Abstract
Imatinib is a tyrosine-kinase inhibitor that binds to ABL proteins and induces cytogenetic remissions in patients with chronic myeloid leukemia (CML). In these patients measuring response by molecular techniques is clearly required. We determined the cytogenetic and molecular response (CgR, MR) to imatinib in 191 patients with late chronic-phase Philadelphia-positive (Ph+) CML, previously treated with interferon α. MR was assessed with real-time quantitative (TaqMan) reverse transcription–polymerase chain reaction and was expressed as the ratio between BCR/ABL and β2-microglobulin × 100, the lowest level of detectability of the method being 0.00001. A complete CgR (CCgR) was achieved in 85 (44%) of 191 patients and was maintained for 2 years in 67 (79%) of 85 patients. A reduction of the transcript level of more than 2 logs was achieved in all but 9 patients with CCgR versus none of 23 with partial CgR. In the CCgRs the median value of the MR was 0.0008 after 12 months and 0.0001 after 24 months, with the transcript level undetectable in 22 cases. We conclude that in CCgRs the degree of MR may vary from 2 to more than 4 logs, and that there is a progressive decrease of transcript level by time. Only 1 of 22 negative cases has had a relapse as yet.
Introduction
The introduction of imatinib may lead to a substantial revolution of the management of Philadelphia-positive (Ph+) chronic myeloid leukemia (CML). Imatinib was rationally and specifically designed to bind to the adenosine triphosphate (ATP)–docking site of tyrosine kinase proteins, including ABL itself (p160) and the hybrid BCR/ABL proteins (p190, p210, p230, etc), which cause Ph+ leukemias.1 Beside ABL, imatinib binds to and inhibits a few other important tyrosine kinases such as cKIT and platelet-derived growth factor receptor β (PDGF-Rβ).2-4 In vitro imatinib inhibits the growth and induces apoptosis in Ph+ cell lines and primary Ph+ hematopoietic cells.5-8 In animal models imatinib inhibits the development of Ph+ leukemias on transplantation of Ph+ cells.9,10 In humans, imatinib induces a rapid and complete hematologic response in almost all Ph+ patients with CML in chronic phase and in about 50% of those who are in accelerated phase and in a smaller but interesting proportion of those who are in blastic phase or have a Ph+ acute leukemia.11-19 More importantly, a major cytogenetic response may be achieved in more than 50% of the patients who begin the treatment in late chronic phase13,19 and in more than 80% of the patients who are treated frontline.18 Most of these cytogenetic responses are complete and are likely to have a beneficial effect on survival. However, their duration cannot yet be determined and the extent to which the leukemic cell burden is reduced is yet unsettled. Molecular biology techniques allow a quantitative assessment of leukemia-specific BCR/ABL transcripts and have already been largely used for the evaluation of residual disease after allogeneic hematopoietic stem cell transplantation (allo-HSCT) and after interferon α (IFN-α) treatment.20-25 In this paper we report on the cytogenetic and molecular response to imatinib in a cohort of patients with late chronic-phase Ph+ CML who have been treated for 2 years.
Patients, materials, and methods
Study protocol
The treatment and study protocol (CML/002/STI571) was designed, sponsored, and operated by the Italian Cooperative Study Group on CML according to good clinical practice and the principles of the Helsinki declaration. Novartis Pharma supplied the drug free of charge and provided in part the support for data and sample collection and for monitoring of adverse events.
The design of the treatment protocol was the same of a prior phase 2 international study of imatinib in late chronic-phase Ph+ CML.13 To be eligible, it was required that a patient was in first chronic phase (< 15% blast cells, < 30% blast cells and promyelocytes, and < 20% basophils in peripheral blood and in bone marrow and a platelet count of more than 100 × 109/L), had already been treated with IFN-α, and was either intolerant of IFN-α or resistant to IFN-α treatment. The patients who were resistant to IFN-α were divided into 2 groups, namely, hematologic resistance (ie, failing to achieve or to maintain a complete hematologic response with IFN-α) and cytogenetic resistance (failing to achieve or to maintain a complete or partial cytogenetic response with IFN-α). Intolerance and resistance were defined exactly as in prior studies.13 All patients had also received hydroxyurea. In addition 57 patients had received arabinosyl cytosine at a low dose, and 13 had received busulfan.
Treatment consisted of imatinib alone, 400 mg, once a day until disease progression. In case of hematologic toxicity grade 3 or 4 the treatment was discontinued until the toxicity had resolved to grade 2 or less. In case of nonhematologic toxicity grade 2 or 3 the treatment was discontinued until the toxicity had resolved to less than grade 2 and was reassumed at 400 mg if toxicity was grade 2 and at 300 mg if toxicity was grade 3. In case of grade 4 nonhematologic toxicity, the study drug was withheld permanently.
The hematologic response (HR) was defined complete (CHR) if the leukocyte count was below 10 × 109/L, if no immature cells were recorded in the differential count, if the platelet count was below 450 × 109/L, and if the spleen was not palpable. Accelerated or blastic phase was defined by any one of the following: more than 15% blast cells or more than 30% blast cells and promyelocytes or more than 20% basophils in peripheral blood or bone marrow.
Cytogenetic studies
Cytogenetic studies were performed at local institutions at baseline, after 3, 6, 9, and 12 months, and every 6 months thereafter, with standard banding techniques; results were not centrally reviewed. The minimum number of marrow cell metaphases required to evaluate the response was 20, but if the results were congruent with prior or later data, the study was accepted if the number of metaphases was between 10 and 20. The cytogenetic response (CgR) was qualified according to the proportion of Ph- metaphases, as complete (Ph- 100%), partial (Ph- 66%-99%), minor (Ph- 34%-65%), and minimal or none (Ph- ≤ 33%). In some analyses, complete CgRs (CCgRs) and partial CgRs (PCgRs) were pooled under the definition of major CgRs (MCgRs).
Molecular studies
Bone marrow samples were collected prior the treatment (baseline), after 3, 6, and 12 months, and after 24 months from the patients who had achieved a CCgR. The samples were shipped by overnight courier to the Group Central Laboratory in Bologna where the cell pellets were isolated and stored. All samples were divided into 3 groups and each group was assigned for molecular assays to one of the 3 reference laboratories of the group, in Bologna, Turin, and Naples. The samples from each patient, from baseline to the end of the study, were analyzed in the same laboratory. The 3 laboratories (Bologna, Turin, and Naples) had been previously involved in the work of a European Union (EU) concerted action aimed at standardizing the protocols for real-time (RT) quantitative analysis of leukemic fusion transcripts.26,27 This standardization program involved a total of 25 laboratories and was performed in 3 distinct phases, namely, selection of primers and probes, standardization of RT quantitative polymerase chain reaction (RTQ-PCR) methods leading to a unique protocol, and development of a quality control program.26,27 When the standardization program was completed, the interlaboratory reproducibility was high, with a coefficient of variation of 11% for BCR/ABL mRNA.
Cell separation and RNA extraction
Leukocyte pellets were isolated from bone marrow aspirates by lysis of red blood cells on their arrival at the Bologna laboratory. Cell pellets were washed twice in saline solution and resuspended in aliquots of 1 × 106 in 600 μL 4 M guanidium isothiocyanate solution (GITC). The aliquots were stored at -20°C until shipment to the other reference laboratories. Total RNA was extracted in the 3 reference laboratories from cells cryopreserved in GITC using ion exchange chromatography on minicolumn (GeneElute, total RNA purification kit, Sigma, St Louis, MO), according to the manufacturer's directions. The quality of RNA was assessed on an ethidium bromide–stained 1% agarose gel containing 2.2 M formaldehyde.
RTQ-PCR assay of minimal residual disease
Minimal residual disease was detected during the follow-up by a standardized RTQ-PCR method that was established in the framework of the EU concerted action.26,27 The method independently measures in each sample by real-time PCR the copy number of mRNA encoding for the p210 BCR/ABL protein and for a control gene, to verify sample-to-sample RNA quality variations. β2-Microglobulin (β2M) was selected and was used as a control gene, among a number of genes that have been assayed in the standardization program for the RQ-PCR in hematopoietic cells, including also ABL, GUS, and G6PDH.27 The reason for using β2M was that the gene is stable and highly expressed in all marrow cells.
For each amplification run, both BCR-ABL and β2M standard curves were independently generated by assaying, in parallel with the samples, 1:10 serial dilutions (from 106 to 102 copies, each in triplicate) of plasmid DNA calibrators containing the target sequences diluted in a solution of Escherichia coli RNA (20 ng/μL). The copy number of BCR-ABL and β2M transcript was derived by the interpolation of threshold cycle (Ct) values to the appropriate standard curve, and the result, for each sample, was expressed as a ratio of BCR/ABL mRNA copies to β2M mRNA. Because the level of β2M mRNA is approximately 2 logs higher than the level of ABL and GUS, the ratio of BCR/ABL to β2M was multiplied by 100. The lowest limit of sensitivity of the method was set at 0.00001 (corresponding to a Ct value of 38 for BCR/ABL and 25.7 for β2M). This ratio would correspond to about 0.001 using ABL or GUS as a control gene.
RTQ-PCR conditions
The reaction conditions were the same for both BCR/ABL and β2M mRNA RTQ-PCR. Briefly, 1 μg total RNA extracted from the patient samples was prewarmed for 10 minutes at 70°C and incubated for 10 minutes at 25°C; the RNA solution was then incubated for 42 minutes at 45°C in a 20-μL reaction mixture containing 10 mM Tris (tris(hydroxymethyl)aminomethane) HCl (pH 8.3), 50 mM KCl, 5.5 mM MgCl2, 1 mM each of deoxyribonucleotide, 20 U RNAsin (Pharmacia, Uppsala, Sweden), 25 mM random examers (Pharmacia), 10 mM of dithiothreitol (DTT; Pharmacia), and 100 U Moloney murine leukemia virus (MoMLV) reverse transcriptase (BRL, Bethesda, MD). After incubation, cDNA solution was diluted 1:5 to a final volume of 50 μL. Junction sequences of p210- and β2M-encoding cDNAs were separately amplified in 5-μL aliquots (each equivalent to 100 ng RNA) of the latter solution. PCR was carried out in a reaction mixture consisting of 1 × Master Mix (Applied Biosystems, Foster City, CA), 300 nM of the appropriate primer pair, and 200 nM of the appropriate probe in a final volume of 25 μL using the following time/temperature profile: 95°C, 15 seconds, and 60°C, 1 minute, for 50 cycles. All amplification reactions were carried out in triplicate and the mean Ct values were used to interpolate standard curves and to calculate the transcript copy number. Primers and probe sequences for RTQ-PCR of BCR-ABL and β2M were designed, tested, and standardized within the EU concerted action (Table 1). Plasmid dilution containing the PCR target sequences of BCR-ABL and β2M, used to generate the standard curves of the assays, were purchased by IPSOGEN (Marseille, France).
Sequences of primers and probes used for the assay of BCR/ABL and β2M mRNA levels
No. . | Sequence (5′-3′) . | Description . |
|---|---|---|
| ENF501 | TCCGCTGACCATCAACAAGGA | Fwd BCR exon 13 |
| ENP541 | Fam - CCCTTCAGCGGCCAGTAGCATCTGA - Tamra | ABL exon 2 |
| ENR561 | CACTCAGACCCTGAGGCTCAA | Rev ABL exon 2 |
| B2M-F | TGCCGTGTGAACCATGTGAC | Fwd β2M exon 2 |
| B2M-P | Fam-TGTCACAGCCCAAGATAGTTAAGTGGGATCG- Tamra | β2M exon 2 |
| B2M-R | ACCTCCATGATGCTGCTTACA | Rev β2M exon 3 |
No. . | Sequence (5′-3′) . | Description . |
|---|---|---|
| ENF501 | TCCGCTGACCATCAACAAGGA | Fwd BCR exon 13 |
| ENP541 | Fam - CCCTTCAGCGGCCAGTAGCATCTGA - Tamra | ABL exon 2 |
| ENR561 | CACTCAGACCCTGAGGCTCAA | Rev ABL exon 2 |
| B2M-F | TGCCGTGTGAACCATGTGAC | Fwd β2M exon 2 |
| B2M-P | Fam-TGTCACAGCCCAAGATAGTTAAGTGGGATCG- Tamra | β2M exon 2 |
| B2M-R | ACCTCCATGATGCTGCTTACA | Rev β2M exon 3 |
To ensure that RNA was not degraded, the samples that gave β2M Ct values higher than 25.7 were discarded. Total number of discarded samples was 27 (7.7%) of 931.
Statistics
The primary efficacy variable of the study was the rate of the CCgR at 1 year. Because it was assumed that the minimum target response probability P1 was 0.30 versus an uninteresting response probability P0 = 0.15, the calculated sample size was 79, with α = 0.025 one-sided and β = 0.90. The main secondary variables were duration of CCgR and molecular response (MR). Comparisons of frequencies were made with the χ2 test or the Fisher exact test as appropriate. Comparisons between groups were performed by the log-rank test.28 Overall survival and time to progression to accelerated or blastic phase were calculated by the product limits method of Kaplan and Meier.29 All statistical calculations were performed by SPSS.9 software (SSPS, Chicago, IL).
Patients
The calculated sample size was 79 cases, based on the expected primary efficacy variable (ie, a CCgR rate of > 30%). This expectation was met in the first 79 cases, but the pressure for the treatment was so strong that before the response could be analyzed another 112 patients were enrolled over 4 months, for a total of 191 cases. Thirty-two patients (17%) had hematologic failure, 103 (54%) had cytogenetic failure, and 56 (29%) were intolerant of IFN-α. These 3 groups were not different for age (median 47, 48, and 52 years), hemoglobin concentration (median, 120, 127, and 131 g/L), white cell count (median, 12, 10, and 11 × 109/L), and platelet count (median, 299, 291, and 283 × 109/L). The spleen was enlarged and palpable in 60% of those with hematologic failure, 27% of those with cytogenetic failure, and 39% of those intolerant to IFN-α. The time lapsed from diagnosis of CML to treatment ranged between 1 and 160 months (median, 38 months) and was longer for cytogenetic failure (median, 50 months) than for hematologic failure (median, 34 months) and intolerance (median, 28 months). All the patients have been observed for a minimum of 12 months (range, 12-36 months; median, 26 months).
Results
HRs and CgRs
The HR and CgR rate during the 1-year study period is reported in Table 2. Eighty-nine percent of patients achieved a CHR, and 80% were in continuous CHR after 1 year. Eighty-five patients (44%) achieved a CCgR at least once and another 33 patients achieved a PCgR, for an MCgR rate of 61%. The rate of the MCgRs did not increase much from 3 months (41%) to 12 months (48%), but the quality of the CgR improved significantly, the CCgR rate doubling from 16% at 3 months to 33% at 12 months. The rates of CHR and CCgR tended to be lower in those with hematologic failure (84% and 37%, respectively) than in those with cytogenetic failure (88% and 44%, respectively) and those intolerant to IFN-α (93% and 50%) but none of these differences reached a level of statistical significance. No relationship was detectable between the response to imatinib and prior exposure to other drugs (low-dose arabinosyl cytosine or busulfan).
Hematologic and cytogenetic response during the 1-year study period
. | Hematologic response . | . | . | Cytogenetic response . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Complete . | . | . | Complete . | . | Major . | . | ||||||
| Time . | NE, no. . | No. . | % . | NE, no. . | No. . | % . | No. . | % . | ||||||
| 3 mo | 0 | 170 | 89 | 36 | 30 | 16 | 79 | 41 | ||||||
| 6 mo | 0 | 171 | 89 | 33 | 51 | 27 | 85 | 44 | ||||||
| 9 mo | 5 | 164 | 86 | 38 | 55 | 29 | 80 | 42 | ||||||
| 12 mo | 6 | 153 | 80 | 27 | 64 | 33 | 91 | 48 | ||||||
| Overall | 0 | 171 | 89 | 2 | 85 | 44 | 118 | 61 | ||||||
. | Hematologic response . | . | . | Cytogenetic response . | . | . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Complete . | . | . | Complete . | . | Major . | . | ||||||
| Time . | NE, no. . | No. . | % . | NE, no. . | No. . | % . | No. . | % . | ||||||
| 3 mo | 0 | 170 | 89 | 36 | 30 | 16 | 79 | 41 | ||||||
| 6 mo | 0 | 171 | 89 | 33 | 51 | 27 | 85 | 44 | ||||||
| 9 mo | 5 | 164 | 86 | 38 | 55 | 29 | 80 | 42 | ||||||
| 12 mo | 6 | 153 | 80 | 27 | 64 | 33 | 91 | 48 | ||||||
| Overall | 0 | 171 | 89 | 2 | 85 | 44 | 118 | 61 | ||||||
The calculations are always based on all 191 cases (intention-to-treat). All cases but 2 were evaluable for the response at least once (overall), whereas several cases were nonevaluable (NE) for cytogenetic response at each time point.
Duration of CgR
Eighty-five patients achieved a CCgR at least once during the 1-year study period. In 9 patients the response was recorded only once, was unstable, and was lost quickly to partial, minor, minimal, or none. One of these 9 patients progressed to accelerated or blastic phase. In the remaining 76 patients the CCgR was stable during the study period. In the subsequent year of follow-up, one of them committed suicide and 8 lost the CCgR. Therefore, 67 (79%) of 85 cases who achieved a CCgR at least once are still in continuous CCgR after 2 years of treatment, corresponding to 35% of all study patients (Table 3).
Stability and duration of CCgRs
Patients . | No. . | % . |
|---|---|---|
| CCgRs | 85/191 | 44 |
| CCgRs unstable or lost in 1 y* | 9/85 | 10 |
| CCgRs lost after more than 1 y† | 9/76 | 12 |
| Patients remaining in CCgR for 2 y | 67/85 | 79 |
| Patients remaining in CCgR for 2 y/all patients | 67/191 | 35 |
Patients . | No. . | % . |
|---|---|---|
| CCgRs | 85/191 | 44 |
| CCgRs unstable or lost in 1 y* | 9/85 | 10 |
| CCgRs lost after more than 1 y† | 9/76 | 12 |
| Patients remaining in CCgR for 2 y | 67/85 | 79 |
| Patients remaining in CCgR for 2 y/all patients | 67/191 | 35 |
The rate of complete response loss was of about 10% per year.
One of 9 progressed to accelerated or blastic phase
One of 9 progressed to accelerated or blastic phase and 1 of 9 committed suicide in CCgR
CgR and prior disease duration
The frequency of stable CCgRs was the same in the patients with a prior disease duration of less than 1 year (10 of 19 or 53%) as in the patients with a prior disease duration of 1 to 2 years (22 of 41 or 54%), but was significantly less in the patients with a prior disease duration of more than 2 years (44 of 131 or 34%; P = .04, χ2 test).
CgR and treatment dose
Imatinib was discontinued permanently in 9 patients and temporarily in 99 (52%), for a total of 228 times (2.3 times for patients). The treatment dose was calculated for all the patients over the entire 1-year study period. For the 76 patients who achieved a stable CCgR the mean daily dose was 375 mg (SD 52), or 94% of scheduled. For the other patients the mean daily dose was 331 mg (SD 88), or 83% of scheduled (P = .001, Student t test).
CgR and other cytogenic abnormalities
Cytogenic abnormalities were identified in 21 (11%) of 191 patients prior to study treatment. These additional abnormalities were always detected in the Ph+ clone and disappeared with the Ph+ clone in all the patients who achieved a CCgR. In this small group of 21 patients, the CgR was not different from that seen in the other patients, because 8 patients achieved a CCgR and 2 a PCgR. None of these responses was lost after 2 years. However, these responses were seen only in the cases with trisomy 8 (6 of 8) and in the cases with miscellaneous abnormalities (4 of 8), but not in cases with trisomy Ph, isochromosome 17, or Y loss (0 of 5).
CgR and molecular data
The type of the transcript was identified in 186 (97%) of 191 patients and was b3a2 (or b3a2/b2a2) in 101 patients, b2a2 in 82, and e19a2 (p230) in 3 cases. The major and the complete CgR rates were similar, 56% and 40% for b3a2 patients versus 49% and 34% for a2b2 patients. One of the 3 patients with e19a2 (p230) achieved a CCgR. The amount of the BCR/ABL transcript prior to the treatment (baseline) was measured in bone marrow cells of 160 cases (84%). It was not different in the patients who achieved a stable CCgR and in those who had a partial or minor or minimal response. However, in the 31 patients with no response at all the transcript level prior to treatment was significantly higher than in any other response category (Table 4). The baseline transcript level was not different in the patients with hematologic or cytogenetic resistance to IFN-α, as well as in the patients who were intolerant of IFN-α.
Transcript level at baseline and cytogenetic response
. | . | Transcript level . | . | . | ||
|---|---|---|---|---|---|---|
| Cytogenetic response . | No. of cases . | Median . | Mean . | SD . | ||
| Complete, stable | 62 | 0.246 | 0.409 | 0.500 | ||
| Partial to minimal | 67 | 0.253 | 0.362 | 0.330 | ||
| None | 31 | 0.583 | 0.631 | 0.558 | ||
. | . | Transcript level . | . | . | ||
|---|---|---|---|---|---|---|
| Cytogenetic response . | No. of cases . | Median . | Mean . | SD . | ||
| Complete, stable | 62 | 0.246 | 0.409 | 0.500 | ||
| Partial to minimal | 67 | 0.253 | 0.362 | 0.330 | ||
| None | 31 | 0.583 | 0.631 | 0.558 | ||
The transcript level is expressed as the ratio between BCR/ABL and β2M × 100. The level was identical in the patients who achieved a response, either complete and stable or partial to minimal. However, the transcript level was significantly higher in the patients who had no response at all (P ≤ .004, Kruskall-Wallis test). Notice that the transcript level could not be measured in 31 patients, including 14 with a complete and stable cytogenetic response.
Molecular response
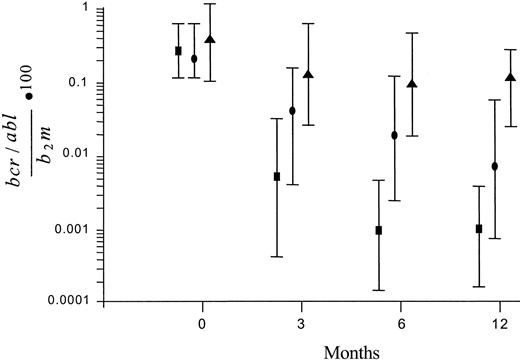
The amount of the BCR/ABL transcript in bone marrow cells was measured prior to treatment (baseline), at intervals during treatment (3 and 6 months), and at the end of the 1-year study period (12 months). In the patients who had achieved a CCgR, the molecular response (MR) was assessed also after 24 months. Figure 1 shows the patterns of MR according to the degree of CgR. In the nonresponders (ie, in the patients who achieved a less than PCgR or an unstable CgR), the level of the transcript remained substantially stable during the treatment. In the complete and stable cytogenic responders the amount of the transcript was already significantly decreased by more than 1 log, from 0.2330 at baseline to 0.0049 after 3 months, and was decreased further to 0.0009 after 6 months and to 0.0008 after 12 months (median values). In the PCgRs the decrease of transcript level was also significant, but the maximum decrease was less than 2 logs.
Patterns of MR according to the degree of CgR. The transcript level is expressed as the ratio of BCR/ABL to β2M × 100. Values are median ± SD. The reduction was small and nonsignificant in the patients who achieved a minor, a minimal, or no CgR (▴), was about 2 logs in the those who achieved a PCgR (•), and was greater, close to 3 logs, in the patients who achieved a stable and CCgR (▪). The number of patients with CCgRs with molecular studies was 61 at baseline, 57 at 3 and 6 months, and 55 at 12 months.
Patterns of MR according to the degree of CgR. The transcript level is expressed as the ratio of BCR/ABL to β2M × 100. Values are median ± SD. The reduction was small and nonsignificant in the patients who achieved a minor, a minimal, or no CgR (▴), was about 2 logs in the those who achieved a PCgR (•), and was greater, close to 3 logs, in the patients who achieved a stable and CCgR (▪). The number of patients with CCgRs with molecular studies was 61 at baseline, 57 at 3 and 6 months, and 55 at 12 months.
The patterns of the MR in the 76 patients who had achieved a complete and stable CgR is shown in Table 5. The results are reported for the whole group of CCgRs and separately, for early and late responders, that is, for the 54 patients who achieved the CCgR in 3 or 6 months (early) and for the 22 patients who achieved the CCgR in 9 or 12 months (late). After 24 months of treatment the difference between early and late responders became insignificant (transcript level was 0.0001 in early responders versus 0.0002 in late responders, median values). In Table 5 are listed also the cases where the amount of the transcript was below the limit of the detection power of the method (≤ 0.00001). The limit was already reached after 3 months in some cases, and after 24 months in 22 cases, which corresponds to 29% of the 76 CCgRs and to 11% of the 191 patients who were evaluable for the study. These MRs were seen with about the same frequency in early cytogenetic responders (16 of 54 or 30%) and in late cytogenetic responders (6 of 22 or 27%; Table 5).
BCR/ABL transcript level in the patients who achieved a complete and stable CgR
. | Transcript level, median . | . | . | No. of cases with a transcript less than 0.00001/ no. of analyzed cases . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time . | Early . | Late . | Total . | Early . | Late . | Total . | ||||
| Baseline | 0.2330 | 0.2490 | 0.2330 | 0/43 | 0/18 | 0/61 | ||||
| 3 mo | 0.0039 | 0.0213 | 0.0049 | 8/38 | 2/19 | 10/57 | ||||
| 6 mo | 0.0003 | 0.0046 | 0.0009 | 10/39 | 2/12 | 12/57 | ||||
| 12 mo | 0.0005 | 0.0034 | 0.0008 | 6/37 | 2/18 | 8/55 | ||||
| 24 mo | 0.0001 | 0.0002 | 0.0001 | 16/30 | 6/16 | 22/46 | ||||
. | Transcript level, median . | . | . | No. of cases with a transcript less than 0.00001/ no. of analyzed cases . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time . | Early . | Late . | Total . | Early . | Late . | Total . | ||||
| Baseline | 0.2330 | 0.2490 | 0.2330 | 0/43 | 0/18 | 0/61 | ||||
| 3 mo | 0.0039 | 0.0213 | 0.0049 | 8/38 | 2/19 | 10/57 | ||||
| 6 mo | 0.0003 | 0.0046 | 0.0009 | 10/39 | 2/12 | 12/57 | ||||
| 12 mo | 0.0005 | 0.0034 | 0.0008 | 6/37 | 2/18 | 8/55 | ||||
| 24 mo | 0.0001 | 0.0002 | 0.0001 | 16/30 | 6/16 | 22/46 | ||||
Complete and stable CgR achieved either early (in 3 or 6 months) or late (in 9 or 12 months). Also listed are the patients in whom the transcript level was below the sensitivity of the method (BCR/ABL to β2M ratio × 100 ≤ 0.00001). Notice that molecular data were missing in 15 patients at baseline and in 30 patients after 24 months.
Table 6 shows the distribution of complete and stable CgRs according to the maximum reduction of the BCR/ABL level. It was less than 2 logs in 9 patients, of whom 3 have lost the CCgR. It was between 2 and 4 logs in 33 patients, of whom 2 have lost the CCgR, and was equal to or more than 4 logs in the others. Although these relationships cannot be significant, due to the small number of cases and events, it is interesting to notice that only one of 18 very good molecular responders lost the CCgR.
Maximum reduction, expressed as a logarithm, of the BCR/ABL level and its relationship with CCgR loss
Maximum reduction of BCR/ABL transcript . | No. of patients . | No. of patients who lost the CCgR . |
|---|---|---|
| Less than 2 logs | 9 | 3 |
| 2-3.9 logs | 33 | 2 |
| 4 logs or more | 18 | 1 |
| NE | 16 | 2 |
| Total | 76 | 8 |
Maximum reduction of BCR/ABL transcript . | No. of patients . | No. of patients who lost the CCgR . |
|---|---|---|
| Less than 2 logs | 9 | 3 |
| 2-3.9 logs | 33 | 2 |
| 4 logs or more | 18 | 1 |
| NE | 16 | 2 |
| Total | 76 | 8 |
NE indicates nonevaluable.
Adverse events
Seventy-seven patients (40%) had at least one hematologic grade 3 or 4 adverse event (AE), which was neutropenia in 45 cases (24%), thrombocytopenia in 16 cases (8%), and both neutropenia and thrombocytopenia in 16 cases (8%). The total numbers of grade 3 and 4 neutropenias were 141 and 18, respectively, with a frequency of 0.74 and 0.09 per patient per year. The total numbers of grade 3 and 4 thrombocytopenias were 54 and 5, respectively, with a frequency of 0.28 and 0.03 per patient per year. The total numbers of nonhematologic AEs grade 1, 2, 3, and 4 were 521, 155, 27, and 4, respectively, with a frequency of 2.73, 0.81, 0.14, and 0.02 AEs per patient per year. These nonhematologic AEs are listed in Table 7.
Number and type of nonhematologic AEs by grade
Type of AE . | Grade 1 . | Grade 2 . | Grade 3/4 . |
|---|---|---|---|
| Constitutional | 158 (0.83) | 45 (0.23) | 9 (0.05) |
| Skin | 47 (0.25) | 21 (0.11) | 4 (0.02) |
| Gastrointestinal | 125 (0.65) | 24 (0.12) | 0 |
| Liver | NR | 9 (0.05) | 9 (0.05) |
| Edema | 77 (0.40) | 14 (0.07) | 2 (0.01) |
| Infections | 33 (0.17) | 12 (0.06) | 0 |
| Neurologic | 8 (0.04) | 8 (0.04) | 0 |
| Psychiatric | 10 (0.05) | 8 (0.04) | 0 |
| Cardiovascular | 10 (0.05) | 4 (0.02) | 3* (0.02) |
| Hemorrhage | 27 (0.14) | 5 (0.03) | 2* (0.01) |
| Other | 36 (0.19) | 6 (0.03) | 2** (0.01) |
| Total AEs | 521 (2.73) | 156 (0.81) | 31 (0.16) |
Type of AE . | Grade 1 . | Grade 2 . | Grade 3/4 . |
|---|---|---|---|
| Constitutional | 158 (0.83) | 45 (0.23) | 9 (0.05) |
| Skin | 47 (0.25) | 21 (0.11) | 4 (0.02) |
| Gastrointestinal | 125 (0.65) | 24 (0.12) | 0 |
| Liver | NR | 9 (0.05) | 9 (0.05) |
| Edema | 77 (0.40) | 14 (0.07) | 2 (0.01) |
| Infections | 33 (0.17) | 12 (0.06) | 0 |
| Neurologic | 8 (0.04) | 8 (0.04) | 0 |
| Psychiatric | 10 (0.05) | 8 (0.04) | 0 |
| Cardiovascular | 10 (0.05) | 4 (0.02) | 3* (0.02) |
| Hemorrhage | 27 (0.14) | 5 (0.03) | 2* (0.01) |
| Other | 36 (0.19) | 6 (0.03) | 2** (0.01) |
| Total AEs | 521 (2.73) | 156 (0.81) | 31 (0.16) |
The numbers of AEs are listed and, in parentheses, the frequency of the AEs (no. of AEs per patient per year). Grade 2 to 4 AEs were reported in 87 (45%) of 191 patients. In the remaining 104 patients AEs were either grade 1 or not reported at all. Constitutional symptoms included muscle cramps, myalgia, arthralgia, musculoskeletal pain, fatigue, fever, and headache. Skin symptoms were rash and related events and pruritus. Gastrointestinal symptoms included nausea, vomiting, mucositis, abdominal pain, and diarrhea. Edema was generalized, superficial, orbital, facial, or weight gain.
NR indicates not reported.
Grade 4 AEs, which were recorded 4 times
AEs led to permanent treatment discontinuation in 9 patients, for thrombocytopenia and liver toxicity (2 patients each), and for gastrointestinal hemorrhage, skin rash, constitutional symptoms (fever and pain), myocardial infarction, and bronchogenic carcinoma (one patient each). The frequency of all the AEs was maximum during the first months of treatment and declined thereafter very rapidly and substantially (Table 8), from 0.20 to 0.10 to 0.03 for hematologic AEs and from 0.76 to 0.31 to 0.13 for nonhematologic AEs.
Number and frequency of hematologic and nonhematologic AEs by time
AEs . | First quarter . | . | Second quarter . | . | Second half year . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Hema AE | |||||||||
| Grade 3 | 100 | 0.17 | 49 | 0.09 | 31 | 0.03 | |||
| Grade 4 | 15 | 0.03 | 4 | 0.007 | 1 | < 0.001 | |||
| Total | 115 | 0.20 | 53 | 0.10 | 32 | 0.03 | |||
| Nonhema AE | |||||||||
| Grade 1 | 324 | 0.56 | 121 | 0.23 | 99 | 0.10 | |||
| Grade 2 | 98 | 0.15 | 37 | 0.06 | 30 | 0.03 | |||
| Grade 3 | 15 | 0.03 | 6 | 0.01 | 6 | 0.006 | |||
| Grade 4 | 1 | 0.001 | 2 | 0.004 | 1 | < 0.001 | |||
| Total | 438 | 0.76 | 166 | 0.31 | 136 | 0.13 | |||
AEs . | First quarter . | . | Second quarter . | . | Second half year . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Hema AE | |||||||||
| Grade 3 | 100 | 0.17 | 49 | 0.09 | 31 | 0.03 | |||
| Grade 4 | 15 | 0.03 | 4 | 0.007 | 1 | < 0.001 | |||
| Total | 115 | 0.20 | 53 | 0.10 | 32 | 0.03 | |||
| Nonhema AE | |||||||||
| Grade 1 | 324 | 0.56 | 121 | 0.23 | 99 | 0.10 | |||
| Grade 2 | 98 | 0.15 | 37 | 0.06 | 30 | 0.03 | |||
| Grade 3 | 15 | 0.03 | 6 | 0.01 | 6 | 0.006 | |||
| Grade 4 | 1 | 0.001 | 2 | 0.004 | 1 | < 0.001 | |||
| Total | 438 | 0.76 | 166 | 0.31 | 136 | 0.13 | |||
The frequency provides an estimate of the number of AEs per patient per month and was calculated dividing, for each time period, the number of AEs by the number of months and by the number of patients at risk (191 in the first quarter, 178 in the second quarter, and 170 in the second half year).
Hema indicates hematologic; nonhema, nonhematologic.
Follow-up
During the trial time, which was 1 year, 21 of 191 patients abandoned the study treatment, one for protocol violation, 11 for progression to accelerated or blastic phase, and 9 for AEs. Two of the latter progressed to accelerated or blastic phase after treatment discontinuation, so that the total progression rate at 1 year was 13 of 191 or 7%. With a median follow-up of 26 months (range, 12-36 months) the overall rate of progression to accelerated or blastic phase is 17 of 191 or 9%, with a small but significant difference in favor of MCgRs by comparison with minor and nonresponders (4% versus 13%; P = .037 by log-rank test; Figure 2). Figure 2 also shows overall survival, which is 97% for MCgRs versus 92% for the others (P = .122 by log-rank test). The number of events (progression or death) was too small to detect any possible difference between PCgRs and CCgRs.
Overall survival and time to progression. Overall survival (top curve) and time to progression to accelerate or blastic phase accelerated or blastic phase (bottom curve) according to the degree of CgR, major (complete plus partial, solid line) versus minor or minimal or none (dotted line). With a median observation time of 26 months, survival is 97% for MCgRs versus 92% for the others (P = .122) and the cumulative rate of progression to accelerated or blastic phase is 4% for MCgRs versus 13% for the others (P = .037; log-rank test, Kaplan-Meier estimates).28,29 The analysis was not corrected for the time to response, because almost all MCgRs were achieved during the first 3 months, and in this period there were no events.
Overall survival and time to progression. Overall survival (top curve) and time to progression to accelerate or blastic phase accelerated or blastic phase (bottom curve) according to the degree of CgR, major (complete plus partial, solid line) versus minor or minimal or none (dotted line). With a median observation time of 26 months, survival is 97% for MCgRs versus 92% for the others (P = .122) and the cumulative rate of progression to accelerated or blastic phase is 4% for MCgRs versus 13% for the others (P = .037; log-rank test, Kaplan-Meier estimates).28,29 The analysis was not corrected for the time to response, because almost all MCgRs were achieved during the first 3 months, and in this period there were no events.
Discussion
This paper reports on a prospective multicenter study of imatinib in patients with late chronic-phase Ph+ CML previously treated with IFN-α. The treatment plan and the study protocol were the same as used in a prior international study reporting on 454 patients with late chronic-phase Ph+ CML in whom treatment with IFN-α failed or who were intolerant of IFN-α.13 In that study the CHR rate was 95%, the MCgR rate was 60%, and the CCgR rate was 41% versus 89%, 63%, and 44%, respectively, in our study. Also the toxicity profile was almost identical; in addition we were able to show that the frequency of all the AEs decreased very substantially with time (Table 8). These results provide independent confirmation of the efficacy and toxicity profile of imatinib in late chronic-phase CML. However, we cannot provide data on the relationship between the response to imatinib and the risk, as suggested in the patients who are treated frontline,18,30 because all our patients were pretreated.
In addition to hematologic and cytogenetic response, this study reports also on the MR and provides data on minimal residual disease after 2 years of treatment. In CML, MRs are rare and transitory with conventional and intensified chemotherapy.31,32 With IFN-α, although several patients achieve a complete and stable CgR, a molecular negativity is rarely seen, but the greater the reduction of BCR/ABL transcripts the better the long-term outcome.22,25,33,34 AlloHSCT induces a CHR and a CCgR in almost all cases. Longitudinal molecular studies after alloHSCT have shown that a small amount of transcript can be detected within 1 year from transplantation, but that once the transcript becomes undetectable, the disease is almost always cured.20,21,35 However, also in the setting of alloHSCT, the use of more refined and more sensitive molecular techniques, including molecular genetics, suggested that the disease may be traceable also in patients who are apparently cured.23
With imatinib, which has several potential advantages over IFN-α and alloHSCT because of a very high response rate and a low toxicity profile, the proportion of CCgR is so high that the attention must be focused on the MR, and a couple of questions require an answer: whether the molecular level of the disease could or should be used to modulate the treatment and whether the disease can become molecularly undetectable, either occasionally as with IFN-α or frequently as with alloHSCT. To date, the MR to imatinib has been discussed at several meetings where it has been reported that it was significantly greater than the MR to IFN-α, although “complete” MRs appear to be rare, as yet.36,37
In this paper we report on the MR to imatinib in a cohort of patients with late chronic-phase Ph+ CML who were under treatment for 2 years, with special attention to those who achieved a CCgR and were therefore candidates for a long survival. Although the findings are still provisional, some points can be made. First, there is no suggestion that the response to imatinib may in part be dependent on the type of the transcript, either b3a2 or b2a2. One of 3 b19a2 (p230) patients achieved a CCgR, but the small number of these patients does not allow us to draw conclusions on this particular and rare transcript. Second, the response to imatinib may be influenced by the baseline molecular level of the disease, the greater the level the lower the chance of achieving a response (Table 4). This may not be surprising because the amplification or the overexpression of the BCR/ABL gene is already recognized as a cause of resistance.38 Third, the grade of MR parallels the grade of CgR (Figure 1). A reduction of 1 or 2 logs is associated only with a PCgR, whereas almost all the complete and stable cytogenic responders have a reduction of more than 2 logs, up to 4 logs and more (Table 6). Another point of interest is the pattern and the rapidity of the response. In some patients the CCgR was shown very quickly, after 3 or 6 months of treatment. In other patients a CCgR could be detected only later, after 9 or 12 months of treatment. We have called these patients early and late complete cytogenic responders, respectively, and we have examined if at subsequent follow-up their behavior was different (Table 5). We found that in late responders the response was only delayed but we could not find any evidence that after 2 years of treatment the MR of late cytogenic responders was worse than that of early cytogenic responders. As a matter of fact, after 24 months of treatment the amount of residual disease was almost identical, as well as the frequency of the cases with a transcript level below the limits of detectability (Table 5). The meaning of these findings is still unclear, but they warn that assessing the sensitivity and the response to imatinib may require at least 1 year and that late responders are not rare.
Other points of major interest are the frequency of molecular cures and the relationship between the degree of MR and the subsequent course of the disease. The frequency of molecular cures cannot be calculated until molecular technologies are standardized and the definition of molecular cure is agreed on. In this study, the lowest limit of transcript detectability was expressed as a BCR/ABL to β2M ratio × 100 equal to or less than 0.00001. In some patients this level was reached as early as after 3 months, but these good MRs became more frequent with time (Table 5). After 2 years they were 22 (29% of the 76 CCgRs and 11% of all 191 evaluable patients). As shown in Table 6, in these good MRs the risk of losing the CCgR is likely to be lower than in the others. Indeed, whereas 3 of 9 cases with a decrease in the BCR/ABL transcript amount of less than 2 logs lost their CCgR, this event occurred in only 3 of 51 cases with a decrease in BCR/ABL of more than 2 logs. These data suggest that the degree of MR may be important to predict the clinical outcome in CML patients treated with imatinib and underline the need of monitoring these patients for the assessment of MR. However, it is not yet clear if the value of MR will be the same as after alloHSCT, where the lowest limit of detectability of the method is reached in 2 years in more than 80% of patients and predicts for cure.
These data were obtained in a heterogeneous cohort of previously IFN-α–treated, late chronic-phase Ph+ CML patients. In patients with previously untreated, early chronic-phase CML the molecular results of imatinib treatment are still pending and may be expected to be better, because the cytogenetic response rate is substantially higher.18 To date, monitoring the MR to imatinib cannot yet substitute for the CgR but there is little doubt that as soon as more information becomes available and the techniques are more standardized, the molecular definition and characterization of residual disease will become mandatory for the management of Ph+ CML.39
Appendix
The following members of the ICSG on CML have actively participated in this study:
E. Pogliani and M. Miccolis (Monza); M. Gobbi and M. Miglino (Genova); M. Lazzarino and S. Merante (Pavia); R. Fanin and M. Tiribelli (Udine); D. Russo (Brescia); G. Alimena and E. Montefusco (Roma); G. Rossi and A. Capucci (Brescia); F. Nobile and M. Martino (Reggio Calabria); A. Bacigalupo and F. Frassoni (Genova); B. Rotoli and L. Luciano (Napoli); F. Ferrara and E. Schiavone (Napoli); M. Martelli and A. Tabilio (Perugia); T. Barbui and R. Bassan (Bergamo); V. Rizzoli and L. Mangoni (Parma); F. Lauria and M. Bocchia (Siena); E. Volpe and F. Palmieri (Avellino); S. Amadori and A. Cantonetti (Roma); M. Pini (Alessandria); G. Specchia (Bari); A. di Tucci (Cagliari); G. L. Scapoli (Ferrara); E. Pungolino (Milano); F. Iuliano (Catanzaro); S. Rupoli (Ancona); P. Guglielmo (Catania); F. Porretto (Palermo); A. Liberati (Perugia); E. Zuffa (Ravenna); M. Cervellera (Taranto); D. Ferrero (Torino); M. Candela (Ancona); C. Bergonzi (Cremona); D. Noli (Nuoro); G. Marini Caracciolo (Palermo); A. Bonati (Parma); F. Papineschi (Pisa); P. Pregno (Torino); and A. Ambrosetti (Verona).
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-07-2575.
A complete list of the members of the ICSG on Chronic Myeloid Leukemia appears in the “Appendix.”
Supported by COFIN 2003 (molecular therapy of Ph+ leukemias), by Fondazione del Monte di Bologna e Ravenna, by the University of Bologna (grants 60%), by the Italian Association for Cancer Research (AIRC), by the National Research Council, and by Regione Campania. D.A. is an employee of Novartis Pharma Italy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The assistance of Katia Vecchi and Maira Marsili is kindly acknowledged.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal