Abstract
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired hemolytic anemia characterized by the increased sensitivity of red blood cells (RBCs) to complement, leading to intravascular hemolysis and hemoglobinuria. PNH is due to the expansion of a cell clone that has acquired a mutation in the PIGA gene. Mice with targeted Piga gene inactivation genetically mimic the human disease and have phosphatidylinositol glycan class A-negative (PIGA-) RBCs with a reduced half-life in circulation. Although PIGA-RBCs are hypersensitive to complement in vitro, their complement sensitivity in vivo is barely detectable. Here we show that the shortened survival of PIGA- RBCs is independent of complement either by using inhibitory C5 antibodies or by transfusion into C5-, C4-, C3-, or factor B-deficient mice. Splenectomy or high-dose cortisone treatment had no effect on the shorter survival of PIGA- RBCs. However, treatment with liposome-encapsulated clodronate, an agent that depletes macrophages in vivo, normalized the half-life of PIGA- RBCs. This indicates that the shortened survival of PIGA- RBCs is due to a novel pathway of PIGA- RBC clearance that is mediated by macrophages, but occurs independently of complement. Future investigations will show whether this novel pathway of PIGA- RBC destruction identified in mice may also operate in patients with PNH. (Blood. 2004;103:2827-2834)
Introduction
The hallmark of paroxysmal nocturnal hemoglobinuria (PNH) is the increased sensitivity of PNH red blood cells (RBCs) to activated complement.1 Blood cells from patients with PNH are deficient in glycosyl phosphatidylinositol (GPI)-linked proteins, due to a somatic mutation in the X-linked PIGA gene causing a block in GPI-anchor biosynthesis.2 Of the missing membrane molecules 2 are complement regulatory proteins: DAF (decay accelerating factor or CD55) and CD59 (membrane inhibitor of reactive lysis or MIRL).3,4 In humans, the deficiency of these 2 molecules is responsible for the increased complement sensitivity of PNH red cells, and thus accounts for intravascular hemolysis and hemoglobinuria characteristic of the disease. DAF is a 70-kDA to 80-kDA glycoprotein that regulates the formation and stability of the C3 and C5 convertases of both the classical and alternative pathways. In humans, DAF is expressed as a GPI-anchored protein on most tissues including red cells, platelets, granulocytes, lymphocytes, and endothelial cells. CD59 is a small 18-kDa to 20-kDa GPI-anchored membrane protein expressed on almost all cell types including blood cells and endothelial cells. CD59 binds to C8 and C9 to inhibit the further assembly of the membrane attack complex necessary for the lysis of the target cell.
Little is known about the biologic or clinical consequences caused by the deficiencies of other GPI-linked proteins. By targeting the inactivation of the Piga gene to hematopoietic progenitor cells, we have generated mice that have blood cells deficient in GPI-linked proteins.5-7 Mice with phosphatidylinositol glycan class A-negative (PIGA-) red blood cells,5 under laboratory conditions, do not develop anemia or have obvious hemoglobinuria. However, in mice with a high percentage of PIGA- RBCs, hemoglobin values are lower, and the reticulocyte counts are higher than in control mice.6,7 In mice with only a proportion of blood cells affected, the proportion of PIGA- RBCs is always lower than the proportion of PIGA- reticulocytes, suggesting that PIGA- RBCs have a reduced half-life in circulation. In order to study the consequences of GPI-anchor deficiency, we further investigated in vitro and in vivo the complement sensitivity of PIGA- RBCs in our genetically engineered animals, and tested whether the reduced half-life of PIGA- RBCs is due to the action of complement.
Complement regulatory molecules, in particular, membrane-bound complement inhibitory molecules, differ between mouse and human. In contrast to humans mice have 2 distinct CD59 genes (CD59a and CD59b).8,9 Both are GPI linked but differ in their expression pattern.9-11 In addition to the GPI-linked form, mice also have a transmembrane form of DAF.12 However, transmembrane DAF has a restricted expression and is not expressed on circulating blood cells or endothelial cells. Mice also express Crry (CR1-related protein y) on all blood cells. Crry is a transmembrane protein that efficiently blocks complement activation at the C3, C4, and C5 levels.13
Here we demonstrate that murine PIGA- RBCs are impaired in the regulation of complement in vitro. However, the reduced half-life of PIGA- RBCs in vivo is complement-independent and is due to increased clearance by fixed macrophages. The identification of a complement-independent pathway of PIGA- RBC clearance in our mice suggests that a similar pathway may contribute to the increased turnover of RBCs in patients with PNH. Future investigations will show whether this novel pathway of PIGA- RBC destruction identified in mice may also operate in patients with PNH. The characterization of this pathway might become important in light of the recent availability of complement inhibitors for the treatment of PNH patients.
Materials and methods
Mice
The mouse strains (LF [loxPiga × cfes-cre] and GL [loxPiga × GATA1-cre]) with PIGA- blood cells have been previously described.6,7 In LF mice the Piga gene is inactivated in hematopoietic stem cells.6 In GL mice recombination of the floxed Piga gene occurs in the erythroid/megakaryocytic lineage.7 Mice carrying only the floxed Piga gene or only the Cre transgene were used as wild-type (wt) controls. All experiments were performed with mice in a C57BL/6 background (N ≥ 10). C5-/- mice (B10.D2-H2-Hc 0) were purchased from Jackson Laboratory (Bar Harbor, ME); C4-/- mice were provided by Michael Carroll, Harvard Medical School Boston14 ;Bf-/- mice were obtained from David Chaplin, Washington University, St Louis, MO15 ; and C3-/- mice were obtained from Harvey Colten, Northwestern University Chicago.16 DAF knock-out mice and DAF-/-, CD59a-/- double-deficient mice were generated as previously reported17,18 and were back-crossed to the C57BL/6 background for more than 6 generations. The animal studies committee of Washington University approved experiments involving animals.
Harvesting serum and plasma
Serum and plasma were collected from 6- and 12-week-old male mice into glass tubes (DispoCulture Glass Tubes; American Scientific Products, McGaw, IL), containing ethylenediaminetetraacetic acid (EDTA; Fisher Scientific, Pittsburgh, PA) to a final concentration of 0.5 mM. Plasma was collected by centrifugation at 4°C and stored at -70°C.19
Flow cytometric analysis
Flow cytometric analysis was performed as reported.5 Fluoroscein isothiocyanate (FITC)-conjugated goat antimouse C3 antibody was purchased from Cappel/ICN (Costa Mesa, CA). All other antibodies were obtained from Pharmingen (San Diego, CA).
Complement media and buffers
An assessment of complement sensitivity of mouse RBCs for lysis by the classical pathway was performed in gelatin veronal buffer (GVB) saline containing 0.1% gelatin, pH 7.35, supplemented with 0.015 mM Ca+2 and 0.05 mM Mg+2 (GVB+2). The buffer used as reaction medium in the alternative pathway system was GVB, pH 7.2, containing 0.5 mM ethyleneglycol-bis-(β-aminoethylether)N′,N′-tetraacetic acid (EGTA) and 0.05 mM Mg+2 GVB/MgEGTA.20 Heat-inactivated serum (30 minutes at 56°C) served as a control.
Sensitivity of RBCs to complement-mediated lysis
In order to determine antibody-induced complement-dependent hemolysis, mouse RBCs at a concentration of 1 × 109 were sensitized for 90 minutes at 37°C with rabbit antimouse red blood cell immunoglobulin G (IgG) antibody (0.1 mg/mL; Accurate Chemical & Scientific, Westbury, NY). Cells were washed 2 times with GVB+2 buffer and suspended into the final concentration of 2 × 108/mL. Using heterologous complement, 50 μL sensitized RBCs were exposed to 100 μL serially diluted male rat serum for 90 minutes at 37°C. Red cell lysis was monitored by the release of hemoglobin measured spectrophotometrically at 415 nm. In experiments using autologous complement, RBCs were labeled with chromium 51 (51Cr) sodium dichromate (ICN Biomedical Research Products, Costa Mesa, CA) for 90 minutes at 37°C.21 Cells were washed 3 times with GVB+2 buffer and suspended to a final concentration of 5 × 107/mL. Sensitized 51Cr-labeled RBCs (50 μL) were exposed for 90 minutes at 37°C to 100 μL serially diluted mouse serum, pooled from wt male C57BL/6 mice. Evaluation of lysis was made by measurement of 51Cr release.
Sensitivity of RBCs to complement-mediated lysis by the alternative pathway was performed similarly. Using heterologous complement, 50 μL RBCs at a concentration of 2 × 108/mL was exposed to 100 μL acidified male rat serum serially diluted in GVB/Mg+2 EGTA. Using autologous complement, 51Cr-labeled RBCs (50 μL) at a concentration of 5 × 107/mL were exposed to the acidified male mouse serum diluted in GVB/Mg+2 EGTA. Incubation conditions were the same as previously described in the classical pathway.22
C3 deposition assays on mouse erythrocytes
In vivo activation of complement by cobra venom factor (CVF)
In vivo survival of transfused RBCs
Packed RBCs (50 μL) from FL mice with about 50% of PIGA- blood cells were stained with PKH26 red fluorescent cell linker according to the manufacturer's instructions (Sigma Aldrich). The labeled cells were injected intravenously into wt and C5-/-, C4-/-, Bf-/-, or C3-/- mice. Host mice were bled and the proportion of PKH26-stained PIGA- and PIGA+ RBCs was analyzed by flow cytometry.
Anti-C5 antibody administration and hemolytic assay
The murine monoclonal antibody BB5.1 against murine C5 and murine 135.8 monoclonal control antibody have been described before.24 FL, GL, and wt mice were injected with 40 mg/kg anti-C5 antibody (BB5.1) or the control antibody (135.8) intraperitoneally biweekly for 3 weeks. The proportion of PIGA- reticulocytes and RBCs was monitored by flow cytometry. The ability of the anti-C5 antibody to block the generation of C5b-9 in vivo was assayed in plasma obtained before, one week after, and at the end of treatment as described before.24 The analysis was performed blinded as to whether the serum was obtained from an anti-C5- or a control antibody-treated mouse.
Methylpredinisolone acetate and clodronate-liposome injections
FL, GL, and wt mice were treated daily with methylpredinisolone acetate (Cortone, 33 mg/kg intraperitoneally for 28 days).25 For the treatment with clodronate-loaded liposomes, wt mice were injected intravenously with 10 mL/kg.26 The injections were repeated 4 times each after a 4-day interval. This regimen has previously shown to efficiently deplete almost all macrophages in spleen, liver, and bone marrow.27 Liposome-encapsulated phosphate-buffered saline (PBS) was used as a control. Clodronate was a gift of Roche Diagnostics (Mannheim, Germany). It was encapsulated into liposomes as described earlier.28
Immunohistochemistry
Spleen, liver, and lung specimens were obtained 48 hours after the injection of liposomes, embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA), and frozen in liquid nitrogen. Cryostat sections were incubated with rat antimouse monoclonal antibody F4/80 (Caltag Laboratories, Burlingame, CA).
Results
PIGA- red blood cells have an increased sensitivity to heterologous complement-mediated lysis in vitro
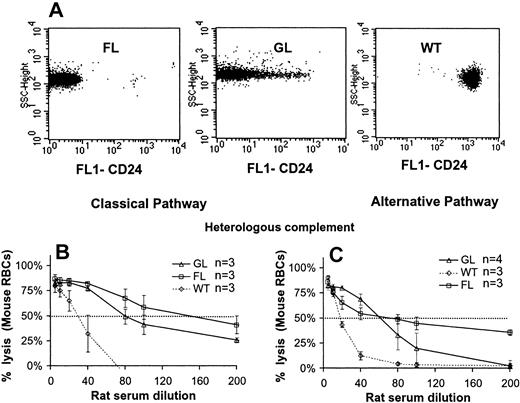
On the basis of the increased proportion of PIGA- reticulocytes relative to PIGA- mature RBCs, we concluded in a previous study that PIGA- red blood cells might have a reduced half-life in circulation.5-7 To test whether the reduced half-life is due to complement-mediated lysis we first assessed the sensitivity of PIGA- RBCs to the lytic action of complement in vitro. RBCs from FL mice completely lack the expression of GPI-linked proteins,6 whereas RBCs from GL mice have residual expression.7 Female FL and GL mice, due to random X inactivation, have about 50% of PIGA- RBCs.6,7 Figure 1A shows the flow cytometric analysis of RBCs from male wt, FL, and GL mice using CD24 as a marker for GPI-linked proteins. To assess the complement sensitivity, RBCs from wt, GL, and FL mice were subjected to a “one-step” complement lytic assay. Figure 1B shows that, over a range of rat serum dilutions, antibody-sensitized PIGA- RBCs were significantly more sensitive to rat complement than RBCs from wt mice. In addition RBCs from FL mice were more sensitive than RBCs from GL mice. Lysis was complement-dependent as no lysis occurred when the serum was heat inactivated. Similarly, PIGA- RBCs were more sensitive to lysis compared with wt RBCs (FL > GL > wt) when complement was activated by the alternative pathway using heterologous complement (Figure 1C). In contrast, no lysis was observed with PIGA- or wt RBCs when autologous mouse serum was used as a source of complement, independent of whether complement was activated by the classical or alternative pathway (data not shown). No lysis was detected even when RBCs were Cr51 labeled, giving a 10-fold increased sensitivity in the detection of RBC lysis compared with the measurement of hemoglobin release. The very low lytic activity of mouse serum on mouse RBCs in our assay is in agreement with previous reports.8,29,30 Most investigators therefore use a heterologous system to assess complement sensitivity of mouse cells.18,22
Increased sensitivity of PIGA- RBCs to complement-mediated lysis. (A) Expression of GPI-linked proteins on RBCs from FL, GL, and wt control animals. RBCs were stained with a fluorescent-labeled monoclonal antibody against CD24, a GPI-linked surface antigen highly expressed on normal RBCs. FL cells lack CD24, whereas a proportion of GL RBCs have some residual CD24 expression. (B-C) Lysis of wt, FL, and GL RBCs by heterologous complement. (B) Activation of rat complement was induced by the classical pathway (antibody sensitized RBCs) or (C) by the alternative pathway. RBCs from FL and GL mice were more sensitive to lysis by heterologous complement, activated by either pathway (FL > GL > wt). Data represent mean ± SD.
Increased sensitivity of PIGA- RBCs to complement-mediated lysis. (A) Expression of GPI-linked proteins on RBCs from FL, GL, and wt control animals. RBCs were stained with a fluorescent-labeled monoclonal antibody against CD24, a GPI-linked surface antigen highly expressed on normal RBCs. FL cells lack CD24, whereas a proportion of GL RBCs have some residual CD24 expression. (B-C) Lysis of wt, FL, and GL RBCs by heterologous complement. (B) Activation of rat complement was induced by the classical pathway (antibody sensitized RBCs) or (C) by the alternative pathway. RBCs from FL and GL mice were more sensitive to lysis by heterologous complement, activated by either pathway (FL > GL > wt). Data represent mean ± SD.
PIGA- RBCs have an increased C3 deposition in vitro
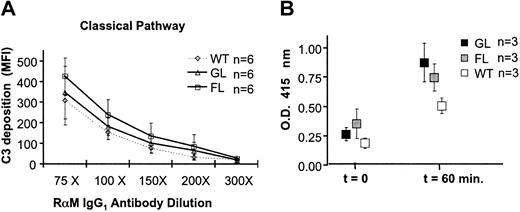
To test whether PIGA- RBCs are impaired in the regulation of autologous complement activation at the C3 level, we incubated PIGA- and wt RBCs sensitized with rabbit antimouse erythrocyte antibody with normal mouse serum and measured C3 deposition on RBCs by flow cytometry using polyclonal antibodies against mouse C3. Figure 2A shows that the C3 deposition on PIGA- RBCs from FL mice is significantly higher at all concentrations of sensitizing antibody when compared with wt RBCs from mice. PIGA- RBCs from GL mice also had a higher deposition of C3 when compared with wt RBCs, however this did not reach statistical significance and was lower than the C3 deposition observed on FL PIGA- RBCs. These findings indicate that in vitro PIGA- RBCs that completely lack GPI-linked proteins are defective in the regulation of autologous complement activated by the classical pathway. However, in vivo an increased amount of C3 was not detected on circulating RBCs (data not shown).
PIGA-RBCs are impaired in the regulation of autologous complement. (A) Increased in vitro C3 deposition on PIGA- RBCs after activation of autologous complement. C3 deposition after complement activation induced by RBCs sensitized with various concentrations of antibody (classical pathway). C3 deposition is assessed measuring the mean fluorescence intensity (MFI) using a fluorescent monoclonal antibody against C3 and by flow cytometry. Data represent mean ± SD. RαM indicates rabbit antimouse RBC antibody. (B) PIGA- RBCs are more susceptible to CVF-induced complement lysis in vivo. Wild type, FL, and GL mice were injected intraperitoneally with 5 mg/kg CVF. Free hemoglobin was measured by spectrophotometry at OD415 before and one hour after administration of CVF. Before injection (t = 0) the OD415 of plasma from wt, FL, and GL was not significantly different. One hour after plasma, OD415 measurements increased in all mice, but were significantly higher in FL and GL mice compared with the measurements in wt mice. Data represent mean ± SD.
PIGA-RBCs are impaired in the regulation of autologous complement. (A) Increased in vitro C3 deposition on PIGA- RBCs after activation of autologous complement. C3 deposition after complement activation induced by RBCs sensitized with various concentrations of antibody (classical pathway). C3 deposition is assessed measuring the mean fluorescence intensity (MFI) using a fluorescent monoclonal antibody against C3 and by flow cytometry. Data represent mean ± SD. RαM indicates rabbit antimouse RBC antibody. (B) PIGA- RBCs are more susceptible to CVF-induced complement lysis in vivo. Wild type, FL, and GL mice were injected intraperitoneally with 5 mg/kg CVF. Free hemoglobin was measured by spectrophotometry at OD415 before and one hour after administration of CVF. Before injection (t = 0) the OD415 of plasma from wt, FL, and GL was not significantly different. One hour after plasma, OD415 measurements increased in all mice, but were significantly higher in FL and GL mice compared with the measurements in wt mice. Data represent mean ± SD.
PIGA- RBCs are more sensitive to autologous complement lysis in vivo during systemic complement activation induced by CVF
Although in mice with 100% PIGA- RBCs the hemoglobin levels and red cell counts were lower and the reticulocyte counts were higher than in wt control mice, no anemia or hemoglobinuria was observed.6,7 Plasma hemoglobin levels were similar to those measured in serum from wt mice (Figure 2B, t = 0), and no evidence of hemoglobinuria was found either by spectrometric analysis or by staining for iron deposition in the kidney (data not shown). To test whether PIGA- RBCs are more susceptible to complement-mediated lysis in vivo under conditions of abnormal complement activation, we induced systemic complement activation by the injection of CVF. CVF is a known potent activator of mouse C3 and C5.31 Figure 2B shows that in all 3 strains of mice, serum hemoglobin levels were elevated after injection with 5 mg/kg CVF. However, mice with PIGA- RBCs (FL and GL mice) had higher free serum hemoglobin levels compared with wt mice. These results indicate that under conditions of abnormal complement activation the regulation of complement on PIGA- red cells is impaired in vivo.
Inhibition of complement using monoclonal antibodies specific for murine C5 is able to completely block complement activity but has no effect on the reduced half-life of PIGA- RBCs
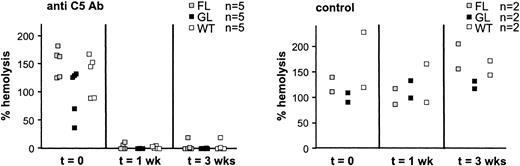
Because complement-loaded RBCs might immediately be lysed or removed from circulation we further investigated the role of complement in the reduced half-life of PIGA- RBCs. To test whether inhibition of the lytic pathway of the complement cascade would prolong the shortened half-life of PIGA- red cells in our animals, we injected wt, FL, and GL mice with a monoclonal antibody specific toward murine C5 (BB5.1). This antibody has previously shown to block complement activation in mice.24 The animals were injected intraperitoneally with 40 mg/kg semiweekly for 3 weeks. The proportion of PIGA- RBCs and reticulocytes was monitored weekly for 5 weeks. Mice with 50% of PIGA- RBCs were used for these experiments. One would expect, if the half-life was normalized by the C5-specific antibody, that the proportion of PIGA- RBCs would approach the proportion of PIGA- reticulocytes. Before antibody treatment in all FL and GL mice, the proportion of PIGA- reticulocytes was higher than the proportion of PIGA- RBCs, consistent with the reduced half-life of circulating mature RBCs.5-7 During the course of antibody injections the proportion of PIGA- RBCs did not change in mice treated with C5-specific antibody and was similar to the proportion of PIGA- RBCs in mice treated with the control antibody. No PIGA- RBCs or reticulocytes were detected in wt mice (data not shown). These results indicate that the anti-C5 antibody did not influence the shortened half-life of PIGA- RBCs.
Serum of the mice was tested for the ability of the anti-C5 antibody to block the generation of C5b-9 before, during, and after treatment.24 Figure 3 shows the lysis of sensitized chicken RBCs by mouse serum clearly demonstrating that in treated FL, GL, and wt mice the anti-C5 antibody potently inactivated almost all hemolytic activity. These results suggested that the terminal pathway of complement activation is not responsible for the reduced half-life of PIGA- RBCs.
Antimouse C5 monoclonal antibodies effectively inhibit complement activation in vivo. Wild-type, FL, and GL mice were injected with a monoclonal antibody specific toward murine C5 (BB5.1) or with a control antibody (135.8), biweekly 40 mg/kg intraperitoneally for 3 weeks. The plasma of treated mice was assessed for C5b-9-mediated hemolysis before, one week into, and at the end of treatment. Hemolytic activity of sera from anti-C5-treated mice were reduced almost to 0 but remained unchanged in the serum of mice treated with the control antibody. Hemolysis has been normalized to hemolysis obtained from normal mouse serum (Sigma Aldrich). Data represent mean ± SD.
Antimouse C5 monoclonal antibodies effectively inhibit complement activation in vivo. Wild-type, FL, and GL mice were injected with a monoclonal antibody specific toward murine C5 (BB5.1) or with a control antibody (135.8), biweekly 40 mg/kg intraperitoneally for 3 weeks. The plasma of treated mice was assessed for C5b-9-mediated hemolysis before, one week into, and at the end of treatment. Hemolytic activity of sera from anti-C5-treated mice were reduced almost to 0 but remained unchanged in the serum of mice treated with the control antibody. Hemolysis has been normalized to hemolysis obtained from normal mouse serum (Sigma Aldrich). Data represent mean ± SD.
PIGA- RBCs have a reduced half-life in mice deficient in the C5, C4, Bf, or C3 complement component
To rule out the possibility that complement inactivation by the anti-C5 antibody might be incomplete and to further investigate the role of complement in the shortened half-life of PIGA- RBCs we measured the half-life of transfused PKH-labeled PIGA- RBCs in wt mice and in mice deficient for the complement component C5, C4, Bf, or C3. RBCs were collected from FL mice with 50% PIGA- RBCs. The half-life of PIGA+ and PIGA- cells derived from the same donor mouse was measured by flow cytometry by following the proportion of PKH-stained RBCs and measuring the expression of the GPI-linked antigen CD24. On the basis of the increased proportion of PIGA- reticulocytes relative to the proportion of PIGA- mature RBCs we have demonstrated that the half-life of PIGA- RBCs is about 7 to 8 days, which is approximately 50% of the half-life of wt RBCs (13-15 days).5 Figure 4A shows the survival of PIGA- and PIGA+ RBCs following transfusion into wt recipient mice. The half-life of PIGA- RBCs is significantly shorter (half-life [t1/2] 5.3 ± 1.7, n = 5, P ≤ .05) when compared with the half-life of PIGA+ RBCs (t1/2 13.0 ± 3.8). The survival of transfused RBCs measured in a wt recipient mouse is in agreement with our previous calculations.
The reduced PIGA- RBC survival is independent of complement. RBCs from FL mice with about 50% of PIGA- RBCs were stained with PKH26 and injected into the tail vein of wt or complement-deficient host mice. Proportion of PKH-stained PIGA- and PIGA+ RBCs was monitored by flow cytometry. (A) PIGA-RBCs after transfusion into wt recipient mice. (B) PIGA- RBCs after transfusion into mice deficient in the complement component C5, (C) C4, (D) factor Bf, or (E) C3. The percentage of stained PIGA- and PIGA+ cells was determined for each time point and normalized to 2 hours after injection time point. Data represent mean ± SD. (F) The proportion of reticulocytes (Retics) in the PIGA- RBC population and PIGA+ population in FL wt mice and FL mice additionally deficient for complement component Bf, C3, C4, and C5. In all animals the proportion of reticulocytes was higher within the PIGA- cells compared with the proportion of reticulocytes in the PIGA+ RBCs, indicating that in all mice PIGA- RBCs have a shortened half-life in circulation. (G) The survival of DAF-/- RBCs compared with simultaneously infused 100% PIGA- RBCs showed a shorter survival of PIGA- RBCs. (H) The survival of DAF-/- CD59a-/- RBCs compared with simultaneously infused 100% PIGA- RBCs showed a shorter survival of PIGA- RBCs. The percentage of stained PIGA- and PIGA+ cells was determined for each time point and normalized to 2 hours after injection time point. Data represent mean ± SD.
The reduced PIGA- RBC survival is independent of complement. RBCs from FL mice with about 50% of PIGA- RBCs were stained with PKH26 and injected into the tail vein of wt or complement-deficient host mice. Proportion of PKH-stained PIGA- and PIGA+ RBCs was monitored by flow cytometry. (A) PIGA-RBCs after transfusion into wt recipient mice. (B) PIGA- RBCs after transfusion into mice deficient in the complement component C5, (C) C4, (D) factor Bf, or (E) C3. The percentage of stained PIGA- and PIGA+ cells was determined for each time point and normalized to 2 hours after injection time point. Data represent mean ± SD. (F) The proportion of reticulocytes (Retics) in the PIGA- RBC population and PIGA+ population in FL wt mice and FL mice additionally deficient for complement component Bf, C3, C4, and C5. In all animals the proportion of reticulocytes was higher within the PIGA- cells compared with the proportion of reticulocytes in the PIGA+ RBCs, indicating that in all mice PIGA- RBCs have a shortened half-life in circulation. (G) The survival of DAF-/- RBCs compared with simultaneously infused 100% PIGA- RBCs showed a shorter survival of PIGA- RBCs. (H) The survival of DAF-/- CD59a-/- RBCs compared with simultaneously infused 100% PIGA- RBCs showed a shorter survival of PIGA- RBCs. The percentage of stained PIGA- and PIGA+ cells was determined for each time point and normalized to 2 hours after injection time point. Data represent mean ± SD.
Transfusion of PIGA- RBCs into C5-deficient mice confirmed our findings using the anti-C5 antibody, indicating that the terminal pathway was not responsible for the shortened half-life in our animals (Figure 4B). To our surprise however, none of the complement component deficiencies resulted in normalization of the PIGA- RBC half-life (P ≤ .05 for all mice; Figure 4C-4E), suggesting that complement has no role in the shortened half-life of PIGA- RBCs. To rule out that this might be due to the transfusion of labeled RBCs we bred our FL mice into a C5-/-, Bf-/-, C4-/-, or C3-/- background. Indeed, in a complement-deficient background too the proportion of PIGA- RBCs remained significantly lower than the proportion of PIGA- reticulocytes in all animals (P < .05 for all strains; Figure 4F), confirming that the shortened half-life of PIGA- RBCs is independent of complement.
Finally, we compared the half-life of RBCs from DAF-/- knock-out mice, deficient for the GPI-linked form of DAF, and RBCs from DAF-/-, CD59a-/- double knock-out mice with the half-life of PIGA- RBCs. After transfusion into a wt host, the survival of DAF-/- RBCs (t1/2 13.5 ± 3.8, n = 4) or DAF-/-, CD59a-/- wt RBCs (t1/2 12.3 ± 2.3, n = 5) was significantly longer than the survival of simultaneously transfused PIGA- RBCs (t1/2 6.0 ± 0.4, P ≤ .05 and t1/2 4.2 ± 2, P ≤ .05, Figure 4G and 4H, respectively), but not significantly different than wt RBCs (P > .05). These results indicate that neither the lack of DAF, nor the lack of both DAF and CD59a, is responsible for the shortened half-life of PIGA- RBCs.
Increased removal by fixed macrophages is responsible for the shortened half-life of PIGA- red cells
Clearance of RBCs occurs mainly in the spleen. To test whether an increased uptake by spleen was responsible for the shortened half-life of PIGA- RBCs in circulation we followed the proportion of PIGA- RBCs and PIGA- reticulocytes in splenectomized and sham-operated GL and FL mice. In both strains of mice splenectomy did not increase the proportion of PIGA- RBCs, and in all splenectomized animals the proportion of PIGA- reticulocytes remained higher than the proportion of PIGA- RBCs, similar to those in sham-operated mice (data not shown). To investigate the possibility that hepatic Kupfer cells might take over RBC clearance after splenectomy or might be the primary cause of the increased turnover of PIGA- RBCs, GL and FL mice were treated daily with 33 mg/kg methylpredinisolone acetate intraperitoneally for 28 days.25 However, the proportion of PIGA- RBCs did not change during the course of the treatment and the discrepancy between PIGA- RBCs and PIGA- reticulocytes was similar to control mice injected with PBS (data not shown). The proportion of PIGA- RBCs also did not change in mice that have previously been splenectomized and treated with steroid thereafter (data not shown). Finally, to deplete macrophages more efficiently we injected mice intravenously with 0.01 mL/g clodronate-loaded liposomes. Clodronate-liposome treatment transiently eliminates macrophages in spleen, liver (Kupfer cells), and bone marrow.26,27 Wt mice were injected with clodronate-liposomes every fourth day for 16 days (5 injections). Liposome-encapsulated PBS was used as a control. Immunohistochemical analysis revealed an almost complete absence of splenic macrophages in animals given clodronate-liposomes but not in PBS-treated animals (data not shown). At 2 days after the first liposome injection, 200 μL packed PKH26-labeled RBCs obtained from a donor mouse with approximate 50% of PIGA- RBCs was transfused. The proportion of PIGA+- and PIGA--labeled RBCs was monitored by flow cytometry. During clodronate-liposome treatment the survival of PIGA+ and PIGA- was almost identical, whereas the treatment with liposome-encapsulated PBS had no effect on the shortened survival of PIGA- RBCs (Figure 5). These results demonstrated that the elimination of fixed macrophages normalizes the half-life of PIGA- RBCs. This indicates that an increased clearance by macrophages is responsible for the shortened half-life of PIGA- RBCs.
Enhanced clearance of PIGA- RBCs by liver macrophages is responsible for their shortened half-life. Wild-type mice received 5 intravenous clodronate-liposome injections (0.01 mL/g) every fourth day. The bold bar below the diagram indicates the time points of injections. PKH-labeled RBCs were injected 2 days after the first injection. The percentage of stained PIGA- and PIGA+ cells was determined for each time point and normalized to 2 hours after injection time point. Liposome-encapsulated PBS was used as a control. Data represent mean ± SD.
Enhanced clearance of PIGA- RBCs by liver macrophages is responsible for their shortened half-life. Wild-type mice received 5 intravenous clodronate-liposome injections (0.01 mL/g) every fourth day. The bold bar below the diagram indicates the time points of injections. PKH-labeled RBCs were injected 2 days after the first injection. The percentage of stained PIGA- and PIGA+ cells was determined for each time point and normalized to 2 hours after injection time point. Liposome-encapsulated PBS was used as a control. Data represent mean ± SD.
Discussion
In patients with PNH, the half-life of PNH RBCs may be as short as 3.5 days32 compared with the normal half-life of 60 days. This has been attributed to the increased sensitivity of PNH RBCs to complement lysis causing intravascular hemolysis and hemoglobinuria. Studying PNH in a mouse model we have identified a novel pathway that causes an increased clearance of RBCs deficient in GPI-linked proteins. This pathway is independent of complement and is mediated by fixed macrophages in spleen, liver, and possibly bone marrow. In patients with PNH this pathway is likely masked by the overwhelming hemolysis caused by complement; however, it might become manifest if the lytic action of complement is inhibited, for example, due to inherited C9 deficiency33 or, more recently, due to treatment with complement inhibitors that currently are tested for the treatment of PNH.34
Mice with PIGA- RBCs have lower hemoglobin levels and RBC counts, whereas the reticulocyte counts are higher compared to wt controls.5-7 This finding is independent of whether all blood cell lineages6 or only the erythroid/megakaryocytic lineage7 is affected, indicating that the reduced half-life is intrinsic to PIGA- RBCs. The most obvious explanation was that, as with human PNH RBCs, murine PIGA- RBCs have a reduced half-life in circulation because of their increased sensitivity to complement. PIGA- RBCs might either be lysed intravascularly or phagocytosed by fixed macrophages due to increased C3 deposition on their cell surface. To test this hypothesis we investigated the complement sensitivity of PIGA- RBCs. Our results demonstrate that murine RBCs deficient in GPI-linked proteins are hypersensitive to heterologous complement in vitro. However, no lysis was detected when autologous complement was used. An increased C3 deposition was found on PIGA- RBCs after complement activation by the classical pathway in vitro. However, in vivo no increased C3 deposition was detected on circulating PIGA- RBCs. Only under extreme conditions of excessive complement activation using CVF did we detect a rather modest increased level of free hemoglobin in mice with PIGA- RBCs (Figure 2B). These results indicate that, although PIGA- RBCs under experimental conditions in vitro are hypersensitive to complement, in vivo, the lack of GPI-linked proteins has minimal effect on the sensitivity of RBCs to spontaneous complement attack. Our findings are in good agreement with the data published for RBCs from DAF-/-17 and from CD59a-/-/DAF-/- double knock-out mice,18,23 suggesting that the remaining transmembrane Crry protein efficiently compensates for the lack of DAF and CD59. Mild intravascular hemolysis in CD59a-/- mice was reported by Holt et al22 but not by Miwa et al.18 These differences were attributed to different genetic backgrounds of the 2 CD59a-/- knock-out mice.18 Our mice, similar to the CD59a-/- mice reported by Miwa et al, are in a C57BL/6 background, and, similar to Miwa et al, no signs of intravascular hemolysis were observed in our animals. Surprisingly, and in contrast to our mice, mice deficient for CD59b (CD59b-/-) have obvious clinical findings of hemolysis, including increased levels of free serum hemoglobin and hemoglobinuria.35 Unlike PIGA- RBCs, CD59b-/- RBCs are highly sensitive to lysis by autologous complement.35 The most likely explanation for the difference between PIGA- RBCs and RBCs from CD59b-/- mice is that RBCs from CD59b-/- mice are deficient in a transmembrane protein that contributes to complement regulation. At the moment it is controversial whether this protein corresponds to CD59b, which was originally reported to be GPI linked and restricted to the testis,10,11 but by Qin et al was found on a variety of cell types including RBCs.35 Our results indicate that the protein missing on CD59b-/- RBCs is not GPI linked.
Although mice with PIGA- RBCs have a similar phenotype regarding complement regulation to C57BL/6 GPI-DAF-/- and GPI-DAF-/-, CD59a-/- double knock-out mice, in contrast to these knock-out mice, mice with PIGA- RBCs have reduced hemoglobin levels and elevated reticulocyte counts.6,7,17,18 To investigate this further, we tested whether inhibition of complement activation would normalize the half-life of PIGA- RBCs in circulation. First, we tested whether antibodies specific for mouse C5 would block the lytic pathway of complement activation and prolong the half-life of PIGA- RBCs. Although the C5 antibody potently inhibited complement activation at the step of C5b-9 formation, treatment with C5 antibody did not alter the half-life of PIGA- RBCs. These data were confirmed when PIGA- RBCs were transfused into C5-deficient recipient mice (Figure 4B), and after breeding of our mice into a C5-deficient genetic background (Figure 4F), clearly indicating that the terminal pathway of complement activation is not responsible for the shortened half-life of PIGA- RBCs. Surprisingly, transfusion of PIGA- RBCs into C4-, Bf-, and C3-deficient mice did not alter the PIGA- RBC half-life either (Figure 4B-E). Breeding of our mice into a Bf-/-, C4-/-, and C3-/- genetic background confirmed these findings, because the half-life of PIGA- RBCs remained shortened (Figure 4F). These data indicate that the shortened half-life of PIGA- RBCs in our mice is independent of complement activation. The demonstration that transfused RBCs deficient for DAF and RBCs deficient for both DAF and CD59a have a significantly longer half-life in circulation than PIGA- RBCs (Figure 4G-H) further confirms our findings that the deficiency of the GPI-anchored forms of DAF and CD59a are not responsible for the shortened half-life of PIGA- RBCs.
In humans, the major cause of the shortened half-life of PNH RBCs is complement-mediated lysis caused by the deficiency of DAF and CD59. However, whether the deficiency of other membrane surface molecules could additionally shorten the half-life of PNH RBCs by a pathway independent of complement has never been considered. Mouse complement in contrast to human complement has a very low lytic activity.8,29,30 Furthermore, mouse RBCs express, in addition to the GPI-linked complement regulatory molecules DAF and CD59, a transmembrane protein, Crry, that potently regulates complement activation.13,36 Thus, although PIGA- RBCs are impaired in the regulation of complement, under steady-state laboratory conditions, this seems not to cause a measurable shortening of their half-life. Our hypothesis is that the absence of a significant pathology due to complement unmasks a novel pathway that shortens the half-life of PIGA- RBCs. Naturally, we cannot exclude the possibility that the increased clearance of PIGA- RBCs observed is specific for mice. The survival curve of labeled PIGA- RBCs is exponential (Figure 4), suggesting that the destruction of PIGA- RBCs by this novel pathway is random. The clearance of RBCs in mice is random and occurs mainly in the spleen. However, splenectomy had no effect on the shortened half-life of PIGA- RBCs. Similarly, high doses of cortisone, known to impair phagocytosis by macrophages in spleen and liver,25 did not alter the half-life of PIGA- RBCs. However, repeated intravenous injections of clodronate-loaded liposomes, which efficiently eliminate macrophages in spleen, liver, and bone marrow, restored the half-life of PIGA- RBCs (Figure 5). These results indicate that increased clearance by macrophages residing in spleen, liver, and possibly bone marrow is responsible for the shortened half-life of PIGA- RBCs. Investigations to identify the GPI-linked protein(s) whose absence leads to the increased RBC destruction are ongoing.
We hypothesize that a similar mechanism might also shorten the half-life of PNH RBCs in humans. The operation of this novel pathway has not so far been apparent because of the overwhelming hemolysis caused by complement. In fact, mild hemolysis was found in a PNH patient with inherited C9 deficiency.33 The cause of hemolysis in this patient is unclear because deficiency of DAF alone does not cause hemolysis.37-39 This suggests that in this C9-deficient PNH patient, as in the mice described here, an increased complement-independent clearance occurs that shortens the half-life of PNH RBCs. The characterization and understanding of this pathway will enable us to delineate its possible clinical importance and possibly determine whether it might also play a role in other diseases with increased RBC clearance. The understanding of this pathway might become particularly important when PNH patients are treated for hemolysis with complement inhibitors,34 which will cause a rise in the proportion of circulating PNH RBCs and possibly unmask this novel route of PNH red cell destruction.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-09-3057.
Supported by National Institutes of Health grants R01 HL63208 and CA89091, the American Heart Association grant 0240037N, and the Digestive Diseases Research Core Center (grant no. P30 DK52574).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Martin Rogers and Deborah LaFlame for technical assistance, John P. Atkinson for help in setting up and interpretation of complement assays, and Philip J. Mason for critical reading of the manuscript. We thank the Digestive Diseases Research Core Center (grant no. P30 DK52574) for the preparation and staining of tissue sections.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal