Abstract
We describe the multiparameter flow cytometric analysis of the relationship between side population (SP) formation and well-characterized, antigen-defined stem cell subsets. We also compared the competitive repopulation ability of subsets defined by the SP profile to those identified by antigenic markers. The vast majority of SP cells possessed a primitive cell phenotype (c-kit+, SCA-1+, Thy-1+, CD31+, CD135neg, lineageneg), but only a minority of antigen-defined subsets were SP cells. Hence, although SP cells are identified independently of antigenic markers, they are not distinct from established stem cell phenotypes, but are a small subset of them. Approximately half of SP cells expressed CD34 at readily detectable levels, and one-third of SP cells possessed the primitive c-kit+, SCA-1+, lineageneg, CD34neg cell phenotype. Since most SP cells are a subset of c-kit+, Thy-1+, lineageneg, SCA-1+ cells (KTLS), we determined whether the SP cell subset represents a further enrichment in long-term repopulating cell content. The SP+ subset of KTLS+ cells was more enriched for competitive repopulation units than the SP- fraction of KTLS+ cells. Hence, the SP cell fraction highlights a subset of the long-term repopulating cells found within the already highly purified KTLS fraction.
Introduction
Stem cells are a rare population of cells with extensive proliferative and self-renewal capabilities that sustain hematopoiesis throughout life. The identification and subsequent isolation of these cells will allow an improved understanding of the interactions between stem cells and their immediate progeny. Stem cell isolation is important for the development of novel strategies such as gene therapy and stem cell expansion. Fractionation of the stem cell compartment may help define populations responsible for stem cell plasticity.1 Resolution of pure stem cell populations from their immediate progeny has proven useful for analysis of relative gene expression profiles.2 Hence, research into the hematopoietic stem cell phenotype has been a very active area in recent years.
Many approaches have been used to isolate hematopoietic stem cells via flow cytometry, the most popular of which are based on the expression of a combination of antigenic markers. Long-term repopulation of lethally irradiated murine hosts has been achieved with cells that are negative or low for markers of differentiated cells (Lineageneg), but positive for SCA-1, Thy-1, and c-kit (CD117).3,4 This rare fraction (KTLS [c-kit+, Thy.1+, Lineageneg, Sca.1+]) is thought to contain virtually all the assessable long-term repopulating cells (LTRCs) and most of the short-term repopulating cells (STRCs) in the murine bone marrow.5 The STRCs within KTLS may be distinguished from the LTRCs via their coexpression of Flt3 (CD135) and Mac-1 (CD11b).6,7 The CD34+ fraction of Lineageneg/SCA-1+/c-kit+ (KLS) cells reportedly contains both LTRCs and STRCs, whereas the CD34- subset of this fraction contains mainly LTRCs and few STRCs.8
In addition to the use of these surrogate markers, alternative methods to identify hematopoietic stem cells have been developed. These often highlight a physiologic property of hematopoietic stem cells that is different to the rest of hematopoiesis. For instance, stem cells have been isolated via their elevated aldehyde dehydrogenase activity or ability to efflux the mitochondrial binding dye Rhodamine 123.9-11
The most promising of these alternative methods involves the identification of the side population (SP), which is formed by preferential efflux of the DNA binding dye Hoechst 33342.12 ABC/G2 transporters selectively expressed on the surface of hematopoietic stem cells are thought to be responsible for this preferential efflux.13,14 The SP reportedly accounts for 0.07% to 0.10% of murine bone marrow and represents a 1000-fold enrichment for LTRCs. The frequency of LTRCs within the SP is thought to be arranged as a hierarchy; the cells which most efficiently efflux the dye are most enriched for LTRCs (3100-fold enrichment of competitive repopulation units [CRUs]), and the cells that efflux the dye least efficiently contain the lowest proportion of LTRCs (250-fold). Although the repopulation potential of the SP has been characterized, its phenotype has yet to be determined exactly. SP cells have been approximated to be negative for lineage markers.
Since the first report in 1996, many papers have been published utilizing SP cells as a source of highly purified hematopoietic stem cells (HSCs) identified independently of surrogate markers.15,16 As the exact frequency of cells that express antigenic markers has not yet been analyzed in a multiparameter format, the relationship between cells capable of SP formation and the popular antigenic marker-defined subsets is still largely unknown. Furthermore, as experiments may be affected by the technique's inherent toxicity, and an anti-murine ABC/G2 antibody is not yet available, the phenotype of these cells needs to be known for more efficient selection. Knowledge of the types of cell present may also give us clues to the function of this highly active pump present on hematopoietic stem cells.
Here, we defined our SP via sensitivity to the ABC/G2-specific inhibitor, reserpine. We then assessed the reserpine-sensitive SP fraction for the presence of cells committed to each particular lineage. In a separate series of experiments, we used 6-color flow cytometry to examine the relationship between SP formation and well-characterized antigenic marker–defined stem cell subsets. Finally, we compared the competitive repopulating potential of subsets defined by SP to those identified by antigenic markers.
Materials and methods
Mouse strains
Strains used in this study were C57Bl6-Thy-1.1, C57Bl6-Thy-1.2, C57Bl6-Ly5.1, and NOD/SCID-Ly5.1 (all originally from Jackson Laboratories, Bar Harbor, ME). Nonobese diabetic–severe combined immunodeficient (NOD/SCID) mice were bred at Charles River (Margate, United Kingdom), before arrival in our animal facility. All C57Bl6 colonies were maintained in our animal facility.
Cell preparation
C57Bl6 bone marrow was flushed from murine femurs and tibias and red blood cells were lysed with ammonium chloride (Stem Cell Technologies, Vancouver, BC, Canada). Nucleated cells were Hoechst labeled for SP analysis as previously described.12 Inhibitor controls involved the addition of either 50 μM verapamil (in distilled water [DW]) or 5 μM reserpine (in acetic acid/DW). Once stained, cells were maintained at 4°C during antibody labeling and until analysis/sorting.
Antibody labeling was performed in phosphate-buffered saline (PBS), 2% fetal calf serum (FCS), 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid) for 30 minutes against appropriate matched-isotype controls. This study used the following antibodies (all from Pharmingen, San Diego, CA): fluorescein isothiocyanate (FITC)–conjugated anti–Thy-1, anti-CD31, and anti-CD34; phycoerythrin (PE)–conjugated anti-CD2, anti-CD3, anti-CD4, anti-CD8, anti-CD11b, anti-CD135, anti-B220, anti–Ter-119, anti–GR-1, and anti-NK1.1; biotinylated anti–stem cell antigen-1 (SCA-1) (subsequently labeled with PE–cyanin-5 (Cy5)–conjugated streptavidin); and allophycocyanin (APC)–conjugated anti-CD117. The lineage cocktail used throughout contained PE-conjugated anti-CD5, anti-CD11b, anti-B220, anti–Gr-1, and anti–Ter-119 antibodies. All Hoechst-stained cells were resuspended in PBS 2% FCS, 10 mM HEPES, 2 μg/mL propidium iodide (PI), and passed through a 70-μm mesh before analysis/sorting.
Flow cytometric analysis
Analysis/sorting was performed on a MoFlo flow cytometer (DAKO-Cytomation, Carpinteria, CA) equipped with laser lines of 350.7 nm to 356.4 nm (for UV), 488 nm and 633 nm. Red and blue fluorescence derived from UV excitation was separated via a 610 dichromatic long-pass (DCLP). Blue Hoechst fluorescence was collected with a 424/44 bandpass (BP) filter and red Hoechst/PI fluorescence via a 620 LP filter. FITC, PE, PE-Cy-5, and APC signals were collected via 530/40, 570/40, 675/20, and 670/40 BP filters. Fluorescence compensation was typically performed during acquisition.
Hoechst/PI red and Hoechst blue fluorescence signals were displayed on a linear, dual-fluorescence dot plot. A rectangular gate was drawn to exclude PI+ dead cells (far right of plot) and unstained debris (origin of plot) as previously described.12 First SCA-1+/Linlow and then CD117+ cells were readily identified as discrete populations within this gate. CD34neg was defined as the fluorescence below the level of background staining. In some analyses, CD34+ events were subdivided into CD34high and CD34low according to fluorescence intensity. All other gates were set above the level of background staining. SP gating was defined as reserpine sensitive during the study.
Sorting gates
The same initial gate as in the previous section was established to exclude dead cells and debris from subsequent analysis. Reserpine-sensitive SP+ cells were then divided into KTLS+ and KTLSneg A non-SP gate was drawn that excluded intermediate SP cells and these were also divided into KTLS+ and KTLSneg. During all sorts performed, the last gate excluded any debris on a forward-versus side-scatter dot plot.
Competitive repopulation assays
In 1 set of experiments, Ly5.1 NOD/SCID mice (8-12 weeks) were sublethally irradiated (375 rads), as previously described for a competitive repopulation assay.17 In a second set of experiments, Ly5.1 C57Bl6 (8-12 weeks) were lethally irradiated (2 doses of 500 rad given 4 hours apart). In both sets of experiments, sorted Ly5.2 C57Bl6 donor cells were administered in PBS via tail-vein injection along with 200 000 recipient-type (Ly5.1) whole bone marrow cells. Bone marrow or peripheral blood from surviving mice was analyzed for donor cell content on the flow cytometer. Analysis involved labeling of red cell lysed, DAPI (4,6-diamidino-2-phenylindole; Sigma)–negative cells with anti–Ly5.1-PE and anti–Ly5.2-FITC antibodies (both from Pharmingen, San Diego, CA). Competitive repopulation units were calculated as (%Ly5.2/%Ly5.1) × (2 × 105/number of donor cells injected).
Results
Definition of SP gating
To accurately analyze and compare SP profiles from different experiments, we established a reliable SP gating strategy. Goodell et al12 described the blocking of SP formation via incubation with 50 μM verapamil, and Zhou et al14 have reported the inhibition of SP formation with 5 μM reserpine. We investigated the possibility of utilizing these inhibitors to define a consistent SP gate. A preliminary inhibitor comparison experiment indicated that a gate drawn on the limit of Hoechst staining during reserpine inhibition included fewer intermediate SPs (I-SP on Figure 2A), and hence accounted for a lower proportion of bone marrow (BM; 0.14% vs 0.21%) than a gate drawn on the limit of verapamil inhibition (data not shown). In this preliminary experiment, we compared the hematopoietic status of cells within each inhibitor-derived gate; red cell–lysed murine BM was Hoechst stained in conjunction with antibodies against various committed cell markers. Reserpine-sensitive SPs contained a lower proportion of T cells (CD2+), B cells (B220+), and granulocytes (GR-1+) than verapamil-sensitive SPs (data not shown). Conversely, reserpine-sensitive SP cells were more enriched for SCA-1+ cells than the verapamil-affected fraction (88.9% of SP vs 74.4%).
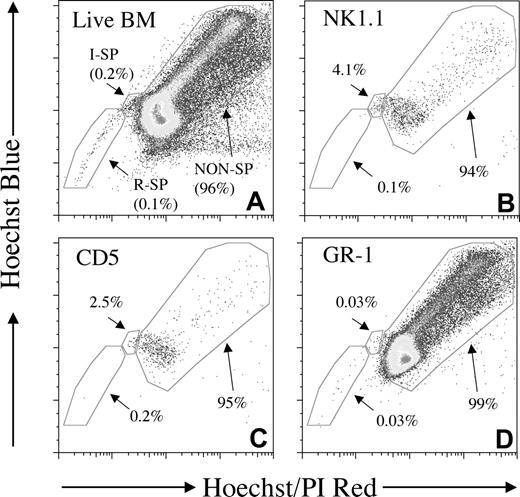
Backgating of lineage marker–positive subsets reveals contrasting SP profiles. Provided for comparison is a corresponding whole BM SP profile with percentage of reserpine-sensitive SP (R-SP), intermediate SP (I-SP), and non-SP populations indicated (A; 100 000 events). Display of NK1.1+ (B; 2087 events) or CD5+ (C; 1154 events) lymphoid cells on Hoechst red/blue plots revealed an enrichment of the rare intermediate SP population. In contrast, Gr-1+ cells (D; 72 800 events) contained very few intermediate or SP cells and mostly non-SP cells.
Backgating of lineage marker–positive subsets reveals contrasting SP profiles. Provided for comparison is a corresponding whole BM SP profile with percentage of reserpine-sensitive SP (R-SP), intermediate SP (I-SP), and non-SP populations indicated (A; 100 000 events). Display of NK1.1+ (B; 2087 events) or CD5+ (C; 1154 events) lymphoid cells on Hoechst red/blue plots revealed an enrichment of the rare intermediate SP population. In contrast, Gr-1+ cells (D; 72 800 events) contained very few intermediate or SP cells and mostly non-SP cells.
As reserpine seemed to be more selective for noncommitted cells and was less toxic (data not shown), the rest of this study is based on reserpine-sensitive SP cells.
Expression of lineage markers on SP cells
Having defined our SP gating strategy, we investigated the presence of lineage-committed BM cells. Hoechst-stained, murine BM cells (n = 5) were labeled with antibodies to SCA-1 and one of the following lineage markers: T cells: CD2, CD4, CD8; B cells: CD5 (T cells as well) and B220; natural killer (NK) cells: NK1.1; myeloid cells: GR-1 and CD11b; erythroid cells: Ter-119.
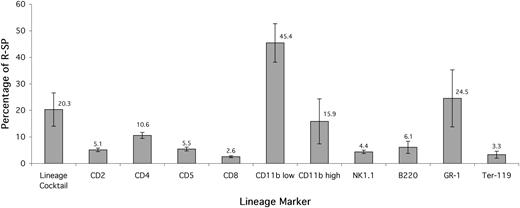
Hoechst staining and subsequent antibody labeling resulted in a viability of 53.1% ± 12.4% (mean ± SD) on the flow cytometer (expressed as the percentage of all nucleated cells that were PI+). The SP accounted for 0.089% ± 0.037% of viable, red cell–lysed, unfractionated murine BM; a similar figure to published values.12,18 A consistently low proportion of these SP cells expressed T-lymphoid cell markers (CD2, CD4, and CD8), B-cell markers (CD5 and B220), and the erythroid marker Ter-119 (Figure 1). Myeloid marker (GR-1) expression accounted for a higher, more variable proportion of SP cells than lymphoid markers. Most CD11b antigen was expressed at a low intensity and coexpressed with SCA-1, the remainder was high-level expression and lacked detectable SCA-1 antigen. Overall, since some of these markers are coexpressed, when they were combined to form a lineage cocktail (CD5, B220, CD11bhigh, GR-1, and Ter-119) only 20.3% ± 14.1% of SP cells were positive for lineage markers (Lin+).
Reserpine-sensitive SP cells contain a low proportion of committed cells. All values are expressed as a mean of 5 separate experiments. Expression of mature cell markers is displayed as percentage of SP. The SP contains a low proportion of lymphoid cells (CD2, 4, 5, 8, B220, and NK1.1) and a slightly higher proportion of mature myeloid cells (GR-1+ and CD11bhigh).
Reserpine-sensitive SP cells contain a low proportion of committed cells. All values are expressed as a mean of 5 separate experiments. Expression of mature cell markers is displayed as percentage of SP. The SP contains a low proportion of lymphoid cells (CD2, 4, 5, 8, B220, and NK1.1) and a slightly higher proportion of mature myeloid cells (GR-1+ and CD11bhigh).
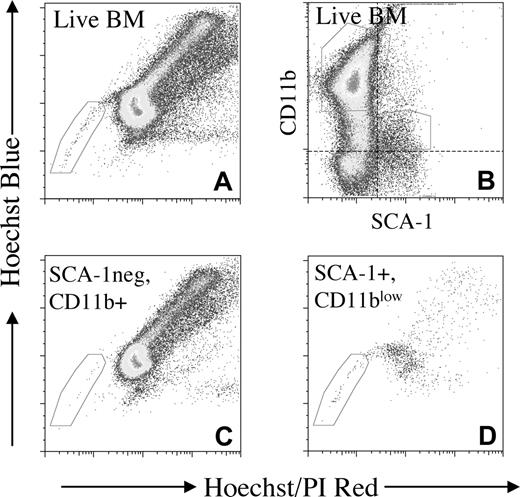
Backgating of committed cell populations onto Hoechst red/blue fluorescence-activated cell sorting (FACS) plots revealed contrasting SP profiles. Typically, a proportion of NK1.1- (Figure 2B), CD2-, CD4-, and CD8- (data not shown) positive cells appeared in the rare, intermediate SP region (I-SP in Figure 2A). A similar but less pronounced pattern was often observed with the B-cell markers CD5 (Figure 2C) and B220 (data not shown). These lymphoid cell patterns were in contrast to the relative infrequency of intermediate SP cells present in Gr-1+ myeloid cell populations (Figure 2D). It was observed during analysis that most CD11blow cells within the SP coexpressed SCA-1. To investigate CD11b/SCA-1 coexpression further, various subpopulations of whole BM were displayed onto Hoechst red/blue fluorescence plots (Figure 3B). The CD11bhigh /SCA-1neg subset of cells gave a very similar profile to GR-1+ cells; mostly non-SP cells with very few intermediate or SP cells (Figure 3C). In contrast, the SCA-1+ subset of CD11b+ cells (which expressed CD11b at a lower level) was enriched for SP cells (3.25% of subset, 46.7% of total SP; Figure 3D) when compared with the whole bone marrow (0.1%; Figure 3A). Thus, all subsequent lineage-negative gating in this study included these SCA-1+/CD11blow events.
SP analysis of subsets defined by SCA-1 and CD11b expression. Analysis of a representative murine marrow that was Hoechst stained and labeled for SCA-1 and CD11b. Subsets defined by SCA-1 and CD11b expression (B) were displayed on Hoechst red/blue dotplots (dotted line indicates level of matched isotype control). SCA-1+/CD11blowcells were more enriched for SP (D; 3.25%), than SCA-1neg/CD11b+ (C; 0.017%) or whole BM (A; 0.1%).
SP analysis of subsets defined by SCA-1 and CD11b expression. Analysis of a representative murine marrow that was Hoechst stained and labeled for SCA-1 and CD11b. Subsets defined by SCA-1 and CD11b expression (B) were displayed on Hoechst red/blue dotplots (dotted line indicates level of matched isotype control). SCA-1+/CD11blowcells were more enriched for SP (D; 3.25%), than SCA-1neg/CD11b+ (C; 0.017%) or whole BM (A; 0.1%).
Comparison between SP and stem cell immunophenotypes
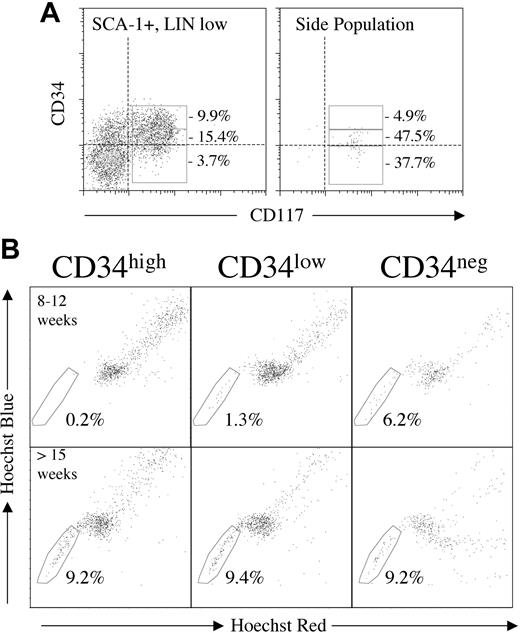
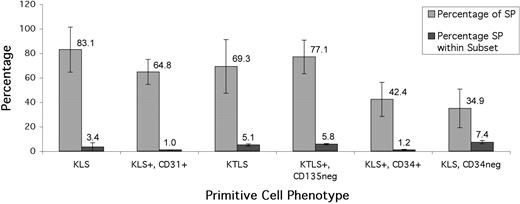
To investigate the presence of noncommitted stem/progenitor cells, Hoechst-stained murine BM (n = 5) was labeled with antibodies to various primitive cell markers. CD117, SCA-1, Thy-1, and CD31 were each expressed on a high proportion of SPs (84.1% ± 18.0%, 79.9% ± 19.7%, 78.8% ± 8.8%, and 72.7% ± 16.9%, respectively). The number of CD34+ cells (including low level; see Figure 5A for an example plot) was approximately equal to the number of CD34neg (56.0% ± 17.1% CD34+). Combining these markers with an antibody cocktail directed against committed cell markers (Lin = CD5, B220, CD11bhigh, GR-1, and Ter-119) allowed the comparison of the SP with established “stem cell” phenotypes (Figure 4). Gating on the CD117+ fraction of SCA-1+/Linlow cells highlighted a primitive cell subset referred to as KLS.3 This combination of markers was expressed on 83.1% ± 18.4% (n = 20) of our SP cells. Most of these cells coexpressed CD31 as KLS+/CD31+ accounted for 64.8% ± 10.2% of SP cells in murine BM. Combining KLS with Thy-1 formed the stem cell phenotype KTLS,5 which comprised most of our SP cells (69.3% ± 22.0%). A similar figure was obtained if only the CD135- fraction (CD135 was added to lineage cocktail) of KTLS was analyzed (77.1% ± 13.8%), indicating that SP+/KTLS+ cells are also the long-term repopulating CD135- subset previously described.6 Cells were classified as KTLSneg when one or more parameters were negative; cells could be positive or negative for the other 3 antigens. Of these SP+/KTLSneg cells, a minority (11.5% ± 10.6%) were Lin+, but the majority of the cells were KLS (48.9% ± 30.7% of SP+/KTLSneg; 11.6% ± 8.9% of total SP, n = 5). Approximately half (42.4% ± 14.0%) of the SP cells were KLS+/CD34+, and most interestingly, 34.9% ± 15.8% of SP cells fell within the primitive KLS+/CD34neg subset.
SP analysis of subsets defined by CD34 expression intensity. Analysis of a representative (8-12 weeks) murine marrow Hoechst stained and labeled for Lin, SCA-1, CD117, and CD34. Linneg/SCA-1+/CD117+ cells (KLS) were divided into subsets of CD34 high, low, and negative intensity (A). Provided for comparison is a CD117/CD34 plot of the SP fraction from the same experiment with the same CD34 high, low, and negative gates. Dotted lines on both plots indicate the level of matched-isotype control. Most SP cells were CD34low or CD34neg in mice 8 to 12 weeks of age (B, upper row). In contrast, significant numbers of KLS+/CD34high were only seen in the older (> 15 weeks) mice. Most interestingly, in all 3 older mice analyzed, the KLS+/CD34neg subset was associated with the cells which most efficiently effluxed the Hoechst dye (B, lower panel).
SP analysis of subsets defined by CD34 expression intensity. Analysis of a representative (8-12 weeks) murine marrow Hoechst stained and labeled for Lin, SCA-1, CD117, and CD34. Linneg/SCA-1+/CD117+ cells (KLS) were divided into subsets of CD34 high, low, and negative intensity (A). Provided for comparison is a CD117/CD34 plot of the SP fraction from the same experiment with the same CD34 high, low, and negative gates. Dotted lines on both plots indicate the level of matched-isotype control. Most SP cells were CD34low or CD34neg in mice 8 to 12 weeks of age (B, upper row). In contrast, significant numbers of KLS+/CD34high were only seen in the older (> 15 weeks) mice. Most interestingly, in all 3 older mice analyzed, the KLS+/CD34neg subset was associated with the cells which most efficiently effluxed the Hoechst dye (B, lower panel).
Reserpine-sensitive SP cells represent a subset of primitive cell phenotypes. All values are expressed as a mean of 5 separate experiments. Expression of cell markers is displayed as percentage of SP. The SP was enriched for the primitive cell phenotypes KLS, KLS/CD31+, KTLS, and KTLS/CD135neg Approximately half of SPs were KLS/CD34+. Displayed for comparison are the small amounts of each antigen-defined subset that fell within the SP gate.
Reserpine-sensitive SP cells represent a subset of primitive cell phenotypes. All values are expressed as a mean of 5 separate experiments. Expression of cell markers is displayed as percentage of SP. The SP was enriched for the primitive cell phenotypes KLS, KLS/CD31+, KTLS, and KTLS/CD135neg Approximately half of SPs were KLS/CD34+. Displayed for comparison are the small amounts of each antigen-defined subset that fell within the SP gate.
Backgating of KLS, KLS+/CD31+, KTLS+, and KTLS+/CD135neg cells onto red/blue Hoechst plots revealed that small proportions of these subsets were in the SP region (Figure 4). Surprisingly, there was no association between these primitive cell phenotypes and their distribution within the range of Hoechst efflux of SP (data not shown).
CD34 expression on SP cells during postnatal development
To investigate a possible relationship between CD34 antigen density and the SP profile, KLS+ events were separated into CD34neg, CD34low, and CD34high (explanation in “Materials and methods” and example in Figure 5A). In 90% of experiments performed (3 at 3 weeks of age; 4 at 8-12 weeks; 3 at > 15 weeks) most CD34 expression within our SP gate was at a low or negative level. KLS+/CD34high SP+ cells were only found in significant numbers in the older mice. Additionally, the absence of KLS+/CD34high SP+ cells was particularly pronounced in the youngest age group. Backgating of KLS+/CD34neg cells from all mice onto Hoechst red/blue plots revealed a possible association with the SP cells with the lowest Hoechst staining in our more-than-15-week age group (Figure 5B).
Competitive repopulation potential of SP-defined subsets
Three phenotypes were chosen for analysis of their repopulation potential: SP+/KTLS+, SP+/KTLSneg, and SPneg/KTLS+. First, SP and non-SP gates were established to avoid any overlap or confusion with the intermediate SP (I-SP on Figure 2A). Cells within these populations were then defined as KTLS-positive or -negative as before.
To investigate 6-week repopulation ability, 10 (SP-defined subsets), 200, or 1000 cells were sorted from C57Bl6 ly5.2 and intravenously injected into sublethally irradiated ly5.1 NOD/SCID mice along with 2 × 105 recipient-type competitor/support cells. Animals were killed 6 weeks after transplantation and bone marrow cells were analyzed via flow cytometry for donor cell content. One third (2/6) of mice injected with 10 SP+/KTLS+ cells engrafted, as did 4 of 9 mice injected with SP+/KTLSneg cells. In contrast, none of the mice (0/6) injected with 10 SPneg/KTLS+ demonstrated any detectable engraftment when analyzed. Engraftment was achieved however, with both the 200 (4/4) and 1000 (5/5) cell dose of SPneg/KTLS+ cells.
In a second set of experiments, we investigated long-term repopulation ability; lethally irradiated ly5.1 C57Bl6 mice were injected with 10 sorted cells and 2 × 105 competitor cells. Peripheral blood was analyzed for donor cell content 4 months after transplantation. All mice (5/5) injected with 10 SP+/KTLS+ cells demonstrated detectable engraftment (up to 55% in one instance), as did half of mice injected with 10 SP+/KTLSneg (1/2; and all mice [3/3] injected with 90 SP+/KTLSneg). No engraftment was detected in the mice injected with 10 SPneg/KTLS+ (0/2), but injection of 1000 cells from this fraction did engraft in all mice (3/3). Calculations derived from the 10 SP+/KTLS+, 90 SP+/KTLSneg, and 1000 SPneg/KTLS+ cohorts revealed a hierarchy of long-term CRU enrichment from 2800-fold for SP+/KTLS+ cells, to 2000-fold for SP+/KTLSneg cells and 250-fold for SPneg/KTLS+.
In summary, the results of both the 6-week and long-term repopulation assays indicate that SP-defined subsets contain a higher proportion of CRUs than SPneg/KTLS+ cells. Although at 6 weeks no difference in CRU content could be detected between the KTLS-positive and -negative fractions of SP, when assessed at 4 months, a slightly higher proportion of CRUs was detected in the SP+/KTLS+ subset than in SP+/KTLSneg cells.
Discussion
This work describes the characterization of murine SP cells in terms of their phenotype and repopulation potential. Before we could compare the SP profile from different experiments we needed to establish our definition of the SP. To this end we compared the effect of inhibitors of SP formation, finally choosing reserpine sensitivity. The SP gate we describe comprises 0.09% murine bone marrow, a fraction similar to that previously reported.12,18 As previously published, the technique is significantly toxic; viability after Hoechst staining and antibody labeling was 55% and even if analyzed immediately after Hoechst staining, viability was only 70% to 80%. Every effort was made to keep this value relatively constant to avoid any effects of variable cell viability.19
Previous reports have stated that the SP gate does not contain a high proportion of lineage-committed cells, although the exact proportion was not reported.12 We can now confirm that most SP cells are indeed negative for the lineage markers CD2, CD4, CD5, CD8, NK1.1, B220, CD11bhigh, Gr-1, and Ter119. When these lineage-committed cell populations were displayed on Hoechst plots, the lymphoid subsets (especially NK1.1+ cells) possessed an intermediate SP population, whereas myeloid subsets did not. Murine NK cells have been reported to contain ABC/G2 mRNA and their intermediate position on the SP plot may be due to this pump's efflux of the Hoechst dye.14
The vast majority of the remaining lineage-negative cells were positive for SCA-1/c-kit and hence were a subset of the KLS fraction. The LTRC fraction of KLS has been reported to be Thy-1+ and CD135-.6 We found that a large proportion of the KLS within SP also coexpressed Thy-1 and furthermore that this proportion was unchanged if only the CD135neg subset of KTLS was analyzed. Although the majority of SP cells did possess the primitive KTLS+/CD135neg phenotype, only a minority of this subset effluxed the Hoechst dye effectively. These data confirm the primitive nature of SP cells and demonstrate that even though SP cells are identified independently of antigenic markers, they are not distinct from established stem cell phenotypes, but are a small subset of them.
Since most SP cells are a subset of KTLS, we set out to determine whether they represented a further enrichment in repopulation cell content within this highly purified stem cell fraction. Even though the technique is inherently toxic, the competitive repopulation potential of the relatively unstained SP seemed unaffected; engraftment could be achieved in recipients with only 10 of the SP+ cells. The SP+/KTLS+ subset was more enriched for CRUs than the SP+/KTLSneg fraction. CRUs were also detected in the SP-negative fraction of KTLS+ cells (mostly KLS) but at a lower frequency. Hence, the SP highlights a subset of the long-term repopulating cells found within the already highly purified KTLS fraction. These results confirm, that as previously reported, not all LTRCs are found in the SP fraction and not all of KTLS are LTRCs.12,20
The subset we classified as SP+ KTLSneg during sorting contained some lineage-positive cells, but the major group was Thy-1–negative KLS cells. Although it has previously been reported that Thy-1+ cells contain all of the repopulating potential in BM,21 we describe a high level of CRU enrichment in our SP+/KTLSneg cells. This SP-positive but Thy-1–negative fraction of the KLS population may represent a previously undescribed, extremely rare, Thy-1–negative stem cell population.
A large proportion of SPs coexpressed CD11b at a low level; always coexpressed with SCA-1. Although it has been previously reported that the vast majority of KTLS+ CD11b+ cells are STRCs, occasional long-term repopulation has been observed in mice injected with both KTLS+/CD11b+ cells and CD34+/CD11b+ cells.5,22 It is possible that the subset of CD11b+ cells that efflux the Hoechst dye is the rare subset responsible for this occasional long-term repopulation. Commonly used depletion cocktails efficiently remove this CD11b low population and may therefore inadvertently deplete important repopulating cells.
Previous reports have described that murine SP cells express low or undetectable amounts of CD34 on their cell surface.18 These data combined with the impressive LTRC enrichment in the SP is important evidence for the existence of CD34- hematopoietic stem cells. We can now confirm that a significant proportion of SP cells are indeed a subset of the KLS+CD34neg LTRCs of Osawa et al,3 but in contrast to previous reports, a large proportion possessed the CD34 antigen at readily detectable levels.18 This difference between our CD34 data and those previously described cannot be explained by our detection method as we used the same directly conjugated RAM-34 clone and similar flow cytometric analysis.18 Furthermore, as can be seen in Figure 5A, our level of CD34 negativity was easily defined due to the presence of unstained c-kit–negative cells within the same Linlow/SCA-1+ population. Although a large proportion of SPs were definitely CD34+, the intensity of expression did indeed seem to be at a lower level than the rest of the bone marrow, as previously reported for primitive cell subsets (TLS).23
Previous research has defined the CD34 expression status of bone marrow LTRCs at different ages. The vast majority of LTRCs are reportedly CD34+ early in murine life (neonate to 5 weeks), both positive and negative during adulthood (7-10 weeks), and become increasingly CD34- as life progresses (> 16 weeks).24,25 In our young mice (3 weeks), primitive KLS+ CD34high cells did not efflux Hoechst dye effectively. In contrast, in the older mouse group (> 15 weeks) primitive SP+KLS+/CD34neg cells were easily detected and were often associated with the lowest Hoechst staining. Combining these age-related data with the changes in CD34 expression reported independently by Ogawa et al24 and Matasuoka et al25 suggests that there are age-related changes in the ability of hematopoietic stem cells to efflux the dye. Specifically, the SP gate in younger mice may not reflect the hematopoietic stem cell as effectively as in older mice. Accordingly, we have also observed a higher proportion of SP cells in mature mice (> 15 weeks) when compared with young mice (3 weeks), although this did not reach significance (P = .055, n = 3 for each). This inverse relationship between age and SP frequency was unexpected considering the very large percentage of SP cells in embryonic tissues.26
One of the most interesting aspects of SP formation is the hierarchy of LTRC content described by Goodell et al in 1997.18 The cell population that most effectively effluxes the dye is the most enriched for LTRCs. We were therefore surprised that the majority of primitive subsets we analyzed (KLS, KTLS, and KTLS+/CD135neg) did not show any preferential localization for the cells with the lowest Hoechst staining. The only subset we analyzed that did seem to be associated with the dimmest Hoechst-stained cells was the KLS+ CD34neg subset (especially in older mice). This association is similar to a previous characterization of SCA-1+ CD34neg SP cells, but we can now confirm that these cells coexpress c-kit, are negative for lineage markers, and hence are a subset of primitive KLS+, CD34neg cells.8,27
Although we have not focused on CD45 expression, we have performed occasional tests and have never detected a significant number of CD45- SP cells. As multipotent mesenchymal stem cells (MSCs) are thought to be CD45-, this suggests that murine MSCs may not be isolated via the SP phenotype.28,29
The SP profile is conserved in multiple species and in various tissues, suggesting that this is a widespread stem cell ability.18,30,31 As a subset of KLS cells have the ability to produce both endothelial and hematopoietic cells, it is possible, considering the CD31 expression of our SP cells, that the SP fraction of KLS offers an enrichment of these multipotent cells.32
The Hoechst profile provides an opportunity to isolate hematopoietic stem cells via a functional ability, independently of antigenic markers. Nevertheless, the SP identifies a subset of the antigen-defined KLS population that is highly enriched for hematopoietic stem cells. This long-term repopulating SP subset contains cells that are positive for CD34, CD11b, Thy-1, and CD31, and cells that are negative for CD34, Thy-1, and CD135. The KLS/CD34neg fraction contained the highest proportion of SPs of any of the subsets we analyzed. If we assume that murine SP cells do indeed represent hematopoietic stem cells arranged in a hierarchy, the KLS+CD34neg subset was the purest, most primitive subset we analyzed directly. Future experiments will investigate the long-term repopulation potential of these CD34-positive and -negative SP cells in murine and human tissues.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-09-3281.
Supported by Cancer Research UK and an Association for International Cancer Research grant no. GA3160 to D.B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Derek Davies, Gary Warnes, Ayad Eddaoudi, and Kirsty Allen of the FACS Lab at Cancer Research UK for their invaluable technical expertise. Furthermore, this study would not have been possible without Julie Bee, Clare Millum, and Ella Smallcombe of our Biological Research Unit.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal