Abstract
Critical checkpoints controlling early thymic T-cell development and homeostasis are set by the proper signaling function of the interleukin 7 receptor (IL-7R) and the pre–T-cell antigen receptor. Although αβ T-cell development is observed in IL-7– and IL-7Rα–deficient mice, the number of thymocytes is significantly reduced, implying a role for the IL-7R in controlling the size of the thymic T-cell compartment. Here, we report the overexpression of IL-7Rα that occurs in the early T-cell compartment from AKR/J mice, animals that are highly susceptible to the spontaneous development of thymoma. Increased IL-7Rα was revealed by surface staining, and increased IL-7Rα mRNA was documented by using reverse transcriptase–polymerase chain reaction (RT-PCR). This resulted in increased survival of AKR/J early thymocytes, shown by the decreased frequency of TUNEL+ (terminal deoxynucleotidyl transferase mediated deoxyuridine triphosphate [dUTP]–fluorescein nick end labeling) cells. In an in vivo thymocyte repopulation model, AKR/J thymocytes had a selective advantage over healthy thymocytes. This advantage occurred at early stages of T-cell development. Our findings support the model that overexpression of growth factor receptors can contribute to proliferation and malignancy.
Introduction
Critical checkpoints controlling early T-cell development and homeostasis are set by the proper signaling function of the interleukin 7 receptor (IL-7R) and the pre–T-cell receptor (pre-TCR).1 The early T-cell compartment accounts for 2% to 5% of the adult thymus and is mainly represented by the CD4–CD8– double-negative (DN) thymocytes.2 DN thymocytes are conventionally fractionated into distinct developmental stages according to the distribution of CD44 versus CD25 markers.3 Four developmental stages have been described in which DN thymocytes progress from CD44+CD25– (stage I) to CD44+CD25+ (stage II) to CD44–CD25+ (stage III), and finally to CD44–CD25– (stage IV) before they progress to the CD4+CD8+ double-positive (DP) stage. Pre-TCR–mediated selection among DN cells for productive TCRβ V(D)J rearrangements, also termed β-selection, has been associated with stage III cells.4,5 Among stage III cells, one cell subset is represented by small-sized cells that either have not completed β gene rearrangement or have out-of-frame rearrangements (“E” cells). The other subset consists of large-sized cells that are often in S phase and show in-frame β chain gene rearrangements (“L” cells). The L fraction represents, therefore, cells that have productively rearranged their β chain gene and expressed the pre-TCR.4 Subsequently, signal through the pre-TCR serves as a first checkpoint in early T-cell development and is critical for further progression and differentiation.6-8 Such progression and differentiation is, however, severely compromised unless there is signaling through the IL-7R.
The IL-7R comprises the IL-7Rα chain (CD127) and the common cytokine receptor γ chain (γc, CD132) that is also a constituent of the IL-2, IL-4, IL-9, IL-15, and IL-21 receptors.9 Although αβ T-cell development is observed in IL-7–, IL-7Rα–, and γc-deficient mice, the number of thymocytes is significantly reduced,10-12 implying a role for the IL-7R in controlling the size of the thymus. Nevertheless, the relationship between checkpoints mediated by IL-7R signals and pre-TCR is still controversial. Three broad kinds of models are that (1) IL-7R signaling has no link to β-selection, but just permits cell survival; (2) β-selection causes IL-7Rα expression, and thus β-selected cells become responsive to IL-7; or (3) IL-7R signaling causes Rag (Recombination-activating gene) expression, which allows β gene rearrangements, which results in β-selection. The expression of Rag-1 and Rag-2 was found to be low among DN cells from IL-7Rα–/– mice,13 which supports this last model. The second model, however, is consistent with the fact that the introduction of a TCRαβ transgene into IL-7Rα–deficient mice was able to rescue thymic T-cell differentiation.14 Finally, the introduction of a bcl-2 transgene into IL-7Rα–/– mice was able to overcome the arrest of stage III DN thymocytes,15,16 demonstrating the role of IL-7R signaling in promoting survival of stage III cells, and this observation favors the first model. Any of these 3 models could account for the importance of IL-7R signaling at different levels of the early T-cell development to control differentiation and maintain homeostasis.
Multiple downstream signaling pathways are linked to IL-7R signaling. Signaling through IL-7R involves Janus kinase 1 (Jak1) and Jak3, 2 members of the Janus family of protein kinases.17 Jak kinases tyrosine phosphorylate signal transducers and activators of transcription (STATs) that dimerize and translocate to the nucleus where they act as regulators of transcription. Activated IL-7Rα also binds phosphatidyl inositol (PI)3-kinase, which ultimately leads to cell-cycle progression and proliferation.18-20 Several protooncogenes have been identified downstream of IL-7Rα signaling such as mel-1821 and pim-1.22 The pim-1 (proviral integration site in Moloney murine leukemia virus [MoMLV]–induced T-cell lymphomas) protooncogene encodes a serine/threonine protein kinase that is a frequent proviral insertion site in T-cell lymphomas.23,24 Pim-1 is constitutively expressed in healthy thymus, and introduction of a pim-1 transgene into IL-7Rα–deficient mice was able to overcome the lack of IL-7R signaling in DN thymocytes.22 Interestingly, introduction of the pim-1 transgene into Rag-deficient mice enabled Rag-deficient stage III cells to bypass the pre-TCR–controlled checkpoint during early T-cell development.22 A key question here is whether a gain of function in the IL-7R pathway can uncouple thymocytes from important checkpoints, leading to abnormal T-cell development that predisposes to thymomas. As a first step in testing this prediction, we investigated the role of IL-7 in abnormal T-cell differentiation by using a murine model known for spontaneous thymoma development: the AKR/J mouse.25 Our results point to a new model by which overexpression of IL-7R on thymocytes predisposes them to leukemia development.
Materials and methods
Mice
AKR/J (Thy1.1, H-2k), B10.BR (Thy1.2, H-2k), and CBA/J (Thy1.2, H-2k) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Rag-1–/– mice on the B10.BR (H-2k) background were crossed and housed in pathogen-free facility. Ex vivo and in vitro experiments were performed by using 4 control strains, B10.BR, C57/Bl6, Balb/c, and CBA/J, with equivalent results, and data are shown from B10.BR mice. In vivo experiments were performed and shown from 2 control B10.BR and CBA/J strains.
BM chimeras
Bone marrow (BM) cells from 8-week-old mice were depleted by treatment with a mixture of anti-CD4, anti-CD8, and anti-CD3 antibodies followed by 1-hour incubation (37°C) with rabbit complement. Eight-week-old Rag-1–/– recipient mice were sublethally irradiated (6 Gy) and injected intravenously with 107 BM cells containing mixtures of AKR/J and B10.BR or AKR/J and CBA/J BM donor cells. Single BM chimeras were generated by injecting 107 BM cells containing 100% AKR/J, 100% B10.BR, or 100% CBA/J BM donor cells to serve as controls. When indicated, 107 BM cells from BM chimeras were secondly transferred intravenously into sublethally irradiated (6 Gy) Rag–/– recipient host. All BM chimeras were analyzed from 3 to 9 weeks after BM transfer.
Treatment with IL-7
Nine-week-old BM chimeras were injected intraperitoneally during 7 consecutive days with phosphate-buffered saline (PBS) plus 0.1% mouse serum as a vehicle control or with mouse recombinant IL-7 (R&D Systems, Minneapolis, MN) diluted in PBS plus 0.1% mouse serum at 10 μg/injection. Mice were analyzed 1 day after treatment completion.
Antibodies and flow cytometry
Anti-CD4 (GK1.5), anti-CD8 (53-6.7), anti-TCRαβ (H7-597), anti-CD44 (IM7), anti-CD25 (PC61), anti-B220 (RA3-6B2), anti-Thy1.1 (OX-7), anti-Thy1.2 (53-2.1), anti–IL-7Rα (CD127; B12-1), and anti-γc (CD132; 4G3) monoclonal antibodies (Pharmingen, San Diego, CA) were used. Antibodies were directly coupled to allophycocyanin (APC), phycoerythrin (PE), fluorescein isothiocyanate (FITC), or biotin, in which case the staining was revealed using streptavidin conjugated to Cychrome (Pharmingen). Thymocytes were analyzed by using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) to determine cell frequency, cell phenotype, and cell size (Forward Scatter Channel, FSC). To ensure reproducibility, FSC was standardized in each experiment by adjusting the gain to normalize forward scatter readings for Flow Check Fluorospheres (Beckman Coulter, Miami, FL). The data were analyzed with FlowJo (Tree Star, San Carlos, CA) software.
TUNEL staining
Thymocytes were isolated from 8-week-old AKR/J or B10.BR mice, and apoptosis was determined in vitro after 12 hours in RPMI medium supplemented with 5% serum. Cells were first labeled by using a mixture of conjugated antibodies, then permeabilized, and fixed in PBS containing 2% paraformaldehyde (PFA) and 0.05% Tween 20 during 20 minutes at 4°C. Next, cells were submitted to TUNEL (terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate [dUTP] in situ nick end labeling) staining in PBS buffer containing 0.5 M acid cacodylic, 0.05 mM DTT (dithiothreitol), 500 μg/mL BSA (bovine serum albumin), 2.5 mM CoCl2, 5 U terminal transferase (Roche, Indianapolis, IN), and 5 μM dUTP biotin (Roche) during 20 minutes at 37°C. TUNEL+ cells were revealed by addition of phycoerythrin (PE)–conjugated streptavidin and analyzed by FACSCalibur cytometer. When indicated, thymocyte cultures were supplemented with 5 ng/mL mouse recombinant IL-7 (R&D System).
BrdU and 7-AAD staining
Three- and 6-week-old BM chimeras were injected intraperitoneally with 1 mg BrdU (bromodeoxyuridine; Sigma, St Louis, MO) at 4-hour intervals and then killed 1 hour after the last injection. Thymocytes were first surface labeled by using a mixture of conjugated antibodies, then permeabilized, and fixed in PBS containing 2% PFA and 0.05% Tween 20 during 20 minutes at 4°C. Next, cells were subjected to DNase I (Sigma) digestion followed by addition of FITC-conjugated anti-BrdU antibody (Becton Dickinson, San Jose, CA) as previously described.26 Cells were subsequently incubated in PBS buffer containing 25 μg/mL 7-AAD (7-aminoactinomycin D; Sigma) diluted in PBS buffer containing 0.5% Tween 20 and 50 μg/mL RNAse (Sigma) during 30 minutes at room temperature before fluorescence activated cell sorting (FACS) analysis. Thymocytes at S phase of the cell cycle were determined by positive staining for BrdU, and thymocytes in the G2/M phase of the cell cycle were identified on the basis of DNA content, detected by 7-AAD staining.
Reverse transcriptase–PCR
Thymocytes and lymphocytes from 8-week-old AKR/J or B10.BR mice were sorted by using FACSVantage Cell sorter (Becton Dickinson, San Jose, CA). Total RNA was first extracted using Trizol solution (Invitrogen, Carlsbad, CA), then 5 μg RNA was reverse transcribed by using a first-strand cDNA synthesis kit (Invitrogen). cDNA products (2 μL) were used for polymerase chain reaction (PCR) reaction to analyze the expression of IL-7Rα mRNA. Primers pair 5′-TCT GAC CTG AAA GTC GTT TAT CGC and 3′-CAT CCT CCT TGA TTC TTG GGT TC was used for IL-7Rα and 5′-GTT GGA TAC AGG CCA GAC TTT GTT G and 3′GAG GGT AGG CTG GCC TAT AGG CT for hypoxanthine phosphoribosyl transferase (HPRT) to confirm equal amount of RNA. PCR reactions were performed in a total volume of 50 μL PCR buffer containing 1.5 mM MgCl2, 0.2 mM deoxynucleotide triphosphate (dNTP), 1 μM of each primer, and 1.75 U DNA polymerase (Invitrogen). The thermal cycling parameters consisted of denaturation at 94°C for 45 seconds, annealing at 55°C for 45 seconds, and extension at 72°C for 45 seconds, during 35 cycles. The PCR products were separated on 1.5% agarose gel and visualized with ethidium bromide.
Genomic PCR
BM cells were isolated from 3- and 9-week-old BM chimeras generated with AKR/J and/or CBA/J BM donor cells. Total DNA was extracted by using Trizol solution (Invitrogen) according to the manufacture's protocol. DNA (0.25 μg) was used for PCR reaction to analyze the expression of microsatellite markers specific to AKR/J versus CBA/J strains. Primers pair 5′-CTG GGT AAG TTC AGT TCT CC and 3′-CTT CAA GGG CAC CTG CA was used to sequence DNA polymorphism located in the D1Mcg165 locus of the chromosome 1 (www.informatics.jax.org). Specific primers for the TCR-δ gene 5′-CAA ATG TTG CTT GTC TGG TG and 3′-GTC AGT CGA GTG CAC AGT TT were used to ensure an equal amount of DNA. PCR reactions and thermal cycling parameters are described as mentioned in “Reverse transcriptase PCR,” and PCR products were separated on 2% agarose gel.
Thymus morphology
Thymuses were removed and weighed, then fixed in 10% formalin and embedded in paraffin. Sections were cut and stained for hematoxylin and eosin, then examined by microscopy.
Statistical analysis
Statistical analysis was performed with the nonparametric unpaired Mann-Whitney U test. A P value less than .05 was considered statistically significant.
Results
Alterations in the early T-cell compartment of leukemic AKR/J mice
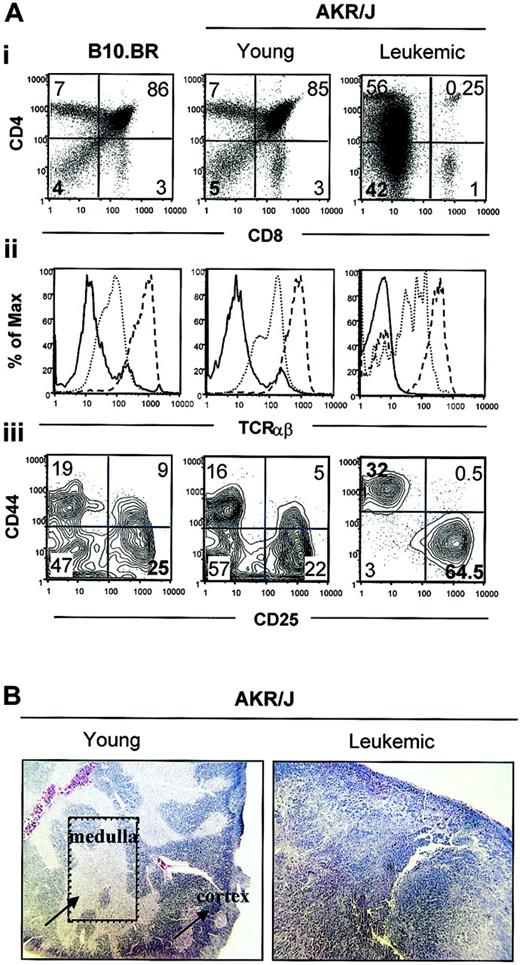
To characterize the abnormalities of T-cell differentiation in the leukemic AKR/J thymus, thymocytes isolated from young (2 months) or leukemic (6 months) AKR/J mice were analyzed (Figure 1A). Three criteria are important to define normal T-cell differentiation in the thymus: (1) CD4 versus CD8 distribution in total thymocytes, (2) the level of expression of TCRαβ in each thymocyte subset, and (3) CD44 versus CD25 expression in the early T-cell compartment. According to these criteria, thymus from young AKR/J mice showed normal T-cell differentiation as compared with age-matched control B10.BR mice. In contrast, leukemic AKR/J mice showed a significant alteration of early T-cell development and homeostasis. First, the leukemic AKR/J thymus as defined by CD4 versus CD8 expression (Figure 1A) was mainly composed by a heterogeneous cell population of CD4–/intCD8– phenotype (90% ± 4.8% versus 4% ± 1.2% in 6-month versus 2-month AKR/J mice, respectively, P = .002). Second, analysis of gated leukemic cells revealed an immature phenotype, because they expressed neither TCRαβ (Figure 1B) nor TCRγδ (data not shown). Third, analysis of thymus structure as revealed by hematoxylin and eosin staining of tissue sections showed disruption of the thymic medulla and dominance of the thymic cortex in leukemic AKR/J thymus (Figure 1B). In line with other studies,27 our results confirmed that T-cell leukemia in AKR/J mice develop in the thymic cortex within the immature thymocyte subsets. However, it was not clear at what exact developmental stage the leukemic immature thymocytes accumulate.
Abnormal early T-cell compartment in leukemic AKR/J mice. (A) Thymus from leukemic (6 months) and young (2 months) AKR/J mice were characterized by FACS analysis in comparison to control B10.BR thymocytes. Numbers shown in FACS profiles denote percentages of cells in each quadrant. Thymocytes were analyzed with 3-color staining by using anti-CD4–PE, anti-CD8–FITC, and anti-TCRαβ–-Cy (i-ii). FACS profiles (i) show analysis of CD4 versus CD8 distribution in total thymocytes. Histograms profiles (ii) show level expression of TCRαβ among gated DN (solid line) versus DP (thin dashed line) and CD4+ SP (thick dashed line) thymocytes. CD44 versus CD25 distribution (iii) was analyzed among gated DN thymocytes by 4-color staining using anti-CD4–PE, anti-CD8–FITC, anti-CD25–APC, and anti-CD44–Cy antibodies. (B) Thymus isolated from leukemic (6 months) and young (2 months) AKR/J mice were examined by hematoxylin and eosin staining. Dark areas represent thymic cortex, whereas light areas show thymic medulla. Results (A-B) are representative of 3 independent experiments with n = 3 mice for each experiment. Original magnification × 40.
Abnormal early T-cell compartment in leukemic AKR/J mice. (A) Thymus from leukemic (6 months) and young (2 months) AKR/J mice were characterized by FACS analysis in comparison to control B10.BR thymocytes. Numbers shown in FACS profiles denote percentages of cells in each quadrant. Thymocytes were analyzed with 3-color staining by using anti-CD4–PE, anti-CD8–FITC, and anti-TCRαβ–-Cy (i-ii). FACS profiles (i) show analysis of CD4 versus CD8 distribution in total thymocytes. Histograms profiles (ii) show level expression of TCRαβ among gated DN (solid line) versus DP (thin dashed line) and CD4+ SP (thick dashed line) thymocytes. CD44 versus CD25 distribution (iii) was analyzed among gated DN thymocytes by 4-color staining using anti-CD4–PE, anti-CD8–FITC, anti-CD25–APC, and anti-CD44–Cy antibodies. (B) Thymus isolated from leukemic (6 months) and young (2 months) AKR/J mice were examined by hematoxylin and eosin staining. Dark areas represent thymic cortex, whereas light areas show thymic medulla. Results (A-B) are representative of 3 independent experiments with n = 3 mice for each experiment. Original magnification × 40.
Next, we investigated the distribution of CD44 versus CD25 markers among DN cells and found that leukemic thymocytes were accumulated exclusively at stage I (32% ± 12%) and stage III (64% ± 19%) of the early T-cell development (Figure 1C). Only a few cells were found in stage IV (3% ± 0.5% versus 50% ± 9.8% in leukemic AKR/J versus control thymocytes, respectively, P < .05), indicating a bias in AKR/J mice that favors leukemia development in the early T-cell compartment before the transition to stage IV. The accumulation of leukemic thymocytes at stages I to III suggests an early disruption point of thymocyte differentiation in young mice that predisposes to leukemia development. We, therefore, speculated that specific events in the young AKR/J thymus selectively promote the survival of thymocytes prior to the transition to stage IV.
Overexpression of IL-7Rα in AKR/J mice
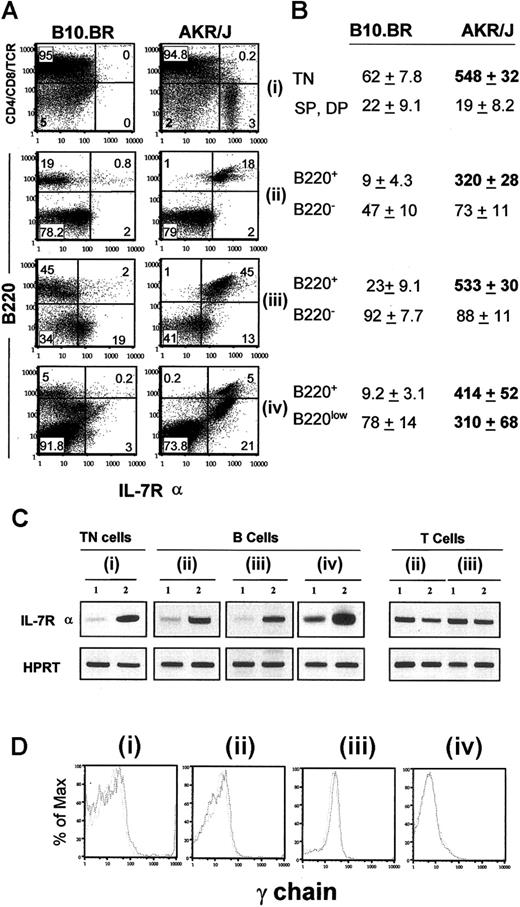
Signaling through IL-7R is known to promote thymocyte survival prior to the transition to stage IV.16,28 Because the profile of IL-7R expression has never been investigated in AKR/J mice, we determined the levels of expression of IL-7Rα and γc by FACS and RT-PCR analysis (Figure 2). First, cells from different organs were isolated from young AKR/J mice and analyzed by FACS using anti–IL-7Rα antibody. Figure 2A shows the levels of surface expression of IL-7Rα in total cells isolated from thymus (Figure 2Ai), lymph nodes (Figure 2Aii), spleen (Figure 2Aiii), and BM (Figure 2Aiv). Compared with control cells, AKR/J cells dramatically overexpressed IL-7Rα on immature CD4–CD8–TCRαβ– triple-negative (TN) thymocytes as well as on B220+ and B220low lymphocytes. Figure 2B summarizes the values of the mean fluorescence intensity of IL-7Rα expression on the indicated cell populations. To confirm this result, we analyzed the expression of IL-7Rα mRNA by RT-PCR using sorted cell populations: CD4–CD8–TCRαβ– (TN) thymocytes, B220+ (B), and TCRαβ+ (T) peripheral lymphocytes. Results in Figure 2C confirm the pronounced increased expression of IL-7Rα mRNA in AKR/J mice specifically in TN thymocytes and B lymphocytes. Nevertheless, FACS analysis of γc expression showed no difference between AKR/J and B10.BR cells (Figure 2D), indicating selective overexpression of the α chain of the IL-7 receptor complex.
Profile of IL-7Rα expression in AKR/J mice. Expression of IL-7Rα was examined at level of surface protein by FACS staining (A,D) and at level of mRNA transcripts by RT-PCR analysis (C). Cells were isolated from thymus (i), lymph nodes (ii), spleen (iii), or BM (iv) of 2-month old AKR/J or control B10.BR mice. (A) FACS analysis was performed in total cells using biotin-conjugated anti–IL-7Rα antibody in combination of a cocktail of PE (anti-CD4, anti-CD8, anti-TCRαβ)–conjugated antibodies (i), or in combination with anti-B220–PE antibody (ii-iv). Numbers shown in FACS profiles denote percentages of cells in each quadrant. Table (B) represents values of the mean fluorescence intensity (MFI) of IL-7Rα expression ± SE in indicated cell population from 4 independent experiments with n = 5 mice for each experiment. All values in bold represent significantly increased MFI (P < .05) on AKR/J cells compared with control B10.BR cells. (C) RT-PCR analysis to investigate expression of IL-7Rα transcripts was performed by using sorted TN (CD4–CD8–TCRαβ–) thymocytes (i), B (B220+) lymphocytes (ii-iv), or T (TCRαβ+) lymphocytes (iii-iv) isolated from 2-month-old B10.BR (lane 1) and AKR/J (lane 2) mice. (D) Histograms represent intensity of γc expression on gated TN thymocytes (i) or B lymphocytes from lymph nodes (ii), spleen (iii), or BM (iii) isolated from 2-month-old B10.BR (dashed line) or AKR/J (solid line) mice. Results (C-D) are representative of 3 independent experiments.
Profile of IL-7Rα expression in AKR/J mice. Expression of IL-7Rα was examined at level of surface protein by FACS staining (A,D) and at level of mRNA transcripts by RT-PCR analysis (C). Cells were isolated from thymus (i), lymph nodes (ii), spleen (iii), or BM (iv) of 2-month old AKR/J or control B10.BR mice. (A) FACS analysis was performed in total cells using biotin-conjugated anti–IL-7Rα antibody in combination of a cocktail of PE (anti-CD4, anti-CD8, anti-TCRαβ)–conjugated antibodies (i), or in combination with anti-B220–PE antibody (ii-iv). Numbers shown in FACS profiles denote percentages of cells in each quadrant. Table (B) represents values of the mean fluorescence intensity (MFI) of IL-7Rα expression ± SE in indicated cell population from 4 independent experiments with n = 5 mice for each experiment. All values in bold represent significantly increased MFI (P < .05) on AKR/J cells compared with control B10.BR cells. (C) RT-PCR analysis to investigate expression of IL-7Rα transcripts was performed by using sorted TN (CD4–CD8–TCRαβ–) thymocytes (i), B (B220+) lymphocytes (ii-iv), or T (TCRαβ+) lymphocytes (iii-iv) isolated from 2-month-old B10.BR (lane 1) and AKR/J (lane 2) mice. (D) Histograms represent intensity of γc expression on gated TN thymocytes (i) or B lymphocytes from lymph nodes (ii), spleen (iii), or BM (iii) isolated from 2-month-old B10.BR (dashed line) or AKR/J (solid line) mice. Results (C-D) are representative of 3 independent experiments.
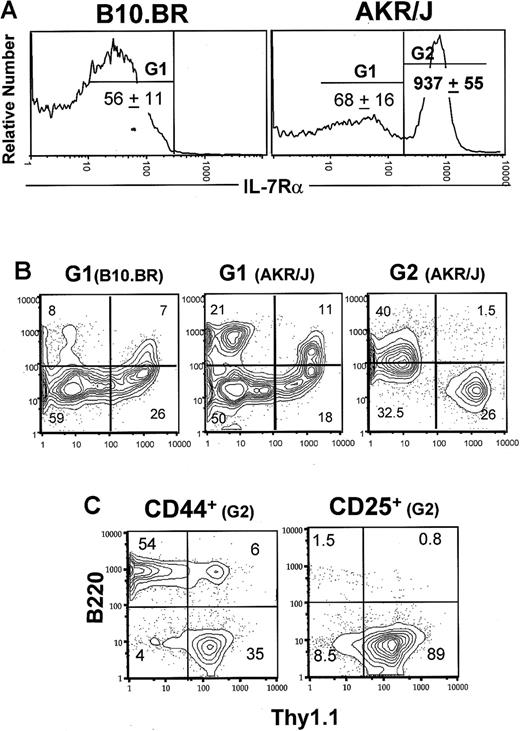
Next, we investigated the distribution of CD44 versus CD25 on gated IL-7Rαlow versus IL-7Rαhigh TN thymocytes (Figure 3). As defined by the G1 gate, IL-7Rαlow–expressing cells from control mice were found mainly among stage III (30% ± 11%) and IV (50% ± 13%) thymocytes. In comparison, AKR/J cells expressing low levels of IL-7Rα were found to be approximately 2-fold increased in stage I thymocytes. The most significant difference, however, was found among IL-7Rαhigh TN thymocytes (G2 gate) which belong specifically to stage I (60% ± 13%) and stage III (23% ± 7.3%) thymocytes. Because CD44+CD25– stage I cells represent a multipotent cell subset, we further analyzed the expression of surface markers specific to T (Thy1.1) and B (B220 and class II) cell lineages as well as markers specific to macrophages (CD11b), dendritic (CD11c) cells, and natural killer (NK1.1) cells. Although CD44–CD25+IL-7Rαhigh cells expressed exclusively the T-cell markers Thy1.1, the CD44+CD25–IL-7Rαhigh cells were composed of both T- and B-cell lineages (Figure 3C). Our data show an important and new feature of the AKR/J thymus, the overexpression of IL-7Rα in early T cells.
Overexpression of IL-7Rα in early T-cell compartment of AKR/J mice. Total thymocytes were isolated from 2-month-old AKR/J or B10.BR mice and depleted from CD4+, CD8+, and TCRαβ+ thymocytes by specific antibodies followed by rabbit complement. (A) Remaining TN cells were stained with anti–IL-7Rα–biotin, anti-CD25–APC, and anti-CD44–FITC antibodies. Gates G1 and G2 define cells expressing normal levels of IL-7Rα, and gate G3 defines cells expressing higher levels of IL-7Rα. Numbers denote the mean of IL-7Rα fluorescence intensity ± SE in each gated cell population. Dot plots (B) show the distribution of CD44 versus CD25 among gated G1 cells. Dot plots (C) show the expression of Thy1.1 versus B220 within CD44-expressing or CD25-expressing IL-7Rαhigh cells. Results are representative of 3 independent experiments with n = 3 mice for each experiment.
Overexpression of IL-7Rα in early T-cell compartment of AKR/J mice. Total thymocytes were isolated from 2-month-old AKR/J or B10.BR mice and depleted from CD4+, CD8+, and TCRαβ+ thymocytes by specific antibodies followed by rabbit complement. (A) Remaining TN cells were stained with anti–IL-7Rα–biotin, anti-CD25–APC, and anti-CD44–FITC antibodies. Gates G1 and G2 define cells expressing normal levels of IL-7Rα, and gate G3 defines cells expressing higher levels of IL-7Rα. Numbers denote the mean of IL-7Rα fluorescence intensity ± SE in each gated cell population. Dot plots (B) show the distribution of CD44 versus CD25 among gated G1 cells. Dot plots (C) show the expression of Thy1.1 versus B220 within CD44-expressing or CD25-expressing IL-7Rαhigh cells. Results are representative of 3 independent experiments with n = 3 mice for each experiment.
Survival advantage of AKR/J TN thymocytes in vitro
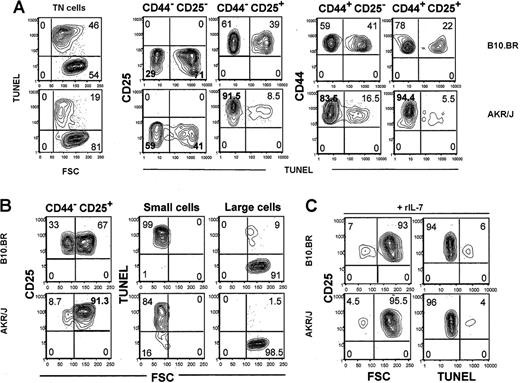
It is established that ex vivo thymocytes undergo massive apoptosis in vitro.29 We, therefore, investigated whether AKR/J thymocytes expressing high levels of IL-7Rα displayed differential susceptibility to cell death in vitro. Apoptosis of thymocytes from young AKR/J mice was assessed by the FACS-TUNEL method and was compared with control strains. Four-color staining allowed exclusion of CD4+, CD8+, and TCRαβ+ thymocytes by using a cocktail of Cy-conjugated anti-CD4, anti-CD8, and anti-TCRαβ antibodies. The frequency of TUNEL+ cells was detected by APC-conjugated streptavidin among gated TN thymocyte subsets as determined by PE-conjugated anti-CD25 and FITC-conjugated anti-CD44 antibodies (Figure 4A). Compared with control TN thymocytes, cells from AKR/J showed a significant decrease in the apoptotic cell frequency (20% ± 6% versus 50% ± 9% TUNEL+ cells from AKR/J versus control cells, respectively, P < .05). Each TN thymocyte subset, as determined by CD44 versus CD25 distribution, showed a decreased frequency of apoptotic cells. Nevertheless, the most significant difference was found among the CD25+ thymocyte subsets. The percentage of TUNEL+ cells among AKR/J thymocytes was approximately 2-fold less in CD44+ cell subsets, whereas it was approximately 4 times less in CD25+ cell populations. Specifically, only 8% ± 3.5% TUNEL+ cells were found among CD44–CD25+ AKR/J thymocytes compared with 38% ± 5.1% among control thymocytes (P = .002).
Decreased in vitro apoptosis of AKR/J TN thymocytes. Total thymocytes were isolated from 2-month-old AKR/J or control B10.BR mice and cultured for 12 hours in medium supplemented with 5% serum. Cells were stained with a cocktail of Cy–(anti-CD4, anti-CD8, anti-TCRαβ) antibodies together with anti-CD25–APC and anti-CD44–FITC. Subsequently, apoptotic cells were detected by FACS-TUNEL staining revealed by addition of PE-conjugated streptavidin. FACS profiles (A) represent frequency of apoptotic cells, as determined by TUNEL positive staining, among gated TN (CD4–CD8–TCRαβ–) thymocytes and among each cell subset within TN thymocytes: CD44–CD25–, CD44–-CD25+, CD44+CD25–, and CD44+CD25+. FACS profiles (B) represent cell size analysis, as determined by FSC channel, among CD44–CD25+ TN thymocytes. This cell subset is divided into small- and large-size cells, and frequency of apoptotic cells among each population is determined by TUNEL staining versus FSC. Results (A-B) are representative of 3 independent experiments with n = 5 mice for each experiments. FACS profiles (C) represent analysis of in vitro apoptosis after addition of 5 ng/mL recombinant mouse IL-7. Analysis of FSC scatter and TUNEL staining are shown among CD44–CD25+ TN thymocytes. Results (C) are representative of 3 independent experiments with n = 3 mice for each experiments. Numbers shown in FACS profiles denote percentages of cells in each quadrant.
Decreased in vitro apoptosis of AKR/J TN thymocytes. Total thymocytes were isolated from 2-month-old AKR/J or control B10.BR mice and cultured for 12 hours in medium supplemented with 5% serum. Cells were stained with a cocktail of Cy–(anti-CD4, anti-CD8, anti-TCRαβ) antibodies together with anti-CD25–APC and anti-CD44–FITC. Subsequently, apoptotic cells were detected by FACS-TUNEL staining revealed by addition of PE-conjugated streptavidin. FACS profiles (A) represent frequency of apoptotic cells, as determined by TUNEL positive staining, among gated TN (CD4–CD8–TCRαβ–) thymocytes and among each cell subset within TN thymocytes: CD44–CD25–, CD44–-CD25+, CD44+CD25–, and CD44+CD25+. FACS profiles (B) represent cell size analysis, as determined by FSC channel, among CD44–CD25+ TN thymocytes. This cell subset is divided into small- and large-size cells, and frequency of apoptotic cells among each population is determined by TUNEL staining versus FSC. Results (A-B) are representative of 3 independent experiments with n = 5 mice for each experiments. FACS profiles (C) represent analysis of in vitro apoptosis after addition of 5 ng/mL recombinant mouse IL-7. Analysis of FSC scatter and TUNEL staining are shown among CD44–CD25+ TN thymocytes. Results (C) are representative of 3 independent experiments with n = 3 mice for each experiments. Numbers shown in FACS profiles denote percentages of cells in each quadrant.
According to previous data, the CD44–CD25+ cell subset is composed of 2 cell populations: small- and large-sized cells. Apoptotic cells are present exclusively among the small-cell population.5 We further analyzed the frequency of apoptotic TUNEL+ cells as a function of the cell size (determined by FSC channel). We confirmed in both AKR/J and control thymocytes that gated large cells were TUNEL–, whereas all small cells were TUNEL+ (Figure 4B). However, a significant decrease in the frequency of small cells was observed in AKR/J thymocytes (8% ± 5%) compared with control thymocytes (33% ± 9%). When thymocytes were cultured in medium supplemented with mouse recombinant IL-7, control CD44–CD25+ thymocytes were rescued from apoptosis as shown by a decreased frequency of both TUNEL+ cells (6% ± 2.3%) and small cells (7% ± 1.9%). Under such a condition, we found similar apoptotic rates between AKR/J and control CD44–CD25+ thymocytes. This result shows that up-regulation of IL-7Rα expression, on AKR/J thymocytes, was associated with enhanced survival in vitro.
Competition between AKR/J and control cells during thymus regeneration
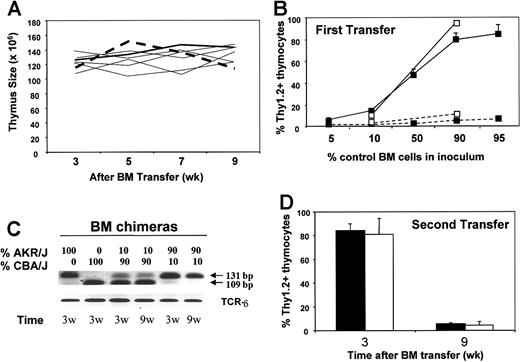
Here, we investigated outcomes of cellular competition between AKR/J and control thymocytes during thymic regeneration. On the basis of previous data that showed radiosensitivity of thymic IL-7 production after BM transplantation,30-32 we constructed mixed BM chimeras in which both AKR/J and control thymocytes would compete for limited IL-7 during thymus regeneration. Five groups of mixed BM chimeric mice were generated by co-injecting into irradiated Rag-1–/––recipient mice a mixture of BM cells containing 5%, 10%, 50%, 90%, and 95% of cells isolated from 8-week-old AKR/J mice together with cells isolated from control B10.BR mice. Donor-derived thymocytes were distinguished by using allelic markers because AKR/J cells express the Thy1.1 marker whereas B10.BR cells express the Thy1.2 marker. As controls, irradiated Rag-1–/––recipient mice were injected either with AKR/J (100%) or B10.BR (100%) cells alone (Figure 5A-B).
In vivo competition between AKR/J and B10.BR thymocytes. BM chimeras were constructed by injecting different ratio of BM cells isolated from 2-month-old AKR/J (Thy1.1) together with control B10.BR or CBA/J (Thy1.2) mice into irradiated Rag-1–deficient hosts. Graph (A) represents numbers of total thymocytes at 3, 5, 7, and 9 weeks after transfer. Dashed line represents results from control single BM chimeras reconstituted with 100% AKR/J cells, solid thick line represents results from control single BM chimeras reconstituted with 100% B10.BR cells, and solid thin lines represent results from mixed BM chimeras. Graph (B) represents the frequency of the control donor-derived thymocytes as a function of the control BM inoculum. Values indicate the frequency of B10.BR (▪) or CBA/J (□) Thy1.2+ donor-derived thymocytes at 3 (solid) and 9 (dashed) weeks after transfer. Results are representative of 5 experiments with n = 3 mice per group of B10.BR-AKR/J and 2 experiments with n = 5 of CBA/J-AKR/J chimeras. Graph (C) shows marrow chimerism of CBA/J versus AKR/J at 3 and 9 weeks after transfer. Results show DNA polymorphism in the chromosome 1 with specific PCR product for AKR/J (131 bp) and CBA/J (109 bp). Data are from a pool of 5 BM chimeras generated either with 0%, 10%, 90%, or 100% AKR/J donor cells. Graph (D) represents second transfer of BM cells isolated from 9-week-old chimeras generated originally with 90% B10.BR (▪) or 90% CBA/J (□) inoculum. Results indicate the percentages of Thy1.2+ thymocytes at 3 and 9 weeks after second transfer. Values represent the mean of n = 5 mice per group.
In vivo competition between AKR/J and B10.BR thymocytes. BM chimeras were constructed by injecting different ratio of BM cells isolated from 2-month-old AKR/J (Thy1.1) together with control B10.BR or CBA/J (Thy1.2) mice into irradiated Rag-1–deficient hosts. Graph (A) represents numbers of total thymocytes at 3, 5, 7, and 9 weeks after transfer. Dashed line represents results from control single BM chimeras reconstituted with 100% AKR/J cells, solid thick line represents results from control single BM chimeras reconstituted with 100% B10.BR cells, and solid thin lines represent results from mixed BM chimeras. Graph (B) represents the frequency of the control donor-derived thymocytes as a function of the control BM inoculum. Values indicate the frequency of B10.BR (▪) or CBA/J (□) Thy1.2+ donor-derived thymocytes at 3 (solid) and 9 (dashed) weeks after transfer. Results are representative of 5 experiments with n = 3 mice per group of B10.BR-AKR/J and 2 experiments with n = 5 of CBA/J-AKR/J chimeras. Graph (C) shows marrow chimerism of CBA/J versus AKR/J at 3 and 9 weeks after transfer. Results show DNA polymorphism in the chromosome 1 with specific PCR product for AKR/J (131 bp) and CBA/J (109 bp). Data are from a pool of 5 BM chimeras generated either with 0%, 10%, 90%, or 100% AKR/J donor cells. Graph (D) represents second transfer of BM cells isolated from 9-week-old chimeras generated originally with 90% B10.BR (▪) or 90% CBA/J (□) inoculum. Results indicate the percentages of Thy1.2+ thymocytes at 3 and 9 weeks after second transfer. Values represent the mean of n = 5 mice per group.
Single BM chimeras reconstituted with 100% AKR/J or 100% B10.BR cells showed normal thymus reconstitution (Figure 5A). In both cases, thymus reconstitution reached an optimum at 3 weeks and remained constant 9 weeks after transfer (132-145 × 106 cells). Similar reconstitution was observed in thymuses isolated from each group of mixed BM chimeras, in which the size of the thymus was constant (125-140 × 106 cells). However, the distribution of AKR/J versus B10.BR cells per thymus showed dramatic alterations (Figure 5B). Rapidly after the equilibrium, the frequency of B10.BR donor-derived thymocytes dramatically decreased in the mixed BM chimeras to constitute less than 1% (n = 75) 9 weeks after transfer. Similar outcomes on thymus reconstitution were observed in mixed BM chimeras constructed with AKR/J cells together with CBA/J cells, used as second control (Figure 5B). We found that AKR/J thymocytes established, 9 weeks after transfer, a strong dominance (94% ± 5%) in all groups of chimeras generated with cotransfers of AKR/J and B10.BR (n = 75) or AKR/J and CBA/J (n = 10) BM cells, regardless of their composition in the BM inoculum (Figure 5B).
Next, we asked whether the advantage of AKR/J thymocytes is due to an advantage occurring at the stem cell level. To this aim, we analyzed the marrow chimerism by using PCR amplification of microsatellite markers known to discriminate between AKR/J and CBA/J strains (Figure 5C). By using primers specific for DNA polymorphism in chromosome 1,33 we were able to detect 2 different PCR products for AKR/J (131 bp) and CBA/J (109 bp) strains. We found that the composition of the BM inoculum proportionally established a marrow chimerism in the mixed BM chimeras at 3 weeks after transfer. But most important, we found that the marrow chimerism was not affected 9 weeks after transfer, indicating no advantage of AKR/J cells at the level of stem cells. For direct demonstration, BM cells from 9-week-old chimeras (constructed originally with 10% AKR/J inoculum) were secondarily transferred to irradiated hosts (Figure 5D). Distribution of Thy1.1 versus Thy1.2 markers in the thymus showed a ratio reflecting the original BM inoculum. Both B10.BR and CBA/J thymocytes exhibited 90% chimerism at 3 weeks after second transfer that again failed to be maintained in the presence of AKR/J thymocytes, 9 weeks later (Figure 5D). Altogether, these data showed a phenomenon of dominance by AKR/J cells that occur specifically in the thymus.
Selective advantage of AKR/J thymocytes in the early T-cell compartment
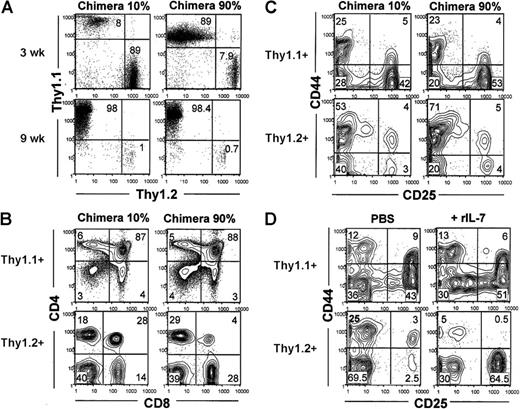
The distribution of Thy1.1 versus Thy1.2 markers in thymuses from chimeras reconstituted with 10% AKR/J versus 90% AKR/J donor cells illustrates AKR/J cell dominance (Figure 6A). Next, we examined thymic cell differentiation among Thy1.1+ (AKR/J) and Thy1.2+ (B10.BR) donor cells by gating on cells expressing the allelic markers. Experiments were performed with all groups of chimeras with similar results, and data are shown from 2 groups of chimeras reconstituted with 10% or 90% of AKR/J cells. Figure 6B-C revealed a blockade of early T-cell development in B10.BR cells, whereas AKR/J thymocytes exhibited normal T-cell development. After 9 weeks, B10.BR thymocytes were blocked at the CD44+CD25+ developmental stage (Figure 6C), and transition beyond that stage was diminished. Indeed, CD44–CD25+ cells represented 3% ± 1.2% versus 44% ± 7.2% in B10.BR (Thy1.2+) versus AKR/J (Thy1.1+) cells in BM chimeras with 10% AKR/J inoculum. Subsequently, the transition to the DP stage was interrupted, as shown in Figure 6B by a decreased frequency of DP cells (< 30%, P = .02). This was not the case in single BM chimeras (with 100% B10.BR inoculum) that showed normal number and frequency of DP and TN thymocytes more than 9 weeks after transfer. Interestingly, this blockade of normal T-cell development is reminiscent of the phenotype provoked by IL-7 deprivation.34 When we administered IL-7 to mixed BM chimeras (at 9 weeks after transfer), the blockade in early T-cell development among B10.BR donor cells was overcome as shown by the transition of precursors to the CD44–CD25+ stage after 7 days of treatment completion (Figure 6D). These data provide evidence that IL-7 is limiting, accounting for the selective advantage of AKR/J thymocytes when present with normal thymocytes in the same thymic microenvironment.
Presence of AKR/J thymocytes induces arrest of early T-cell development within control thymocytes that is overcome by addition of exogenous IL-7. (A) Total thymocytes were isolated from mixed BM chimeras reconstituted with 10% or 90% of AKR/J inoculum at 3 and 9 weeks after transfer and stained using biotin–anti-Thy1.1 and PE–anti-Thy1.2. FACS profiles show the distribution of AKR/J (Thy1.1+) versus B10.BR (Thy1.2+) donor-derived thymocytes. (B) Total thymocytes were isolated from mixed BM chimeras reconstituted with 10% or 90% of AKR/J BM cells at 6 weeks after transfer and analyzed by 4-color staining using biotin–anti-Thy1.1, PE–anti-Thy1.2, APC–anti-CD4, and FITC–anti-CD8. FACS profiles show the distribution of CD4 versus CD8 within gated Thy1.1+ or Thy1.2+ thymocytes. (C) Total thymocytes were isolated from mixed BM chimeras reconstituted with 10% or 90% of AKR/J BM cells at 6 weeks after transfer and analyzed by 4-color staining using a cocktail of Cy–(anti-CD4, anti-CD8, anti-TCRαβ) antibodies together with APC–anti-CD25, FITC–anti-CD44, and biotin–anti-Thy1.1 or biotin–anti-Thy1.2. FACS profiles show the distribution of CD25 versus CD44 among gated Thy1.1+ or Thy1.2+ thymocytes within TN (CD4–CD8–TCRαβ–) cell population. Results (A-C) are representative of 5 independent experiments with n = 3 mice per group of BM chimeric mice. (D) At 9 weeks after transfer, mixed BM chimeras were injected intraperitoneally with PBS or mouse recombinant IL-7 (10 μg/mouse) during 7 consecutive days. Subsequently, cells were recovered from thymus and stained as described in panel B. FACS profiles show the distribution of CD25 versus CD44 within gated Thy1.1+ or Thy1.2+ thymocytes within TN (CD4–CD8–TCRαβ–) cell population in PBS versus IL-7–treated mice. Results (D) are representative of 2 individual experiments with 2 mice per group of BM chimeric mice. Numbers shown in FACS profiles denote percentages of cells in each quadrant.
Presence of AKR/J thymocytes induces arrest of early T-cell development within control thymocytes that is overcome by addition of exogenous IL-7. (A) Total thymocytes were isolated from mixed BM chimeras reconstituted with 10% or 90% of AKR/J inoculum at 3 and 9 weeks after transfer and stained using biotin–anti-Thy1.1 and PE–anti-Thy1.2. FACS profiles show the distribution of AKR/J (Thy1.1+) versus B10.BR (Thy1.2+) donor-derived thymocytes. (B) Total thymocytes were isolated from mixed BM chimeras reconstituted with 10% or 90% of AKR/J BM cells at 6 weeks after transfer and analyzed by 4-color staining using biotin–anti-Thy1.1, PE–anti-Thy1.2, APC–anti-CD4, and FITC–anti-CD8. FACS profiles show the distribution of CD4 versus CD8 within gated Thy1.1+ or Thy1.2+ thymocytes. (C) Total thymocytes were isolated from mixed BM chimeras reconstituted with 10% or 90% of AKR/J BM cells at 6 weeks after transfer and analyzed by 4-color staining using a cocktail of Cy–(anti-CD4, anti-CD8, anti-TCRαβ) antibodies together with APC–anti-CD25, FITC–anti-CD44, and biotin–anti-Thy1.1 or biotin–anti-Thy1.2. FACS profiles show the distribution of CD25 versus CD44 among gated Thy1.1+ or Thy1.2+ thymocytes within TN (CD4–CD8–TCRαβ–) cell population. Results (A-C) are representative of 5 independent experiments with n = 3 mice per group of BM chimeric mice. (D) At 9 weeks after transfer, mixed BM chimeras were injected intraperitoneally with PBS or mouse recombinant IL-7 (10 μg/mouse) during 7 consecutive days. Subsequently, cells were recovered from thymus and stained as described in panel B. FACS profiles show the distribution of CD25 versus CD44 within gated Thy1.1+ or Thy1.2+ thymocytes within TN (CD4–CD8–TCRαβ–) cell population in PBS versus IL-7–treated mice. Results (D) are representative of 2 individual experiments with 2 mice per group of BM chimeric mice. Numbers shown in FACS profiles denote percentages of cells in each quadrant.
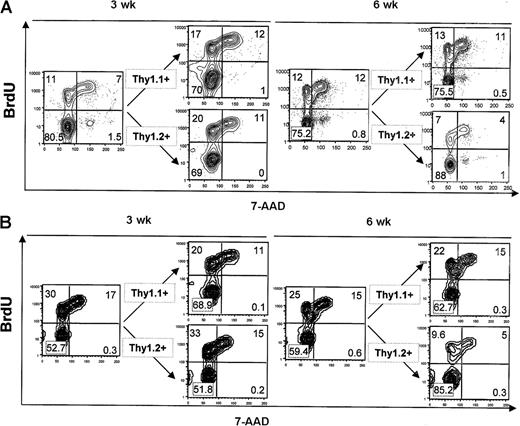
Next, we asked whether the dominance of AKR/J cells that occurs in mixed BM chimeras was the result of an advantage in cell proliferation. To establish this, mixed BM chimeras were injected with BrdU at 3 and 6 weeks after transfer, and the frequency of cycling cells was determined by a combination of BrdU and 7-AAD staining (Figure 7). Cells positive for BrdU staining represent cell populations that have undergone S phase of the cell cycle, whereas 7-AAD staining revealed cell subsets in the G2/M phase of the cell cycle. Analysis of the frequency of cycling cells at 6 weeks after transfer (a time point that coincides with the developing dominance of AKR/J donor cells) in comparison to analysis at 3 weeks after transfer (a time point that represents the equilibrium between both donor cell types) provided 3 important observations. First, the frequency of cycling cells among total thymocytes was constant (20% ± 3.2%), thereby explaining the constant size of thymuses described in Figure 5A. Second, the frequency of cycling cells among gated AKR/J (Thy1.1+) donor cells was constant at 3 versus 6 weeks after transfer, indicating that AKR/J cell dominance was not because of excessive proliferation. This was shown in total AKR/J thymocytes (27 ± 2.9) as well as in gated TN thymocytes (33% ± 4.1%). Third, the frequency of cycling cells among gated B10.BR (Thy1.2+) donor cells showed a decrease from 3 to 6 weeks after transfer both on total thymocytes (from 29% ± 3.2% to 8% [plumsn] 1.9%, P < .05) as well as on gated TN thymocytes (from 40% ± 5.4% to 12% ± 4.1%, P < .05). This decreased cycling cell frequency in B10.BR donor cells was due to the loss of cells among CD4+CD8+ DP and CD44–CD25+ TN thymocytes (Figure 6B-C), known to be the most important cycling cells of the thymus.35,36
Advantage of AKR/J thymocytes is not due to increased proliferation capability. Mixed BM chimeras reconstituted with 50% of AKR/J inoculum were analyzed for in vivo incorporation of BrdU at 3 and 6 weeks after transfer. BrdU (1 mg) was twice injected intraperitoneally at 4-hour intervals, and analysis was performed 1 hour after the last injection. (A) Thymocytes were analyzed by 4-color staining by using biotin–anti-Thy1.1, PE–anti-Thy1.2, FITC–anti-BrdU, and 7-AAD. FACS profiles show BrdU versus 7-AAD staining in total thymocytes and in gated Thy1.1+ or Thy1.2+ cells. (B) Thymocytes were analyzed by 4-color staining by using a cocktail of PE–(anti-CD4, anti-CD8, anti-TCRαβ) antibodies together with FITC–anti-BrdU, 7-AAD, and biotin–anti-Thy1.1 or biotin–anti-Thy1.2. FACS profiles show BrdU versus 7-AAD staining in gated TN (CD4–CD8–TCRαβ–) thymocytes and in gated Thy1.1+ or Thy1.2+ TN thymocytes. Results (A-B) are representative of 4 independent experiments with n = 3 mice per group of BM chimeric mice. Numbers shown in FACS profiles denote percentages of cells that fall into each quadrant.
Advantage of AKR/J thymocytes is not due to increased proliferation capability. Mixed BM chimeras reconstituted with 50% of AKR/J inoculum were analyzed for in vivo incorporation of BrdU at 3 and 6 weeks after transfer. BrdU (1 mg) was twice injected intraperitoneally at 4-hour intervals, and analysis was performed 1 hour after the last injection. (A) Thymocytes were analyzed by 4-color staining by using biotin–anti-Thy1.1, PE–anti-Thy1.2, FITC–anti-BrdU, and 7-AAD. FACS profiles show BrdU versus 7-AAD staining in total thymocytes and in gated Thy1.1+ or Thy1.2+ cells. (B) Thymocytes were analyzed by 4-color staining by using a cocktail of PE–(anti-CD4, anti-CD8, anti-TCRαβ) antibodies together with FITC–anti-BrdU, 7-AAD, and biotin–anti-Thy1.1 or biotin–anti-Thy1.2. FACS profiles show BrdU versus 7-AAD staining in gated TN (CD4–CD8–TCRαβ–) thymocytes and in gated Thy1.1+ or Thy1.2+ TN thymocytes. Results (A-B) are representative of 4 independent experiments with n = 3 mice per group of BM chimeric mice. Numbers shown in FACS profiles denote percentages of cells that fall into each quadrant.
Discussion
Leukemia in AKR/J mice develops in the thymic cortex within the immature thymocyte subsets.27,37,38 Here, we described the specific stages of thymic development at which leukemic thymocytes accumulate. These stages belong to an early T-cell compartment, revealed by the distribution of CD44 versus CD25 expression. We searched for specific events that might promote survival and/or division of thymocytes within the early T-cell compartment and found a novel feature of AKR/J mice: overexpression of IL-7Rα within the early T-cell compartment. Such an overexpression was confirmed at the surface protein level by FACS staining and at the mRNA level by RT-PCR analysis. The fact that B cells from AKR/J mice showed a similar overexpression of IL-7Rα argues against the possibility that such a phenomenon may result from a physiologic event during thymocyte development. Instead, the up-regulation of IL-7Rα found in (AKR/J × B10.BR) F1 mice (data not shown) favors the possibility of a dominant mutation, leading to overexpressed IL-7Rα. Such a mutation is strongly suggested by the high frequency of proviral insertions previously described in the genome of AKR/J mice,39 and evidence of provirus insertion (MoMuLV) in c-myc exon 1 resulting in high expression of c-myc in AKR/J mice support our hypothesis.40,41 Nevertheless, the exact molecular events leading to overexpression of IL-7Rα in AKR/J mice remain to be determined.
The IL-7Rα subunit has been shown to be obligatory for the formation of high-affinity signaling competent IL-7R complex, as well as for thymic stromal lymphopoietin receptor (TSLPR) complex.42 Indeed, the sharing of IL-7Rα by both IL-7 and TSLP helped to explain why the phenotype of IL-7R–/– mice appears more severe than the phenotype of IL-7–/– mice, given that mutation of IL-7Rα abrogates signaling by both IL-7 and TSLP. Therefore, the role of IL-7Rα in thymic development appears to be attributed to the importance of its ligands, IL-7 and TSLP, known to share a closely overlapping profile of biologic activities, including promotion of T-cell precursor growth and survival.42 Here, we investigated the outcomes of such an overexpression of IL-7Rα in thymic development and asked whether it may confer an advantage of cell survival or growth of immature thymocytes, which subsequently develop into tumors in AKR/J mice. We used 2 experimental strategies to approach this question. First, we investigated in vitro apoptosis of thymocytes by using the TUNEL technique. Our data provide clear evidence of increased survival of AKR/J thymocytes as shown by a decreased frequency of TUNEL+ cells among TN thymocytes as well as a decreased frequency of small-sized cells among CD44–CD25+ thymocytes, known to be apoptotic cells. Whether enhanced AKR/J thymocyte survival is mediated by IL-7 remains uncertain. The fact that normal levels of γc expression were found on AKR/J thymocytes argues in favor of survival mediated by TSLP. Nevertheless, we do not exclude the possibility that overexpressed IL-7Rα may act to retain and/or stabilize IL-7 on the cell surface and thereby optimize the duration of signaling. Second, we established a model of in vivo cell competition between AKR/J and healthy thymocytes for thymus regeneration in the presence of a limited amount of IL-7.30-32 We developed mixed BM chimeras in which both AKR/J and control BM cells were cotransferred into irradiated Rag-1–/––recipient mice. We found that the presence of AKR/J thymocytes induced alterations in the frequency and absolute number of control donor-derived thymocytes. The dominance by AKR/J cells was specific to the thymus because marrow chimerism was not affected, and subsequent transfers into second hosts showed similar kinetic of equilibrium, reflecting unaffected marrow chimerism. The outcome of AKR/J cell dominance on normal T-cell differentiation was a blockade at the CD44+CD25+ developmental stage, leading to interruption of the subsequent transition into the DP stage. Interestingly, the blockade of the T-cell development of B10.BR or CBA/J cells observed in the presence of AKR/J thymocytes was reminiscent of the phenotype induced by in vivo treatment with anti–IL-7 antibody.34 This blockade was overcome by IL-7 administration, suggesting that high levels of IL-7Rα on AKR/J thymocytes may act as a decoy that deprived “normal” thymocytes from access to the limited amount of IL-7. Moreover, the advantage of AKR/J thymocytes was not due to preferential expansion of AKR/J cells because AKR/J thymocytes displayed a similar frequency of cycling cells at 3 versus 6 weeks after transfer. Together, results from in vitro and in vivo approaches provide direct evidence that high levels of IL-7Rα on TN thymocytes can act as a decoy to provide cells with a survival or maintenance advantage during the events of early T-cell development.
What are the outcomes of these results in the understanding of T-cell leukemia development? Originally, it was thought that the mink cell focus-forming (MCF) virus presumably integrates into many sites of individual thymocytes of AKR/J thymus, one of which grows out to become a tumor. Following this reasoning all thymocyte subsets would have an equal likelihood of being transformed that should result in a majority of CD4+CD8+ tumor phenotype because DP cells represent 80% of healthy thymus. Because leukemia in AKR/J develops, instead, in the thymic cortex within immature thymocytes argues against this possibility and rather suggested specific events that occur during the early T-cell development.27 Consequently, Li and Baltimore43 have suggested a scenario of cytokine-driven leukemogenesis models in which MCF gp70 would induce the proliferation or the survival of specific stages of TN thymocytes through stimulation of a cytokine receptor, at that time not yet identified. Our data identify a new feature of overexpressed IL-7Rα on TN thymocytes in AKR/J mice and provide evidence in support to the model that overexpression of growth factor receptors can contribute to malignancy.
The potential role of γc-receptor in cytokine-driven leukemogenesis models has been widely discussed in many human cancers.44-48 IL-7R expression was described in more than 20% of lymphoid cells in 50% to 75% of cutaneous T-cell lymphomas and in more than 20% of lymphoid cells in 40% of nodal large T-cell lymphomas.49 In thyroid lymphomas, IL-7R was expressed in germinal centers, interfollicular cells, and lymphomas cells.50 In Hodgkin disease, Reed-Sternberg cells were found positive for IL-7R expression.51 In B-lineage acute lymphoblastic leukemia, overexpression of IL-7R was reported.52 And in acute lymphoblastic lymphoma, 2 alternatively spliced transcripts encoding truncated IL-7R proteins have been described.53,54 Despite accumulation of evidence that favors a role of γc-receptor family in leukemogenesis, the exact mechanisms by which cytokines mediate T-cell leukemia in humans are still unclear. As a consequence, the occurrence of leukemia following γc gene therapy of X-linked severe combined immune deficiency (SCID) brought these trials to a halt, pending further investigation. Because the X-linked SCID is caused by mutations in the γc gene, the trial by Cavazzana-Calvo et al55 aimed to transduce cells in an effort to achieve higher levels of the human γc expression. Unfortunately, this trial was compromised in 2 of 10 patients as a result of the γc vector integration near the protooncogene LMO2 and cellular transformation in a prethymic stem or progenitor cell.56 Now, the hypotheses point to a role of the γc transgene product itself with particular interest toward the IL-7R pathway because high levels of IL-7 were found in patients with SCID.57 Increased signaling through the IL-7R could have primed cells of patients with SCID to transformation, and additional up-regulation of LMO2 could compound this effect, leading the cells one step further to transformation. Our finding that higher levels of IL-7Rα expression may promote thymic oncogenesis has potentially important implications for such trials.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-06-2126.
Supported by an American Diabetes Association Mentor-Based Postdoctoral Fellowship (Y.L.) and by a grant from the National Institutes of Health (grant GM56689) (I.N.C.). R.A.F. is an investigator of the Howard Hughes Medical Institute.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr Elizabeth Eynon for critical reading of this manuscript and Dr Patrick Fields for helpful discussions. We also thank Tom Taylor for cell sorting and Fran Manzo for help with manuscript preparation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal