect, both during synchronous cell cycle re-entry and during exponential growth.18,21 However, the mechanism of action of Gas1 is still elusive. Cell sensitivity to Gas1 was related to p53 activity and expression. By the use of p53 mutants it was found that the transactivating function of p53 was dispensable for Gas1-induced growth arrest.19,20
All the observations reported here suggest that the biologic meaning of Gas1 induction in the endothelium is different from that previously described.19,20 In this cell type, Gas1 does not affect cell growth but its expression parallels inhibition of apoptosis, and this is the first report in which an antiapoptotic effect of Gas 1 is reported.
The lack of effect of Gas1 on cell proliferation is not fully surprising, since other cell lines were unresponsive to this mediator.31 In this article we compared endothelial cells with the 293 cell line, which, reportedly,31 is insensitive to Gas1-induced growth suppression. Interestingly, this cell line behaved like the endothelium and was strongly protected from apoptosis by Gas1 expression. This suggests that the antiproliferative and antiapoptotic effect of Gas1 may be inversely related. An attractive speculation is that Gas1 selectively modulates p53 signaling by inducing growth inhibition while blocking apoptosis. Alternatively, in cells in which p53 level is low or inactive as in 293,31 other signaling pathways induced by Gas1 may prevail.
Besides VE-cadherin, another potent antiapoptotic agent such as VEGF was able to up-regulate Gas1. This was true in both sparse and confluent cells and was reduced by addition of an anti–VE-cadherin antibody, suggesting that the clustering and adhesive activity of this protein is required for optimal VEGF intracellular signaling.
In previous work we found that VE-cadherin can associate to VEGF receptor 2 (VEGF-R2) and that this is required for induction of PI3-kinase activation and Akt phosphorylation.15 This pathway is responsible, to a large extent, for the survival signal transferred by VEGF.26 Consistently, here we report that Gas1 induction by VEGF is VE-cadherin and PI3-kinase dependent. Accordingly, the truncated mutant of VE-cadherin, which is unable to activate PI3-kinase, was also ineffective in inducing Gas1 synthesis and in inhibiting apoptosis.15
Confluent endothelial cells are unresponsive to proliferation induced by VEGF but can still respond to its survival signals. It is possible that Gas1, induced through PI3-kinase activation, participates in the antiapoptotic activity of VEGF and acts as a stabilizing factor reducing the sensitivity of confluent endothelial cells to apoptotic stimuli.
The precise molecular mechanism through which PI3-kinase activation induces Gas1 is unknown. However, it is likely that PI3-kinase, induced by VE-cadherin clustering and VEGF, activates Rac,36 which in turn may activate the nuclear factor κB (NFkB) system and directly or indirectly induce Gas1 transcription45 (Figure 8).
Schematic representation of the pathway through which VE-cadherin and VEGF-R2 might induce Gas1 synthesis. As reported previously15,44 VEGF-R2 may form a complex with VE-cadherin in confluent endothelium. VE-cadherin clustering at junctions and activation of VEGF-R2 leads to PI3-kinase and Rac activation,36,44 which, in turn, may induce NFkB nuclear translocation45 and induction of Gas1 transcription.
Schematic representation of the pathway through which VE-cadherin and VEGF-R2 might induce Gas1 synthesis. As reported previously15,44 VEGF-R2 may form a complex with VE-cadherin in confluent endothelium. VE-cadherin clustering at junctions and activation of VEGF-R2 leads to PI3-kinase and Rac activation,36,44 which, in turn, may induce NFkB nuclear translocation45 and induction of Gas1 transcription.
The role of Gas1 in maintaining endothelial cell homeostasis may be cell specific. This protein exerts a complex pattern of activities in other cellular and organ systems. A recent article shows that Gas1 has an opposite effect on neuronal cells promoting their apoptosis after excitotoxic insults.46 In vivo, studies on Gas1-null animals showed that it is required for growth of granule and glial cells and for the complete development of the cerebellum.23 Overexpression of Gas1 leads to growth arrest in embryonic limb cells and promotes interdigital cell death.25 A novel and surprising finding is that during embryogenesis Gas1 is induced by Wnt and is able to bind Sonic hedgehog reducing its availability within dorsal somites.22
It is difficult to combine all of these observations in a common picture but it is possible that Gas1 exerts cell-specific activities depending on cell differentiation and presence of specific intracellular mediators. Although the mechanism of action of Gas1 is still elusive, its role in protecting endothelial cells from apoptosis may be important in cardiovascular diseases. This possibility is supported by the data reported here using the in vitro allantois organ culture. In this system, inhibition of Gas1 synthesis by siRNA reduces the protective effect of VEGF. Inhibition of Gas1 synthesis increased apoptosis in both the existing vascular structures that were maintained by the addition of VEGF and in the endothelial cells of newly forming vessels that were induced by VEGF.
Besides activation of PI3-kinase–Akt/PKB signaling and induction of bcl-2,47,48 VEGF mediates survival by up-regulating members of the newly discovered family of antiapoptotic proteins (IAPs) such as XIAP and survivin.49-52 However, survivin up-regulation is prevented by arresting the cell cycle. This behavior differs from Gas1, which can be induced by VEGF even more effectively in confluent/nonproliferating cells. Targeting one or the other of these pathways may help modulate endothelial sensitivity to apoptosis (ie, increasing Gas1 expression may protect endothelial cells from damage in ischemic injury), whereas inhibition of its synthesis may increase endothelial death and induce vascular regression in proliferative diseases.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-07-2459.
Supported by the Associazione Italiana per la Ricerca sul Cancro; European Community (QLRT-2001-02059); Agenzia Spaziale Italiana; Associazione Duchenne Parent Project; Italian Ministry of Health; Ministry of University, Scientific and Technological Research; Telethon Italy; Consiglio Nazionale delle Ricerche/Ministero dell'Istruzione, dell'Universita' e della Ricerca (MIUR) (CNR.02.731.DEJA); MIUR/Fondo per gli investimenti della Ricerca di Base (RBNE01MAWA_009 and RBNE01F8LT_007); Cofin 2002 (2001053777-002). R.S. and L.Z. were recipients of FIRC fellowships.
R.S. and M.C. contributed equally to this work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors are grateful to Paul A. Fleming for the precious technical help in setting up the in vitro allantois organ culture. We thank the Italian Foundation for Cancer Research (FIRC) for providing support for Affymetrix Gene Chip analysis.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal