Abstract
Macrophage inflammatory protein–1α (MIP-1α) gene expression is abnormally regulated in multiple myeloma (MM) owing to imbalanced expression of the acute myeloid leukemia–1A (AML-1A) and AML-1B transcription factors. We hypothesized that the increased expression ratios of AML-1A to AML-1B also induced abnormal expression of other hematopoietic and bone-specific genes that contribute to the poor prognosis of MM patients with high levels of MIP-1α. We found that interleukin-3 (IL-3) was also induced by the imbalanced AML-1A and AML-1B expression in myeloma. IL-3 mRNA levels were increased in CD138+ purified myeloma cells with increased AML-1A–to–AML-1B expression from MM patients, and IL-3 protein levels were significantly increased in freshly isolated bone marrow plasma from MM patients (66.4 ± 12 versus 22.1 ± 8.2 pg/mL; P = .038). IL-3 in combination with MIP-1α or receptor activator of nuclear factor–kappa B ligand (RANKL) significantly enhanced human osteoclast (OCL) formation and bone resorption compared with MIP-1α or RANKL alone. IL-3 stimulated the growth of interleukin-6 (IL-6)–dependent and IL-6–independent myeloma cells in the absence of IL-6, even though IL-3 did not induce IL-6 expression by myeloma cells. These data suggest that increased IL-3 levels in the bone marrow microenvironment of MM patients with imbalanced AML-1A and AML-1B expression can increase bone destruction and tumor cell growth.
Introduction
Multiple myeloma (MM) is an incurable hematologic malignancy, in which bone destruction is the major source of morbidity.1 Recently, we identified macrophage inflammatory protein–1α (MIP-1α) as an osteoclast (OCL)–activating factor, which is produced by freshly isolated myeloma cells from patients who have extensive bone disease and that induces human OCL formation independently of receptor activator of nuclear factor–kappa B ligand (RANKL).2 MIP-1α also enhances the OCL-inducing activity of RANKL and interleukin-6 (IL-6).3 We demonstrated that blocking MIP-1α expression in an in vivo model of human MM profoundly decreases disease progression and bone destruction4 and that patients with elevated levels of MIP-1α have an extremely poor prognosis. Blocking MIP-1α activity inhibited both MM cells homing to the marrow as well as tumor growth. Consistent with our observations is the recent report by Abe et al,5 who showed that elevated levels of MIP-1α are present in 15 of 20 MM patients, and that MIP-1α induced rabbit OCL formation. Uneda et al6 also reported that the MIP-1α levels produced by myeloma cells correlated with the number of bone lesions in 16 of 18 MM patients expressing elevated MIP-1α levels. Recently, Oyajobi et al7 reported that MIP-1α increased osteolysis and tumor burden in the murine 5TGM1 model of myeloma bone disease. In addition, Magrangeas et al8 have reported that MIP-1α is one of the key factors that is overexpressed in MM and that has a strong association (P = .0001) with active myeloma bone disease. Taken together, these data demonstrated that MIP-1α is an important mediator of myeloma bone disease and that blocking MIP-1α activity has profound effects on both tumor burden and bone destruction in in vivo models of MM.
Recently, we reported that abnormal transcriptional regulation of MIP-1α gene expression occurs in MM owing to imbalanced expression of the acute myeloid leukemia–1A (AML-1A) and AML-1B transcription factors (TFs), and this imbalance results in enhanced MIP-1α expression.9 Therefore, we determined whether imbalanced AML-1A and AML-1B expression induces expression of other genes that may contribute to the poor prognosis of MM by enhancing the adhesion of tumor cells to stromal cells, the growth of myeloma cells, or the stimulation of osteoclastic bone resorption. Candidate genes that may be regulated by the AML-1 class of TFs in myeloma cells are c-fms, IL-3, IL-7, GM-CSF, and the MDR1 gene, since the AML-1 class of TFs is known to regulate the expression of these genes.10-19 We found that IL-3 mRNA is increased by the abnormal expression ratios of AML-1A to AML-1B in myeloma cell lines and is overexpressed in purified myeloma cells from patients with MM at both the mRNA and the protein levels, and that IL-3 induces OCL formation and myeloma cell growth.
Patients, materials, and methods
Materials and myeloma cell lines
Lipofectamine Plus transfection kits, restriction enzymes, and Taq polymerase were purchased from Invitrogen (Carlsbad, CA). MIP-1α, IL-3, and IL-3 enzyme-linked immunosorbent assay (ELISA) kits were obtained form R&D Systems (Minneapolis, MN). RANKL was kindly provided by Immunex (Seattle, WA), and chemicals were purchased from Sigma (St Louis, MO). Methyl thiazolyl tetrazolium (MTT) assay kits were purchased from ATCC (Manassas, VA). Myeloma-derived ARH-77, MCQ/2, IM9, and MM.1S cells were grown in RPMI 1640 media containing 10% fetal calf serum (FCS) (Invitrogen). The IL-6–dependent myeloma cell lines KAS6/1 and ANBL6 were cultured in RPMI 1640 in the presence of 1 to 5 ng/mL recombinant human IL-6 (R&D Systems). The ARH-77 cell line contains Epstein-Barr virus (EBV) while the other cell lines are EBV free.
Generation of ARH-77 and MM.IS cell lines stably transfected with AML-1A (ARH-A, MM.IS-A), AML-1B (ARH-B, MM.IS-B), or empty vector (ARH-EV, MM.IS-EV)
To identify a gene whose expression was increased by imbalanced AML-1A and AML-1B expression in myeloma cells and that could contribute to the poor prognosis of myeloma, AML-1A, AML-1B, or empty vector (pcDNA3) was transfected into ARH-77 or MM.IS cells by means of a Lipofectamine Plus kit (Invitrogen). Stable clones were isolated as described previously,9 with minor modifications. Briefly, AML-1A, AML-1B, or empty vector cDNAs (2 μg) were diluted with 100 μL fetal calf serum and antibiotic-free Dulbecco minimum essential media (DMEM) and then 6 μL Plus reagent was added. After 15-minute incubation, Lipofectamine diluted with 100 μL serum and antibiotic-free DMEM was added, and the mixture was incubated for 15 minutes. Transfection reagents were added to 106 ARH-77 and MM.IS cells per well in 6-well plates with serum and antibiotic-free RPMI 1640 medium. After 3-hour incubation, transfection media were removed, and complete media containing serum and antibiotics were added. ARH-77 and MM.IS cells transfected with AML-1A, AML-1B, or empty vector were then treated with 500 μg/mL G418 every 4 days and cultured for 3 weeks. Single-cell clones were isolated by serial dilution, and each clone was tested for its capacity to express AML-1A or AML-1B by reverse-transcription polymerase chain reaction (RT-PCR) with the use of specific primer sets described below.
Human bone marrow samples from patients with multiple myeloma and healthy donors
Bone marrow aspirates were collected into heparinized syringes from patients with MM and healthy donors. All patients gave informed consent, and these studies were approved by the Institutional Review Board of the University of Pittsburgh Medical Center and General Clinical Research Center (GCRC) at the University of Pittsburgh (Pittsburgh, PA), and at the University of Texas at San Antonio (San Antonio, TX). Bone marrow (2 mL per aspirate) was collected from each subject, and an aliquot of the bone marrow sample was stained with Wright-Giemsa for histologic examination. The percentage of plasma cells present in the sample was determined by morphologic criteria. The bone marrow was pelleted by centrifugation at 1000g at 4°C immediately after collection, and the bone marrow plasma was collected and stored at -80°C for subsequent studies.
Purification of CD138+ myeloma cells
CD138+ plasma cells from patients with MM and healthy donors were purified by CD138 (syndecan-1) microbeads by means of a Miltenyi magnetic cell sorting system (Miltenyi Biotec, Auburn, CA) as described by Draube et al.20 Bone marrow mononuclear cells (108) were incubated with CD138 microbeads for 15 minutes and loaded onto a positive selection column placed in the magnetic field. The column was washed with 1 mL buffer (phosphate-buffered saline [PBS] containing 0.5% bovine serum albumin [BSA] and 2 mM EDTA [ethylenediaminetetraacetic acid)]) 3 times. After removal of the column from the magnetic field, CD138+ cells were eluted with 1 mL buffer with the use of a plunger and washed 2 times with PBS. The purity of the myeloma cells, as assessed by CD138/CD45 staining and morphology,21 was greater than 95%.
RT-PCR analysis
Myeloma-derived ARH-77 and MM.1S cells and bone marrow mononuclear cells from patients with MM and healthy donors separated by gradient centrifugation, and CD138+ plasma cells isolated from the mononuclear cell fraction were resuspended in PBS at 5 × 106/mL. Total RNA was extracted from the cells with the use of RNAzol according to the manufacturer's protocol (Biotecx Laboratories, Houston, TX). RNAs were precipitated with isopropanol, and the pellets washed with 70% ethanol, briefly air dried, dissolved in diethylpyrocarbonate-treated water and stored at -80°C. RT-PCR analysis was performed by means of the PerkinElmer (Branchburg, NJ) PCR apparatus. After RT, the PCR was carried out under the following conditions: 94°C for 30 seconds, 60°C for 30 seconds, and 72°C for 1 minute for 24 to 34 cycles depending on relative amount of PCR products for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was used with the same PCR condition as an internal control. The PCR primers for MIP-1α, AML-1A, AML-1B, IL-3, and GAPDH used were as follows: AML-1A sense strand (SS), 5′-CTG GTC ACT GTG ATG GCT GG-3′; AML-1A antisense strand (AS), 5′-CTG CCT TAA CAT CTC CAG GG-3′; AML-1B SS, 5′-CAC CGA CAG CCC CAA CTT CC-3′; AML-1B AS, 5′-AGG TGG CGA CTT GCG GTG GG-3′; MIP-1α SS, 5′-ACA TTC CGT CAC CTG CTC AG-3′; MIP-1α AS, 5′-CGG TTG TCA CCA GAC GCG G-3′; IL-3 SS, 5′-CTT CAA CAA CCT CAA TGG GG-3′; IL-3 AS, 5′-AAT TCA TCT GAT GCC GCA GG-3′; GAPDH SS, 5′-ACC ACA GTC CAT GCC ATC AC-3′; and GAPDH AS, 5′-TCC ACC ACC CTG TTG CTG TA-3′. The PCR products were subcloned into the TA cloning vector and sequence analyzed to confirm their identity.
Human OCL formation assay
Nonadherent healthy human marrow mononuclear cells were prepared as previously described22 and resuspended in α–minimum essential media (α-MEM) (Gibco, Grand Island, NY) with 20% horse serum (Hyclone, Logan, UT) at 106/mL. The marrow cells (1 × 105/100 μL) were plated in 96-well plates in the presence or absence of varying concentrations of recombinant human MIP-1α, IL-3, and/or RANKL. Cultures were maintained in an atmosphere of 5% CO2 and air at 37°C for 3 weeks. The cultures were fed biweekly by replacing half the media with an equal volume of fresh media containing the cytokine of interest. In selected experiments, IL-3 was only added for the first week, the first 2 weeks, the last 2 weeks, or the last week of 3-week culture period. After 3 weeks of culture, cells were fixed with 2% formaldehyde in PBS, and the number of OCL-like multinucleated cells (more than 3 nuclei) that cross-reacted with the 23c6 monoclonal antibody (generously provided by Dr Michael Horton, St Bartholomew's Hospital, London, England), which identifies OCL-like cells, were scored.23 Binding of the 23c6 monoclonal antibody was assessed with biotin-conjugated rabbit antimouse immunoglobulin G (IgG) coupled to alkaline phosphatase (Vector Laboratories, Burlingame, CA). The cells were counterstained with methylgreen. In selected experiments, human bone marrow mononuclear cells were treated with marrow plasma from MM patients in the absence and presence of a murine monoclonal antihuman IL-3–neutralizing antibody (500 ng/mL) as follows: Bone marrow plasma from MM patients containing known amounts of IL-3 and/or MIP-1α were dialyzed against α-MEM for 48 hours and added to these cultures at 30% (vol/vol) concentration (biweekly), and the cultures were continued for 3 weeks. Cultures not treated with bone marrow plasma were used as controls, and an isotype-specific mouse IgG (500 ng/mL) was used as a control for anti–IL-3 antibody treatment.
Measurement of IL-3 in marrow plasma from patients with MM and healthy donors and conditioned media from CD138+ purified plasma cells from patients with MM
The concentrations of IL-3 in bone marrow plasma from patients with MM and healthy donors, and 96-hour conditioned media from CD138+ purified plasma cells, were determined by means of ELISA kits according to the manufacturers' protocols. The ELISA kit for human IL-3 could detect as little as 7.4 pg/mL IL-3 in bone marrow plasma.
Effects of IL-3 on the growth of MM cells
To test the effects of IL-3 on the growth of myeloma cell lines, IL-6–independent myeloma cell lines (ARH-77, MM.1S, MCQ/2, and IM9) and IL-6–dependent myeloma cell lines (KAS6/1,ANBL6) were treated with 0.01 to 1 ng/mL IL-3 for 96 hours in the presence and absence of IL-6, and the number of viable cells that excluded trypan blue was scored with a hemocytometer. Alternatively, myeloma cell lines were treated with 1 μg/mL anti–IL-6 neutralizing antibody (R&D Systems) and 0.1 to 1 ng/mL IL-3 for 4 days. Samples were transferred into 96-well plates, and myeloma cell proliferation was assayed by means of a MTT assay kit (ATCC). Briefly, the insoluble purple formazan crystals formed by cellular dehydrogenase, were solubilized by detergent, and optical density was quantified with a spectrophotometer.
Statistical analysis
In vitro and in vivo results are reported as the mean ± standard error of the mean (SEM) for typical experiments done in 5 replicate samples and were compared by the Student t test. Results were considered significantly different for P < .05.
Results
IL-3 mRNA expression is down-regulated in ARH-77 and MM.IS cells stably transfected with AML-1B
We determined if imbalanced AML-1A and AML1B expression regulated the expression of other genes associated with tumor growth or increased the bone-resorbing activity produced by myeloma cells. An extensive literature search suggested that c-fms, IL-3, IL-7, GM-CSF, and MDR1 were candidate genes that might be increased by the abnormal AML-1A and AML-1B expression in myeloma cells.10-19 Therefore, we evaluated the mRNA expression levels of these genes in myeloma-derived ARH-77 and MM.IS cells stably transfected with AML-1A, AML-1B, or empty vector by RT-PCR using specific primer sets. Among these genes, we found that IL-3 was one of the genes that were increased by imbalanced AML-1A and AML-1B expression in myeloma cells. As shown in Figure 1A, MIP-1α mRNA was down-regulated in ARH-77 cells stably transfected with AML-1B as we previously reported.9 IL-3 mRNA expressions levels were also down-regulated in 3 independent ARH-77 clones stably transfected with AML-1B compared with ARH-77 cells stably transfected with either AML-1A or EV. IL-3 mRNA levels were markedly increased in 2 of 3 ARH-77 clones stably transfected with AML-1A, while an empty vector–transfected ARH-77 clone or parent ARH-77 cell weakly expressed IL-3 mRNA. Three of 3 AML-1B–transfected ARH-77 clones did not express MIP-1α or IL-3 mRNA (Figure 1A). Similarly, MIP-1α and IL-3 mRNA was down-regulated in 2 independent MM.1S cells stably transfected with AML-1B compared with MM.1S cells stably transfected with either AML-1A or EV. IL-3 mRNA levels were markedly increased in 2 of 2 MM.1S single-cell clones stably transfected with AML-1A, compared with an empty vector–transfected MM.1S clone or parental MM.1S cells (Figure 1B).
The mRNA expression levels of AML-1A, AML-1B, MIP-1α, and IL-3 in myeloma cell lines. The mRNA expression levels of AML-1A, AML-1B, MIP-1α, and IL-3 in myeloma cell lines expressing AML-1A or AML-1B. ARH-77 (A) and MM.1S (B) cell lines were stably transfected with AML-1A, AML-1B, or empty vector (EV), and the mRNA expression levels of MIP-1α and IL-3 were determined. Three independent ARH-77 single-cell clones (A) and 2 independent MM.1S single-cell clones (B) stably transfected with AML-1A or AML-1B were examined by RT-PCR analysis as described in “Materials and methods.” GAPDH was used as an internal control.
The mRNA expression levels of AML-1A, AML-1B, MIP-1α, and IL-3 in myeloma cell lines. The mRNA expression levels of AML-1A, AML-1B, MIP-1α, and IL-3 in myeloma cell lines expressing AML-1A or AML-1B. ARH-77 (A) and MM.1S (B) cell lines were stably transfected with AML-1A, AML-1B, or empty vector (EV), and the mRNA expression levels of MIP-1α and IL-3 were determined. Three independent ARH-77 single-cell clones (A) and 2 independent MM.1S single-cell clones (B) stably transfected with AML-1A or AML-1B were examined by RT-PCR analysis as described in “Materials and methods.” GAPDH was used as an internal control.
IL-3 expression is up-regulated in bone marrow from patients with MM compared with healthy donors
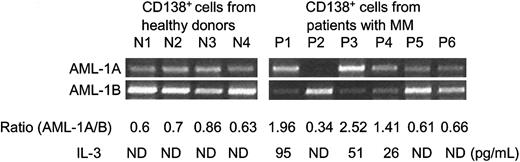
We then determined the IL-3 mRNA expression levels in bone marrow samples from patient with MM versus healthy donors by RT-PCR. As shown in Figure 2A, IL-3 mRNA was consistently overexpressed in 6 of 8 bone marrow samples from MM patients, and 4 of 6 patients that showed decreased AML-1B and increased MIP-1α expression. Marrow from healthy donors expressed very low amounts of MIP-1α or IL-3 mRNA and had increased expression of AML-1B. Since total bone marrow cells contain multiple cell types and the number of myeloma cells varies depending on the stage of the disease, we then assessed IL-3 mRNA expression levels in CD138+ purified plasma cells from patients with MM versus healthy donors. As shown in Figure 2B, in purified cells from 9 patients, 7 of 9 patients showed increased IL-3 mRNA and 8 of 9 showed decreased AML-1B expression. Seven of 8 patients who had decreased AML-1B mRNA levels had increased IL-3 expression. IL-3 mRNA was increased in 4 of 5 plasma cells from MM patients who showed increased MIP-1 α and AML-1A expression. We then calculated the correlation coefficients (r values) for the relative mRNA expression levels of IL-3 versus MIP-1α and AML-1A detected by scanning the x-ray films. IL-3 expression levels in purified plasma cells from patients with MM correlated with enhanced MIP-1 α and AML-1A expression levels (r = 0.753 for IL-3 versus MIP-1 α; r = 0.62 for IL-3 versus AML-1A). As shown in Figure 2C, IL-3 protein levels were significantly increased in marrow plasma from 12 of 16 MM patients compared with 10 healthy donors (66.4 ± 12 versus 22.1 ± 8.2; P = .038). We then measured IL-3 protein expression levels in conditioned media from purified CD138+ plasma cells of MM patients using commercially available IL-3 ELISA kits. Purified CD138+ and CD138- cells (2 × 106) were cultured in RPMI1640 media containing 10% fetal calf serum for 96 hours. Three of 6 purified CD138+ plasma cells (2 × 106) from MM patients secreted 95, 51, and 26 pg/mL IL-3 into their conditioned media, and the IL-3 expression levels are correlated with increased mRNA expression ratios of AML-1A to AML-1B in purified CD138+ plasma cells from patients with MM (Figure 3).
The mRNA expression levels of AML-1A, AML-1B, MIP-1α, and IL-3 in bone marrow samples from patients with MM and healthy donors. Relative mRNA expression levels of AML-1A, AML-1B, MIP-1α, and IL-3 were determined by RT-PCR analysis. (A) (B) Total bone marrow mononuclear cells (A) and CD138+ purified plasma cells (B) from healthy donors and MM patients were examined. The mRNA expression levels of MIP-1α and IL-3 were significantly enhanced in total bone marrow samples (A) as well as purified plasma cells (B) from patients with MM as compared with healthy donors. IL-3 expression levels in purified plasma cells from patients with MM correlated with enhanced MIP-1α and decreased AML-1B expression (r = 0.753 for IL-3 versus MIP-1α; r = 0.62 for IL-3 versus AML-1A). (C) IL-3 protein expression levels in bone marrow plasma from MM patients and healthy donors were measured by commercial ELISA kits. Expression levels of IL-3 were significantly increased in 12 of 16 bone marrow plasma samples from MM patients as compared with healthy donors.
The mRNA expression levels of AML-1A, AML-1B, MIP-1α, and IL-3 in bone marrow samples from patients with MM and healthy donors. Relative mRNA expression levels of AML-1A, AML-1B, MIP-1α, and IL-3 were determined by RT-PCR analysis. (A) (B) Total bone marrow mononuclear cells (A) and CD138+ purified plasma cells (B) from healthy donors and MM patients were examined. The mRNA expression levels of MIP-1α and IL-3 were significantly enhanced in total bone marrow samples (A) as well as purified plasma cells (B) from patients with MM as compared with healthy donors. IL-3 expression levels in purified plasma cells from patients with MM correlated with enhanced MIP-1α and decreased AML-1B expression (r = 0.753 for IL-3 versus MIP-1α; r = 0.62 for IL-3 versus AML-1A). (C) IL-3 protein expression levels in bone marrow plasma from MM patients and healthy donors were measured by commercial ELISA kits. Expression levels of IL-3 were significantly increased in 12 of 16 bone marrow plasma samples from MM patients as compared with healthy donors.
IL-3 expression levels by purified CD138+ plasma cells from MM patients and healthy donors. CD138+ plasma cells were purified from patients with MM and healthy donors. First, 2 × 106 cells were cultured in RPMI media containing 10% fetal calf serum for 96 hours. IL-3 expression levels in these culture media were measured by commercially available IL-3 ELISA kits. At the end of culture period, cells were harvested, and mRNA expression levels of AML-1A and AML-1B were determined by RT-PCR analysis. Three of 6 purified CD138+ plasma cells from MM patients who showed enhanced AML-1A mRNA expression secreted 26 to 95 pg/mL IL-3 into culture media. IL-3 was not detected (ND) in media conditioned by CD138+ cells from healthy donors and patients 2, 5, and 6 (P2, P5, and P6).
IL-3 expression levels by purified CD138+ plasma cells from MM patients and healthy donors. CD138+ plasma cells were purified from patients with MM and healthy donors. First, 2 × 106 cells were cultured in RPMI media containing 10% fetal calf serum for 96 hours. IL-3 expression levels in these culture media were measured by commercially available IL-3 ELISA kits. At the end of culture period, cells were harvested, and mRNA expression levels of AML-1A and AML-1B were determined by RT-PCR analysis. Three of 6 purified CD138+ plasma cells from MM patients who showed enhanced AML-1A mRNA expression secreted 26 to 95 pg/mL IL-3 into culture media. IL-3 was not detected (ND) in media conditioned by CD138+ cells from healthy donors and patients 2, 5, and 6 (P2, P5, and P6).
In contrast, purified CD 138+ plasma cells from healthy donors did not secrete IL-3 protein.
IL-3 acts at the early stages of OCL formation
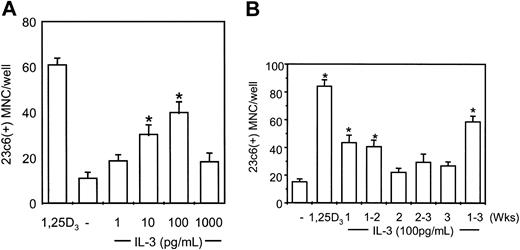
We then tested the effects of IL-3 on OCL formation. Bone marrow mononuclear cells prepared from human marrow aspirates from healthy donors were cultured at 2 × 105/mL in the presence of 1 to 1000 pg/mL IL-3. As shown in Figure 4A, IL-3 dose-dependently increased OCL formation from 1 to 100 pg/mL. As seen with other cytokines in this assay,24,25 OCL formation induced by very high concentrations of IL-3 (1000 pg/mL) was less than OCL formation induced by 100 pg/mL IL-3, but was still greater that control levels.
IL-3 induces osteoclast formation in human marrow cultures and acts at the early stages of OCL differentiation. (A) Long-term human marrow cultures were treated with varying concentrations of recombinant human IL-3 (hIL-3). Controls for these experiments were cultures treated with 1,25–(OH)2D3 (10-8 M). IL-3 at concentrations of 10 to 100 pg/mL significantly increased osteoclast formation in a dose-dependent manner. (B) Human bone marrow cultures were treated with hIL-3 (100 pg/mL) for varying periods of time. IL-3 significantly increased OCL formation only when present during the first 2 weeks of the 3-week culture period. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 4 independent experiments (*P < .05), compared with culture lacking IL-3.
IL-3 induces osteoclast formation in human marrow cultures and acts at the early stages of OCL differentiation. (A) Long-term human marrow cultures were treated with varying concentrations of recombinant human IL-3 (hIL-3). Controls for these experiments were cultures treated with 1,25–(OH)2D3 (10-8 M). IL-3 at concentrations of 10 to 100 pg/mL significantly increased osteoclast formation in a dose-dependent manner. (B) Human bone marrow cultures were treated with hIL-3 (100 pg/mL) for varying periods of time. IL-3 significantly increased OCL formation only when present during the first 2 weeks of the 3-week culture period. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 4 independent experiments (*P < .05), compared with culture lacking IL-3.
We have previously demonstrated that OCL precursors proliferate during weeks 1 to 2 of marrow cultures and then differentiate and fuse during weeks 2 to 3.22 To determine at which phase of OCL formation IL-3 was acting, IL-3 (100 pg/mL) was added to human bone marrow cultures at different times, and OCL formation was assessed. As shown in Figure 4B, addition of IL-3 for only the first week or first 2 weeks of the culture period significantly increased OCL formation. Addition of IL-3 for second week or last 2 weeks of the culture period did not increase OCL formation.
IL-3 increases OCL formation and bone resorption in combination with MIP-1α and RANKL
Since IL-3 appeared to act at the early stages of OCL formation and MIP-1α and RANKL act at the later stages of OCL formation2,26 and their expression levels are increased in bone marrow sample from patients with MM, we tested the effects of IL-3 in combination with MIP-1α or RANKL on OCL formation. As shown in Figure 5A, treatment of bone marrow mononuclear cells with IL-3 (100 pg/mL) in combination with MIP-1α (200 pg/mL) or low concentration of RANKL (10 ng/mL) for 3 weeks significantly increased OCL formation compared with MIP-1α, RANKL, or IL-3 alone.
Effects of IL-3 in combination with MIP-1α or RANKL on OCL formation. Human bone marrow mononuclear cells were cultured with IL-3 in combination with MIP-1α and RANKL. (A) Treatment of human bone marrow cells with 100 pg/mL IL-3 in combination with 200 pg/mL hMIP-1α or 10 ng/mL RANKL for the entire culture period significantly increased OCL formation (23c6+ multinucleated cells [MNCs]) compared with control cultures that were treated with only IL-3, MIP-1α, or RANKL. (B) Treatment of human bone marrow cells with 100 pg/mL IL-3 for the first week of culture period in combination with 200 pg/mL hMIP-1α or 10 ng/mL RANKL at the second and third weeks of the culture period further increased OCL formation compared with control cultures that were treated with media for the first week of the culture period. (C) The numbers of large OCLs (more than 10 nuclei per OCL) were quantified in these cultures. IL-3 markedly increased the number of large OCLs. (D) Pit formation on dentine slices by OCLs formed in cultures treated with 100 pg/mL IL-3 for the first week and followed by 200 pg/mL MIP-1α or 10 ng/mL RANKL was increased compared with that of control cultures treated with MIP-1α or RANKL alone. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 4 independent experiments (*P < .05).
Effects of IL-3 in combination with MIP-1α or RANKL on OCL formation. Human bone marrow mononuclear cells were cultured with IL-3 in combination with MIP-1α and RANKL. (A) Treatment of human bone marrow cells with 100 pg/mL IL-3 in combination with 200 pg/mL hMIP-1α or 10 ng/mL RANKL for the entire culture period significantly increased OCL formation (23c6+ multinucleated cells [MNCs]) compared with control cultures that were treated with only IL-3, MIP-1α, or RANKL. (B) Treatment of human bone marrow cells with 100 pg/mL IL-3 for the first week of culture period in combination with 200 pg/mL hMIP-1α or 10 ng/mL RANKL at the second and third weeks of the culture period further increased OCL formation compared with control cultures that were treated with media for the first week of the culture period. (C) The numbers of large OCLs (more than 10 nuclei per OCL) were quantified in these cultures. IL-3 markedly increased the number of large OCLs. (D) Pit formation on dentine slices by OCLs formed in cultures treated with 100 pg/mL IL-3 for the first week and followed by 200 pg/mL MIP-1α or 10 ng/mL RANKL was increased compared with that of control cultures treated with MIP-1α or RANKL alone. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 4 independent experiments (*P < .05).
We then treated human bone marrow mononuclear cells with 100 pg/mL IL-3 for only the first week of the culture and added 200 pg/mL MIP-1α or 10 ng/mL RANKL for the last 2 weeks of culture. As shown in Figure 5B, IL-3 treatment for the first week followed by MIP-1α or RANKL treatment for the last 2 weeks of culture significantly enhanced OCL formation compared with the control culture treated with MIP-1α or RANKL for 3 weeks. Interestingly, OCLs formed in these cultures were markedly increased in both size and nuclear number compared with control culture treated with MIP-1α or RANKL (Figure 5C). Furthermore, OCLs formed in the cultures treated with IL-3 for only the first week of the culture and followed by MIP-1α or RANKL demonstrated increased pit formation on dentine slices compared with OCLs in the culture treated with MIP-1α or RANKL alone (Figure 5D).
Anti–IL-3 neutralizing antibody only inhibits OCL formation stimulated by bone marrow plasma containing IL-3 from patients with MM
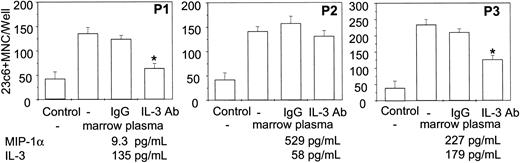
To test whether anti–IL-3 antibody inhibits OCL formation stimulated by bone marrow plasma from patients with MM, we treated human OCL formation cultures with plasma from MM patients in the absence and presence of anti–IL-3 neutralizing antibody. Bone marrow plasma from MM patients containing known amounts of IL-3 and MIP-1α, were dialyzed against α-MEM for 48 hours and added on these cultures at the 30% (vol/vol) concentration for 3 weeks. Bone marrow plasma from patients with MM stimulated OCL formation compared with the nontreated control cultures. Furthermore, OCL formation stimulated by plasma from MM patients that contained more than 100 pg/mL IL-3 (more than 30 pg/mL in the cultures) was inhibited by anti–IL-3 antibody treatment as shown in P1 and P3 in Figure 6 compared with the cultures not treated with anti–IL-3 or treated with isotype-specific mouse IgG. In contrast, OCL formation stimulated by marrow plasma containing high levels of MIP-1α and lower levels of IL-3 levels (patient 2) was not significantly blocked by anti–IL-3 antibody treatment compared with the isotype-specific IgG controls, possibly reflecting the effects of the very high levels of MIP-1α on OCL formation.
Effects of an anti–IL-3 neutralizing antibody on OCL formation stimulated by bone marrow plasma from patients with MM. Bone marrow plasma from MM patients containing varying amounts of IL-3 and MIP-1α were dialyzed against α-MEM for 48 hours. Human bone marrow culture was treated with dialyzed bone marrow plasma from patients with MM at the 30% (vol/vol) concentration for 3 weeks in the presence and absence of anti–IL-3 neutralizing antibody (500 ng/mL). Cultures not treated with bone marrow plasma or cultures treated with isotype IgG were used as controls for these experiments. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment (*P < .05). Similar results were seen in 2 independent experiments.
Effects of an anti–IL-3 neutralizing antibody on OCL formation stimulated by bone marrow plasma from patients with MM. Bone marrow plasma from MM patients containing varying amounts of IL-3 and MIP-1α were dialyzed against α-MEM for 48 hours. Human bone marrow culture was treated with dialyzed bone marrow plasma from patients with MM at the 30% (vol/vol) concentration for 3 weeks in the presence and absence of anti–IL-3 neutralizing antibody (500 ng/mL). Cultures not treated with bone marrow plasma or cultures treated with isotype IgG were used as controls for these experiments. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment (*P < .05). Similar results were seen in 2 independent experiments.
IL-3 enhances the proliferation of myeloma cells
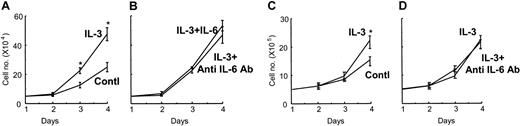
To determine the effects of IL-3 on myeloma cell growth, we treated various myeloma cell lines (MM.1S, ARH-77, MCQ/2, IM9, and KAS6/1) with 10 pg/mL to 1 ng/mL IL-3 for 96 hours. The growth rates of myeloma-derived IL-6–dependent KAS6/1 cells were significantly enhanced by treatment with 1 ng/mL IL-3 in the absence of IL-6 (Figure 7A) compared with nontreated control cultures. The enhanced growth rates of KAS6/1 cells by IL-3 were not further increased by the addition of 1 ng/mL anti–IL-6 (Figure 7B) and were not blocked by 100 ng/mL anti–IL-6 neutralizing antibody. Furthermore, 1 ng/mL IL-3 significantly stimulated the growth of MM.1S cells, which are IL-6 independent (Figure 7C), and IL-3–enhanced growth rates of MM.1S cells were not blocked by anti–IL-6 neutralizing antibody (Figure 7D). We then measured IL-6 expression levels in conditioned media from KAS6/1 cells treated with 1 ng/mL IL-3 for 96 hours. We could not detect increased IL-6 expression by KAS6/1 cells treated with IL-3.
Effects of IL-3 on the growth of myeloma cells. The IL-6–dependent myeloma-derived KAS6/1 cells were treated with 1 ng/mL IL-3 in the absence (A) and presence (B) of IL-6 (1 ng/mL). IL-3 significantly increased the growth of KAS 6/1 cells in the absence of IL-6 compared with nontreated control culture. IL-6 (1 ng/mL) did not further increase the growth of KAS6/1 cells by induced IL-3, and addition of an anti–IL-6 neutralizing antibody (100 ng/mL) to the cultures did not block the growth of KAS6/1 cells induced by IL-3 (B). IL-3 also increased the growth of the IL-6–independent MM.1S cells (C), and the enhanced growth was not blocked by an anti–IL-6 neutralizing antibody (D). Results represent the mean ± SEM for triplicate determinations for a typical experiment. Similar results were seen in 2 independent experiments (*P < .05).
Effects of IL-3 on the growth of myeloma cells. The IL-6–dependent myeloma-derived KAS6/1 cells were treated with 1 ng/mL IL-3 in the absence (A) and presence (B) of IL-6 (1 ng/mL). IL-3 significantly increased the growth of KAS 6/1 cells in the absence of IL-6 compared with nontreated control culture. IL-6 (1 ng/mL) did not further increase the growth of KAS6/1 cells by induced IL-3, and addition of an anti–IL-6 neutralizing antibody (100 ng/mL) to the cultures did not block the growth of KAS6/1 cells induced by IL-3 (B). IL-3 also increased the growth of the IL-6–independent MM.1S cells (C), and the enhanced growth was not blocked by an anti–IL-6 neutralizing antibody (D). Results represent the mean ± SEM for triplicate determinations for a typical experiment. Similar results were seen in 2 independent experiments (*P < .05).
Discussion
Recently, we reported that abnormal expression of AML-1A and AML-1B regulates MIP-1α expression in myeloma cells9 and that enhanced MIP-1α expression results in stimulation of OCL formation and tumor cell homing and adhesion in an in vivo model of myeloma bone disease.4 We now report that enhanced IL-3 expression also results from abnormal AML-1A and AML-1B expression in myeloma cells and that IL-3 markedly increases OCL formation in combination with MIP-1α and RANKL, as well as myeloma cell growth. These results are consistent with previous reports that IL-3 levels are increased in 70% of MM patients and that IL-3 can induce OCL formation and enhance the proliferation and differentiation of promyeloma cells from MM patients.27-30
Highly purified MM cells produce IL-3. MM cell production of IL-3 appears to result from decreased AML-1B expression analogous to our previous finding with MIP-1α. Transduction of AML-1B cDNA into ARH-77 and MM.IS cells markedly decreased both IL-3 and MIP-1α mRNA expression. AML-1B induces low levels of expression of many hematopoietic genes such as GM-CSF, G-CSF receptor, c-fms, and Bcl-2,10-19 and increases transcription of these genes to higher levels in combination with other tissue-specific regulators such as activator protein 1 (AP-1), CCAAT/enhancer-binding protein–α (C/EBP), ets-1, PU.1, and c-Myb.31 However, in ARH77 and MM.IS cells, transduction of AML-1B markedly down-regulated IL-3 expression, and transduction of ARH 77 and MM.IS cells with AML-1A up-regulated IL-3 mRNA expression. Uchida et al32 reported that AML-1A and AML-1B can transactivate the human IL-3 promoter, demonstrating that although AML-1A lacks a putative transactivation domain, it can transactivate the IL-3 promoter in T cells nearly as effectively as AML-1B. It is unclear how AML-1B down-regulates IL-3 expression in myeloma cells. Possibly a repressor gene is also activated by AML-1B near the adjacent GATA-2 site as reported by Zhang et al.33 However, to date no such factor has been identified in myeloma cells.
Multiple studies have suggested that IL-3 can increase the proliferation of myeloma cells in combination with IL-1β, IL-5, tumor necrosis factor–β (TNF-β), macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage CSF (GM-CSF), G-CSF, and IL-6.34-38 Our results demonstrate that IL-3 levels are increased in marrow plasma samples from the majority of patients with MM whom we tested. Consistent with our results, Merico et al28 reported that IL-3 is the only cytokine consistently increased in serum samples from MM patients while IL-1β, IL-4, IL-6, IL-7, IL-8, and tumor necrosis factor–α (TNF-α) are not increased. Lauta29 and Goto et al30 also reported that serum IL-3 levels are increased in MM patients compared with patients with monoclonal gammopathy of undetermined significance (MGUS) and that IL-3 can differentiate peripheral blood nonadherent mononuclear cells from MM patients but not from MGUS patients into mature plasma cells. Furthermore, our data demonstrate that IL-3 stimulation of myeloma cell growth was not due to induction of IL-6, since increased IL-6 levels were not detected in conditioned media from myeloma cells treated with IL-3. Additionally, anti–IL-6 antibody did not block the effects of IL-3 on the myeloma cell growth, and IL-3 stimulated the growth of IL-6–independent cell lines.
We found that recombinant IL-3 induced OCL formation, as did IL-3, in marrow plasma samples from MM patients and acted at the early stage of OCL differentiation. Barton et al27 also reported that treatment of murine bone marrow monocytic cells with IL-3 enhanced formation of multinucleated cells that express tartrate-resistant acid-phosphatase activity, an OCL marker enzyme, in the absence of 1,25–(OH)2D3. Consistent with our result that IL-3 increases early OCL precursors, Lorenzo et al39 have reported that combined treatment of IL-3 and GM-CSF induced OCL differentiation via the stimulation of M-CSF production by human monocytes. M-CSF increases the proliferation of OCL precursors.40 However, IL-3 can induce OCL formation independently of M-CSF. Myint et al41 have reported that IL-3 can induce OCL formation in osteopetrotic op/op mice, which do not produce functional M-CSF. They showed that treatment of young op/op mice with 5 ng recombinant murine GM-CSF (rmGM-CSF) and 100 ng rmIL-3 expanded their bone marrow cavities at 2 weeks after administration and significantly increased the number of tartrate-resistant acid phosphatase–positive (TRAP+) cells and bone marrow macrophages. These results demonstrate that GM-CSF and IL-3 induce the development of osteoclasts, which can correct osteopetrosis in the op/op mice and suggest that IL-3 has an important role in OCL development in the bone marrow microenvironment. However, Barton and Mayer42 found that IL-3 does not induce bone resorption as measured by 45Ca release in neonatal mouse calvaria, even though IL-3 induced formation of osteoclast-like cells. These data suggest that IL3 induces only partial maturation of osteoclasts and that additional factors produced in the bone marrow microenvironment are necessary to activate osteoclastic bone resorption. Our observations that IL-3 stimulated bone resorption in combination with MIP-1α and RANKL, factors that act at the later stages of OCL development and are also present in marrow plasma from MM patients, suggest that IL-3 increases the number of immature OCLs, which then are further differentiated into active mature OCLs by MIP-1α and RANKL.2,28 More importantly, OCLs formed in the cultures treated with IL-3 and MIP-1α or RANKL showed markedly increased OCL size, number of nuclei per OCL, and bone-resorbing activity compared with control cultures treated with MIP-1α or RANKL alone. Consistent with our results, Lees et al43 reported that increased OCL size correlated with enhanced bone resorption.
Interestingly, treatment of human bone marrow culture with low concentrations of RANKL (10 ng/mL), which are suboptimal concentrations for OCL formation, can stimulate formation of 23c6+ OCL-like multinucleated cells in vitro that do not form pits on dentine slices. This most likely reflects that 10 ng/mL RANKL is not sufficient for activation of c-Jun N-terminal kinase (JNK), which prolongs the survival of mature OCLs, and permits them to actively resorb bone.44 These data suggest that increased IL-3, MIP-1α, and RANKL expression may cooperatively enhance bone resorption in the marrow microenvironment of MM patients in the presence of low levels of RANKL.
Patients with increased levels of MIP-1α have a poor prognosis, and MIP-1α and IL-3 expression is increased by abnormal AML-1A and AML-1B expression in myeloma cells. These factors in combination increase osteoclastic bone resorption as well as the proliferation of myeloma cells. Recently, Lentzsch et al45 have reported that MIP-1α also increases the growth and survival of myeloma cells. These results suggest that other genes that contribute to the poor prognosis of MM in addition to MIP-1α and IL-3 may also be increased or decreased by abnormal AML-1A and AML-1B expression. Puig-Kroger et al46 have reported that CCAAT-binding factor (CBF)/AML-1 decreases transcription of the CD11a integrin gene through recognition of its binding element, suggesting that lymphocyte function–associated antigen–1 (LFA-1) (CD11a/CD18) expression may be decreased in acute myeloid and B-lineage acute lymphoblastic leukemias by AML-1B and contribute to their altered adhesion and metastatic potential.47 Thus, the imbalanced AML-1A and AML-1B expression in myeloma may also affect myeloma cell homing and adhesion to stromal cells, in addition to the growth of myeloma cells and osteoclastic bone resorption.
Prepublished online as Blood First Edition Paper, November 13, 2003; DOI 10.1182/blood-2003-06-1992.
Supported by grants from the International Myeloma Foundation, Veterans Administration Merit Review Award, and the Multiple Myeloma Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to acknowledge the General Clinic Research Center at the University of Pittsburgh, and the University of Texas at San Antonio for their support of these studies. We also thank Donna Gaspich and Theresa Casciato for preparation of the manuscript.



![Figure 5. Effects of IL-3 in combination with MIP-1α or RANKL on OCL formation. Human bone marrow mononuclear cells were cultured with IL-3 in combination with MIP-1α and RANKL. (A) Treatment of human bone marrow cells with 100 pg/mL IL-3 in combination with 200 pg/mL hMIP-1α or 10 ng/mL RANKL for the entire culture period significantly increased OCL formation (23c6+ multinucleated cells [MNCs]) compared with control cultures that were treated with only IL-3, MIP-1α, or RANKL. (B) Treatment of human bone marrow cells with 100 pg/mL IL-3 for the first week of culture period in combination with 200 pg/mL hMIP-1α or 10 ng/mL RANKL at the second and third weeks of the culture period further increased OCL formation compared with control cultures that were treated with media for the first week of the culture period. (C) The numbers of large OCLs (more than 10 nuclei per OCL) were quantified in these cultures. IL-3 markedly increased the number of large OCLs. (D) Pit formation on dentine slices by OCLs formed in cultures treated with 100 pg/mL IL-3 for the first week and followed by 200 pg/mL MIP-1α or 10 ng/mL RANKL was increased compared with that of control cultures treated with MIP-1α or RANKL alone. Results represent the mean ± SEM for quadruplicate determinations for a typical experiment. Similar results were seen in 4 independent experiments (*P < .05).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/6/10.1182_blood-2003-06-1992/6/m_zh80060458320005.jpeg?Expires=1767785503&Signature=tgelcG20Z89-NEaavjDCyIbPHTzxaK4HBl0idcBb~Y8a~T-hNXJU8TgnuBEou6GWGCM0vRvfFA0acQm4nR4JJnCgMpPZiznN1DF7XFYaxZ4uv3~EhGRCFMe-vWSVY8sMl3yQ75FS7t-jzAuupC~MnZ4fLvVVV5-TNxJan9KidJ0SD4znwo2Qnskw0GhTdV9BJpBgMO-DdD4w6carz8OHEdnHIrxynpIpLxfb0lwQ0RaBpyCUGb702dFUUS3ML54WneyY2KzYqdKRH~4MnU1NNOoe5xp75q2mkw0LhmTs2~K54hhtJTKh~OZCNBZTZsHoOx9zX7WbOt-xq6~ufzfx0Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal