Abstract
The virulence of the malaria parasite, Plasmodium falciparum, is due in large part to the way in which it modifies the membrane of its erythrocyte host. In this work we have used confocal microscopy and fluorescence recovery after photo-bleaching to examine the lateral mobility of host membrane proteins in erythrocytes infected with P falciparum at different stages of parasite growth. The erythrocyte membrane proteins band 3 and glycophorin show a marked decrease in mobility during the trophozoite stage of growth. Erythrocytes infected with a parasite strain that does not express the knob-associated histidine-rich protein show similar effects, indicating that this parasite protein does not contribute to the immobilization of the host proteins. Erythrocytes infected with ring-stage parasites exhibit intermediate mobility indicating that the parasite is able to modify its host prior to its active feeding stage.
Introduction
The malaria parasite, Plasmodium falciparum, invades human erythrocytes and induces dramatic changes in membrane fluidity, permeability, deformability, and adhesiveness of the host cell.1 Knobby protrusions are formed at the erythrocyte membrane by the association of the knob-associated histidine-rich protein (KAHRP) with the erythrocyte cytoskeleton.2,3 These knobs represent the points of adhesion to other host cell types1 and are responsible for much of the pathology of malaria. KAHRP knock-out parasites do not cytoadhere effectively under conditions of flow and produce less dramatic changes in erythrocyte membrane deformability.2,4
The technique of fluorescence recovery after photobleaching (FRAP) has been widely used to study protein dynamics in cells.5 Studies of the diffusional mobility of integral proteins within human erythrocyte membranes have provided important insights into the molecular organization of eukaryotic membranes.5-7 Measurements of lateral mobility can report on interactions both within the plane of the bilayer and with underlying cytoskeletal proteins. In this work, we have used the FRAP technique with a confocal microscope to study the mobility of fluorescently labeled host membrane proteins in infected erythrocytes at different stages of parasite growth and to assess the specific role of KAHRP in these mobility changes.
Study design
P falciparum (3D7 and a KAHRP knockout line) were cultured as previously described.2,8 Band 3 and glycophorin in washed control or parasitized erythrocytes were labeled with eosin-5-maleimide or fluorescein-5-thiosemicarbazide (Molecular Probes, Eugene, OR),8,9 and the specificity of labeling confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Intact erythrocytes exhibited a tendency to lyse during imaging. The labeled cells (3 × 108 cells in 300 μL phosphate-buffered saline [PBS]) were therefore lysed prior to imaging by incubating with 12 hemolytic units of activated streptolysin O10 (SLO; Sigma, St Louis, MO) and by pelleting and resuspending in PBS to maintain isotonic conditions. This procedure does not completely remove the hemoglobin, thus minimizing the adherence of the cells to the glass slide and coverslip used for imaging.
The lysed cells (2 μL, 10% hematocrit) were sandwiched between the slide and coverslip, sealed with paraffin wax, and imaged using an inverted Leica SP2 laser scanning confocal microscope as described previously (Leica Microsystems, Heidelberg, Germany).5 Under these conditions, the membrane surface closest to the coverslip appears as a smooth disk and was used for photobleaching measurements. This surface could be clearly distinguished from the surface adjacent to the glass slide.
The general considerations for acquiring and analyzing images following point bleaching on the confocal microscope have been previously discussed.5 For each image obtained after bleaching, the average fluorescence intensity within the bleached region was background corrected using a region outside the cell and normalized by dividing by the average fluorescence intensity of the whole cell (Fcorr). The kinetics of the recovery were analyzed by fitting equation 10 in Klonis et al5 to the data to provide the diffusion coefficient (DL) and the mobile fraction (fmob) of the labeled species. For each parameter cited, we report the average mean value from n series of experiments, the total number (t) of individual determinations, and the SE of the average mean.
Results and discussion
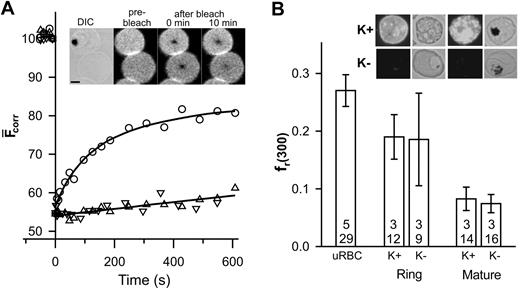
Band 3 and glycophorin are the major integral proteins of the erythrocyte membrane and can be specifically labeled with fluorescent probes.7 In this work, we have used SLO lysis to selectively lyse infected erythrocytes without affecting the parasite.10 Differential interference contrast and phase contrast images were used to distinguish parasitized erythrocytes from uninfected erythrocytes. The inset in Figure 1A shows that the fluorescence recovery into the bleached region of a band 3–labeled erythrocyte infected with a mature parasite (trophozoite) is reduced compared to that in an adjacent uninfected erythrocyte and exhibits different kinetics (Figure 1A). The fluorescence within the bleached region of the uninfected erythrocyte plateaus after 10 minutes, permitting reliable determination of DL and fmob (0.0028 ± 0.0005 μm2 /s and 0.49 ± 0.05, respectively; n = 5, t = 26), which is consistent with literature reports.6,7,11 This value of DL is 2 orders of magnitude slower than that predicted theoretically for a freely diffusing membrane-embedded protein5 and reflects the presence of higher order oligomers, linkage via ankyrin to spectrin,7,12 and steric impediments imposed by the underlying cytoskeletal meshwork.13
FRAP analysis of eosin-labeled band 3 in control and parasitized erythrocytes. Aliquots of cultures of wild-type (K+) and KAHRP knock-out (K-) 3D7 strain malaria parasites in erythrocytes (∼5% parasitemia) were treated with eosin-maleimide to label band 3, then permeabilized with SLO. (A) The inset shows a differential interference contrast (DIC) image of an erythrocyte infected with a mature-stage parasite containing the dark hemozoin pigment with an uninfected erythrocyte below it. The fluorescence images of the same cells are shown before, immediately after, and 10 minutes after application of the bleach pulse at the center of each cell. Scale bar is 2 μm. The graph shows the typical kinetics of fluorescence recovery into the bleached region for an uninfected erythrocyte (○) and for erythrocytes infected with mature stage K+ (▵) or K- (▿) parasites. Data points shown at negative times represent fluorescence intensities prior to the bleach event. (B) The fr(300) values for uninfected erythrocytes, K+ ring stage- and mature stage-infected erythrocytes and K- ring stage- and mature stage-infected erythrocytes. The top number reflects the number of separate experiments and the bottom number the total number of individual measurements. Inset shows immunofluorescence analysis of KAHRP in K+ and K- strains carried out using the B89 monoclonal anti-KAHRP antibody. Shown are bright field and corresponding fluorescence images of erythrocytes infected with ring (non–hemozoin-containing) and mature (hemozoin-containing) parasites. Scale as in panel A. Error bars indicate standard error.
FRAP analysis of eosin-labeled band 3 in control and parasitized erythrocytes. Aliquots of cultures of wild-type (K+) and KAHRP knock-out (K-) 3D7 strain malaria parasites in erythrocytes (∼5% parasitemia) were treated with eosin-maleimide to label band 3, then permeabilized with SLO. (A) The inset shows a differential interference contrast (DIC) image of an erythrocyte infected with a mature-stage parasite containing the dark hemozoin pigment with an uninfected erythrocyte below it. The fluorescence images of the same cells are shown before, immediately after, and 10 minutes after application of the bleach pulse at the center of each cell. Scale bar is 2 μm. The graph shows the typical kinetics of fluorescence recovery into the bleached region for an uninfected erythrocyte (○) and for erythrocytes infected with mature stage K+ (▵) or K- (▿) parasites. Data points shown at negative times represent fluorescence intensities prior to the bleach event. (B) The fr(300) values for uninfected erythrocytes, K+ ring stage- and mature stage-infected erythrocytes and K- ring stage- and mature stage-infected erythrocytes. The top number reflects the number of separate experiments and the bottom number the total number of individual measurements. Inset shows immunofluorescence analysis of KAHRP in K+ and K- strains carried out using the B89 monoclonal anti-KAHRP antibody. Shown are bright field and corresponding fluorescence images of erythrocytes infected with ring (non–hemozoin-containing) and mature (hemozoin-containing) parasites. Scale as in panel A. Error bars indicate standard error.
The small degree of recovery and the approximate linear kinetics of recovery in the trophozoite-infected erythrocyte (Figure 1A) do not permit reliable determination of DL and fmob. However, the data indicate restricted mobility of band 3 and are consistent with previous reports of decreased rotational mobility8 and increased aggregation14 of band 3 in parasitized erythrocytes. To compare band 3 mobility in different cells, we compared the fractional recovery of fluorescence 300 seconds after the bleach event (fr(300)). A reduction of fr(300) is evident in trophozoite-infected erythrocytes compared to uninfected erythrocytes (Figure 1B). In an effort to determine the molecular basis for this restriction of band 3 mobility, we examined a parasite strain that does not express KAHRP2 (Figure 1B inset). Erythrocytes infected with a KAHRP- strain show a similar fluorescence recovery profile (Figure 1A) indicating that KAHRP is not a major determinant of the restriction of band 3 mobility. The fr(300) values of erythrocytes infected with young parasites (rings) were intermediate between those of uninfected and trophozoite-infected erythrocytes and were similar in both KAHRP- and KAHRP+ strains (Figure 1B). This suggests that the changes in band 3 mobility begin during the ring stage of infection.
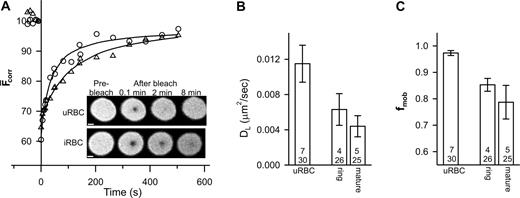
We also examined the mobility of glycophorin. Analysis of the recovery kinetics revealed some variation in the value of DL for glycophorin (average values per experiment ranged from 0.005 to 0.025 μm2/s). The mean values of DL and fmob (n = 12, t = 52) were 0.014 ± 0.002 μm2/s and 0.96 ± 0.02, respectively. The fmob value is consistent with previous reports6,7,11 and its greater magnitude compared to band 3 is consistent with the absence of specific interactions of glycophorin with the underlying cytoskeleton. In contrast, the DL value of glycophorin is higher than previous reports (0.003 μm2/s7,11 ), although 50-fold increases in glycophorin mobility have been noted in ghosts incubated at 37°C for 24 hours.15 The rate of fluorescence recovery appeared to decrease in trophozoite-infected erythrocytes compared to uninfected erythrocytes (Figure 2A) and reflects a reduction in both DL and fmob (Figure 2B-C).
FRAP analysis of fluorescein-labeled glycophorin in control and parasitized erythrocytes. Aliquots of cultures of parasitized erythrocytes (approximately 5% parasitemia) were treated with fluorescein thiosemicarbazide to label glycophorin and then permeabilized with SLO. (A) Inset shows fluorescence images of an uninfected red blood cell (uRBC) and of an erythrocyte with a mature-stage parasite (iRBC) during a FRAP measurement. Scale bar is 2 μm. The graph shows the typical kinetics of fluorescence recovery into the bleached region for an uninfected erythrocyte (○) and for erythrocytes infected with mature-stage parasites (▵). Data points shown at negative times represent fluorescence intensities prior to the bleach event. Average mean DL and fmob values for uninfected erythrocytes and ring stage- and mature stage-infected erythrocytes obtained from a number of measurements are shown in panels B and C, respectively. Numbers in bars as defined in Figure 1B. Error bars represent standard error.
FRAP analysis of fluorescein-labeled glycophorin in control and parasitized erythrocytes. Aliquots of cultures of parasitized erythrocytes (approximately 5% parasitemia) were treated with fluorescein thiosemicarbazide to label glycophorin and then permeabilized with SLO. (A) Inset shows fluorescence images of an uninfected red blood cell (uRBC) and of an erythrocyte with a mature-stage parasite (iRBC) during a FRAP measurement. Scale bar is 2 μm. The graph shows the typical kinetics of fluorescence recovery into the bleached region for an uninfected erythrocyte (○) and for erythrocytes infected with mature-stage parasites (▵). Data points shown at negative times represent fluorescence intensities prior to the bleach event. Average mean DL and fmob values for uninfected erythrocytes and ring stage- and mature stage-infected erythrocytes obtained from a number of measurements are shown in panels B and C, respectively. Numbers in bars as defined in Figure 1B. Error bars represent standard error.
As with band 3, the effects on glycophorin mobility are already obvious in ring-infected erythrocytes (Figure 2B-C). It has been suggested that the parasite makes relatively few alterations to the gross characteristics of the erythrocyte membrane during the first 24 hours of intraerythrocytic growth, although a small decrease in membrane deformability has been observed.16,17 Our data suggest that the effects on the organization of the erythrocyte membrane proteins begin in the ring stage but become more pronounced at later stages. Although KAHRP does not appear to be a major determinant of these changes, other proteins exported by the parasite may affect host-membrane protein organization. Another contribution may be from oxidative stress induced by parasite growth.14,18 The consequences of oxidative stress include the deposition of oxidized hemoglobin (hemichromes) at the cytoplasmic surface of the erythrocyte membrane, which induces band 3 aggregation18,19 and binding of autoantibodies to the external surface.19 Both band 3 and glycophorin exhibit a reduced mobility in density-fractionated sickle cells20 that is attributed to an accumulation of oxidative damage including the deposition of hemichromes. The relative roles of both oxidative stress and parasite-exported proteins in modulating the mobilities of host membrane proteins need to be delineated.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-08-2692.
Supported by the National Health and Medical Research Council, Australia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Expert technical assistance was provided by Ms Emma Fox. We thank Prof Alan Cowman and Dr Brendan Crabb for supplying KAHRP knockout parasites and Dr Diane Taylor, Georgetown University, Washington, DC for donating the B89 monoclonal anti-KAHRP antibody.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal