Abstract
The CCAAT/enhancer binding protein alpha (C/EBPα) is an essential transcription factor for granulocytic differentiation. C/EBPα mutations are found in approximately 8% of acute myeloid leukemia (AML) patients. Most of these mutations occur in the N-terminal coding region, resulting in a frame shift and the enhanced translation of a dominant-negative 30-kDa protein, which may be responsible for the differentiation block observed in AML. To test this hypothesis, we introduced a cDNA encoding an N-terminal mutated C/EBPα (mut10) into primary hematopoietic progenitors using a retroviral vector. Expression of mut10 in human CD34+ cord blood cells dramatically inhibited differentiation of both myeloid and erythroid lineages. Immunohistochemical analysis demonstrated coexpression of both myeloid and erythroid markers in the immature transformed cells. Surprisingly, mut10 did not block myelocytic differentiation in murine progenitors but did alter their differentiation kinetics and clonogenicity. Experiments were performed to confirm that the differential effect of mut10 on murine and human progenitors was not due to species-specific differences in C/EBPα protein sequences, expression levels, or inefficient targeting of relevant cells. Taken together, our results underline the intrinsic differences between hematopoietic controls in mouse and human and support the hypothesis that mutations in CEBPA are critical events in the disruption of myeloid differentiation in AMLs. (Blood. 2004;103:2744-2752)
Introduction
The hallmark of acute leukemia is the clonal, neoplastic proliferation of immature hematopoietic cells. The observed inhibition or complete arrest in differentiation can conceivably be due to an indirect mechanism, in which genetic mutations that disrupt cell cycle controls support proliferation at the expense of differentiation, and/or due to genetic abnormalities that directly interfere with the differentiation program. In support of the latter hypothesis, growing evidence suggests that the transcription factors that play pivotal roles in lineage-specific differentiation may be important targets of transformation in acute leukemias.1
The basic zipper (B-ZIP) transcription factor CCAAT/enhancer binding protein α (C/EBPα) and the ETS-family transcription factor PU.1 are 2 major regulators of myeloid differentiation.2,3 Unlike PU.1, which governs transcription of a wide spectrum of myeloid-specific genes found in both early hematopoietic stem cells and mature monocytes and granulocytes, C/EBPα regulation has a more specific function in granulopoiesis. Indeed, C/EBPα knockout mice show a selective block in the differentiation of granulocytes, including neutrophils and eosinophils.4 Furthermore, the constitutive expression of C/EBPα in myeloid progenitors results in the induction of granulocytic development and partial inhibition of monocytic and erythroid development.5-7
Several independent studies have demonstrated mutations in the CEBPA gene encoding C/EBPα in approximately 8% of acute myeloid leukemia (AML).8-11 These mutations are found primarily in AMLs with a myeloblast phenotype (French-American-British [FAB]-M1 and -M2 subtypes), consistent with the importance of C/EBPα in granulocytic differentiation. In addition, these mutations are almost exclusively found in AMLs grouped as intermediate risk based on cytogenetic analysis.8,10,11 The absence of mutations in AMLs within other risk groups associated with specific cytogenetic markers suggests that some genetic abnormalities may disrupt differentiation controls by interfering with C/EBPα regulation at different levels. This later hypothesis is supported by the finding that the AML1-ETO fusion product of t(8;21), a common cytogenetic abnormality within the favorable risk group, disrupts C/EBPα function and thus the autoregulation of the CEBPA gene.12,13 Down-regulation of CEBPA might thus account for the impaired myeloid differentiation observed in primary hematopoietic cells expressing AML1-ETO.14-17
The CEBPA mutations can be largely divided into 2 groups: (1) C-terminal mutations that disrupt the B-ZIP region, containing both DNA-binding as well as dimerization domains18 ; and (2) N-terminal mutations that disrupt the reading frame, resulting in premature termination of the normal 42-kDa and enhanced translation of a 30-kDa protein initiated at an internal AUG start codon. The 30-kDa protein lacks the transactivating enhancers 1 and 2 (TE1 and TE2, also known to as TAD119,20 ) and was shown to inhibit DNA binding and transactivation by wild-type C/EBPα.10 Thus, whereas C-terminal mutations result in loss of function,9,10 the N-terminal mutations give rise to a dominant-negative protein. Significantly, AML patients often carry both C-terminal and N-terminal CEBPA mutations,11 suggesting that the proposed dominant-negative effect of the N-terminal mutant is weak, giving a selective advantage to cells that have lost the second allele.
To evaluate the significance and role of N-terminal CEBPA mutations in the development of AML, we expressed a C/EBPα mutant in primary hematopoietic progenitors isolated from either human umbilical cord blood (CB) or murine bone marrow (BM) and evaluated its effect on differentiation. Our results underline intrinsic differences between hematopoietic controls in mouse and human and present strong evidence that mutations in CEBPA may be critical events leading to the disruption of myeloid differentiation observed in many AMLs.
Materials and methods
Retroviral vectors and generation of infectious pseudotyped viral particles
Either C/EBPα wild-type or mutant 10 (mut10) cDNAs10 were cloned into the Friend murine embryonic-stem cell virus (FMEV) retroviral vector FMEV-GFP (R780)14 containing the enhanced green fluorescent protein (eGFP), to generate FMEV-C/EBPα (R890) and FMEV-mut10 (R891), respectively. The cDNAs extended from nucleotide (nt) 562 to 1746 (sequencing numbering as in GenBank no. U34070), which includes a short open reading frame upstream of the AUG that initiates translation of the wild-type 42-kDa protein. A murine equivalent of mut10 was generated by amplifying the identical region from a genomic clone isolated from 129/Ola mouse liver cells and introducing a 4-bp deletion at position 610 (numbering as in GenBank no. M62362) by using the AatII restriction site. The sequence of our mouse clone differs from that deposited in the GenBank as follows: 604delC, 679-680insC, 1164A>G, 1583delG. The murine C/EBPα-AatII mutant was inserted into FMEV-YFP (R930) to generate FMEV-mutAatII (R1005). FMEV-YFP is similar to FMEV-GFP, except the eGFP coding sequence is replaced with that of the enhanced yellow fluorescent protein (eYFP; BD Biosciences, Heidelberg, Germany). To generate a Cre-recombinase expression vector (FMEV-CRE), a cassette containing the simian virus (SV)-40 promoter and the Cre coding region21 was placed downstream of the eGFP gene in a FMEV-vector. Retroviral pseudotypes were prepared as previously described14 with either the envelope (Env) protein of the Moloney murine leukemia virus22 (for infection of mouse cells) or the feline endogenous retrovirus RD11423 (for infection of human cells). To confirm excision of the Cebpa locus after FMEV-CRE infection, polymerase chain reaction (PCR) on genomic DNA was performed as previously described.24
Enrichment of hematopoietic progenitors from murine BM and human CB cells, infections, and transplantation
BM cells were isolated from tibiae and femora of untreated female C57Bl/6J (B6)-Ly5.2 or Cebpafl/fl Swiss Black (SWB) mice between 12 and 16 weeks of age and enriched for early progenitors (Linneg; > 95% purity) by depletion of cells expressing lineage-specific antigens using magnetic-activated cell separation (MACS) antibiotin MicroBeads (Militenyi Biotec, Bergisch-Gladbach, Germany). Human CB samples were collected after normal full-term delivery with informed consent of the mothers, using an approved protocol of the “Ärztekammer Hamburg” ethics commission. For the enrichment of human progenitors, CD34+ (> 98% purity) cells were isolated by magnetic cell sorting as previously described.25
Cells were prestimulated for 3 days (murine Linneg BM) and 2 days (human CD34+ CB) in serum-free medium (StemSpan; StemCell Technologies, St Katharinen, Germany) supplemented with 50 ng/mL murine stem cell factor (mSCF), 10 ng murine interleukin 3 (mIL3), and 100 ng/mL each human fetal liver tyrosine kinase 3 (hFLT3) and IL11 for murine cells or in 100 ng/mL each hSCF and hFLT3 and 20 ng/mL each hIL6 and thrombopoietin (TPO) for human cells. Recombinant mSCF and mIL3 were obtained from Strathman AG (Hamburg, Germany), all other recombinant growth factors were purchased from StemCell Technologies. Infections were carried in 6-well plates coated with RetroNectin (CH-296; Cambrex, Verviers, Belgium), as previously described.25,26 For in vivo experiments, 1 × 106 murine cells were transplanted into lethally irradiated (9 Gy) B6-Ly5.1 or SWB recipients 1 day after transduction.
Western blot analysis
Total cellular protein extracts were prepared as described previously27 and were size separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). After transfer to an Immunobilon-P polyvinylidene fluoride (PVDF) membrane (Millipore, Schwalbach, Germany), a 1:100 dilution of the polyclonal anti-C/EBPα antibody (C-18; Santa Cruz Biotechnology, Heidelberg, Germany) was used to detect protein expression. After incubation with the first antibody, the bound antibody was detected with donkey antigoat immunoglobulin G (IgG) conjugated with horseradish peroxidase (HRP; SC-2056; Santa Cruz Biotechnology) and visualized using the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Freiburg, Germany).
In vitro culture and colony assays
Either 6 days or 1 day after retroviral transduction, murine Linneg BM and human CD34+ CB cells, respectively, were sorted for GFP-positive expression using a MoFlow Cell Sorter (Cytomation Bioinstruments, Freiburg, Germany). Single-cell suspensions were either maintained in liquid cultures or plated in methylcellulose. Liquid cultures containing serum-free medium (StemSpan; StemCell Technologies) were supplemented as described for the transduction procedure. To induce differentiation of liquid cultures, cells were washed twice with phosphate-buffered saline (PBS) and plated at a concentration of 3 × 105/mL to 5 × 105/mL in 50 ng/mL each human granulocyte-macrophage colony-stimulating factor (hGM-CSF), hSCF, and hIL3 with or without G-CSF for human cells or 20 ng/mL mGM-CSF for murine cells. For colony assays, murine cells were plated in methylcellulose at concentrations of 1 × 104 and 5 × 104 cells/mL either in the presence of G-CSF alone (20 ng/mL; MethcultTM, StemCell Technologies) or in a cytokine cocktail containing IL3, IL6, SCF, and erythropoietin (Epo; MethCultTM GF M3434). Human CD34+ CB cells were plated in the presence of SCF, GM-CSF, hIL3, and Epo (MethCultTM GF H4434). Individual colonies were counted after 8 days for murine cells or after 10 to 14 days for human cells, as indicated. Cytospins were made from representative colonies or from total plates. Cells from liquid cultures were analyzed for lineage markers using a FACScalibur (BD Biosciences), as previously described.14
Morphologic methods
For the microscopic analysis of in vitro cultures, methanol-fixed cytospins were stained with a modified Wright-Giemsa stain and periodic acid Schiff (PAS) reaction (Sigma, Deisenhofen, Germany). For demonstrating the hematologic marker enzymes alpha-naphthyl acetate esterase (unspecific esterase), naphthol AS-D chloroacetate esterase, and acid phosphatase, commercially available kits were used according to the manufacturers' instructions (Sigma). The intracellular presence of myeloperoxidase, glycophorin A, lysozyme, and mast cell tryptase was immunocytochemically revealed by an indirect immunoperoxidase labeling technique. Briefly, acetone-fixed cytospins were incubated with appropriately diluted monoclonal antihuman myeloperoxidase, glycophorin A, and mast cell tryptase antibodies (DAKO Diagnostika, Hamburg, Germany) followed by the peroxidase-conjugated, mouse-specific Envison detection system (DAKO). Peroxidase activity was visualized by the help of commercial diaminobenzidine (DAB) or AEC (3-amino-9-ethylcarbazole) substrates (DAKO). The same method was applied for the immuno-tracing of intracellular lysozyme (muramidase) by using a polyclonal rabbit antihuman lysozyme antibody (DAKO). For differential counts of hematopoietic cells on cytospins, at least 300 cells were counted using a microscope front lens with a × 100 magnification.
Results
Transduction of murine BM cells with eGFP and C/EBPα (mut10)-expressing vectors
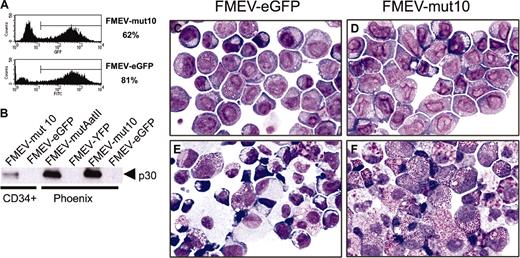
To test the effect of N-terminal C/EBPα mutants on myeloid differentiation, the previously characterized CEBPA mutant 10 cDNA (mut10)10 was inserted into an FMEV retroviral vector (Figure 1A), which coexpresses eGFP and thus allows the identification and isolation of transduced cells. Previous studies have demonstrated that 2 proteins, a 20-kDa protein initiating at the first AUG and a 30-kDa protein initiating at an internal AUG, are expressed from mut10 cDNA (Figure 1B) and that the 30-kDa protein is responsible for the dominant-negative effect on DNA binding and transactivation by wild-type C/EBPα.10 BM cells enriched for early progenitors (Linneg) were infected with vector/viral pseudotypes. As determined by eGFP expression, efficient transduction with both FMEV-mut10 and the control vector was obtained (32%-62% and 38%-69%, respectively; range of 3 independent experiments; Figure 1C). Cells were then either transplanted into irradiated donors or sorted for eGFP expression and analyzed in vitro. Western blot analysis was performed on cell extracts prepared from either sorted cells of infected bone marrow cultures or Phoenix virus-producing cells (Figure 1D). As a control, Phoenix cells expressing an FMEV vector carrying the wild-type C/EBPα gene were also examined. As predicted, cells infected with FMEV-C/EBPα expressed the full-length 42-kDa protein and, at much reduced levels, the 30-kDa protein. In contrast, only the 30-kDa protein could be detected in cells infected with FMEV-mut10 using a C-terminal antibody (Figure 1D).
Mut10 C/EBPα expresses enhanced levels of a 30-kDa protein. (A) Schematic depiction of the vectors used for these studies. The cDNA for mut10 C/EBPα was inserted into the FMEV-GFP retroviral vector. Cotranslation of eGFP is mediated by an internal ribosomal entry site (I). A posttranscriptional regulatory element (PRE) from the woodchuck hepatitis virus increases expression levels. (B) Schematic representation of predicted proteins expressed by wild-type or mut10 C/EBPα cDNAs. Due to a 7-bp deletion resulting in a frameshift at amino acid 39 and a termination codon at amino acid 159, the full-length 42-kDa protein is truncated and enhanced expression of a 30-kDa protein is observed. (C) Coexpression of eGFP in the vectors allows the detection of both transduced and nontransduced cells. The eGFP fluorescence was measured by flow cytometry in BM cells infected with either the control “empty” vector (FMEV-GFP) or the vector containing mut10 C/EBPα cDNA (FMEV-mut10). Transduction efficiencies obtained with BM cells are indicated. (D) Expression of the 30-kDa protein was confirmed by Western blot analysis. Protein extracts were prepared from either infected BM cells or transient transfected Phoenix packaging cells, from which virus supernatants were obtained. Protein (3 μg/lane) was separated by SDS-PAGE and immunoblotted with anti-C/EBPα antibody that detects an epitope on the C-terminus.
Mut10 C/EBPα expresses enhanced levels of a 30-kDa protein. (A) Schematic depiction of the vectors used for these studies. The cDNA for mut10 C/EBPα was inserted into the FMEV-GFP retroviral vector. Cotranslation of eGFP is mediated by an internal ribosomal entry site (I). A posttranscriptional regulatory element (PRE) from the woodchuck hepatitis virus increases expression levels. (B) Schematic representation of predicted proteins expressed by wild-type or mut10 C/EBPα cDNAs. Due to a 7-bp deletion resulting in a frameshift at amino acid 39 and a termination codon at amino acid 159, the full-length 42-kDa protein is truncated and enhanced expression of a 30-kDa protein is observed. (C) Coexpression of eGFP in the vectors allows the detection of both transduced and nontransduced cells. The eGFP fluorescence was measured by flow cytometry in BM cells infected with either the control “empty” vector (FMEV-GFP) or the vector containing mut10 C/EBPα cDNA (FMEV-mut10). Transduction efficiencies obtained with BM cells are indicated. (D) Expression of the 30-kDa protein was confirmed by Western blot analysis. Protein extracts were prepared from either infected BM cells or transient transfected Phoenix packaging cells, from which virus supernatants were obtained. Protein (3 μg/lane) was separated by SDS-PAGE and immunoblotted with anti-C/EBPα antibody that detects an epitope on the C-terminus.
BM cells transduced with mut10 rarely contribute to long-term engraftment of irradiated donors
Approximately 3 months after receiving transplants of either FMEV-eGFP or FMEV-mut10 Linneg BM cells, mice were bled and/or killed and the proportion of transduced cells in the blood and bone marrow was determined. As expected, blood and BM cells were of donor origin as determined by expression of the Ly5.2 and not Ly5.1 alloantigen (data not shown). Surprisingly, however, in 39 of 42 mice receiving BM cells infected with FMEV-mut10 with either 32% (n = 19) or 62% (n = 23) transduction efficiency, no eGFP-positive cells in the blood or bone marrow could be detected by fluorescence-activated cell sorter (FACS) analysis. In 3 mice receiving the 62% eGFP-positive BM, low frequencies of eGFP-positive cells (2 to 9%) were detectable. However the levels of eGFP expression in these cells were approximately 1% to 2% of that observed in the BM cells used for transplantations, as determined by mean fluorescence. This is in striking contrast to 3 sets of controls in which all 16 mice receiving FMEV-eGFP-infected BM cells with either a transduction efficiency of 68% (n = 4), 38% (n = 4), or 15% (n = 8) contained transduced cells in the bone marrow 3 months after transplantation, albeit with various frequencies (2% to 79%). Importantly, the mean fluorescence of eGFP was reduced on the average by only 20% in control mice. Variations in expression levels can reflect the strength of FMEV retroviral enhancer in different lineages; however, we saw no significant difference in the distribution of lineages within the FMEV-eGFP- or FMEV-mut10-transduced cell populations as determined by antigen expression in FACS analysis (data not shown). Thus, the consistently low eGFP levels in mut10 mice are most likely due to selection for cells with low retroviral expression levels. Taken together, these results suggest that high levels of expression of mut10 in early progenitors impairs their ability to contribute to long-term engraftment. This could be due to defects in homing, loss of self-renewal capacity, cell cycle block, increased apoptosis, or other cytotoxic effects.
Mut10 expression in murine BM cells does not block granulocytic differentiation but alters the kinetics of differentiation and reduces the self-renewal potential
As the in vivo studies did not allow us to make conclusions with regard to the effect of mut10 on differentiation, several in vitro assays were performed on infected Linneg BM cells sorted for eGFP expression. We first performed colony assays under conditions that promote differentiation in granulocytic, monocytic, and erythroid differentiation. Consistent with a loss of self-renewal of early progenitors, significantly reduced numbers of colony-forming cells (CFCs) were observed in FMEV-mut10-infected cultures versus FMEV-GFP-infected cultures (Table 1). However, no significant difference was observed in the ratio of lineage-specific CFCs, suggesting that mut10 did not alter the differentiation potential along any specific lineage (Table 1). Furthermore, microscopic examination of cells within mature colonies (day 10), confirmed the presence of mature forms of both myeloid (granulocyte/monocyte) and erythroid lineages. These results support the hypothesis that mut10 expression decreases the number of early progenitors but does not impair differentiation.
Reduced levels but normal lineage distribution of CFCs in murine BM cells expressing mut10-C/EBPα
. | No. of colonies per 104 cells . | Mean distribution of mature colonies, % . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vector . | . | CFU-Mix . | CFU-GM . | CFU-G . | CFU-M . | BFU-E . | ||||
| FMEV-mut10 | 39 ± 7 | 2.5 ± 1.4 | 60 ± 9.7 | 2.5 ± 0.4 | 28 ± 15.2 | 7.5 ± 5.4 | ||||
| FMEV-GFP | 88 ± 18 | 2.5 ± 1.4 | 65 ± 1.7 | 5.1 ± 2.8 | 23 ± 1.9 | 3.8 ± 1.6 | ||||
| Mock infected | 95 ± 6 | 1.0 ± 1 | 53 ± 1.2 | 4.1 ± 1.2 | 29 ± 7.3 | 13 ± 7.7 | ||||
. | No. of colonies per 104 cells . | Mean distribution of mature colonies, % . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vector . | . | CFU-Mix . | CFU-GM . | CFU-G . | CFU-M . | BFU-E . | ||||
| FMEV-mut10 | 39 ± 7 | 2.5 ± 1.4 | 60 ± 9.7 | 2.5 ± 0.4 | 28 ± 15.2 | 7.5 ± 5.4 | ||||
| FMEV-GFP | 88 ± 18 | 2.5 ± 1.4 | 65 ± 1.7 | 5.1 ± 2.8 | 23 ± 1.9 | 3.8 ± 1.6 | ||||
| Mock infected | 95 ± 6 | 1.0 ± 1 | 53 ± 1.2 | 4.1 ± 1.2 | 29 ± 7.3 | 13 ± 7.7 | ||||
Mock-infected or vector-infected Linneg BM cells sorted for GFP expression were plated in methylcellulose at 2 different cell concentrations and scored for colony formation and morphology after 7 days. Results shown are from one experiment performed in triplicate. Similar values were obtained in a second independent experiment. CFU-Mix indicates mixed colony-forming unit; CFU-GM, granulocyte macrophage colony-forming unit; and BFU-E, erythroid burst-forming unit.
Previous studies have shown that IL3 and GM-CSF can override the block in granulocytic differentiation in cell lines established from Cebpa-/- mice.28,29 We thus tested the number of granulocytic colonies in the presence of G-CSF alone. As a positive control for a differentiation block, bone marrow cells from Cebpafl/fl mice were also used. In these mice the Cebpa gene is flanked by loxP sites recognized by the Cre recombinase. BM cells (Linneg) from these mice were infected with either a retroviral vector expressing the Cre recombinase (FMEV-CRE) or eGFP alone (FMEV-GFP) with a transduction efficiency of 40% and 66%, respectively. Cells were sorted for GFP expression and then plated in methylcellulose containing G-CSF alone. As predicted, no or only single granulocyte colony-forming units (CFU-Gs) were observed in Cebafl/fl cultures infected with FMEV-CRE, in contrast to controls where an average of 27 CFU-Gs per 5 × 104 cells were present (Figure 2), confirming that C/EBPα expression is important for granulocytic differentiation under these conditions. Furthermore, these results demonstrate that the transduction protocol is able to target cells at a stage where C/EBPα expression is necessary for further differentiation. In contrast, FMEV-mut10 infection of BM cells did not block the formation of CFU-Gs in the presence of G-CSF. Although the absolute number of colonies was reduced compared with controls, these colonies were morphologically identical to that obtained in control-infected cultures. These results demonstrate that constitutive overexpression of the 30-kDa protein in these murine progenitors reduces the absolute number of colonies but does not block granulocytic differentiation, even when stimulated solely by G-CSF, whose receptor is regulated by C/EBPα.4,30
G-CSF stimulates the formation of CFU-G colonies in mut10-transduced BM cells but not in BM in which Cebpa has been excised. To determine the differentiation potential of mut10-transduced cells in response to G-CSF alone, Linneg BM cells from wild-type B6 mice were infected with either FMEV-mut10 or control vector FMEV-GFP, sorted for GFP expression, and then plated in methylcellulose containing G-CSF. As a control, Linneg BM were isolated from SWB mice homozygous for floxed Cebpa alleles. BM cells were infected with either FMEV-CRE or FMEV-GFP, sorted for GFP expression, and plated in G-CSF. Colonies were scored 7 days after plating. Results presented are from a single experiment performed in triplicate. Error bars indicate SEM.
G-CSF stimulates the formation of CFU-G colonies in mut10-transduced BM cells but not in BM in which Cebpa has been excised. To determine the differentiation potential of mut10-transduced cells in response to G-CSF alone, Linneg BM cells from wild-type B6 mice were infected with either FMEV-mut10 or control vector FMEV-GFP, sorted for GFP expression, and then plated in methylcellulose containing G-CSF. As a control, Linneg BM were isolated from SWB mice homozygous for floxed Cebpa alleles. BM cells were infected with either FMEV-CRE or FMEV-GFP, sorted for GFP expression, and plated in G-CSF. Colonies were scored 7 days after plating. Results presented are from a single experiment performed in triplicate. Error bars indicate SEM.
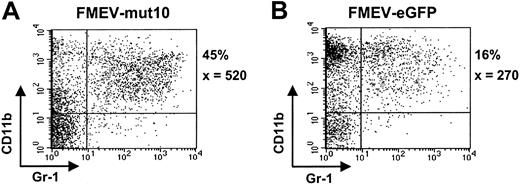
To further analyze the differentiation potential of mut10-infected cells, liquid cultures were maintained in the presence of an “expansion” cocktail (SCF, FLT3, IL11, and IL3) or under myeloid differentiation conditions (GM-CSF). Somewhat surprisingly, although control cultures under “expansion” conditions contained many cells of blast cell morphology (> 75%) after 6 days in culture, mut10 cultures already showed extensive differentiation (< 5% blast cells), consistent with the loss of self-renewal and increased differentiation in these cultures. FACS analysis at various time points confirmed the increased levels of myelocytic differentiation (CD11b/Gr1-positive cells) in these cultures (Figure 3A-B; data not shown). The percentage of CD11b/Gr1-positive cells was 3-fold higher than parallel control-infected (FMEV-eGFP) cultures in 2 independent experiments. Interestingly, after 14 days of culture, when the control cultures contained almost exclusively macrophages and mast cells, cultures infected with mut10 showed continued differentiation of monocytic and granulocytic forms (Figure 4A-B). The altered kinetics of myelopoiesis in mut10-expressing cells was also evident in cultures containing GM-CSF alone (data not shown). Expression levels of eGFP in the FMEV-mut10 cultures were comparable to that in control cultures (data not shown), ruling out that a selection for low-expressing mut10 clones occurred. In conclusion, although myelocytic differentiation appears to be initially increased in cultures expressing mut10, terminal differentiation of the culture is delayed.
FMEV/mut10-infected cells still contain a high percentage of granulopoiesis after 14 days in culture. Flow cytometric profiles of cells from FMEV-mut10 (A) or control FMEV-eGFP (B) cultures 14 days after infection and maintenance in SCF, IL3, FLT3, and IL11. The percentage of cells double-positive for CD11b and Gr1 is indicated for each culture, as well as the mean fluorescence intensity of Gr1, of which the expression level correlates with granulocytic maturation. Double-negative cells were c-kit positive and confirmed to be mast cells by microscopic examination of cytospins.
FMEV/mut10-infected cells still contain a high percentage of granulopoiesis after 14 days in culture. Flow cytometric profiles of cells from FMEV-mut10 (A) or control FMEV-eGFP (B) cultures 14 days after infection and maintenance in SCF, IL3, FLT3, and IL11. The percentage of cells double-positive for CD11b and Gr1 is indicated for each culture, as well as the mean fluorescence intensity of Gr1, of which the expression level correlates with granulocytic maturation. Double-negative cells were c-kit positive and confirmed to be mast cells by microscopic examination of cytospins.
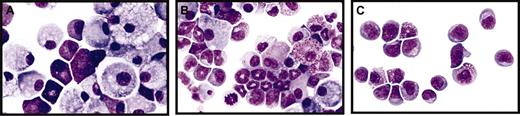
Mut10-expression alters the kinetics but does not block the differentiation of murine BM cells. (A-C) Giemsa-stained cytospins of liquid cultures maintained for 14 days after infection with either control FMEV-GFP (A), FMEV-mut10 (B), or MPEV-CRE vectors (C) in SCF, IL3, FLT3, and IL11. (A) Cultures infected with a control vector consist predominantly of macrophages and mast cells, typical for the end-stage of long-term cultures of hematopoietic cells. (B) Cells infected with the FMEV-mut10 vector display a spectrum of maturing monocytes/macrophages and promyelocytes/metamyelocytes. (C) Cells lacking C/EBPα due to recombination of the conditional gene targeted alleles remain in an undifferentiated myeloblastic or early promyelocytic stage. Original magnification, × 560.
Mut10-expression alters the kinetics but does not block the differentiation of murine BM cells. (A-C) Giemsa-stained cytospins of liquid cultures maintained for 14 days after infection with either control FMEV-GFP (A), FMEV-mut10 (B), or MPEV-CRE vectors (C) in SCF, IL3, FLT3, and IL11. (A) Cultures infected with a control vector consist predominantly of macrophages and mast cells, typical for the end-stage of long-term cultures of hematopoietic cells. (B) Cells infected with the FMEV-mut10 vector display a spectrum of maturing monocytes/macrophages and promyelocytes/metamyelocytes. (C) Cells lacking C/EBPα due to recombination of the conditional gene targeted alleles remain in an undifferentiated myeloblastic or early promyelocytic stage. Original magnification, × 560.
Consistent with the important role of C/EBPα in granulopoiesis, “expansion” cultures containing BM cells in which the C/EBPα locus was excised by CRE resulted in the accumulation of blastlike cells, including cells characteristic of myeloblasts (Figure 4C). GM-CSF cultures of the same CRE-infected BM cells were composed of cells at various stages of monopoiesis, but no granulocytic progenitors or precursors were observed (data not shown).
Taken together, these results demonstrate that expression of mut10 cDNA and thus overexpression of the 30-kDa C/EBPα protein does not block granulocytic differentiation of primary murine hematopoietic progenitors under any of the conditions tested but alters the kinetics of the differentiation process. This is in contrast to cultures in which the Cebpa gene was excised, where our results confirm the importance of C/EBPα in terminal granulopoietic differentiation.
Similarly to the human mut10, a murine C/EBPα mutant does not block myelopoietic differentiation of murine progenitors but inhibits their clonogenic potential
For the FMEV-mut10 vector used to infect mouse BM cells, a cDNA derived from human cells was used. Although the murine and human C/EBPα proteins share 91.9% identity, it is conceivable that the human protein cannot completely substitute for the mouse protein. We thus generated a murine equivalent of the human mut10 cDNA, which we termed mutAatII, and cloned it into an FMEV-YFP vector. As predicted and consistent with previous results of a similar mutant,31 the 30-kDa but not the 42-kDa protein could readily be detected with a C-terminal antibody (Figure 5B). In 2 independent experiments, mouse Linneg BM cells were infected with either the control FMEV-YFP vector (42% and 71% transduction efficiencies) or with FMEV-mutAatII (25% and 60% transduction efficiencies). Cells sorted on the basis of YFP expression were either seeded into methylcellulose cultures or maintained in liquid cultures in either expansion or differentiation conditions. Completely consistent with results obtained from the human mut10 cDNAs, we observed a 39% ± 8% reduction in the total number of CFCs in the FMEV-AatIImut cells compared with control infections (average of 2 independent experiments). Furthermore, no difference in the distribution of lineage-committed CFCs was observed between the 2 cultures (data not shown). Finally, perturbation of the differentiation kinetics were again characterized by increased myelopoiesis and increased numbers of immature cell types at late stages, as judged by microscopic examination of cytospins and parallel FACS analysis. These results show that both the human C/EBPα mut10 and the equivalent murine mutAatII do not block granulocytic differentiation of murine progenitors but alter the kinetics of myeloid differentiation.
Transduction of mut10 into human CD34+CB cells abrogates myeloid differentiation. CD34+ CB cells were infected with either FMEV-mut10 or control FMEV-GFP as described in “Materials and methods.” (A) Flow cytometric measurements of eGFP-positive cells and their mean fluorescence was determined for each cell population. The transduction efficiency for one experiment is indicated. (B) Western blot analysis confirmed the expression of a 30-kDa protein from both the human mut10 cDNA and the murine mutAatII cDNA from the FMEV vectors. Similar levels of mut10 C/EBPα protein were observed in CD34+ CB cells and murine Linneg BM cells (Figure 1D); the protein from FMEV-mut10 Phoenix cells serves as an internal control between blots, as this is the identical protein extract. The high levels observed in Phoenix cells are due to high copy numbers of the DNA transiently expressed in these cells. In contrast, the amount of protein in the transduced CD34+ CB cells and murine Linneg BM represents stable expression of an estimated 1 to 3 copies of the vector per cell. (C-F) Cytomorphology of human CD34+ CB cells infected with the control FMEV-eGFP (C,E) or FMEV-mut10 vector (D,F), sorted for eGFP expression, and cultured in GM-CSF, SCF, and IL3. Immediately after sorting (C-D), both cell cultures consisted of a minor fraction of erythroid progenitors (proerythroblasts and basophilic macroblasts) and a major fraction of myeloblasts and promyelocytes. The nuclear morphology of the mut10-positive cells (D) is obviously changed compared with the control (C). (E-F) In 14-day cell cultures, the control culture contains a wide spectrum of maturing erythroid and granulocytic cells, as well as macrophages (E), while the mut10-transduced hematopoietic cell culture consists predominantly of neutrophilic and eosinophilic promyelocytes (F). Giemsa staining. Original magnification, × 560.
Transduction of mut10 into human CD34+CB cells abrogates myeloid differentiation. CD34+ CB cells were infected with either FMEV-mut10 or control FMEV-GFP as described in “Materials and methods.” (A) Flow cytometric measurements of eGFP-positive cells and their mean fluorescence was determined for each cell population. The transduction efficiency for one experiment is indicated. (B) Western blot analysis confirmed the expression of a 30-kDa protein from both the human mut10 cDNA and the murine mutAatII cDNA from the FMEV vectors. Similar levels of mut10 C/EBPα protein were observed in CD34+ CB cells and murine Linneg BM cells (Figure 1D); the protein from FMEV-mut10 Phoenix cells serves as an internal control between blots, as this is the identical protein extract. The high levels observed in Phoenix cells are due to high copy numbers of the DNA transiently expressed in these cells. In contrast, the amount of protein in the transduced CD34+ CB cells and murine Linneg BM represents stable expression of an estimated 1 to 3 copies of the vector per cell. (C-F) Cytomorphology of human CD34+ CB cells infected with the control FMEV-eGFP (C,E) or FMEV-mut10 vector (D,F), sorted for eGFP expression, and cultured in GM-CSF, SCF, and IL3. Immediately after sorting (C-D), both cell cultures consisted of a minor fraction of erythroid progenitors (proerythroblasts and basophilic macroblasts) and a major fraction of myeloblasts and promyelocytes. The nuclear morphology of the mut10-positive cells (D) is obviously changed compared with the control (C). (E-F) In 14-day cell cultures, the control culture contains a wide spectrum of maturing erythroid and granulocytic cells, as well as macrophages (E), while the mut10-transduced hematopoietic cell culture consists predominantly of neutrophilic and eosinophilic promyelocytes (F). Giemsa staining. Original magnification, × 560.
Mut10 abrogates differentiation of human CD34+ CB cells
The effect of mut10 on the differentiation of murine progenitors is somewhat incongruous with the confirmed importance of C/EBPα in myeloid differentiation and the dominant-negative effect of mut10 expression on wild-type C/EBPα with respect to DNA binding and transactivation of C/EBPα-regulated genes.10 To determine if this was attributable to inherent differences between human and murine regulatory controls of differentiation that have evolved in these 2 species, we tested the effect of mut10 expression in primary human hematopoietic progenitors using the same retroviral vectors. CD34+ CB cells were efficiently infected with FMEV-eGFP and FMEV-mut10 (74% to 81% and 51% to 63%, respectively; range of 3 independent experiments) and sorted on the basis of eGFP expression. Notably, transcription levels of the FMEV-mut10 vector in human progenitors were equivalent to that observed in murine progenitors as measured by GFP fluorescence (x = 383 ± 40, n = 3; and x = 390 ± 98, n = 2, respectively; Figure 5A). Western blot analysis also confirmed that expression levels of the 30-kDa in the FMEV-mut10-infected CD34+ CB cells were equivalent to that observed in infected murine BM cells (Figure 5B).
Immediately after cell sorting, immature, blastlike cell elements predominated both FMEV-GFP and FMEV-mut10 infected CB cultures, although distinct differences could already be noted (Figure 5C-D). The number of immature erythroid cells (proerythroblasts and basophilic macroblasts) was significantly reduced in FMEV-mut10 cultures (7%) compared with FMEV-GFP control cultures (30%). In addition, the nuclear cytoplasmic ratio was higher in cells transduced with FMEV-mut10 than in controls. Furthermore, the FMEV-mut10 cultures were characterized by the presence of larger nuclei, the formation of more irregular nuclear contours (indented or lobulated) and delicate nuclear folds and creases, and the persistence of internuclear chromatin bridges and the development of pseudopods. After 14 days in differentiation medium (GM-CSF, IL3, and SCF), the differences became even more apparent (Figure 5E-F). Whereas control cultures exhibited a spectrum of maturing forms of normal erythroid (28%), monocytes/macrophages (38%), granulocytes (7%), and mast cells (27%), mut10-transduced cultures contained predominantly myeloblasts and neutrophilic (and fewer eosinophilic) promyelocytes with nuclear atypias (79%) admixed with some macrophages (15%) and rare erythroblasts.
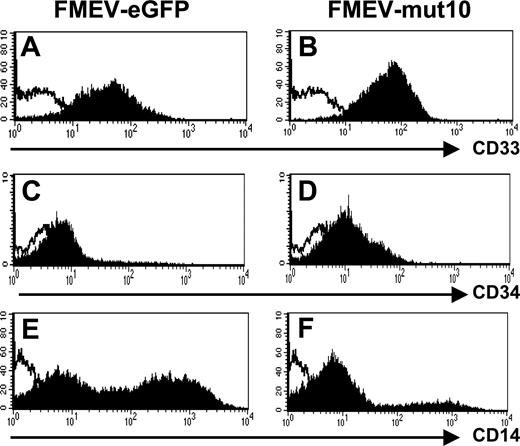
Consistent with an immature myeloid phenotype, FACS analysis showed that all 9-day FMEV-mut10-infected cells were CD33+ (Figure 6A-B). Similarly, the FMEV-mut10 culture showed overall higher mean fluorescence intensity of CD34 antigen compared with differentiated control cells (Figure 6C-D). Only a minor population of the cells expressing mut10 were positive for CD14 antigen (20% versus 59% in controls), indicative of early to late stages of monocytic differentiation (Figure 6E-F). CD15 levels were similar to control-infected cells (data not shown). Consistent with their morphology, these cells were negative for dendritic cell markers (CD1a and CD86; data not shown).
Impaired differentiation of human CD34+progenitors expressing mut10 C/EBPα documented by flow cytometric profiles. Control-infected and mut10-transduced CD34+ CB cells were incubated with antibody against the indicated antigens and analyzed by flow cytometry. Depicted are the results from day-9 liquid cultures maintained with GM-CSF, SCF, and IL3. Results are representative of 2 independent experiments.
Impaired differentiation of human CD34+progenitors expressing mut10 C/EBPα documented by flow cytometric profiles. Control-infected and mut10-transduced CD34+ CB cells were incubated with antibody against the indicated antigens and analyzed by flow cytometry. Depicted are the results from day-9 liquid cultures maintained with GM-CSF, SCF, and IL3. Results are representative of 2 independent experiments.
Differentiation block observed in both erythroid and myeloid lineages
Colony assays of FMEV-GFP- and FMEV-mut10-infected cultures were also performed to assess the cloning potential of the transduced cells and the differentiation potential under conditions that support differentiation in both myeloid and erythroid lineages. Although cells from control cultures gave rise to typical colonies from unipotent to multipotent CFCs (Table 2), almost all colonies from mut10-infected cultures were of a single morphology, closely resembling but distinct to CFU-G colonies in controls. After 12 days, colonies were disrupted and cells were spun onto glass slides and fixed. Whereas cells derived from FMEV-eGFP-infected colonies were composed of mature erythrocytes, macrophages, and granulocytes, cells from FMEV-mut10-infected colonies resembled those from liquid cultures, containing predominantly myeloblasts and neutrophilic promyelocytes. These results confirmed that both myeloid and erythroid differentiation was inhibited by expression of mut10.
Human CD34+ CB cells expressing mut10-C/EBPα do not give rise to normal colonies
. | No. of colonies per 103 cells . | Mean distribution of colonies, % . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vector . | . | CFU-GEMM . | CFU-GM . | CFU-G . | CFU-M . | BFU-E . | ||||
| FMEV-mut10 | 154 ± 14 | 0 | 0 | 0 | 0 | 0.3 ± 0.2 | ||||
| FMEV-GFP | 149 ± 20 | 2.2 ± 1.6 | 6.3 ± 0.4 | 14 ± 8 | 29.5 ± 12 | 48 ± 15 | ||||
. | No. of colonies per 103 cells . | Mean distribution of colonies, % . | . | . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vector . | . | CFU-GEMM . | CFU-GM . | CFU-G . | CFU-M . | BFU-E . | ||||
| FMEV-mut10 | 154 ± 14 | 0 | 0 | 0 | 0 | 0.3 ± 0.2 | ||||
| FMEV-GFP | 149 ± 20 | 2.2 ± 1.6 | 6.3 ± 0.4 | 14 ± 8 | 29.5 ± 12 | 48 ± 15 | ||||
Four days after isolation of CD34+ CB cells, mock-infected, or vector-infected cells sorted for GFP expression were plated in methylcellulose at 2 different cell concentrations and scored for colony formation and morphology after 11 days. Results are the average of 2 independent experiments, each performed in triplicate. CFU-GEMM indicates granulocyte-erythrocyte-megakaryocyte-macrophage colony-forming unit.
Promiscuous expression of both myeloid and erythroid markers in differentiation-impaired mut10-transduced cells
To better characterize the phenotype of the mut10-transduced cells, lineage-specific cell markers were examined by histochemical or immunohistochemical analysis. For these studies, both cells from liquid cultures or from day-12 colony assays were examined. Consistent with a myeloid origin, all mut10-infected cells were positive for acid phosphatase (data not shown). Furthermore, although a few rare cells expressed mast cell tryptase, the majority were negative or very weakly positive for this lineage-specific enzyme, thus unequivocally ruling out the possibility that the culture was composed of mast cells, often found in long-term in vitro cultures supplemented with SCF (data not shown). Quite strikingly, in contrast to control cells that showed lineage-specific staining for glycophorin A (erythroid), lysozyme (predominately monocytic), and myeloperoxidase (predominately granulocytic), approximately 70% of the mut10-expressing cells expressed simultaneously 2 or 3 of these lineage markers (Figure 7A-F). These results demonstrate the complex interplay of the lineage differentiation programs, which can be disrupted by a single aberrant transcription factor.
Immunocytochemical demonstration of lineage-specific cell marker expression of infected cultures. Cytospins of day-12 colony assays from human CB cells infected with a control (A,C,E) or mut10 carrying vector (B,D,F) were subjected to immunocytochemical staining. Mut10-transduced cells with an abnormal promyelocytic morphology exhibit a promiscuously positive immunoreactivity for myeloperoxidase (99% positive; B), lysozyme (76% positive; D), and glycophorin A (71.6% positive; F). The control cells show a lineage restricted immunoreactivity of myeloperoxidase (A), lysozyme (C), or glycophorin A (E) in promyelocytic, monocytic, and erythroid cells, respectively. Original magnification, × 560.
Immunocytochemical demonstration of lineage-specific cell marker expression of infected cultures. Cytospins of day-12 colony assays from human CB cells infected with a control (A,C,E) or mut10 carrying vector (B,D,F) were subjected to immunocytochemical staining. Mut10-transduced cells with an abnormal promyelocytic morphology exhibit a promiscuously positive immunoreactivity for myeloperoxidase (99% positive; B), lysozyme (76% positive; D), and glycophorin A (71.6% positive; F). The control cells show a lineage restricted immunoreactivity of myeloperoxidase (A), lysozyme (C), or glycophorin A (E) in promyelocytic, monocytic, and erythroid cells, respectively. Original magnification, × 560.
Discussion
Mutations in the CEBPA gene have been found in approximately 8% of AML in several independent studies. In vitro studies have shown that the 30-kDa protein, predominately expressed from CEBPA genes incurring mutations in the N-terminal coding region, acts dominantly to inhibit DNA binding and C/EBPα-regulated transcription.10 Due to the importance of C/EBPα in regulating granulopoiesis, it was proposed that inhibition of C/EBPα function would block granulocytic differentiation. Indeed, our results show that expression of an N-terminal C/EBPα mutant (mut10) abrogates normal myeloid differentiation in primary human progenitors. Importantly, however, the impaired differentiation is not limited to the granulocytic lineage but also affects both monopoiesis and erythropoiesis. The majority of CD34+ CB cells infected with FMEV-mut10 are blocked in differentiation at an abnormal promyelocyte-like stage.
In addition to abrogating differentiation and maturation, our analysis revealed the concomitant expression of several lineage-specific markers (eg, the erythroid marker glycophorin A and myeloid marker myeloperoxidase) in the mut10-transduced human cells. Several studies support the hypothesis that multipotent cells concomitantly express a number of lineage-specific genes, which are progressively extinguished during lineage commitment.32-36 Thus, we postulate that the coexpression of several genes normally found in one lineage or another is not necessarily due to the up-regulation of their expression but rather the inability of the differentiation program to extinguish expression. Significantly, promiscuous expression of lineage-specific markers is a recognized hallmark of leukemic cells.32
Quite surprisingly, expression of mut10 did not abrogate myelocytic differentiation in murine progenitor cells, although the kinetics of the differentiation program were perturbed. In addition, the clonogenic potential was impaired in these cells and they were no longer able to contribute to long-term repopulation in vivo. The murine equivalent (mutAatII) to the human mut10 C/EBPα also did not abrogate differentiation but resulted in a similar loss of clonogenicity and shift in differentiation kinetics as the human protein, demonstrating that species-specific differences in the protein sequence were not responsible for the differential effect. This contention is further supported by preliminary results showing that the murine mutAatII blocks differentiation of human cells similarly to mut10 (B. Niebuhr, M.S., unpublished results, November 2003). It is also unlikely that the observed difference between human and murine progenitors is due to dissimilar expression levels of the protein, as we found equivalent levels of both mut10 and the coexpressed GFP protein in cells of both origins. Finally, to verify that the appropriate target cells were infected in our approach, we took advantage of a transgenic mouse model, in which the Cebpa gene was flanked by loxP sites. Using the same protocol for infection, BM cells were infected with a FMEV-CRE retroviral vector. In this setting, a granulocytic block was observed, leading to the outgrowth of blast cells in liquid culture. Consistent with results from knockout mice,4 monocytic differentiation was still apparent.
How can the opposing effects of mut10 expression on hematopoietic progenitors from these 2 species be best explained? The loss of both transactivating domains TE1 and TE2 but the retention of the B-ZIP domain would theoretically enable the 30-kDa protein expressed by mutated CEBPA to form heterodimers with other C/EBPs and inhibit their ability to transactivate gene expression in a dominant-negative fashion. Thus it would be expected that the target genes of C/EBPα are down-regulated in mut10-transduced cells. The genes for lysozyme, myeloperoxidase, and the receptor for G-CSF (G-CSFR) are directly regulated by C/EBPα during myeloid differentiation.4,30,37,38 Surprisingly, we did not observe a down-regulation of the genes encoding either lysozyme or myeloperoxidase in human cells expressing mut10. This is consistent with recent studies of Wang and Friedman38 and Friedman et al39 that demonstrated that although a repressor-coupled dominant-negative C/EBPα directly down-regulates these 2 genes, they are not down-regulated by the 30-kDa protein in a murine progenitor cell line. Significantly, however, they have observed down-regulation of G-CSFR in their system, consistent with the work of Pabst et al10 using human U937 cells. As we observed a significant decrease in colony formation in the presence of G-CSF alone, G-CSFR gene may also be partially inhibited by mut10 in primary cells. The inhibition of G-CSFR stimulation would, however, not explain the differentiation block in human progenitors, as differentiation was induced in a cocktail containing GM-CSF, IL3, and SCF. Conceivably, mut10 may inhibit only a small spectrum of C/EBPα target genes, depending on their interplay with other transcription factors. Thus, the inhibition of a critical C/EBPα target gene in human cells but not in murine cells may explain the differential effects observed here. The evolution of 2 common but distinct mechanisms by which C/EBPα exerts its function is found in the regulation of its own gene—whereas the mouse gene is directly regulated by C/EBPα by DNA binding, the human gene is regulated indirectly by C/EBPα binding to other transcription factors.40-42 Using microarrays to identify direct target genes of C/EBPα in these 2 systems7 may help to identify genes responsible for the differential dominant-negative effects of mut10.
In contrast to our study, 2 groups have recently shown that artificial dominant-negative C/EBP mutants blocked both monocytic and granulocytic differentiation but not erythroid differentiation in murine and human progenitors.6,38 Both of these dominant-negative mutants interfere with C/EBP transcriptional activation, either by actively repressing transcription or by forming stable heterodimers that abolish DNA binding.6,38 Thus, the inability of mut10 to efficiently repress transcription of all C/EBPα target genes may explain why differentiation was not blocked in murine cells and why the differentiation block in human cells was distinct from that observed by Iwama et al.6 In their study, expression of the artificial dominant-negative C/EBPα in human CD34+ CB cells resulted in the accumulation of dendritic cells and no block in erythropoiesis.6
In addition to disrupting the transcriptional control of lineage-specific genes, C/EBPα mutants may impair or alter differentiation by disrupting cell cycle controls, a separable function of C/EBPα.43 Importantly, C/EBP-related transcription factors have been shown to regulate the balance between cell proliferation and mitotic growth arrest during terminal differentiation in several cell systems.44 Although different mechanisms by which C/EBPα inhibits proliferation have been proposed, including down-regulation of p21 and inhibition of cyclin-dependent kinases,45,46 recent studies have shown the importance of C/EBPα inhibition of the E2F pathway and the consequent down-regulation of c-myc in granulocytic differentiation.47-49 Two domains in C/EBPα are important for E2F inhibition, one that directly binds E2F and maps to the non-DNA-binding face of the basic region of B-ZIP domain49-51 and a second domain that indirectly inhibits E2F and maps to the TE1 domain.49-51 The presence of both domains is critical for inducing granulocytic differentiation.51,52 Can some of the effects we observe with mut10 expression in hematopoietic progenitors be due to the sequestering of E2F by the B-ZIP domain, thereby inhibiting progression from G1 to the S phase of the cell cycle? It is tempting to speculate that an increased incidence of growth cycle arrest would alter the balance of self-renewal and differentiation, leading to the loss of long-term repopulating cells in vivo, the reduction of CFCs in vitro, as well as the altered differentiation kinetics observed in murine cells. Further work, however, is required to determine if all of the effects observed in mut10-transduced murine progenitors are governed by a common mechanism and what molecular events are at play. Conversely, can the differentiation block observed in human cells expressing mut10 be due to the inhibition of cell cycle arrest, a stringent requirement for terminal granulopoietic differentiation?49,50 We can presently only speculate how mut10 may differentially control growth arrest.
Somewhat unexpectedly, mut10 inhibited both erythroid and myeloid (monocytic and granulocytic) differentiation in human cells. In light of the accumulating evidence that C/EBPα may dictate granulocytic differentiation by inhibiting differentiation of monocytic and erythroid lineage by several different mechanisms, this finding is perhaps not so surprising. Interestingly, this regulation has been shown to occur at 2 levels: (1) transcriptional regulation of monocytic or erythroid transcription factors (eg, Jun-B53 and Id-17 ), or (2) direct binding and impairment of such factors (eg, PU.16,54 and c-Jun55 ). The protein interactions between the monocytic regulator PU.1 and erythroid regulator GATA-binding protein-1 (GATA-1) that dictate lineage commitment is well established.56-59 As the B-ZIP domain of C/EBPα is necessary for both PU.1 and c-Jun down-regulation, mut10 would also be expected to interact with these transcription factors. Thus, it is tempting to speculate that interference with the extensive cross-talk between these lineage-specific transcription factors may underlie the block in multiple lineages observed in mut10-infected human progenitors.
In summary, our results clearly demonstrate that expression of an N-terminal C/EBPα mutant disrupts myeloid differentiation in primary human progenitors, supporting the importance of such mutations in the development of AML. Furthermore, they demonstrate intrinsic differences between hematopoietic controls in mouse and human, signaling the need for caution in interpreting and transferring results between these 2 species. Further studies are required to test the domain in mut10 that is important for impairing myelopoiesis and to determine what mechanisms in mouse cells circumvent this differentiation block. Understanding such mechanisms may benefit the development of therapeutic molecules that override the differentiation block observed in AMLs.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-07-2280.
Supported by grants from the Deutsche José Carreras-Leukaämie-Stiftung (RO1/04) and the National Institutes of Health (RO1 CA88046). The Heinrich-Pette-Institut is supported by the Freie und Hansestadt Hamburg and the Bundesministerium für Gesundheit.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge the technical support of Ulla Bergholz, Karin Heigl, Susanne Roscher, and Marion Ziegler.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal