Abstract
Endothelial progenitor cells (EPCs) were shown to be present in systemic circulation and cord blood. We investigated whether EPCs display specific properties compared with mature endothelial cells. Human cord blood CD34+ cells were isolated and adherent cells were amplified under endothelial conditions. Expression of specific markers identified them as endothelial cells, also called endothelial progenitor-derived cells (EPDCs). When compared to mature endothelial cells, human umbilical vein endothelial cells (HUVECs) and human bone marrow endothelial cells (HBMECs), endothelial markers, were expressed to the same extent except for KDR, which is expressed more in EPDCs. They display a higher proliferation potential. Functional studies demonstrated that EPDCs were more sensitive to angiogenic factors, which afford these cells greater protection against cell death compared with HUVECs. Moreover, EPDCs exhibit more hematopoietic supportive activity than HUVECs. Finally, studies in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice demonstrated that human circulating EPCs are able to colonize a Matrigel plug. EPDCs display the morphology and phenotype of endothelial cells. Their functional features indicate, however, that although these cells have undergone some differentiation steps, they still have the properties of immature cells, suggesting greater tissue repair capabilities. Future use of in vitro amplified peripheral blood EPDCs may constitute a challenging strategy for cell therapy.
Introduction
Until recently, it was believed that neovascular formation in adults resulted exclusively from proliferation, migration, and remodeling of preexisting vessels, a process referred to as angiogenesis, whereas, in contrast, vasculogenesis was defined as the formation of new blood vessels from endothelial progenitor cells (EPCs) during embryogenesis. The discovery of EPCs in adults1 has led to the new concept that vasculogenesis and angiogenesis may occur simultaneously. Extensive data support the existence of these cells, their bone marrow origin, and their contribution to the formation of new blood vessels in adults.2 Circulating EPCs (CEPCs) differ from the circulating endothelial cells that are randomly detached from the vessel walls and enter the circulation as a result of vascular injury. When incubated in vitro with appropriate medium in the presence of specific growth factors, CEPCs have a high proliferating potential and within 10 to 20 days produce colonies of cells expressing endothelial cell markers. Previous studies have established that CEPCs express the cell surface markers CD34,2,3 CD133,4 and KDR.5
These cells may be recruited and incorporated into sites of active neovasularization2,6 and have the ability to be mobilized from bone marrow in response to ischemia or cytokine therapy.7 Moreover, human EPCs isolated from peripheral blood and expanded ex vivo have been shown to be activated in different models of vascular diseases. For example, these cells have been shown to promote successfully neovascularization of ischemic tissues in nude mice8 and to develop into neovessels in vivo when seeded in decellularized grafts.9
The physiologic role of CEPCs is not yet clearly established, but several studies suggest that these cells are involved in the protection against tissue degeneration due to vascular dysfunction. The number of CEPCs is inversely correlated with the risk factor for coronary artery disease.10 These results open up prospects for the use of CEPCs as a prognostic tool for cardiovascular diseases. In contrast, an increase in angiogenesis could have critical consequences in several diseases. Indeed, the hypothesis that tumor growth was angiogenesis dependent11 and its subsequent confirmation by genetic methods12 provided a strong incentive to try to target the vascular bed of tumors. Id mutant mice that do not develop tumors because of defective angiogenesis have been shown to develop tumors after bone marrow transplantation from wild-type mice,13 suggesting that CEPCs also participate in tumor vascularization. More studies are necessary to measure the real risk of tumor development after CEPC injection. Meanwhile, the potential presence of microtumors should be scrutinized with peculiar attention before treating patients with highly angiogenic cell therapy products.
Until now, the bone marrow stem cells that produce CEPCs have not been clearly identified. Over recent years, much attention has focused on bone marrow stem cell potentiality. It has been reported that bone marrow contains cells termed multipotent adult progenitor cells (MAPCs), which, at a single-cell level, can be differentiated into a large number of cell types, including endothelial cells.14 The existence of a common precursor for both hematopoietic and endothelial cells (hemangioblast) was thought to be restricted to embryonic development. However, recent studies have identified a postnatal hemangioblast.15 Other studies support the existence of a bipotent progenitor giving rise to both endothelial cells and smooth muscle cells.16 The relationship between these different bone marrow stem cells is not clear. It is also possible that CEPCs mobilized from the bone marrow are in fact progenitors or stem cells with broader differentiation potential, among which endothelial differentiation is revealed at sites of neovascularization that provide an adequate microenvironment.
The hypothesis that bone marrow is the reservoir of EPCs prompted several groups to test the potential therapeutic effect of autologous cells of medullar origin in human ischemic disease. Bone marrow mononuclear cells injected locally in patients with lower limb or cardiac ischemia seems to contribute to the regeneration of the vasculature.17-19 However, these studies constitute pioneering clinical trials and it is likely that more purified cell therapy product will be used in the near future, thus limiting possible toxicity and side effects. In this context, autologous CEPCs purified from peripheral blood and expanded in vitro appear to be the best alternative to crude bone marrow mononuclear cells.
Before envisaging the use of differentiated EPCs for therapeutic purposes, it is first essential to investigate whether they have particular in vitro behavior compared with mature vessel wall cells. The aim of our study was to increase knowledge of endothelial progenitor-derived cells (EPDCs) by characterizing their specific phenotypic and functional properties.
Materials and methods
Isolation and culture of CD34+ mononuclear cells from cord blood, HUVECs, HBMECs, and L87-4
Human umbilical cord blood samples were collected in blood packs (Maco-Pharma, Tourcoing, France) from donors in compliance with French legislation. Mononuclear cells (MNCs) were isolated by centrifugation on Pancoll (Dutscher, Burmath, France). CD34+ MNCs were isolated by a magnetic beads separation method following the manufacturer's instructions (MACS; Miltenyi Biotec, Paris, France). CD34+ cells were plated onto gelatin-coated wells (Sigma-Aldrich, Poole, United Kingdom) and maintained in endothelial basal medium-2 (EBM2) supplemented with EGM2-MV SingleQuots (Clonetics Cambrex, Ermerainville, France). Human umbilical vein endothelial cells (HUVECs) were isolated according to the method of Jaffe et al.20 HUVECs and human bone marrow endothelial cells (HBMECs),21 both used as endothelial cell positive controls, were cultured in EGM2-MV. The fibroblastic cell line L87-422 was used as negative control.
FACS analysis
Cells were detached with nonenzymatic cell dissociation medium to avoid the destruction of cell membrane markers. Staining was performed with monoclonal antibodies against CD31-phycoerythrin (PE; PharMingen, San Diego, CA), CD146-fluorescein isothiocyanate (FITC; Chemicon, Temecula, CA), CD45-FITC (Immunotech, Marseille, France), and CD34-peridinin chlorophyll protein (PerCP; Becton Dickinson, Heidelberg, Germany), or with unconjugated antibodies against intercellular adhesion molecule 2 (ICAM-2), endoglin (Immunotech), KDR (Sigma-Aldrich), CD144 (Immunotech), integrin subunits β1, β2, α1, α2, α5, ICAM-1 (Becton Dickinson, Le Pont de Claix, France), complex αvβ3, complex αvβ5 (Chemicon), vascular cell adhesion molecule 1 (VCAM-1; Diaclone), FLT-1, fibroblast growth factor receptor 2 (FGFR-2), nitric oxide synthetase III (NOSIII; Santa Cruz Biotechnology, Santa Cruz, CA) followed by FITC- or PE-conjugated secondary antibodies (Sigma-Aldrich). Quantitative fluorescence analysis was performed using a fluorescence-activated cell sorting (FACS) Calibur flow cytometer and WinMDI software (Becton Dickinson).
Immunocytochemistry analysis
Cells seeded and fixed onto gelatin-coated 8-chamber Lab-Tek slides (Nunc, Roskilde, Denmark) were sequentially incubated with primary antibodies against CD31, von Willebrand factor (VWF; Dako, Glostrup, Denmark), CD144, or collagen IV (SBA, Birmingham, AL) and with appropriate secondary antibodies. To detect acetylated low-density lipoprotein (LDL) uptake, cells were incubated with Dil-ac-LDL. Slides were examined and photographed under a fluorescence microscope (Leica, Rueil-Malmaison, France).
Transmission electron microscopy analysis
Cells were fixed in 2.5% (vol/vol) glutaraldehyde, postfixed in 2% (wt/vol) osmium tetroxide, dehydrated in graded ethanol, and embedded in epoxy resin. Thin sections (76 nm) were cut and examined with a JEOL 100C electron microscope (Jeol, Croissy sur Seine, France), after uranyl acetate and lead citrate staining.
Semiquantitative RT-PCR analysis
RNA extraction was performed by the Trizol method, according to the manufacturer's instructions (Gibco, Karlsruhe, Germany). The reverse transcription–polymerase chain reaction (RT-PCR) products were hybridized with an internal 32P-labeled probe to allow a semiquantitative analysis. The oligonucleotides used were: CD133, 5′ probe: 5′GCCACCGCTCTAGATACTGC3′; 3′ probe: 5′TGTTGTGATGGGCTTGTCAT3′; internal probe: 5′CGGCTCTAATTTTTGCGGTA3′. KDR, 5′ probe: 5′AATACCAGTGGATGTGATGC3′; 3′ probe: 5′CTGGCATGGTCTTCTGTGAAG3′; internal probe: 5′TCCCAGTTGAAGTCAATCCC3′. CD144, 5′ probe: 5′CCCACCGGCGCCAAAAGAGA3′; 3′ probe: 5′CTGGTTTTCCTTCAGCTGGA3′; internal probe: 5′GCCAGGTATGAGATCGTGGT3′. Tie-2, 5′ probe: 5′CCAAACGTGATTGACACTGG3′; 3′ probe: 5′TGTGAAGCGTCTCACAGGTC3′; internal probe: 5′TGAGCCTTACTTTGGGGATG3′. Flt-1 5′ probe: 5′GTCACAGAAGAGGATGAAGG3′; 3′ probe: 5′CACAGTCCGGCACGTAGGTG3′; internal probe: 5′GGCTCTGTGGAAAGTTCAGC3′. GAPDH, 5′ probe: 5′CCACCCATGGCAAATTCCAT3′; 3′ probe: 5′GGTGGACCTGACCTGCCGTC3′; internal probe: 5′TGTGGTCATGAGTCCTTCCA3′.
Cell proliferation potential
At subconfluence, cells were detached and counted. The number of divisions was estimated using the following equation: (Ln (number of cells counted/number of cells at the beginning of the assay)/Ln2).
Cell proliferation assay
EPDCs or HUVECs were seeded and incubated overnight in EGM2-MV medium (day 0). At day 1, cells were incubated in EGM2-MV medium depleted in fibroblast growth factor 2 (FGF-2) and vascular endothelial growth factor (VEGF) with or without 2.5% fetal bovine serum (FBS) as control or supplemented with 2, 4, 8, 12.5, 25, or 50 ng/mL VEGF, FGF-2, or placental growth factor (PlGF; R&D Systems, Minneapolis, MN). At day 3, the medium was changed. At day 4, cells were counted in a particle counter (CoulterZ1; Coultronics, Margency, France).
Cell survival assay
Cells were stained with annexin V–FITC and propidium iodide (PI) according to the manufacturer's protocol (R&D Systems) and subjected to FACS analysis. To exclude necrotic cells, only PI- cells were counted.
Wound healing assay
Cell migration was performed by using the method described by Sato and Rifkin23 with modifications. One linear scar was drawn in a confluent cell monolayer. A set of digital photographs was taken. The border between cells and dish surface was then marked by a line on a computer using LuciaG of Metech version 4.61 (Nikon, Paris, France). Dishes were incubated for 18 hours in EGM2-MV medium containing 0.1% bovine serum albumin (BSA), without FBS, FGF-2, and VEGF as control, or supplemented with 10 ng/mL of VEGF, FGF-2, or PlGF. A second set of photographs was taken. Photographs were superimposed on the computer screen, and nuclei of cells that migrated across the line drawn at the border of the scar in the first set of photographs were counted.
Capillary tube formation assay in Matrigel
For analysis of capillary tube formation, 150 μL Matrigel (Becton Dickinson) was laid into a 48-well plate (Falcon, Heidelberg, Germany) and incubated at 37°C for 30 minutes. EPDCs or HUVECs were trypsinized and 4 × 104 cells were suspended in 300 μL EGM2-MV medium and plated onto Matrigel. Twenty-four hours later, the medium was removed and the cells were incubated under various conditions: EGM2-MV medium depleted in FBS, FGF-2 and VEGF as control conditions, or control conditions supplemented with 10 ng/mL VEGF, FGF-2, PlGF. Capillary tube formation in Matrigel was observed under an inverted microscope after 6 hours of incubation.
Cobblestone area–forming cell assay
CD34+ sorted cells were seeded at limiting dilutions (ranging from 200 to 6.25 cells/well) on a preestablished confluent monolayer of EPDCs or HUVECs in a long-term culture medium consisting of 50% (vol/vol) Myelocult (StemCell Technologies, Vancouver, BC, Canada), 35% (vol/vol) α-minimal essential medium (α-MEM), 15% (vol/vol) FBS with 10-6 M hydrocortisone. Wells were screened weekly for the presence of hematopoietic colonies. After 5 weeks, the incidence of cobblestone area–forming cells (CAFCs) were estimated by plotting the proportion of negative wells versus the number of cells per well. As control, to ensure that the long-term culture medium used did not affect the proliferation of HUVECs or EPDCs, we cultivated both types of cells in this medium for 5 weeks and compared their proliferation to that of HUVECs and EPDCs in EGM2-MV medium.
Matrigel plug assay
Nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were engrafted in the retro-orbital vein with 5 × 105 cord blood CD34+ or 5 × 105 cord blood CD34-. Four weeks later, Matrigel maintained at 4°C was mixed with 1 μg FGF-2 and injected subcutaneously into NOD/SCID mice. The experiment was performed 3 times with 5 mice under each set of conditions. Ten days after injection, tissues containing the Matrigel plug were removed and frozen. Matrigel cryosections were prepared. At the same time, spleen, liver, lung, and heart of each mouse were frozen and cryosections were prepared. May-Grünwald-Giemsa staining was performed to visualize the Matrigel invasion. Human cells were visualized by using the MOM kit (AbCys, Paris, France) following the manufacturer's instructions on Matrigel sections and with primary antibody against human CD31 (Chemicon) and then with an appropriate secondary antibody. Finally, an FITC-labeled antibody against human CD45 (Immunotech) or against CD144 (Bender MedSystems, Vienna, Austria) was added. Cryosections of organs were stained using same antibodies. Staining with α-smooth muscle actin (ASMA) was also performed as positive control. 4,6-diamino-2-phenylindole (DAPI) staining was used to visualize the cell nuclei. Sections were examined and photographed under a fluorescence microscope (Leica).
Statistical analysis
Results are given as mean ± SEM. Comparisons between means were made using Student t test and the nonparametric Mann-Whitney U test.
Results
CD34+ MNCs differentiate into cells with a typical endothelial phenotype
Cord blood CD34+ cells were isolated (purity 93%-98%) and cultivated under endothelial conditions, yielding a monolayer of cells displaying morphology similar to that of endothelial cells. We first characterized by flow cytometry the expression of a selected set of markers and compared it to that obtained in HUVECs and HBMECs and to the L87-4 cell line. Endothelial cells derived from CD34+ cells, HUVECs, and HBMECs are strongly positive for endothelial markers such as CD31, ICAM-2, endoglin, and FGFR-2. All these cells express CD146, KDR, and CD144, but at a low level. The culture no longer contained any hematopoietic cells at this stage (day 40) as attested by the absence of CD45+ cells (Figure 1). These cells are thus endothelial cells. One of the important functions of endothelial cells is to produce nitric oxide (NO). This NO production is mediated by NOS. Among these, NOSIII is constitutively expressed in endothelial cells. We showed that NOSIII is expressed in EPDCs, HUVECs, and HBMECs (Figure 1). This suggests that EPDCs are functional in terms of NO production.
FACS analysis of CD31, ICAM-2, endoglin, CD146, KDR, CD144, CD45, FGFR-2, Flt-1, and NOSIII in EPDCs, HUVECs, and HBMECs at 40 days of culture. Plots show isotype control IgG staining (white histograms) versus specific antibody staining (gray histograms). Each analysis shown is a representative profile.
FACS analysis of CD31, ICAM-2, endoglin, CD146, KDR, CD144, CD45, FGFR-2, Flt-1, and NOSIII in EPDCs, HUVECs, and HBMECs at 40 days of culture. Plots show isotype control IgG staining (white histograms) versus specific antibody staining (gray histograms). Each analysis shown is a representative profile.
Relative mean fluorescence intensity (MFI) was compared in EPDCs, HUVECs, HBMECs, and L87-4 cells (Table 1). This revealed that EPDCs expressed KDR at a significantly higher level than HUVECs and HBMECs (4.9 ± 0.3 versus 2.3 ± 0.1 and 1.5 ± 0.2, P < .001, n = 3). CD31, CD144, CD146, Flt-1, endoglin, ICAM-1, ICAM-2, αvβ3, α vβ5, and integrin β1, α2, and α5 were uniformly expressed on the 3 endothelial cell types. VCAM-1 and integrins α1 and β2 were not expressed. CD133, a marker of immature hematopoietic cells and EPCs, was not detected on EPDCs, HUVECs, or HBMECs, confirming previous observations.
MFI of EPDC, HUVEC, HBMEC, and L87-4 phenotypic markers analyzed by flow cytometry
. | EPDCs, MFI ± SEM . | HUVECs, MFI ± SEM . | HBMECs, MFI ± SEM . | L87-4, MFI ± SEM . |
|---|---|---|---|---|
| KDR | 4.9 ± 0.3 | 2.3 ± 0.1 | 1.5 ± 0.2 | 1.5 ± 0.4 |
| CD31 | 31.1 ± 3.2 | 23.7 ± 1.3 | 27.8 ± 10.8 | 1.1 ± 0.1 |
| CD144 | 6.9 ± 2.4 | 5.7 ± 0.2 | 2.0 ± 0.9 | 1.2 ± 0.2 |
| CD146 | 5.43 ± 1.7 | 8.9 ± 1.3 | 10.9 ± 0.3 | 0.9 ± 0.2 |
| Flt-1 | 8 ± 0.3 | 7.8 ± 0.5 | 9.6 ± 0.7 | 1.1 ± 0.1 |
| Endoglin | 17.9 ± 5.2 | 25.3 ± 5.8 | 15.7 ± 2.8 | 9.2 ± 3.2 |
| ICAM-1 | 7.7 ± 3.6 | 14.2 ± 4.5 | 9.2 ± 1.6 | 7.2 ± 0.3 |
| ICAM-2 | 8.3 ± 3.3 | 7.6 ± 0.2 | 11.0 ± 3.9 | 1.3 ± 0.2 |
| αvβ3 | 7.1 ± 1.0 | 12.5 ± 3.4 | 4.3 ± 0.6 | 2.4 ± 0.2 |
| αvβ5 | 7.7 ± 0.4 | 15.0 ± 2.1 | 6.4 ± 1.5 | 1.9 ± 0.3 |
| VCAM-1 | 1.6 ± 0.5 | 1.0 ± 0.1 | 1.6 ± 0.6 | 1.2 ± 0.1 |
| Integrin β1 | 28.9 ± 3.8 | 25.6 ± 8.9 | 32.1 ± 7.4 | 18.1 ± 0.9 |
| Integrin β2 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.4 |
| Integrin α1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 2.3 ± 1.3 | 1.7 ± 0.9 |
| Integrin α2 | 12.9 ± 6.3 | 16.2 ± 6.5 | 12.0 ± 5.8 | 1.7 ± 0.5 |
| Integrin α5 | 20.5 ± 7.2 | 14.4 ± 5.0 | 18.4 ± 2.3 | 4.1 ± 0.1 |
| CD133 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.2 |
. | EPDCs, MFI ± SEM . | HUVECs, MFI ± SEM . | HBMECs, MFI ± SEM . | L87-4, MFI ± SEM . |
|---|---|---|---|---|
| KDR | 4.9 ± 0.3 | 2.3 ± 0.1 | 1.5 ± 0.2 | 1.5 ± 0.4 |
| CD31 | 31.1 ± 3.2 | 23.7 ± 1.3 | 27.8 ± 10.8 | 1.1 ± 0.1 |
| CD144 | 6.9 ± 2.4 | 5.7 ± 0.2 | 2.0 ± 0.9 | 1.2 ± 0.2 |
| CD146 | 5.43 ± 1.7 | 8.9 ± 1.3 | 10.9 ± 0.3 | 0.9 ± 0.2 |
| Flt-1 | 8 ± 0.3 | 7.8 ± 0.5 | 9.6 ± 0.7 | 1.1 ± 0.1 |
| Endoglin | 17.9 ± 5.2 | 25.3 ± 5.8 | 15.7 ± 2.8 | 9.2 ± 3.2 |
| ICAM-1 | 7.7 ± 3.6 | 14.2 ± 4.5 | 9.2 ± 1.6 | 7.2 ± 0.3 |
| ICAM-2 | 8.3 ± 3.3 | 7.6 ± 0.2 | 11.0 ± 3.9 | 1.3 ± 0.2 |
| αvβ3 | 7.1 ± 1.0 | 12.5 ± 3.4 | 4.3 ± 0.6 | 2.4 ± 0.2 |
| αvβ5 | 7.7 ± 0.4 | 15.0 ± 2.1 | 6.4 ± 1.5 | 1.9 ± 0.3 |
| VCAM-1 | 1.6 ± 0.5 | 1.0 ± 0.1 | 1.6 ± 0.6 | 1.2 ± 0.1 |
| Integrin β1 | 28.9 ± 3.8 | 25.6 ± 8.9 | 32.1 ± 7.4 | 18.1 ± 0.9 |
| Integrin β2 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.4 |
| Integrin α1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 2.3 ± 1.3 | 1.7 ± 0.9 |
| Integrin α2 | 12.9 ± 6.3 | 16.2 ± 6.5 | 12.0 ± 5.8 | 1.7 ± 0.5 |
| Integrin α5 | 20.5 ± 7.2 | 14.4 ± 5.0 | 18.4 ± 2.3 | 4.1 ± 0.1 |
| CD133 | 1.2 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.2 |
MFI was considered positive at 2.
The expression of all these markers was measured in L87-4 cells. These cells did not express KDR, CD31, ICAM-2, CD144, CD146, α1, α2, β2 integrins, αvβ5, VCAM-1, Flt1, or CD133. However, integrin β1 was markedly expressed, whereas endoglin, ICAM-1, αvβ3, and integrin α5 were detected (Table 1).
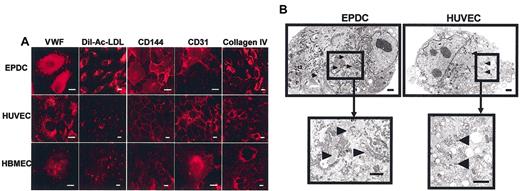
Immunocytochemistry analysis was performed to characterize further the phenotype of EPDCs. Figure 2 demonstrates that at day 40 of culture, EPDCs stained positively for CD144 and CD31. Both have a membrane expression and have been observed in lamellipods and at cellular junctions. All cells incorporate Dil-Ac-LDL, one of the characteristic functions of endothelial cells. The same results were observed in HUVECs and HBMECs (Figure 2A). The expression of different matrix molecules was also analyzed. EPDCs and both positive control cells were stained with collagen IV (Figure 2A) as well as the matrix network. L87-4 control cells did not exhibit positive staining for CD31 and CD144 and did not incorporate Dil-Ac-LDL (data not shown). Without primary antibody, no fluorescence was observed (data not shown). The granular staining obtained with VWF antibody suggested the presence of Weibel-Palade bodies, typical secretion granules present in endothelial cells. This was confirmed by electron microscopy analysis. Figure 2B shows that EPDCs, as well as HUVECs, cultured for 40 days displayed organelles morphologically corresponding to Weibel-Palade bodies. Furthermore, EPDCs exhibited a higher density of endoplasmic reticulum network in comparison with HUVECs, indicating an increased metabolic activity.
Immunofluorescence analysis and ultrastructural studies. (A) Immunofluorescence analysis of EPDCs, HUVECs, and HBMECs. Cells were stained with antibody against VWF, CD144, CD31, or collagen IV. EPDCs as well as HUVECs and HBMECs take up Dil-Ac-LDL and were positively stained with VWF, CD144, CD31, or collagen IV antibodies. Scale bar equals 5 μm. (B) Ultrastructural studies of EPDCs and HUVECs. EPDCs and HUVECs were fixed and prepared for transmission electron microscopy. Scale bar equals 1 μm. Arrowheads indicate Weibel-Palade bodies.
Immunofluorescence analysis and ultrastructural studies. (A) Immunofluorescence analysis of EPDCs, HUVECs, and HBMECs. Cells were stained with antibody against VWF, CD144, CD31, or collagen IV. EPDCs as well as HUVECs and HBMECs take up Dil-Ac-LDL and were positively stained with VWF, CD144, CD31, or collagen IV antibodies. Scale bar equals 5 μm. (B) Ultrastructural studies of EPDCs and HUVECs. EPDCs and HUVECs were fixed and prepared for transmission electron microscopy. Scale bar equals 1 μm. Arrowheads indicate Weibel-Palade bodies.
Variations in marker expression during EPDC differentiation
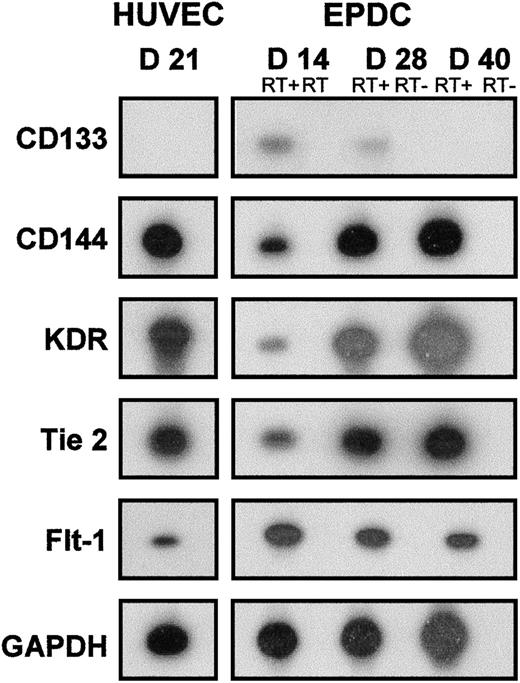
The expression of markers was monitored by RT-PCR followed by hybridization of PCR products by an internal labeled probe. As shown in Figure 3, the expression of CD133, a marker of immature EPDCs, was still detectable at day 14 and decreased thereafter. This marker was not detectable in HUVECs even at the beginning of the culture (data not shown). Expression of endothelial markers such as CD144, KDR, and Tie-2 was detected from day 14 and increased throughout maturation, reaching levels comparable to that of HUVECs at day 40. The expression of Flt-1 was detected at day 14 and had decreased slightly by day 40, but was still detectable.
RT-PCR analysis of EPDCs and HUVECs. Expression of CD133, CD144, KDR, Tie-2, Flt-1, and GAPDH mRNA was determined with a 32P-labeled internal probe at days 14, 28, and 40 of culture for EPDCs and at day 21 of culture for HUVECs. RT- was the control realized without reverse transcription before PCR. D indicates day.
RT-PCR analysis of EPDCs and HUVECs. Expression of CD133, CD144, KDR, Tie-2, Flt-1, and GAPDH mRNA was determined with a 32P-labeled internal probe at days 14, 28, and 40 of culture for EPDCs and at day 21 of culture for HUVECs. RT- was the control realized without reverse transcription before PCR. D indicates day.
EPDCs display a higher proliferation potential than HUVECs
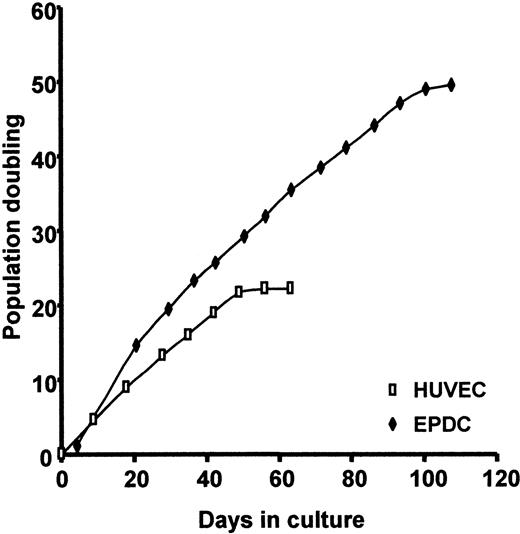
Figure 4 compares EPDC proliferation potential to that of HUVECs. The population doubling curve demonstrates that EPDCs have a higher proliferation potential. After a period of latency (day 1 to day 5), the cells grew in clusters. Serial dilutions showed that these clusters appeared at a frequency of 1:4 × 105 to 5 × 105 seeded CD34+ cells. The number of EPDCs rapidly increased to 6.9 × 013 cells per outgrowth progenitor cell, corresponding to up to 45 population doublings at day 94. Outgrowth of the EPDCs then slowed down. EPDCs reached 49 divisions and could be maintained in culture for more than 3 months. In contrast, HUVECs started proliferating immediately after seeding. The number of cells reached 5.1 × 106/seeded cell, corresponding to 22 population doublings under the same culture conditions. Outgrowth subsequently slowed down. HUVECs could only be cultured for 2 months under our conditions.
Proliferation potential of EPDCs compared with HUVECs under EGM2-MV conditions. Cells were enumerated at each passage.
Proliferation potential of EPDCs compared with HUVECs under EGM2-MV conditions. Cells were enumerated at each passage.
Effect of angiogenic factors on EPDC and HUVEC proliferation in the presence of serum
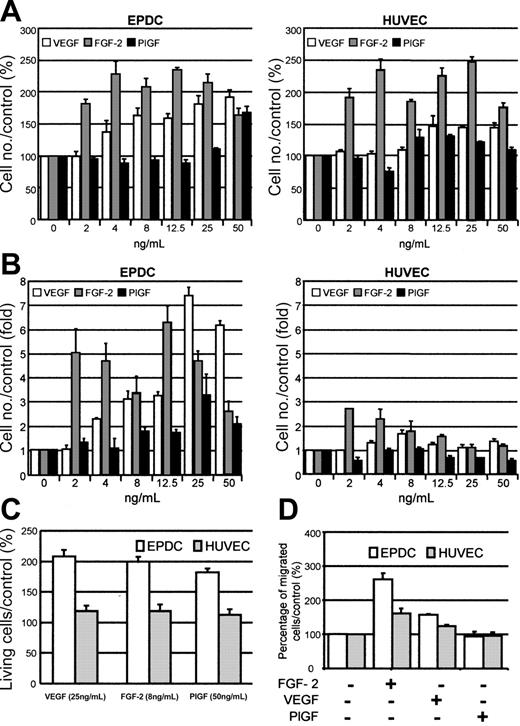
We first tested to what extent VEGF, FGF-2, and PlGF induce cell proliferation when incubated with EPDCs or HUVECs in the presence of 2.5% FBS. Figure 5A shows that VEGF induced EPDC proliferation from 4 ng/mL. Proliferation increased up to 50 ng/mL in a concentration-dependent manner. In contrast, HUVECs were less sensitive to VEGF induction because they did not respond at concentrations below 12.5 ng/mL. Higher concentrations of VEGF did not further increase their proliferation. Furthermore, EPDCs and HUVECs responded strongly to 2 ng/mL FGF-2. The maximal response was reached at 4 ng/mL FGF-2. PlGF stimulated EPDC proliferation only at 50 ng/mL and was not efficient in inducing a significant increase in HUVEC proliferation.
Effect of VEGF, FGF-2, or PlGF on EPDC and HUVEC proliferation, survival, and mobility. Cell proliferation was analyzed (A) in the presence of 2.5% of FBS or (B) under serum starvation conditions. (C) The percentage of living cells compared with the control (EGM2-MV medium depleted in FBS, FGF-2, and VEGF) was estimated by annexin V/PI staining. The annexin V-/PI- population was representative of living cells. (D) Effect of 10 ng/mL FGF-2, VEGF, or PlGF on EPDC and HUVEC mobility compared with control conditions (EGM2-MV medium containing 0.1% BSA, depleted in FBS, FGF-2, and VEGF).
Effect of VEGF, FGF-2, or PlGF on EPDC and HUVEC proliferation, survival, and mobility. Cell proliferation was analyzed (A) in the presence of 2.5% of FBS or (B) under serum starvation conditions. (C) The percentage of living cells compared with the control (EGM2-MV medium depleted in FBS, FGF-2, and VEGF) was estimated by annexin V/PI staining. The annexin V-/PI- population was representative of living cells. (D) Effect of 10 ng/mL FGF-2, VEGF, or PlGF on EPDC and HUVEC mobility compared with control conditions (EGM2-MV medium containing 0.1% BSA, depleted in FBS, FGF-2, and VEGF).
Effect of angiogenic factors on EPDCs and HUVECs in serum-free conditions
Important differences between EPDCs and HUVECs were revealed when cells were cultured with the same growth factors in the absence of FBS (Figure 5B). Under these conditions, the number of HUVECs moderately increased in the presence of VEGF. The maximal effect (1.63-fold compared with unstimulated cells) was observed at 8 ng/mL. In contrast, EPDCs were more sensitive to growth factors. In the presence of 4 ng/mL VEGF, cell number was 2.32-fold higher than in the control. This effect was even more striking at 12.5 ng/mL VEGF (7.39-fold increase). The maximal response of HUVECs to FGF-2 was obtained with 2 ng/mL (2.72-fold). At higher concentrations, the effect of FGF-2 decreased. FGF-2 was much more active on EPDCs (5.05-fold increase at 2 ng/mL). This effect was even more exacerbated at 12.5 ng/mL (6.31-fold increase). Finally, PlGF was effective only on EPDCs. The maximum effect was observed at 25 ng/mL (3.32-fold increase).
We compared the response of subconfluent cells cultivated under serum starvation conditions for 72 hours in the absence or presence of increasing concentrations of VEGF, FGF-2, or PlGF. The annexin V-/PI- population represented the surviving cells. In the concentration range tested, a protective effect of each growth factor was seen in EPDCs as well as in HUVECs against serum starvation–induced cell death. This effect was 2-fold higher in EPDCs, whatever the growth factor used (Figure 5C).
Effect of angiogenic factors on EPDC and HUVEC mobility and vascular tube formation
This was tested by incubating EPDCs or HUVECs with 10 ng/mL VEGF, FGF-2, or PlGF. VEGF and FGF-2 significantly induced EPDC and HUVEC mobility (Figure 5D). The effect was greater for EPDCs than for HUVECs (262% for EPDCs versus 162% for HUVECs). PlGF had no effect (Figure 5D).
We analyzed vascular tube formation in the presence of VEGF or FGF-2 or PlGF. The EPDC network was denser than that observed for HUVECs, especially with PlGF (data not shown).
Effect of EPDCs on human hematopoiesis
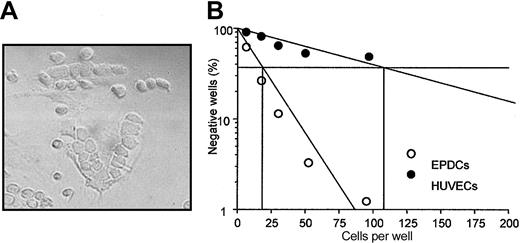
Between day 10 and 20 of culture, we observed in 15% to 20% of our primary culture of EPDCs that hematopoietic cells spontaneously formed cluster structures underneath the endothelial cell monolayer (Figure 6A), resembling CAFCs observed when hematopoietic progenitor cells were cocultured with stromal cells. These structures maintain hematopoietic progenitors in long-term culture. We followed the generation of CAFCs from human cord blood CD34+ cells seeded at limiting dilutions and cocultured for 5 weeks with EPDCs or HUVECs as feeder layers. Figure 6B shows a typical representative experiment of limiting dilutions for CAFCs generated on these different feeders. After 5 weeks, the CAFCs observed correspond to early progenitors. Their frequency was 1/21.2 ± 1/2.69 (P < .005) for EPDCs versus 1/95.8 ± 1/10.32 (P < .005) for HUVECs. This difference was significant (P < .03) and was observed from the first week, suggesting that EPDCs were better able than HUVECs to support all kinds of CAFCs.
Hematopoietic supportive activity of EPDCs and HUVECs. (A) CAFC-like structure observed in primary culture of CEPDCs. Original magnification, × 250. (B) Cord-blood CD34+ cells were seeded at limiting dilutions onto a preestablished EPDC or HUVEC monolayer. EPDCs and HUVECs were seeded at passage 2. CAFC frequencies (determined at 37% of negative wells) were measured at week 5 of culture. Data shown are the means of 3 experiments.
Hematopoietic supportive activity of EPDCs and HUVECs. (A) CAFC-like structure observed in primary culture of CEPDCs. Original magnification, × 250. (B) Cord-blood CD34+ cells were seeded at limiting dilutions onto a preestablished EPDC or HUVEC monolayer. EPDCs and HUVECs were seeded at passage 2. CAFC frequencies (determined at 37% of negative wells) were measured at week 5 of culture. Data shown are the means of 3 experiments.
Even though the medium used for coculture with hematopoietic cells is not fully optimized for endothelial cell cultures, we verified that this medium does not affect HUVEC and EPDC proliferation and survival (data not shown).
Human EPDCs migrate toward an angiogenic site and participate in neovascularization in NOD/SCID mice
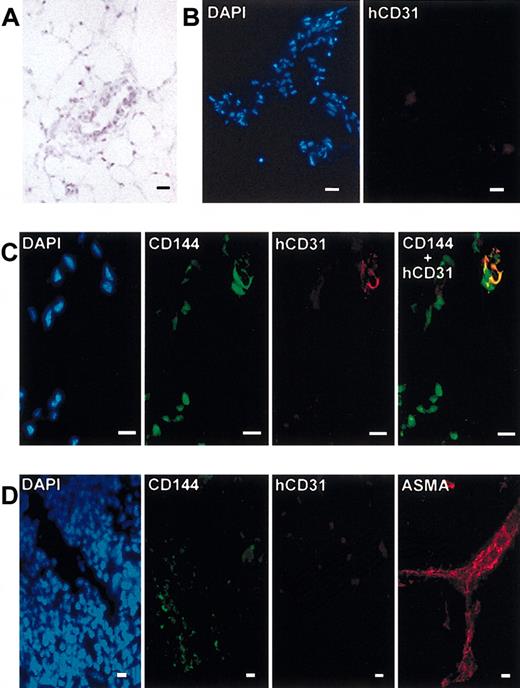
NOD/SCID mice engrafted with cord blood CD34+, CD34-,ornot engrafted were then injected with Matrigel plug and killed 10 days later. May-Grünwald-Giemsa staining of Matrigel cryosections showed vessel-like structures in the invaded Matrigel (Figure 7A). The presence of human endothelial cells was analyzed by the immunofluorescence of cryosections of the Matrigel plugs. No human CD31+ cells or CD45+ cells were detected in the Matrigel plug of the mice that were not injected (used as negative staining control; Figure 7B) or were injected with the CD34- population (data not shown). However, the presence of human CD31+ cells was detected in vascular structure present in the Matrigel plug. These CD31+ cells were CD45- as evidenced by double staining. However, these CD31+ cells coexpressed the CD144 marker, confirming their endothelial phenotype. This demonstrates that the injected CD34+ population contained functional EPCs that had the capacity to reach an angiogenic site and to participate in a neoangiogenic process (Figure 7C). The staining of the spleen cryosections did not reveal the presence of human endothelial cells in this organ. No positive staining was obtained with antibody against human CD31. Murine endothelial cells were detected by CD144 staining (Figure 7D). As positive control, cells stained with ASMA were detected (Figure 7D). Staining of liver, lung, and heart cryosections did not reveal the presence of human endothelial cells (data not shown). The same results were obtained with mice injected with CD34- cells.
Presence of human endothelial cells in Matrigel plug. (A) May-Grünwald-Giemsa staining of Matrigel cryosection. (B) DAPI (blue) and human CD31 (red) staining of Matrigel cryosections from negative control mice (no CD34+ transplants). (C) DAPI (blue), CD144 (green), human CD31 (red), and double CD144 and human CD31 (yellow) staining of Matrigel cryosection from mice receiving CD34+ transplants. (D) DAPI (blue), CD144 (green), human CD31 (red), and ASMA (red) staining of spleen cryosections. Scale bars equal 10 μm.
Presence of human endothelial cells in Matrigel plug. (A) May-Grünwald-Giemsa staining of Matrigel cryosection. (B) DAPI (blue) and human CD31 (red) staining of Matrigel cryosections from negative control mice (no CD34+ transplants). (C) DAPI (blue), CD144 (green), human CD31 (red), and double CD144 and human CD31 (yellow) staining of Matrigel cryosection from mice receiving CD34+ transplants. (D) DAPI (blue), CD144 (green), human CD31 (red), and ASMA (red) staining of spleen cryosections. Scale bars equal 10 μm.
Discussion
The use of EPCs as a tool for cell therapy in diseases involving defects of angiogenesis, such as ischemia, was envisioned as soon as these cells were discovered. Many experiments performed in different models confirmed that these cells were very potent in the revascularization of ischemic tissues and their specific tropism for the diseased tissues was very encouraging.17-19 However, the feasibility of using these cells as a cell therapy product in patients with ischemic diseases was hampered by the rarity of the cells in the peripheral blood. Expansion of these cells is thus necessary, but until now all the ensuing technical problems were unresolved. Among these problems is the determination of the extent to which these cells could be expanded without losing their migration/vascularization capacities. Meanwhile, increasing numbers of clinical trials have started, using bone marrow mononuclear cells in cell therapy of ischemic diseases.24 It is likely that EPCs play a major role in the beneficial effect of cell therapy, but until now this hypothesis was unproven.
It is therefore important to enhance our knowledge of the specific features of EPDCs. We thus undertook a systematic study of the phenotypic and functional properties of EPDCs and compared them to those of mature endothelial cells (HUVECs or HBMECs). We found that from 14 to 40 days of culture, EPDCs express all the markers of mature endothelial cells, whereas CD133 expression decreases gradually during maturation. At day 40, quantitative analysis of these markers in EPDCs, HUVECs, and HBMECs did not reveal striking differences. However, KDR was expressed at higher levels in EPDCs than in the other endothelial cells. Like mature endothelial cells, EPDCs express NOSIII, suggesting that these cells have the capacity to produce NO, one of the important functions of endothelial cells. The morphology of EPDCs is typical of endothelial cells, as revealed by immunofluorescence and electron microscopy. EPDCs also express high levels of VWF, which is localized in granular structures resembling Weibel-Palade bodies. The presence of these granules was further confirmed by electron microscopy. These phenotypic similarities between EPDCs and mature endothelial cells of different origins confirm that EPCs are able to be fully differentiated in vitro into endothelial cells, thus validating the differentiation culture conditions that have been used.
More striking differences between EPDCs and mature endothelial cells were revealed by functional studies. First, EPDCs could be maintained in culture for more than 3 months, whereas senescence of HUVECs was observed after 55 to 65 days of culture. We observed that EPDCs were able to perform more divisions than HUVECs. Thus, we confirm previous observations showing that EPDCs are more proliferative than HUVECs, and quantified this difference. We then investigated the individual influence of VEGF, FGF-2, and PlGF on the proliferation of EPDCs and HUVECs. In the presence of serum, HUVECs and EPDCs responded to FGF-2 to a comparable extent. However, EPDCs were more sensitive to VEGF than HUVECs. This observation correlates with the higher KDR expression observed in EPDCs and may contribute to their higher proliferative capacities compared with HUVECs. More striking differences were revealed when the cells were cultured without serum. When 12.5 ng/mL FGF-2, 25 ng/mL VEGF, or 25 ng/mL PlGF was added to the culture, the number of EPDCs increased 6.5-, 7.5-, and 3.2-fold, respectively, whereas the number of HUVECs was almost stable at the same concentrations. Because the experiment was performed over 72 hours, it is possible that this differential effect of growth factor could be explained by a survival advantage of EPDCs compared with HUVECs. Indeed, the percentage of living EPDCs at 72 hours (annexinV-/PI-) was twice that of HUVECs. The number of apoptotic EPDCs (annexinV+/PI-) was also half that of HUVECs (data not shown). This greater sensitivity to growth factors displayed by EPDCs compared with HUVECs confirms that, even when differentiated, they still retain the properties of immature cells. Another important function assumed by endothelial cells is tissue repair. This function needs both migrating and proliferative capacities. We measured the ability of the cells to repair a linear scar cut in a confluent monolayer of endothelial cells. This ability reflects both motility and proliferation potential. We show that in the presence of FGF-2, the repair of the artificial wound was more efficient with EPDCs than with HUVECs.
Converging evidence indicates that hematopoietic and endothelial cells establish interactions in the bone marrow.25 In this context, we observed cobblestone-forming structures in the EPDC culture that were very similar to those obtained with hematopoietic progenitors cultured on a stromal cell monolayer. Hematopoietic cells niche underneath the stromal monolayer forming CAFCs. This interaction results in the long-term maintenance of the immature and proliferative properties of the hematopoietic progenitors. This observation prompted us to investigate whether EPDCs display the property of “stromal-like cells” that could maintain the long-term survival of hematopoietic immature progenitors. We used a classical test that has been established for stromal cells and demonstrated that the stromal ability was much greater for EPDCs than for HUVECs. This may reflect an intrinsic property and suggests that, under certain conditions, endothelial progenitors may cooperate with stromal cells in supporting hematopoiesis. Likewise, in the context of leukemia, malignant hematopoietic cells could stimulate the formation of new vessels in the bone marrow by producing high levels of angiogenic growth factors such as VEGF.26
Moreover, it has been shown that endothelial and hematopoietic progenitors share common markers suggesting a common progenitor. In particular, circulating endothelial cells and chronic myeloid leukemia blasts may be of clonal origin because they both carry the Philadelphia chromosome.27
Finally, we checked in NOD/SCID mice the ability of human EPCs contained in the engrafted cord blood CD34+ population to colonize a Matrigel plug diffusing FGF-2, constituting an in vivo model of neoangiogenesis. We found that EPCs contained in the CD34+ engrafted cell population were able specifically to reach the Matrigel plug and to participate in vascular-like structures. We analyzed whether EPCs were localized only in the Matrigel plug, thus confirming the specific affinity of these cells for angiogenic targets. No human cells were detected in the vascular structures of organs such as liver, lung, heart, or spleen.
In summary, we show that in vitro–differentiated EPDCs display the morphologic, phenotypic, and functional features of endothelial cells. In addition, even differentiated, these cells still retain properties of immature cells as compared to mature endothelial cells isolated from blood vessels. Injected EPCs have the capacity to reach a neoangiogenic site and there to differentiate into endothelial cells, suggesting tissue repair capabilities. Another specific feature of EPDCs is their ability to support long-term survival of hematopoietic progenitors. This capacity could be used to target pharmacologic agents to the angiogenic bone marrow environment of the leukemic clone.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-08-2770.
Supported by research grants from the Association Française contre la Myopathie/Institut National de la Santé et de la Recherche Médicale (AFM/INSERM No. 4CS14F).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank the Centre Hospitalier de Longjumeau and the Clinique Ambroise-Paré of Bourg-la-Reine for providing us with cord blood samples, and Sophie Darquenne for her technical advice.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal