Abstract
Hepcidin is a 25-amino acid peptide involved in iron homeostasis in mice and humans. It is produced in the liver from a larger precursor, and it is detectable in blood and urine. In contrast to the human genome, which contains only one copy of the gene, the mouse genome contains 2 highly similar hepcidin genes, hepc1 and hepc2, which are, however, considerably divergent at the level of the corresponding mature 25-amino acid peptide. This striking observation led us to ask whether hepc1 and hepc2 performed the same biologic activity with regard to iron metabolism in the mouse. We recently described the severe iron-deficient anemia phenotype in transgenic mice overexpressing hepc1 in the liver. Here we report that, in contrast to the hepc1-transgenic mice, none of the 7 founder hepc2-transgenic animals suffered from anemia. They all developed normally with hematologic parameters similar to the nontransgenic littermates. Hepc2 transgenic mRNA level was found to be very high for all lines compared with the level of hepc1 transgene mRNA necessary to produce severe anemia. These data provide evidence that hepc2 does not act on iron metabolism like hepc1 and give clues for the identification of amino acids important for the iron-regulatory action of the mature 25-amino acid peptide. (Blood. 2004;103:2816-2821)
Introduction
Hepcidin, a liver-specific regulatory peptide, was recently demonstrated to play a key role in regulating iron homeostasis. Although not demonstrated, hepcidin is likely acting on iron metabolism by limiting intestinal iron absorption and iron release from macrophages (for reviews, see Nicolas et al1 and Ganz2 ). This function is fundamental for maintaining iron homeostasis, in particular to avoid accumulation of excess iron that leads to organ dysfunction. This is the case in hereditary hemochromatosis (HH), a prevalent genetic disorder of iron hyperabsorption leading to hyperferremia, tissue iron deposition, and complications including cirrhosis, hepatocellular carcinoma, heart disease, endocrinopathies, and diabetes (for review see Fleming and Sly3 ). Most patients with HH are homozygous for a missense mutation C282Y in the atypical major histocompatibility complex (MHC) class I molecule HFE.4 A more severe form of the disease, known as juvenile hemochromatosis (JH), is characterized by rapid iron loading and clinical presentation of hypogonadism and cardiomyopathy at a young age.5 The major responsible gene is linked to chromosome 1q21 but it has not yet been identified. Recently, 2 families with homozygous mutations of the hepcidin gene on chromosome 19 have been reported.6 The affected individuals presented all the clinical signs of JH, which highlights the irreplaceable regulatory role of hepcidin in maintaining iron balance in humans. In mice, complete hepcidin deficiency has also been reported to be associated with a severe iron overload phenotype.7 Interestingly, along with the uncommon JH due to complete hepcidin deficiency, partial hepcidin deficiency has been described in the most common form of HFE-related hemochromatosis in mice and humans.8-12 We recently proposed that the failure of hepcidin induction in HFE-related hemochromatosis might contribute to the pathogenesis of the disease; this was suggested by our finding that constitutive hepcidin expression prevented iron overload in a murine model of hemochromatosis.11
In contrast to the unique copy of the hepcidin gene in the human genome, the hepcidin locus has undergone duplication in mice leading to 2 hepcidin genes, hepc1 and hepc2.7,13 The 2 genes are located in the same region on mouse chromosome 7, but so far the exact distance between them has been difficult to establish due to the many discrepancies between contigs from cosmids and mouse genome sequences. The hepc1 and hepc2 nucleotide sequences (93% identity between the full-length cDNAs), as well as the regulatory region (up to 1000-bp sequence of the 5′-flanking genomic region of hepc1 and hepc2 genes), are highly similar. Hepc1 cDNA fragment detected both hepc1 and hepc2 transcripts in Northern blot analysis; however, it was possible to differentiate them by reverse transcriptase-polymerase chain reaction (RT-PCR).7 It has been demonstrated that both hepc1 and hepc2 genes are up-regulated in iron-loaded mice (Ilyin et al14 and N.G., personal observations, 2002) and both are completely silenced in the liver of hepcidin-deficient mice.7 This latter model is unusual in that hepcidin deficiency was not engineered experimentally but was found unexpectedly as a consequence of the targeted disruption of the gene immediately upstream in the chromosome, namely the Usf2 gene.7
We recently established the role of hepcidin in iron metabolism by generating transgenic mice expressing hepc1 under the control of a liver-specific promoter active throughout development.15 The majority of the transgenic mice were born with pale skin and died within a few hours after birth. Three transgenic founders were kept alive by iron injection and were used to produce progeny. The former transgenic mice and the progeny of the 3 founders presented with decreased body iron levels and anemia, clearly demonstrating that hepc1 strongly impairs iron homeostasis. At present, there are no data on the functional role of hepc2 in mice. Due to the high homology between hepc1 and hepc2, it was reasonable to assume that both genes were similarly regulated and that both proteins were playing the same function in mice. To gain insights into these questions, which are of importance for determining the structural requirement for iron-regulatory action of hepcidin, we sought, first, to compare hepcidin sequences from mammals and fish species and to analyze the 3-dimensional (3D) structure of hepc1, hepc2, and human hepcidin peptides, and, second, to develop transgenic mice overproducing hepc2, exactly as we had for hepc1, in order to determine the effect of hepc2 on iron metabolism.
Materials and methods
Animals
All animals used in these experiments were cared for in accordance with criteria outlined in the “European Convention for the Protection of Laboratory Animals.” Animals were maintained in a temperature- and light-controlled environment and were given free access to tap water and food (standard laboratory mouse chow, AO3; Usine d'Alimentation Rationelle, Epinay-sur-Orge, France).
Generation of transgenic mice
Transgenic mice had a mixed genetic background that included contributions from C57BL/6 and B6D2 strains as previously described.16 Full-length murine hepc2 cDNA was amplified from total mouse pancreas RNAs using the forward and reverse primers 5′-GGGGGATATCAGGCCTCTGCACAGCAGAACAGGAGG-3′ and 5′-GGGGGATATCAGGCCTCTATTCTTCACAACAGATACC-3′, respectively. The hepc2 PCR fragment was introduced between the transthyretin (TTR) sequences (consisting of the 3 kilobase (kb) of the mouse TTR regulatory regions 5′ to the cap site, the first exon, first intron, and most of the second exon) and the simian virus (SV) 40 small-T poly(A) signal sequence.17 The construct was checked by DNA sequencing. The 4.7-kbp TTR-hepc2 transgene was separated from the plasmid sequence by digestion with HindIII and used for pronuclear microinjection.
Genotyping of transgenic mice by PCR and Southern blotting
Southern blotting was done as described.7 BamHI-digested DNA was electrophoresed, transferred to a nylon membrane (Hybond-N+; Amersham, Arlington Heights, IL), and hybridized to a probe corresponding to the 1.7-kbp BglII-HindIII fragment of the TTR plasmid.17 The probe was labeled with (32P) deoxycytosine triphosphosphate (dCTP) by random priming using a commercially available kit (Megaprime DNA labeling systems; Amersham). The 5.3-kbp-labeled fragment corresponds to the endogenous TTR gene, and the 4.7-kbp-labeled fragment corresponds to the transgene.
For PCR, genomic DNA (0.5-1 μg) was used in 50-μL reactions that included 2 primers. The TTR-hepc2 transgene was amplified using the forward primer 5′-GAGTCAGGAAGTATGTGAGGG-3′ (annealing in intron 1 of TTR) and the reverse primer 5′-AACAGATACCACAGGAGGGT-3′ (annealing in hepc2 cDNA). PCR was performed as follows: 27 cycles (each cycle consisting of 40 s at 94°C, 40 s at 56°C, and 40 s at 72°C), with an initial denaturation step at 94°C for 4 minutes and a final elongation step at 72°C for 5 minutes, in 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 0.05% W-1, 2 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate [dNTP], 0.2 μM each primer, and 4 units of Taq polymerase (GIBCO/BRL; Carlsbad, CA). The 705-base pair (bp)-specific product corresponds to the TTR-hepc2 transgene. The reaction was analyzed on 1.5% to 2% agarose gel containing ethidium bromide. This PCR method for mouse genotyping was found to give the same results as the Southern blot method.
Northern blotting and RT-PCR analysis
Twenty micrograms of liver RNA was denatured in formaldehyde-containing buffer and electrophoresed in 1% agarose, 2.2 M formaldehyde gels. Northern blot was performed as described.7 After electrophoresis, RNA was transferred to a nylon membrane (GeneScreen Plus; Dupont-NEN Life Science Products, Boston, MA) in 20 × standard saline citrate (SSC) buffer. The probe used to detect hepcidin mRNAs was prepared from the plasmid isolated by suppressive subtractive hybridization pT-Adv/hepc1.7 Due to the presence of the SV40 small-T poly(A) sequence, the size of the transgenic hepcidin transcript is greater than that of the endogenous one. Each blot was probed simultaneously with ribosomal 18S cDNA to check for the integrity and the amount of loaded RNAs. For RT-PCR analysis, double-stranded cDNA was synthesized in 20 μL, with 2 μg total RNA (either from liver or pancreas), in the presence of 0.25 mM of each dNTP, 200 ng of random hexanucleotide primers, 20 units RNAsin (Promega, Madison, WI), 10 mM dithiothreitol (DTT), and 200 units Moloney murine leukemia virus (M-MLV) reverse transcriptase (GIBCO/BRL). After denaturation of RNA at 70°C for 10 minutes in a thermal cycler (Perkin Elmer Cetus; Perkin Elmer, Shelton, CT), the reaction was performed for 1 hour at 42°C before reverse transcriptase was inactivated for 6 minutes at 96°C. At the end of the reaction 80 μL of 10 mM Tris-HCl (pH 8.0) and 0.1 mM EDTA (ethylenediaminetetraacetic acid; pH 8.0) were added. PCR amplification was performed with 5 μL reverse transcriptase reaction mixture in 50 μL 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2, 0.05% (vol/vol) W-1, 0.2 mM of each dNTP, 1 pmol forward and reverse specific primers (listed below), 1 pmol forward and reverse control β-actin primers, and 2 units Taq polymerase (GIBCO/BRL). PCR conditions were 21 cycles of denaturation at 94°C for 20 seconds, annealing at 50°C for 20 seconds, and primer extension at 72°C for 20 seconds. Following PCR, the amplified products (171 bp for hepc1 or hepc2 and 250 bp for β-actin) were separated by electrophoresis on 1.5% agarose gel. Sequences of the primers were as follows: hepc1, 5′-CCTATCTCCATCAACAGATG-3′ (forward) and 5′-AACAGATACCACACTGGGAA-3′ (reverse); hepc2, 5′-CCTATCTCCAGCAACAGATG-3′ (forward) and 5′-AACAGATACCACAGGAGGGT-3′ (reverse); β-actin, 5′-AGCCATGTACGTAGCCATCC-3′ forward) and 5′-TTTGATGTCACGCACGATTT-3′ (reverse).
Hematologic analysis of mice
Blood was obtained by retroorbital phlebotomy before the mice were killed and was collected in heparinized tubes (capiject T-MLH; Terumo Medical, Ann Arbor, MI). Blood cell counts and erythrocyte parameters (red blood cell count, hemoglobin level, hematocrit, and mean cell volume) were determined using a MaxM Coulter automatic analyzer (Beckman Coulter, Hialeah, FL).
Determination of genomic organization of the mouse Usf2/hepcidin locus
The mouse genomic clone RP23-22G9 (GenBank accession no. AC087143) and the WGS supercontig Mm7 sequence (GenBank accession no. NW_000310) were organized with respect to hepc1, hepc2, Usf 2, and truncated Usf 2 by sequence comparison with the corresponding cDNA. A series of PCR analysis was performed to determine the genomic organization of the locus in the murine 129/Sv genome. The sequences of the primers are available upon request.
Three-dimensional structure analysis of mouse hepc1 and hepc2 and human hepcidin peptides
Graphic modeling of the 3D structure of the peptides was performed using the Centre de Biochimie Structurale (CBS) (http://bioserv.cbs.cnrs.fr) modeling tools (Meta-Server, Modeller18 ) with human hepcidin (PDB 1M4F19) as reference. Visualization and analysis of mouse hepc and 1M4F were carried out using Swiss-PdbViewer software (http://www.expasy.org/spdbv/).
Results
Comparison of various hepcidin sequences
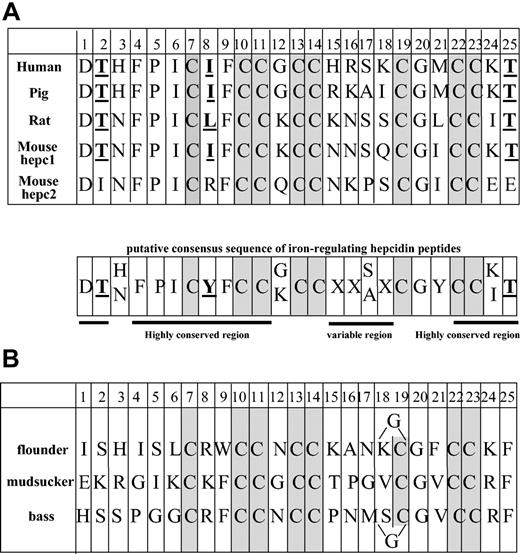
Human hepcidin is a small cysteine-rich peptide derived from the C-terminus of an 84-amino acid prepropeptide. It was originally isolated from urine20 and blood ultrafiltrate.21 Although the predominant form of hepcidin in human urine contains 25 amino acids (hepc-25), 2 peptides shorter at the amino terminus were also found, hepc-22 and hepc-20. Hepcidin (hepc-21) has also been purified and characterized from bass gills,22 and several hepcidin peptides have been predicted from mRNA analysis in rat, mouse, and various fish species. Figure 1 compares hepcidin peptides from mammals (Figure 1A) and fish (Figure 1B). The most striking observation is that they all share 8 cysteines at very conserved positions. These cysteines were shown to be engaged in the formation of 4 internal disulfide bridges.19 Besides the conserved cysteines (3 CC doublets and 2 isolated C), the similarities are weak between mammal and fish hepcidin-like peptides since only G20 is absolutely constant, with 3 other positions conserved in flounder peptide (H3, K24, and T25). In contrast, the similarities are considerable between human, pig, rat, and mouse hepc1 hepcidin peptides since, in addition to the 8 cysteines, 9 residues are invariant (positions 1, 2, 3, 4, 5, 6, 9, 20, and 25) and 5 are of 2 types only (positions 3, 8, 12, 17, and 24). In general, the first 14 and the last 4 residues are highly conserved, while the 15-21 domain is conserved only for the cysteines and G20. In contrast, mouse hepc2 is much more divergent, in particular in the conserved domains. Residue T2 is replaced by I, I-L8 by R, and T25 by E. Furthermore, the presence of a proline at position 17 and of 2 glutamic acids instead of neutral or basic residues at positions 24 and 25, as well as the change of I-Y to R in position 8 are likely to have profound consequences on the structure, charge, and, therefore, function of hepc2.
Comparison of the 25-amino acid peptide sequences. The sequences for hepcidin are from mammals (A) and various fish species (B). In the putative consensus amino acid sequence, the X is for any residue and Y for a neutral residue. The conserved cysteines are shaded and the bold underlined residues are those characteristics of the iron-regulatory peptides. In the flounder and bass hepcidin peptides, an additional G residue is present between residues 18 and 19.
Comparison of the 25-amino acid peptide sequences. The sequences for hepcidin are from mammals (A) and various fish species (B). In the putative consensus amino acid sequence, the X is for any residue and Y for a neutral residue. The conserved cysteines are shaded and the bold underlined residues are those characteristics of the iron-regulatory peptides. In the flounder and bass hepcidin peptides, an additional G residue is present between residues 18 and 19.
Three-dimensional structure analysis of mouse hepc1 and hepc2 and human hepcidin peptides
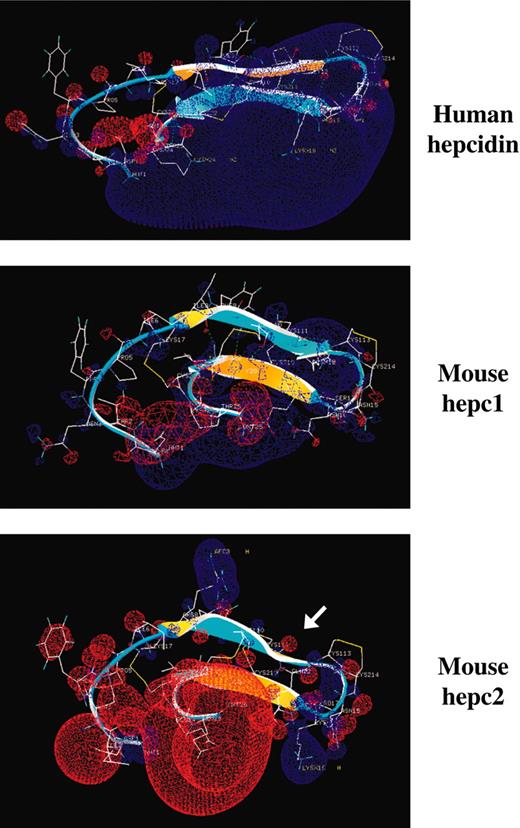
To gain insight into the functional and/or structural differences between hepc1, hepc2, and human hepcidin, the 3D structure of the peptides was compared using the CBS modeling tools. While the specific tridimensional hairpin structure (β1 sheet, coil, β2 sheet) of the molecule appeared to be highly conserved between the 3 peptides, including the length and the location of the 2 β sheets (Figure 2), hepc2 presented specific features (in particular electrostatic charge and hydrophobicity) that confer different environmental constraints on the peptide compared with human hepcidin and hepc1. (1) Hepc2 shows a torsion of the β1 sheet at its C-terminal (Figure 2, arrow), most likely due to the specific Q12-S18 H-bond passing behind the β1 sheet. In addition, S18 (first position of β2) is tightly linked by H-bonds to the adjacent K16 and P17 residues. This conformation appears to “lock” the hairpin coil (comprising the critical vicinal disulfide bond C13-C14), and possibly the β2 strand, and this structure may be rigidified by the presence of a proline (P17) in this triad (K16P17S18). (2) Another striking observation is the very specific electric field of hepc2 compared with human hepcidin and hepc1. Figure 2 clearly shows that hepc2 displays a considerable loss of electrostatic potential, specifically at the C-terminal of the molecule. Indeed, a marked electric field (Figure 2, blue), surrounding part of the coil and β2 sheet, is observed in the human molecule due to H15, R16, K18, and K24 residues. This potential is lowered in hepc1, where only T2, K12, and K24 are positively charged. In hepc2, R8 and K16 are weakly positive, since E24 and E25 make a significant negative block. (3) The invariant amino acids shown in Figure 1A in human, pig, rat, and mouse hepc1, which are different in mouse hepc2, most likely also contribute to the change in electrostatic charge and hydrophobicity: the T2I change introduces a hydrophobic potential at the N-terminal of the hepc2 molecule, whereas the I-L8R change induces a hydrophilic potential. This R residue disrupts the hydrophobic region spanning from I6 to C11-C14. Finally, as mentioned, the double change K24E and T25E at the C-terminal of the hepc2 molecule results in a very hydrophilic zone.
Three-dimensional structural analysis of human hepcidin and mouse hepc1 and hepc2 peptides. The 3D structure of mouse hepc1 and hepc2 was obtained by reference to human hepcidin (PDB 1M4F19) using the CBS modeling tools. The 2 β sheets of the peptides are represented by arrows, the β1 sheet comprises amino acids 7 to 12, and the β2 sheet comprises amino acids 18 to 22. These β sheets are antiparallel and the structure is stabilized by disulfide bridges (7-23, 10-22, 11-19) and H-bonds (8-22, 10-20, 12-18). The upper convex β-sheet face is colored in blue and the lower concave face in yellow. The convex/concave faces in hepc1 and hepc2 point in opposite directions to that of human hepc. The hepc2 β1 strand shows a marked torsion most likely due to the Q12-S18 H-bond passing behind the β2 sheet. The electrostatic potential was mapped on the molecule using the Poisson-Boltzmann equation (Swiss-Pdb Viewer tools). The positive potentials are drawn in blue and negative ones in red. The arrow is pointing out the torsion in the β1 sheet.
Three-dimensional structural analysis of human hepcidin and mouse hepc1 and hepc2 peptides. The 3D structure of mouse hepc1 and hepc2 was obtained by reference to human hepcidin (PDB 1M4F19) using the CBS modeling tools. The 2 β sheets of the peptides are represented by arrows, the β1 sheet comprises amino acids 7 to 12, and the β2 sheet comprises amino acids 18 to 22. These β sheets are antiparallel and the structure is stabilized by disulfide bridges (7-23, 10-22, 11-19) and H-bonds (8-22, 10-20, 12-18). The upper convex β-sheet face is colored in blue and the lower concave face in yellow. The convex/concave faces in hepc1 and hepc2 point in opposite directions to that of human hepc. The hepc2 β1 strand shows a marked torsion most likely due to the Q12-S18 H-bond passing behind the β2 sheet. The electrostatic potential was mapped on the molecule using the Poisson-Boltzmann equation (Swiss-Pdb Viewer tools). The positive potentials are drawn in blue and negative ones in red. The arrow is pointing out the torsion in the β1 sheet.
Hepc2 gene expression in the pancreas
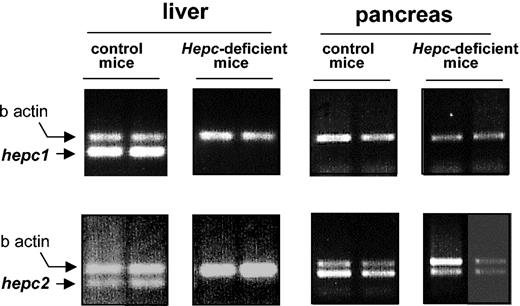
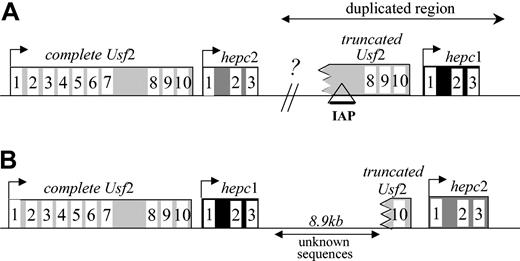
In a preliminary screening using hepc1- and hepc2-specific reverse transcription assays, we found that while hepc1 was almost specifically expressed in the liver, hepc2 was also strongly expressed in the pancreas (Figure 3), in agreement with Ilyin et al.14 The expression of hepc1 and hepc2 was further investigated in the iron-loaded hepcidin-deficient mice. Interestingly, while, as previously reported,7 both hepc1 and hepc2 expression was repressed in the liver of the hepcidin-deficient mice, hepc2 expression was not affected in the pancreas of hepcidin-deficient mice (Figure 3). This result strongly suggests that, if secreted, the pancreatic hepc2 product is not able to supplement the lack of liver hepcidin production to overcome the iron overload phenotype of the hepcidin-deficient mice. The difference in the pancreatic expression of hepc1 and hepc2 in hepcidin-deficient mice is surprising but might be explained by the presence, in the region of interest, of a retroviral intracisternal A-particle (IAP) element as recently reported.14 Indeed, this IAP element is a retrotransposon able to promote the ectopic tissue-specific expression of the adjacent genes.23 However, the exact location of the IAP is a matter of discussion due to, as previously mentioned, the major discrepancies between the cosmid sequences and the genomic sequences retrieved from the mouse sequencing genome project. Figure 4 shows the controversial genomic organization of the region encompassing the Usf 2 and hepcidin genes in mouse chromosome 7. It is worth noting that the duplication event cuts off the end of the Usf 2 gene (referred to as truncated Usf 2). According to the clone RP23-22G9 (GenBank accession no. AC087143), the hepc 2 gene is downstream of the complete Usf 2 gene, and hepc1 corresponds to the duplicated gene lying downstream of the truncated Usf 2 gene (Figure 4A). This organization corresponds to the one we previously published.7 In this configuration, as reported by Ilyin et al,14 the IAP element is located 5′ to the hepc1 gene. In contrast, according to the WGS supercontig Mm7 sequence (GenBank accession no. NW_000310) the hepc1 gene is located downstream of the complete Usf 2 gene, hepc2 being the duplicated gene (Figure 4B). For further insight into the genomic arrangement of this region, we performed a series of PCR analyses on genomic DNA and confirmed the sequence of the mouse genome (NW_000310; Figure 4B) (ie, that hepc1 is downstream of the Usf 2 gene and that hepc2 is the duplicated gene; data not shown).
The mRNA level ofhepc1andhepc2as determined by RT-PCR using liver and pancreatic mRNA from control and hepcidin-deficient mice. Following PCR, the amplified products (171 bp for hepc1 and hepc2, 250 bp for β-actin7 ) were separated by electrophoresis on 1.5% agarose gel.
The mRNA level ofhepc1andhepc2as determined by RT-PCR using liver and pancreatic mRNA from control and hepcidin-deficient mice. Following PCR, the amplified products (171 bp for hepc1 and hepc2, 250 bp for β-actin7 ) were separated by electrophoresis on 1.5% agarose gel.
Genomic organization of the locus encompassinghepcidinandUsf2genes. The genomic organization was determined according to the sequences of the RP23-22G9 clone (A) and the WGS Mm7 supercontig (B) on chromosome 7. The GenBank accession numbers are AC087143 and NW_000310 for panels A and B, respectively. The genes are represented by rectangles (the numbering of the exons is indicated), while the lines correspond to intergenic regions. The arrows represent the direction of transcription. Our analysis (data not shown) confirms genomic organization as represented in panel B.
Genomic organization of the locus encompassinghepcidinandUsf2genes. The genomic organization was determined according to the sequences of the RP23-22G9 clone (A) and the WGS Mm7 supercontig (B) on chromosome 7. The GenBank accession numbers are AC087143 and NW_000310 for panels A and B, respectively. The genes are represented by rectangles (the numbering of the exons is indicated), while the lines correspond to intergenic regions. The arrows represent the direction of transcription. Our analysis (data not shown) confirms genomic organization as represented in panel B.
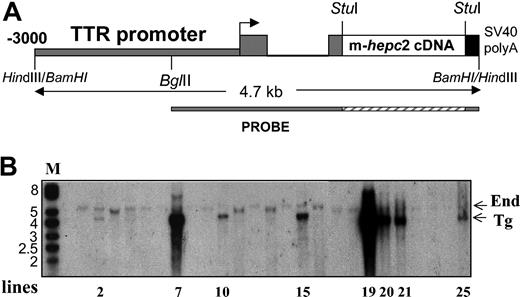
Generation of the TTR-hepc2 transgenic mice
The transgenic construct was made exactly as for hepc1 (ie, by introducing the murine PCR-made hepc2 cDNA fragment between the -3 kb mouse TTR promoter region and the SV40 small-T poly(A) signal).17 The construction is schematized in Figure 5A. After standard microinjection of the linearized construct, a total of 8 independent transgenic founders (F0) was obtained. Figure 5B shows the Southern blot of the BamHI-digested genomic DNA from these different F0 mice. The probe revealed a band at 4.7 kbp corresponding to the transgene (Tg) and one at 5.3 kbp corresponding to the transthyretin endogenous gene (End). This latter band, used as an internal reference corresponding to 2 allele copies, showed that all the founders contained more than 2 transgene copies, except founder 2, which contained approximately 2 copies of the transgene. This founder, for some undetermined reasons, died at the age of 7 weeks before any possible phenotype exploration. The other founders were regularly checked for blood parameters, which were all normal up to 8 weeks. Furthermore, none of the phenotypic traits observed in hepc1-transgenic mice, in particular reduced body size, pallor, hairlessness, and crumpled skin, were visible.
Generation of TTR-hepc2transgenic mice. (A) Schematic representation of the TTR-hepc2 construct. The murine hepc2 cDNA was introduced between the transthyretin sequences (consisting of the 3 kb of the mouse TTR regulatory regions 5′ to the cap site, the first exon, first intron, and most of the second exon) and the SV40 small-T poly(A) signal sequence.17 (B) Southern blot analysis of tail DNA from transgenic founders. Genomic DNA was digested by BamHI and hybridized with the TTR probe shown in panel A. Two bands of the expected size, 5.3 kbp and 4.7 kbp, representing the endogenous TTR gene (End) and the transgene (Tg), respectively, were detected.
Generation of TTR-hepc2transgenic mice. (A) Schematic representation of the TTR-hepc2 construct. The murine hepc2 cDNA was introduced between the transthyretin sequences (consisting of the 3 kb of the mouse TTR regulatory regions 5′ to the cap site, the first exon, first intron, and most of the second exon) and the SV40 small-T poly(A) signal sequence.17 (B) Southern blot analysis of tail DNA from transgenic founders. Genomic DNA was digested by BamHI and hybridized with the TTR probe shown in panel A. Two bands of the expected size, 5.3 kbp and 4.7 kbp, representing the endogenous TTR gene (End) and the transgene (Tg), respectively, were detected.
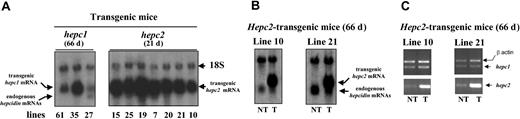
Level of hepc2-transgenic expression in F1 progeny
At the age of 8 weeks, the founders were mated to give F1 offspring. In contrast to the very obvious pallor of the hepc1-transgenic newborns, no difference was observed in the littermates between hepc2-transgenic and nontransgenic mice. At 21 days of age, F1 hepc2-transgenic animals were killed and the level of hepc2 transgene mRNA expression in liver was determined by Northern blot. As shown in Figure 6A, transgenic hepc2 transcripts were found in all F1 animals analyzed. Importantly, the level of hepc2 transgene mRNA expression was very high compared with the level of hepc1 transgene mRNA expression in hepc1-transgenic animals (lines 61, 35, and 27) that resulted in an iron-deficient anemia phenotype. Furthermore, in the liver of older mice (66 days of age), when the endogenous hepcidin gene expression begins to be detectable, we found no difference in the expression of the endogenous hepcidin genes between hepc2-transgenic animals and nontransgenic animals (Figure 6B). This result is in contrast to the marked reduced expression of endogenous hepcidin genes we previously reported in hepc1-transgenic animals.15 Finally, the mRNA levels of hepc1 and hepc2 were assessed individually by semiquantitative RT-PCR analysis (Figure 6C) in the liver of hepc2-transgenic and nontransgenic mice. We found that the level of hepc1 transcripts was unmodified in transgenic versus nontransgenic animals (confirming the Northern blot experiment of Figure 6B) and that, as expected, the level of hepc2 transcripts was greatly increased due to the presence of both endogenous and transgenic hepc2. Taken together, these results clearly suggest that the hepc2 product is not as active as hepc1 is (or human hepcidin) in iron metabolism.
Hepcidin mRNA level in transgenic mice constitutively expressinghepc2as determined by Northern blot and RT-PCR analyses. Twenty micrograms of total liver RNA purified from 66-day-old transgenic mice expressing hepc111 and from 21-day-old transgenic mice expressing hepc2 (A) or from 66-day-old nontransgenic (NT) mice compared with age-matched hepc2-transgenic (T) mice (B) were electrophoresed, blotted, and hybridized with the (32P)-labeled hepcidin probe. This probe reveals both the transgenic hepcidin mRNAs (transgenic hepc1 mRNA and transgenic hepc2 mRNA, top band) and the endogenous hepcidin mRNAs. Note that in the 66-day-old hepc1-transgenic mice the endogenous genes (bottom band) are repressed or reduced (line 27) most likely due to, as reported,15 the hepc1-transgene-induced anemia. (C) Individual expression of hepc1 and hepc2 mRNAs was measured by RT-PCR, as described in “Materials and methods,” in 66-day-old nontransgenic (NT) mice compared with age-matched hepc2-transgenic (T) mice. Following PCR, the amplified products (171 bp for hepc1 or hepc2 and 250 bp for β-actin) were separated by electrophoresis on 1.5% agarose gel.
Hepcidin mRNA level in transgenic mice constitutively expressinghepc2as determined by Northern blot and RT-PCR analyses. Twenty micrograms of total liver RNA purified from 66-day-old transgenic mice expressing hepc111 and from 21-day-old transgenic mice expressing hepc2 (A) or from 66-day-old nontransgenic (NT) mice compared with age-matched hepc2-transgenic (T) mice (B) were electrophoresed, blotted, and hybridized with the (32P)-labeled hepcidin probe. This probe reveals both the transgenic hepcidin mRNAs (transgenic hepc1 mRNA and transgenic hepc2 mRNA, top band) and the endogenous hepcidin mRNAs. Note that in the 66-day-old hepc1-transgenic mice the endogenous genes (bottom band) are repressed or reduced (line 27) most likely due to, as reported,15 the hepc1-transgene-induced anemia. (C) Individual expression of hepc1 and hepc2 mRNAs was measured by RT-PCR, as described in “Materials and methods,” in 66-day-old nontransgenic (NT) mice compared with age-matched hepc2-transgenic (T) mice. Following PCR, the amplified products (171 bp for hepc1 or hepc2 and 250 bp for β-actin) were separated by electrophoresis on 1.5% agarose gel.
Discussion
In contrast to other mammalian species, mice contain 2 homologous duplicated genes that encode for hepc1 and hepc2. The aim of this study was to investigate the relative roles of these hepc1 and hepc2 peptides in iron metabolism by analyzing their 3D structures and comparing the phenotypes of transgenic mice overexpressing hepc2 to that of already characterized transgenic mice overexpressing hepc1 at a similar level.15
During the course of this study, we have been brought to reexamine the genomic organization of the Usf2/hepcidin locus due to the discrepancies between the cosmid sequences and the genomic sequences obtained from the mouse sequencing genome project. In contrast to the genomic configuration we previously reported7 and more recently reported by Ilyin et al14 for hepc1 and hepc2, our genomic data analyses determined that the mouse hepc1 gene is downstream of the Usf2 gene and that hepc2 is the duplicated gene lying downstream of the truncated Usf2 gene. Interestingly, Ilyin et al14 reported the presence of a mouse retroviral IAP element in the intron 7 of the truncated Usf2. According to our data, the presence of this IAP element is expected to be 5′ to the hepc2 gene and not to the hepc1 one as reported by Ilyin et al.14 This location was, however, not confirmed due to a 8.9-kb gap between hepc1 and the truncated Usf2 gene in the NW_000310 contig (Figure 4B). We hypothesized that the presence of this IAP element in the regulatory region of hepc2 could contribute to the observed deregulation of hepc2 in the pancreas of hepcidin-deficient mice, since this sequence was previously reported to be able to alter the transcription of adjacent genes. However, the difference between regulation of the hepc1 and hepc2 genes was not limited to constitutive deregulation in the pancreas, as illustrated by the differential response of these genes to inflammation, a well-characterized situation known to induce hepcidin gene expression.13,22,24 Indeed, we found that turpentine injection in mice induced an increase in hepc125 but repression of hepc2 gene expression in the liver (G.N., unpublished observation, 2002). Consequently, even if IAP sequences are really localized 5′ to the hepc2 gene, it is uncertain whether they are responsible for all of the differential regulation of the hepc1 and hepc2 genes, or whether such regulation reflects more subtle changes in the regulatory regions of these genes.
In the present paper, we report that, in contrast to the severe iron-deficient anemia characterized in hepc1-transgenic mice, hepc2-transgenic mice animals develop normally with hematologic parameters similar to the nontransgenic mice. Such a differential effect of hepc1 and hepc2 on iron homeostasis appears to be consistent with the specific structural differences in hepc2 compared with hepc1 and other hepcidin peptides that have either proved to be active on iron metabolism (in humans) or are most likely active because they are produced by a single hepcidin gene (rats and pigs). From these peptide sequences, we can propose a tentative consensus sequence of iron-regulatory active hepcidin (Figure 1A) differing from hepc2 in the most conserved regions. As for the fish hepcidin-like peptides, in which conservation is limited to the cysteine positions, we can speculate that they are archetypes of the ancestral peptides from which mammalian hepcidin peptides have derived, maybe corresponding to a special type of β-defensin endowed with antimicrobial activity.26
The specific role of hepc2 in mice remains an open question. On one hand, it is obvious that a second hepc gene is not indispensable since hepcidin duplication has so far been characterized only in mice. On the other hand, hepc2 does not appear to be an inactive pseudogene, because its global structure is well conserved and because hepc2 mRNA is present at a high level and apparently subjected to specific regulation. In fact hepc2 shares some common features with fish hepcidin-like peptides, in particular at position 8, an isoleucine in iron-regulatory active hepcidins and which is either arginine or lysine in hepc2 and fish peptides. We can therefore speculate that hepc2 could also share common functions with these fish peptides, most likely in the innate immunity. According to this hypothesis, the ancestral antimicrobial hepcidin-like gene would have been recruited for iron regulation in mammals. However, a gene duplication phenomenon may have permitted conservation of a selectively advantageous antimicrobial gene in mice.
Further investigations, in particular selective knockout of the hepc1 and hepc2 genes in mice, should allow us to directly test these hypotheses. It will also be possible to directly test in vivo our hypothesis concerning the consensus sequence of the iron-regulatory peptides and, therefore, to obtain further information on the residues crucial for their activities. These data will be essential for designing agonistic and antagonistic peptides of potentially great therapeutic interest for patients suffering from different diseases involving abnormal iron homeostasis.
Supported by a grant from Debiopharm (D.-Q.L., J.-C.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-07-2524.
We thank Carole Beaumont and Bernard Grandchamp for fruitful discussions, Terry Van Dyke for the TTR vector, and Alan Strickland for the careful revision of the text.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal