Abstract
Derangement of cellular immunity is central in the pathophysiology of adult autoimmune/idiopathic thrombocytopenic purpura (ITP). Herein we investigated cytokine gene expression in peripheral blood mononuclear cells (PBMCs) of adult chronic ITP patients and attempted to correlate cytokine polarization with the degree of thrombocytopenia. We used semiquantitative reverse-transcriptase–polymerase chain reaction (RT-PCR) to measure the expression of type-1 (interleukin-2 [IL-2], interferon γ [IFN-γ]) and type-2 (IL-4, IL-5, IL-10, IL-3, IL-13) cytokines by PBMCs from 21 patients and 11 controls. Plasma transforming growth factor β1 (TGF-β1) levels were measured by enzyme-linked immunoassay (ELISA). T helper 1 (Th1)/Th2 ([IL-2 + IFN-γ]/[IL-4 + IL-5]) cytokine mRNA ratios, thought to reflect the Th deviation of the pathogenic disease-specific T cells, and type-1/type-2 mRNA ratios, thought to reflect the overall immune response polarization, were significantly increased in ITP patients. The Th1/Th2 ratio was inversely correlated with platelet counts. TGF-β1 levels appeared suppressed in patients with active disease, though not significantly. Our findings show a clear type-1 cytokine polarization of the autoimmune response in adult ITP that persists irrespective of disease status.
Introduction
Adult idiopathic thrombocytopenic purpura (ITP) is a chronic acquired organ-specific autoimmune hemorrhagic disease.1 The thrombocytopenia of ITP is mainly attributed to the early destruction of platelets by the activated reticuloendothelial system, following their sensitization by antiplatelet glycoprotein autoantibodies.2 Other mechanisms contribute, such as complement mediated lysis,3 ineffective thrombopoiesis,2 or direct T-cell cytotoxicity.4
Dysfunctional cellular immunity is considered important in ITP pathophysiology.5 Several studies have shown the presence of activated platelet-specific autoreactive T cells that recognize and respond to autologous platelet antigens and drive the generation of platelet reactive autoantibodies by B cells6-8 in the peripheral blood of ITP patients. The autoimmune process is believed to be sited in the spleen9 ; memory platelet-specific T cells are released into the peripheral circulation.
Several studies have found evidence supporting a T helper 0 (Th0)/Th1 polarization of the immune response in ITP,10-12 whereas others have yielded inconsistent13 or opposing14 results. We studied the expression of type-1 and type-2 cytokines in adult ITP patients at the peripheral blood mononuclear cell (PBMC) population mRNA level, and our results support the concept that adult ITP is the manifestation of a type-1 polarized immune process.
Study design
Patients
There were 21 adult chronic ITP patients (18 women and 3 men; median age, 64 years; range, 25-86 years) studied. Patients were treated and followed up in the Hematology Division of Patras University Hospital (PUH). Of the patients, 13 were in remission (platelet [PLT] count > 100 × 109/L [> 100 000/mm3]) and 8 were in active disease at the time of blood sample collection. At the time of blood sample collection, 4 patients were receiving glucocorticoids, 1 intravenous immunoglobulin G (IVIg), 5 danazol, and 1 hydroxychloroquine. A control group consisted of 11 adult healthy volunteers (6 women and 5 men; median age, 40 years; range, 18-65 years). Informed consent was obtained from each participating patient. PUH abides by the Helsinki Declaration on ethical principles for medical research involving human subjects.
Cell cultures
PBMCs isolated from each subject were processed immediately (ex vivo) or cultured for 8 hours in the presence of 20 ng/mL phorbol myristate acetate (PMA) and 1 μM ionomycin.15
Reverse-transcriptase–polymerase chain reaction (RT-PCR)
Total cellular RNA extracted from PBMCs was reverse transcribed. Of each RT-reaction mixture, 2 μL was submitted to PCR.15,16 Electrophoresis bands were quantified using Scion Image for Windows software based on NIH Image for Macintosh by Wayne Rasband of the National Institutes of Health (Rockville, MD; http://www.scioncorp.com/frames/fr_download_now.htm). Data analysis was done with GraphPad Prism version 3.00 for Windows (GraphPad Software, San Diego, CA; http://www.graphpad.com/prism/Prism.htm). β2-Microglobulin mRNA band intensity was used as baseline.
TGF-β1 ELISA
Determination of transforming growth factor β1 (TGF-β1) levels in the plasma was performed by an enzyme-linked immunoassay (ELISA).15
Results and discussion
The expression of cytokines by PBMCs was measured ex vivo and after in vitro activation by mitogens in short-term cultures. Both patient and control PBMCs produced small or nondetectable amounts of cytokines ex vivo, with the exception of interferon γ (IFN-γ) for the patients and interleukin-10 (IL-10) for both patients and controls (Figure 1). PMA/ionomycin were used for the induction of the cytokine genes studied because they induce the expression of cytokine genes—not randomly—but according to the differentiation program of each Th phenotype.17 The differences in cytokine gene expression between patients and controls described in this study were thus better elicited after in vitro activation of PBMCs by PMA/ionomycin.
Cytokine gene expression profiles in adult chronic ITP patients. Expression (mean ± SEM) of the cytokines studied at the mRNA level in control subjects, patients in active disease phase (PLT count < 100 × 109/L [< 100 000/mm3]), and patients in hematologic remission by peripheral blood mononuclear cells ex vivo and after in vitro stimulation with PMA and ionomycin (P/I). Asterisks denote significance using unpaired 2-tail t test (*P < .05; **P < .01). Comparisons among subject groups (controls, patients with active disease, and patients in remission) were made under the same experimental conditions (ex vivo PBMCs or stimulated PBMCs) for each cytokine separately.
Cytokine gene expression profiles in adult chronic ITP patients. Expression (mean ± SEM) of the cytokines studied at the mRNA level in control subjects, patients in active disease phase (PLT count < 100 × 109/L [< 100 000/mm3]), and patients in hematologic remission by peripheral blood mononuclear cells ex vivo and after in vitro stimulation with PMA and ionomycin (P/I). Asterisks denote significance using unpaired 2-tail t test (*P < .05; **P < .01). Comparisons among subject groups (controls, patients with active disease, and patients in remission) were made under the same experimental conditions (ex vivo PBMCs or stimulated PBMCs) for each cytokine separately.
We observed elevated Th1 cytokine (IL-2, IFN-γ) mRNA levels in ITP patients (Figure 1), in accordance with previous reports of increased serum Th1 cytokine levels in ITP patients10-12 but not with other studies.13,14 The observed tendency of lower Th1 cytokine expression levels in patients with active disease compared with patients in remission may be accounted for by the phenomena of activated memory T cells homing to the spleen in active disease9 and in vivo T-cell exhaustion18 due to prolonged in vivo activation.
We found suppressed expression levels of the Th2 cytokines IL-4 and IL-5 (significant only for IL-5) in patients with active disease relative to patients in remission (Figure 1). Significantly reduced IL-4 levels have been reported for ITP patients in vivo10,13 and in vitro,8 whereas opposing findings are described elsewhere.14
The expression of IL-10 was found greatly suppressed in active disease following in vitro mitogenic stimulation (Figure 1). Significantly reduced serum IL-10 levels have been found in ITP patients with active disease compared with clinically stable patients and healthy controls.13 Conversely, raised serum IL-10 levels have been described in pediatric patients with chronic ITP,10,15 whereas in another study19 IL-10 levels were found to be similar between patients and controls.
Expression levels of IL-3 and IL-13 in ITP patients fell within the control range (Figure 1). Significantly increased serum IL-3 levels have been described in chronic ITP patients,20 but in another study21 serum IL-3 levels were just above the detection limit.
Although patients tended to have lower circulating TGF-β1 levels, the difference was not statistically significant (data not shown). Serum TGF-β1 concentration has been shown to vary inversely with disease activity.13,22 TGF-β1 levels were measured in plasma and not analyzed at the gene level, because TGF-β1 activity is mainly regulated at the level of the activation of the latent factor by proteases outside the cell,23 and, unlike the other cytokines studied,24 a direct relation of the circulating levels of the active factor with intracytoplasmic mRNA levels has not been established.
Treatment effects on autoimmune polarization could not be detected (data not shown) probably because of the small number of patients in each treatment group or because the treatments used do not seem to change the natural history of the disease.25
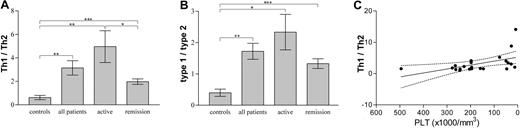
Absolute individual cytokine levels are physiologically less important than relative concentrations of antagonizing cytokines, as expressed with calculated Th1/Th2 and type-1/type-2 ratios, which showed much less dispersion of values. The Th1/Th2 ratio is considered to reflect the profile of the pathogenic disease-specificT helper cells, whereas the type-1/type-2 ratio is thought to give an overall picture of their immune response, since it includes cytokines—the expression of which is not restricted to specific Th cell populations. We found that patients had significantly increased Th1/Th2 and type-1/type-2 ratios compared with controls irrespective of clinical severity of their disease, indicating a type-1 polarization in ITP patients (Figure 2A-B), and a permanent derangement of the immune function in ITP patients regardless of the clinical manifestations of the disease. Patients with active disease exhibited significantly higher Th1/Th2 ratios than patients in remission (Figure 2A), and lower peripheral platelet numbers correlated with higher Th1/Th2 ratios (Figure 2C).
Th1/Th2, type-1/type-2 cytokine expression ratios and relevance to platelet concentration in adult chronic ITP patients. (A) Th1 cytokines (IL-2, IFN-γ) over Th2 cytokines (IL-4, IL-5) expression ratios (mean ± SEM) in controls, patients, patients with active disease, and patients in remission. Asterisks show statistical significance (*P < .05; **P < .01; ***P < .005). Unpaired 2-tail t test was used for comparison among groups. (B) Type-1 cytokines (IL-2, IFN-γ) over type-2 cytokines (IL-4, IL-5, IL-10, IL-3, IL-13) expression ratios (mean ± SEM) in controls, patients, patients with active disease, and patients in remission. Asterisks show statistical significance (*P < .05; **P < .01; ***P < .005). Unpaired 2-tail t test was used for comparison among groups. (C) Th1/Th2 cytokine expression ratio versus peripheral blood platelet concentration linear regression line (solid) with 95% confidence intervals (dotted lines) in ITP patients. r2 = 0.2515; P = .0174.
Th1/Th2, type-1/type-2 cytokine expression ratios and relevance to platelet concentration in adult chronic ITP patients. (A) Th1 cytokines (IL-2, IFN-γ) over Th2 cytokines (IL-4, IL-5) expression ratios (mean ± SEM) in controls, patients, patients with active disease, and patients in remission. Asterisks show statistical significance (*P < .05; **P < .01; ***P < .005). Unpaired 2-tail t test was used for comparison among groups. (B) Type-1 cytokines (IL-2, IFN-γ) over type-2 cytokines (IL-4, IL-5, IL-10, IL-3, IL-13) expression ratios (mean ± SEM) in controls, patients, patients with active disease, and patients in remission. Asterisks show statistical significance (*P < .05; **P < .01; ***P < .005). Unpaired 2-tail t test was used for comparison among groups. (C) Th1/Th2 cytokine expression ratio versus peripheral blood platelet concentration linear regression line (solid) with 95% confidence intervals (dotted lines) in ITP patients. r2 = 0.2515; P = .0174.
A Th1 or Th0/Th1 cytokine gene bias with persistent IL-10 expression has been described in childhood ITP that persists after IVIg treatment and evolves into a chronic form of ITP with late recurrences, whereas children with a normal or Th2 expression profile, without IL-10, either at presentation or after treatment, remained relapse-free and were probably cured.15 It appears that a persistent Th0/Th1 deviation of the immune response is closely associated with the chronic/relapsing nature of chronic ITP in adults and children.
Overall, the findings of the present study support the concept that chronic adult ITP is the manifestation of a type-1 polarized immune response. The polarized immune activity detected in PBMCs is thought to reflect the nature of the localized autoimmune process taking place in the target organs of ITP (spleen, bone marrow). Understanding the pathophysiology of adult ITP can lead to more effective clinical interventions, especially in refractory cases.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-07-2268.
Supported in part by a grant from Novartis (Basel, Switzerland).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Cytokine gene expression profiles in adult chronic ITP patients. Expression (mean ± SEM) of the cytokines studied at the mRNA level in control subjects, patients in active disease phase (PLT count < 100 × 109/L [< 100 000/mm3]), and patients in hematologic remission by peripheral blood mononuclear cells ex vivo and after in vitro stimulation with PMA and ionomycin (P/I). Asterisks denote significance using unpaired 2-tail t test (*P < .05; **P < .01). Comparisons among subject groups (controls, patients with active disease, and patients in remission) were made under the same experimental conditions (ex vivo PBMCs or stimulated PBMCs) for each cytokine separately.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/7/10.1182_blood-2003-07-2268/6/m_zh80070459070001.jpeg?Expires=1769174795&Signature=cLzl3td4vh39jgisB0jK79jYXzRVP6o2lm4EFJtP0SToOwhPwWzY6SGqlFgGgXMF0a8BLV88~Xb~sFKaqud2pS4YD~l7ksslcT~EXlLAdYbqFWAHgXWE6krs2FDxrW6Sg7HE-~ke8YFFC5k9Pgjih-i5atRK2iXQm4Onesb6rlb1ogDX0AZO1tptDOmREhEJjHSVx3tLpliWVYiVia~gCxMPuJxLPi8yx9vEJ6OaexMeIpy6J4YZip0Bh~xD3Y02z8aw9x9hHAfivG4htNo2KYWftQnnFZ1v7kEeUpjx6fGz3dz2VV3T5jDhwKfy62V5m4YnJGcBTEc8089UubRAPw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal