Abstract
In the current era of effective prophylactic and preemptive therapy, cytomegalovirus (CMV) is now a rare cause of early mortality after hematopoietic stem cell transplantation (HSCT). However, the ultimate goal of completely eliminating the impact of CMV on survival remains elusive. Although the direct effects of CMV (ie, CMV pneumonia) have been largely eliminated, several recent cohort studies show that CMV-seropositive transplant recipients and seronegative recipients of a positive graft appear to have a persistent mortality disadvantage when compared with seronegative recipients with a seronegative donor. Recipients of T-cell–depleted allografts and/or transplants from unrelated or HLA-mismatched donors seem to be predominantly affected. Reasons likely include both incomplete prevention of direct and indirect or immunomodulatory effects of CMV as well as consequences of drug toxicities. The effect of donor CMV serostatus on outcome remains controversial. Large multicenter cohort studies are needed to better define the subgroups of seropositive patients that may benefit from intensified prevention strategies and to define the impact of CMV donor serostatus in the era of high-resolution HLA matching. Prevention strategies may require targeting both the direct and indirect effects of CMV infection by immunologic or antiviral drug strategies.
Introduction
Prior to the arrival of effective antiviral therapy in the early 1990s, cytomegalovirus (CMV) disease was the leading infectious cause of death among CMV-seropositive recipients of hematopoietic stem cell transplantation (HSCT). The initial goal of prevention strategies was to eliminate CMV disease, which presents as often-fatal pneumonia despite combination treatment with ganciclovir and immunoglobulin. Thus, both prophylaxis and preemptive therapy strategies have been extensively studied (reviewed in Boeckh et al1 ) and prevention protocols based on these strategies are now used in transplant centers around the world. As a result, CMV disease during the first 3 months after HSCT has been reduced from 20% to 30% to less than 5% in most studies.1 Despite these accomplishments, several lines of investigation indicate that overall survival remains inextricably linked to the pretransplant CMV serostatus of the patient. Thus, the overriding goal of antiviral supportive care—to reduce the treatment-related mortality for the CMV-seropositive patient to the level of a seronegative patient—has remained elusive (Figure 1).
Schema demonstrating the impact of pretransplant CMV infection on overall survival after allogeneic HSCT. Despite the near-complete elimination of early CMV disease with current strategies, a survival disadvantage persists for high-risk, CMV-seropositive patients (R+) compared with D–/R– patients.
Schema demonstrating the impact of pretransplant CMV infection on overall survival after allogeneic HSCT. Despite the near-complete elimination of early CMV disease with current strategies, a survival disadvantage persists for high-risk, CMV-seropositive patients (R+) compared with D–/R– patients.
Additional studies2-4 have also examined the impact of donor serostatus among seropositive recipients; these studies have come to conflicting conclusions. The issue of donor CMV serostatus is important, as it may be relevant for donor selection for both seronegative and seropositive transplant recipients. The CMV serostatus of the donor, however, must be considered as one selection factor among many that are important for transplantation outcome including HLA compatibility, donor age, and sex matching.5
Herein, we will review the effect of CMV serostatus of both the recipient and donor on transplant outcome in the current era of preemptive antiviral therapy. Directions for future research will be proposed.
CMV serostatus of the recipient
Despite the significant decline in the incidence of CMV disease among CMV seropositive HSCT recipients early after transplantation, several recent studies have indicated that a substantial mortality disadvantage still exists for the CMV-seropositive patient (Table 1). The studies differ with regard to sample size, time of follow-up, endpoints, underlying disease, and graft manipulation (ie, T-cell depletion); the multitude of factors known to be associated with mortality raises the issue of residual confounding in many of these studies. Large studies of CMV serostatus and mortality in more homogeneous populations, however, demonstrate similar findings.6,8
Impact of recipient CMV serostatus on outcome after HSCT: recent studies
Reference, by first author . | No. patients . | Underlying disease . | T-cell-depleted donors, % . | Unrelated donors, % . | Results among CMV-seropositive recipients compared with CMV-seronegative recipients with a seronegative donor . |
|---|---|---|---|---|---|
| Broers7 * | 115 | Mixed | 95 | 0 | 24% absolute decline in OS (P = .01) |
| McGlave8 | 1423 | CML | 23 | 100 | 20% relative decline in DFS (P = .002) |
| Cornelissen9 * | 127 | ALL | 26 | 100 | 38% relative decline in DFS (P = .05) |
| Craddock6 † | 106 | CML | 100 | 100 | 22% absolute decline in OS (P = .006) |
| Kroger10 | 125 | Mixed | 100 | 100 | 41% absolute decline in OS (P < .001) |
| Castro-Malaspina11 | 510 | MDS | 24 | 100 | 46% relative decline in DFS (P = .001) |
| Nichols4 | 1750 | Mixed | 0 | 57 | 26% relative decline in OS (P = .03) |
| Kollman3 | 6978 | Mixed | 25 | 100 | 7% absolute decline in OS (P < .001) |
| Meijer12 | 48 | Mixed | 100 | 100 | 41% absolute rise in TRM (P < .001) |
| Doney13 | 182 | ALL | 0 | 52 | 99% relative rise in TRM (P = .01) |
Reference, by first author . | No. patients . | Underlying disease . | T-cell-depleted donors, % . | Unrelated donors, % . | Results among CMV-seropositive recipients compared with CMV-seronegative recipients with a seronegative donor . |
|---|---|---|---|---|---|
| Broers7 * | 115 | Mixed | 95 | 0 | 24% absolute decline in OS (P = .01) |
| McGlave8 | 1423 | CML | 23 | 100 | 20% relative decline in DFS (P = .002) |
| Cornelissen9 * | 127 | ALL | 26 | 100 | 38% relative decline in DFS (P = .05) |
| Craddock6 † | 106 | CML | 100 | 100 | 22% absolute decline in OS (P = .006) |
| Kroger10 | 125 | Mixed | 100 | 100 | 41% absolute decline in OS (P < .001) |
| Castro-Malaspina11 | 510 | MDS | 24 | 100 | 46% relative decline in DFS (P = .001) |
| Nichols4 | 1750 | Mixed | 0 | 57 | 26% relative decline in OS (P = .03) |
| Kollman3 | 6978 | Mixed | 25 | 100 | 7% absolute decline in OS (P < .001) |
| Meijer12 | 48 | Mixed | 100 | 100 | 41% absolute rise in TRM (P < .001) |
| Doney13 | 182 | ALL | 0 | 52 | 99% relative rise in TRM (P = .01) |
CML indicates chronic myelogenous leukemia; ALL, acute lymphocytic leukemia; MDS, myelodysplastic syndrome; OS, overall survival; DFS, disease-free survival; TRM, transplant-related mortality.
The seropositive group includes some seronegative recipients with a seropositive donor.
The difference in OS is between seropositive recipients and seronegative recipients (some of whom have seropositive donors); the difference between seropositive recipients and D—/R— patients is 14% at 5 years.
Analysis of published studies suggests that specific subgroups of CMV-seropositive HSCT recipients appear to be at particularly high risk for treatment-related mortality in the current era. The use of T-cell depletion (either via graft manipulation ex vivo or via the use of anti–T-cell antibodies in vivo) appears to be one such factor. Indeed, most of the studies performed to date have focused on these patients (Table 1). A small study addressed the particular method of T-cell depletion, suggesting that the dose of antithymocyte globulin (ATG) is an important factor in the observed effect on survival.14 Larger studies are needed to confirm this hypothesis. CMV-seropositive recipients of transplants from unrelated donors may also be at higher risk for mortality; this association was demonstrated in a study by McGlave et al8 (where CMV seronegativity was still associated with a 20% lower hazard for mortality after controlling for graft manipulation) and the studies by Nichols et al4 and Meijer et al.12 A recently completed subanalysis of the study by Nichols et al indicates that the survival disadvantage associated with CMV seropositivity in T-cell–replete patients is restricted to recipients of HLA-mismatched or unrelated transplants (Table 2).4
Impact of donor HLA status on overall mortality in a cohort of T-cell-replete transplant recipients
Donor/recipient CMV serostatus . | Mortality for matched related donors (hazard ratio, 95% CI) . | Mortality for mismatched related or unrelated donors (hazard ratio, 95% CI) . |
|---|---|---|
| D-/R- | 1.0 (reference) | 1.0 (reference) |
| D+/R- | 0.98 (0.70-1.38) | 1.36 (1.06-1.74) |
| D-/R+ | 0.90 (0.65-1.24) | 1.29 (1.04-1.60) |
| D+/R+ | 1.07 (0.81-1.42) | 1.26 (1.01-1.58) |
Donor/recipient CMV serostatus . | Mortality for matched related donors (hazard ratio, 95% CI) . | Mortality for mismatched related or unrelated donors (hazard ratio, 95% CI) . |
|---|---|---|
| D-/R- | 1.0 (reference) | 1.0 (reference) |
| D+/R- | 0.98 (0.70-1.38) | 1.36 (1.06-1.74) |
| D-/R+ | 0.90 (0.65-1.24) | 1.29 (1.04-1.60) |
| D+/R+ | 1.07 (0.81-1.42) | 1.26 (1.01-1.58) |
Data from cohort previously published by Nichols et al.4 Shown are results of multivariable models, which controlled for patient/donor age, underlying disease and disease risk, conditioning regimen, cell source, GVHD prophylaxis, cell dose, and year of transplantation. Recipients of HLA-mismatched transplants from relatives and unrelated donors had similar outcomes (data not shown). For matched related donors, N = 749; for mismatched related or unrelated donors, N = 1001.
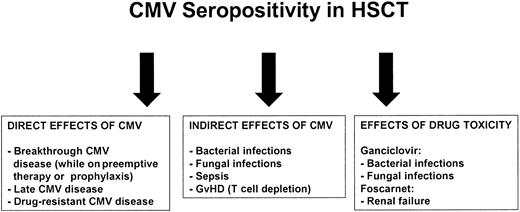
The mechanism for increased mortality among CMV-seropositive patients is often not presented, though transplant-related mortality rather than relapse accounts for the majority of the findings. Factors likely responsible for the poor outcome in the current era are illustrated in Figure 2. Some indirect effects (eg, nonviral infections) may be difficult to separate from consequences of drug toxicity (eg, ganciclovir-associated neutropenia). Both Broers et al7 and Cornelissen et al9 documented a higher incidence of acute graft-versus-host disease (GVHD) in CMV-seropositive recipients, which resulted in a high incidence of opportunistic infections and respiratory insufficiency. Craddock et al6 found no difference in GVHD, but more infectious death in the CMV-seropositive group. Meijer also failed to find a difference in acute GVHD according to serostatus, but a significant increase in infectious mortality was detected among seropositive recipients of unrelated donor transplants.12 The putative immunomodulatory effects of CMV infection have long been recognized in the setting of solid organ transplantation and HIV/AIDS, and have recently been reviewed.15
Possible reasons for association of CMV with poor outcome in high-risk patients.
Possible reasons for association of CMV with poor outcome in high-risk patients.
CMV serostatus of the donor
Selection of an appropriate HSCT donor usually begins with a search for an HLA-matched sibling. If one is not available, the use of stem cells from an HLA-matched unrelated donor is pursued. Outcome after unrelated donor transplantation has been associated with similar outcomes for some underlying diseases.16,17 Searches of donor registries may identify multiple suitable matches; in such instances, donor selection may be based on other factors, including CMV serostatus. It is known that many transplant physicians prefer CMV-seronegative donors.3 In theory, however, the selection of donor according to CMV serostatus may depend on the serostatus of the HSCT recipient. For the CMV-seronegative recipient, it may be preferable to utilize CMV-seronegative donors in order to reduce the possibility of primary CMV infection associated with a seropositive allograft.18 In contrast, the use of stem cells from a seropositive donor may be preferred in the seropositive recipient due to the potential for active transferred immunity, a phenomenon shown to be operative in the case of herpes simplex virus.19 As detailed below, however, the data to support these hypotheses have been conflicting.
In a landmark study that examined the effect of donor factors on transplant outcomes among almost 7000 patients receiving unrelated marrow transplants in the National Marrow Donor Program (NMDP) Registry from 1987 to1999, Kollman et al3 showed that donor age and HLA mismatch (but not donor CMV serostatus) were the primary drivers of transplant-related mortality. Although CMV seropositivity in the recipient was clearly associated with poorer outcome, the survival curves for donor-positive versus donor-negative were virtually superimposed for both the CMV-positive and -negative recipient (Figure 3, Table 3).3 In contrast, Ljungman et al2 conducted a similar analysis of over 7000 CMV-seropositive patients in the European Blood and Marrow Transplant Registry who received transplants over more than 2 decades, and found somewhat different results. No significant effect of donor serostatus could be discerned among the almost 6000 patients who received transplants from HLA-identical siblings, but transplant-related mortality and overall mortality were higher among 542 CMV-seropositive recipients of stem cells from seronegative unrelated donors when compared with the 566 seropositive recipients of stem cells from seropositive unrelated donors in both univariate and multivariable models (Figure 3, Table 3).2 Donor serostatus did not affect the risk for acute or chronic GVHD in that study, but there was a trend toward a higher rate of death associated with infections among patients who received grafts from CMV-seronegative donors (28% vs 23%, P = .07).
Effect of donor and recipient CMV serostatus on survival after HSCT in 2 large cohorts. Donor serostatus did not affect survival among seropositive or seronegative HSCT recipients in the NMDP study (curve on the left). In contrast, donor CMV serostatus affected overall survival among recipients of unrelated allografts in the European Group for Blood and Marrow Transplantation (EBMT) study (curve on the right). Figures used with permission.2,3
Effect of donor and recipient CMV serostatus on survival after HSCT in 2 large cohorts. Donor serostatus did not affect survival among seropositive or seronegative HSCT recipients in the NMDP study (curve on the left). In contrast, donor CMV serostatus affected overall survival among recipients of unrelated allografts in the European Group for Blood and Marrow Transplantation (EBMT) study (curve on the right). Figures used with permission.2,3
Recently published studies of donor CMV seropositivity's impact on mortality
. | . | . | Percent of patients with T-cell depletion . | Percent of transplants from unrelated donors . | Effect on mortality . | . | |
|---|---|---|---|---|---|---|---|
| Author . | N . | Setting . | . | . | Positive recipient . | Negative recipient . | |
| Kollman et al3 | 6878 | Mixed | 25 | 100 | None | None | |
| Nichols et al4 | 1001* | Mixed | 0 | 100 | None | Increased | |
| Ljungman et al2 | 1108 | Mixed | 27 | 100 | Decreased | NA | |
. | . | . | Percent of patients with T-cell depletion . | Percent of transplants from unrelated donors . | Effect on mortality . | . | |
|---|---|---|---|---|---|---|---|
| Author . | N . | Setting . | . | . | Positive recipient . | Negative recipient . | |
| Kollman et al3 | 6878 | Mixed | 25 | 100 | None | None | |
| Nichols et al4 | 1001* | Mixed | 0 | 100 | None | Increased | |
| Ljungman et al2 | 1108 | Mixed | 27 | 100 | Decreased | NA | |
Data restricted to unrelated donor transplants.
NA indicates not analyzed.
Also included recipients of HLA-mismatched transplants from related donors (see Table 2).
Reconciling these results is difficult, but of significant clinical importance. Given the option of an equally matched CMV-seronegative or -seropositive donor for a seropositive recipient, Ljungman et al2 suggest that selecting the seropositive unrelated donor for the seropositive patient decreases the hazard for overall mortality by up to 35%. Interestingly, donor serostatus appeared to alter the hazard of death in some diseases (eg, chronic myeloid leukemia [CML]) but not others (eg, acute leukemia); this may be due to the competing risks of relapse and noninfectious mortality, both of which are higher in patients with acute leukemia. Indeed, Ljungman and colleagues2 suggest that by adjusting for disease state (rather than examining disease groups separately), the NMDP study may have missed this interaction between CMV serostatus and disease. Other subgroups of interest (the influence of T-cell depletion or mismatched transplants, for example) did not appear to have been specifically analyzed in the context of donor serostatus in the NMDP study. Residual confounding may also explain some of the results, given that increasing donor age (the primary predictor of poor outcome in that study) was associated with CMV seropositivity. Finally, by including a significant number of patients from the pre–ganciclovir era (20%), the NMDP study may have also attenuated the true relationship between donor serostatus and outcome. Results were adjusted for time periods in both studies,2,3 however, it is possible that important differences in posttransplant prevention strategies and supportive care were not accounted for. The study by Nichols et al4 (which was performed exclusively in the era of effective prophylaxis and preemptive therapy) did not find a difference in survival between seropositive and seronegative donors for the seropositive transplant recipient, though all patients in that series received T-cell–replete transplants (Table 3).
For the CMV-seronegative recipient of allogeneic HSCT, it has been demonstrated that the receipt of stem cells from a seropositive donor (D+/R–) is associated with a significant increase in the hazard for overall mortality when compared with transplants from a seronegative donor, findings that have been offered as further evidence of the immunomodulatory properties of primary CMV in the HSCT setting.4 Notably, this difference has been particularly apparent in unrelated donor transplants (Table 2). Cwynarski et al20 also showed that seronegative children receiving stem cells from seropositive donors had decreased overall survival when compared with D–/R– patients. Kollman et al,3 however, did not confirm these findings in the setting of unrelated transplantation. Given the risk of primary infection due to the seropositive allograft, however, the prevailing practice of selecting a seronegative donor for the seronegative recipient seems prudent.
Conclusions and future work
Recipient CMV serostatus continues to be an important variable for overall survival after transplantation in the era of antiviral preemptive therapy and prophylaxis. This survival disadvantage appears largely restricted to highly immunosuppressed patients, such as recipients of T-cell–depleted grafts or grafts from HLA-mismatched or unrelated donors. Mechanistically, the increased mortality in seropositive recipients appears to be due to both direct and indirect effects of CMV as well as drug toxicities (Figure 1). The impact of donor seropositivity, however, remains controversial, particularly for seropositive recipients.
These results raise several questions that are in urgent need of investigation in order to optimize supportive care for the HSCT recipient.
1. What groups of CMV-seropositive and D+/R– patients are at highest risk for mortality in the current era?
Studies to date suggest that the survival disadvantage is limited to high-risk patients, yet these patients are not very well characterized. T-cell depletion and HLA-mismatched or unrelated donor status appear to be important variables for poor survival (Tables 1 and 2), but the impact of more sensitive HLA matching strategies,14,21-23 posttransplant antiviral prevention strategies, and the impact of underlying disease remain to be investigated. The method and degree of T-cell depletion may also be an important variable.14 To more accurately define groups of patients who might benefit from improved prevention strategies, analysis of large cohorts of patients who received transplants under current supportive care protocols (including preemptive or prophylactic anticytomegalovirus strategies and effective antifungal prophylaxis) are needed. The impact of high-resolution HLA matching strategies on CMV-related mortality is also likely to be important, and should be a focus of investigation. Also, the effect of serostatus with use of nonmyeloablative as well as reduced-toxicity conditioning regimens24,25 as well as socioeconomic status or ethnicity as confounding factors26,27 should be studied.
2. What is the mechanism of excess mortality among high-risk, CMV-seropositive patients?
Although the studies cited above show that seropositivity in the recipient is associated with increased mortality, less accurate and mostly retrospective information is available on the causes of death. Knowledge of the causes of death in seropositive recipients (relative to D–/R– patients) may provide important leads regarding operative mechanisms, and may also offer opportunities for intervention.
3. What strategies are available to improve outcomes in high-risk recipients?
If survival among D–/R– patients is used as the gold standard, the current literature indicates that preemptive therapy (the predominant strategy for allogeneic transplant recipients28,29 ) is less effective for high-risk recipients of HSCT. Thus, improved prevention strategies are needed and the issue of preemptive therapy versus prophylaxis should be revisited. By suppressing reactivation, prophylaxis may also decrease the indirect immunomodulatory effects of viral replication. However, significant disadvantages are ascribed to this strategy. Ganciclovir given at engraftment historically caused prolonged neutropenia and was associated with a higher risk of invasive bacterial and fungal infections.30-32 A delayed recovery of CMV-specific immune responses compared with patients who receive culture-based preemptive therapy has also been described,33 which may lead to an increased risk of late-onset CMV disease.34 An effect of ganciclovir prophylaxis on recovery of CMV-specific immunity was not seen when compared with antigenemia-based preemptive strategies.35
Thus, new anti-CMV drugs with an improved toxicity profile are needed to provide effective and well-tolerated prophylaxis in high-risk patients. Although drug development is being pursued,36-38 these new compounds are early in development and may not be readily available for several years. Immunologic strategies including adoptive immunotherapy and vaccine strategies are another option as they may overcome issues of drug toxicity by hastening immune reconstitution.41,42 There have been major laboratory improvements in T-cell therapy, which may make this therapy more readily available in the near future.41-46 The use of cytokines such as interleukin 7 (IL-7) may also prove useful.47 One potential obstacle for widespread use of immune-based strategies may be the use of systemic steroids.36 Clinical trials are needed to evaluate these strategies.
A look at presently available drugs may also be useful. A review of recently conducted studies indicates that the sequelae of ganciclovir-induced neutropenia (ie, bacterial and fungal infections) may not be as common as they once were with current management of neutropenia (via the use of growth factors and/or early discontinuation of ganciclovir in the setting of toxicity and/or alternative use of foscarnet) and better antifungal agents. In 2 randomized trials the incidence of severe nonviral infections was not increased between the ganciclovir and the control arms.30,48 Thus, it is possible that ganciclovir or valganciclovir prophylaxis may be of benefit in subgroups of highly immunosuppressed transplant recipients, where even preemptive strategies based on highly sensitive polymerase chain reaction (PCR)–based surveillance often fail.47 With improvements in supportive care (ie, early use of growth factors), use of alternative agents with different toxicity profiles (eg, foscarnet) in cases of neutropenia, improved diagnostics for fungal infections, and better agents for antifungal prophylaxis and treatment, it may be possible to design an effective and well-tolerated long-term antiviral prophylaxis strategy aimed at both the direct and indirect effects of CMV. These intensified strategies should be studied in a randomized fashion. Valacyclovir has not shown a strong antiviral effect in high-risk patients50 and is thus unlikely to be useful as a single agent in this setting. Whether it could be part of a strategy could be tested in randomized trials.
Finally, the available data suggest that CMV seropositivity is primarily associated with poor outcome in patients receiving T-cell–depleted HSCT, yet appears to be relatively benign among T-cell–replete transplants from related donors. This raises the question if alternatives to T-cell depletion should be pursued for the CMV-seropositive recipient with a family donor until improved prevention strategies are developed. Although evidence in support of this position stems from retrospective analyses rather from randomized trials, we believe that recognition and open debate of this issue are warranted. Recipients of T-cell–depleted grafts, however, may be particularly good candidates for adoptive transfer studies due to their lower risk of GVHD and thus lower utilization of steroid therapy. At the present time, however, no data exist on the efficacy of this approach in affecting outcome.
4. What priority should donor CMV serostatus assume in the process of donor selection?
As summarized above, the selection of a CMV-seronegative donor for the seronegative HSCT recipient seems prudent.4,49 For seropositive recipients, however, the impact of donor CMV status remains controversial.2,3 The European study had fewer numbers of “high-risk” transplant recipients, and was not able to entirely control for donor age; importantly, both studies also included patients from the era prior to effective CMV therapy, which may confound their results, and did not include high-resolution HLA typing. Future studies should focus on cohorts exclusively from the current era, and should include other important variables such as antiviral prevention strategy and degree of HLA match using contemporary techniques.
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-10-3616.
Supported in part by the National Institutes of Health (grant nos. CA18029, AI 41754, HD 40540, and AI 01839).
The authors received consulting fees and/or lecture fees from Bayer AG, Glaxo-Smith-Kline, MedImmune Inc, Roche Laboratories, Vical Inc, and Viropharma Inc (M.B.); and from Roche Laboratories and Vical Inc (W.G.N.).
We thank Ted Gooley for performing the subanalysis presented in Table 2.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal