Abstract
The 1D10 antigen is the target for Hu1D10 (apolizumab), a humanized HLA-DR β-chain–specific antibody that is currently in clinical trials for hematologic malignancies. We demonstrate that Hu1D10 induces caspase-independent apoptosis following secondary cross-linking in primary chronic lymphocytic leukemia (CLL) cells. Generation of reactive oxygen species (ROS) and signal transduction, as evidenced by phosphorylation of Syk and AKT, were noted. The source of the Hu1D10-induced ROS was examined using the Raji lymphoblastic cell line with engineered defects in the mitochondrial respiratory chain. Hu1D10 treatment of clones with deficient mitochondrial respiration produced ROS suggesting a cytoplasmic source. Administration of ROS scavengers to primary CLL cells prior to Hu1D10 treatment diminished AKT activation. Treatment with Hu1D10 and the phosphatidylinositol 3-kinase inhibitor LY294002 demonstrated in vitro synergy with enhanced apoptosis. In conjunction with an ongoing clinical trial, blood samples were collected following intravenous infusion of Hu1D10 and analyzed for phosphorylation of AKT. Two of 3 patient samples showed a sustained increase in AKT phosphorylation following Hu1D10 administration. These data suggest that Hu1D10 ligation in CLL cells induces death and survival signals for which combination therapies may be designed to greatly enhance efficiency of both Hu1D10 and other class II antibodies in development.
Introduction
Chronic lymphocytic leukemia (CLL) is one of the most common types of leukemia diagnosed in the Western Hemisphere, with a median survival of 18 months to 3 years in patients with advanced stage disease.1-3 Therapeutic options for the initial treatment of CLL can include either alkylator therapy (chlorambucil or cyclophosphamide) or a nucleoside analog (fludarabine or cladribine),4,5 with recent studies demonstrating the ability of fludarabine to prolong progression-free survival.6-8 Attempts to improve outcome further by combining fludarabine with cyclophosphamide have shown promising results9-11 and are currently being evaluated in phase 3 testing. Even this combination yields a complete response rate of less than 50% in all series reported,9-11 emphasizing the need for pursuit of new therapies for the treatment of CLL.
Immunotherapy with unconjugated monoclonal antibodies in CLL represents one new potentially exciting mode of therapy for CLL. Of the many antibodies tested in CLL, rituximab and alemtuzumab have the highest reported success rate to date. Studies with rituximab in small lymphocytic lymphoma (SLL) and CLL12-17 initially demonstrated marginal results, although subsequent studies directed at overcoming the adverse pharmacokinetic parameters13 have led to improved efficacy.18,19 Rituximab has also been demonstrated to improve complete response to fludarabine-based therapy.20 The alemtuzumab antibody has similarly demonstrated activity in patients with CLL refractory to fludarabine,21-25 but use has been somewhat limited by a high frequency of serious infections. Studies by our group have demonstrated that both rituximab and alemtuzumab induce caspase-dependent apoptosis in vivo,26,27 similar to that induced by fludarabine and other chemotherapeutic agents. Neither of these therapies produces consistent complete remissions, providing support for identifying therapeutic targets, particularly ones that induce apoptosis in a different manner.
Class II major histocompatibility complex (MHC) antigens are expressed only on a subset of immune effector cells, including B cells, monocytes, and dendritic cells and represent one such potential novel target. It is notable that several groups have reported that murine antibodies targeting either the α or β chain of HLA-DR induce apoptosis in B-cell lymphoma cell lines,28-33 normal B cells, and mature B-cell malignancies. Interrogation of the pathway leading to apoptosis in cell lines has demonstrated varied results, with some studies noting caspase-8 activation and others noting use of a caspase-independent pathway.28-36 These studies examined different murine and humanized antibodies targeting several different components of the HLA-DR α or β chain in several cell models, possibly explaining the discordant results.
Introduction and preliminary successful application of the humanized antibody Hu1D10 (apolizumab) in the treatment of non-Hodgkin lymphoma (NHL) has prompted renewed interest in therapeutic targeting of class II antigens. Hu1D10 is a humanized IgG1 monoclonal antibody directed at the 1D10 antigen.33,37 The 1D10 antibody binds to an epitope on a variant of the HLA-DR β chain.33 This antigen is expressed on a proportion of NHL patient cells and also on normal B lymphocytes, monocytes, and dendritic cells.33 The 1D10 antigen expression on CLL cells was reported as frequent in one small cohort of patients with CLL,33 but has not been well characterized relative to clinical features associated with this disease. In vitro studies against NHL cell lines demonstrate that Hu1D10 induces toxicity by antibody-dependent cellular cytotoxicity (ADCC), complement-mediated cell lysis, and apoptosis in the absence of Fc subunit cross-linking.33,36 A phase 1 study of Hu1D10 in NHL has been completed with responses noted in 5 patients with low-grade lymphoma.38 Based on these data, we sought to determine if Hu1D10 induced apoptosis in CLL cells in vitro and to ascertain if transmembrane signaling may contribute to this cytotoxic effect.
Materials and methods
Cell culture and cell lines
The human B-lymphoblastic Raji cell line was cultured at 37°C in an atmosphere of 5% CO2 in RPMI 1640 media (Media Tech, Herndon, VA) supplemented with 10% fetal bovine serum (FBS; Hyclone Laboratories, Logan, UT). Respiration-deficient subclones (C2, C6, and C8) were derived from Raji cells by chronic exposure to a low concentration of ethidium bromide (50 ng/mL) for approximately 30 cell cycles in the RPMI culture medium supplemented with 10% FBS, 0.47% glucose, 50 μg/mL uridine, and 1 mM pyruvate (Sigma-Aldrich, St Louis, MO), and then subcloned by the limited dilution method as described previously.39-41 Deletion of specific mtDNA and defects in mitochondria respiration function of the subclones (ρ– cells) were confirmed by polymerase chain reaction and oxygen consumption assay, respectively, as we previously described.41 Due to the lack of mitochondrial respiration, the ρ– cells depend on glycolysis as the energy source and are unable to produce superoxide radicals through the defective respiration chain, as evidenced by substantially lower overall generation of reactive oxygen species (ROS) regardless of the presence or absence of a respiration chain inhibitor.41
CLL sample processing
Blood was obtained from patients with B-cell CLL with informed consent under a protocol approved by the hospital internal review board. All patients examined in this series had immunophenotypically defined CLL as outlined by the modified 96 National Cancer Institute criteria.42 CLL cells were isolated immediately following donation using Ficoll density gradient centrifugation (Ficoll-Paque Plus; Amersham Biosciences, Piscataway, NJ). Isolated mononuclear cells were incubated (37°C and 5% CO2) in RPMI 1640 media supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine (Invitrogen, Carlsbad, CA), and penicillin (100 U/mL)/streptomycin (100 μg/mL; Sigma-Aldrich) for up to 18 hours prior to use in experiments. Samples used were more than 90% B cells as determined by CD19 surface staining and fluorescence-activated cell sorting (FACS) analysis. Freshly isolated CLL cells were used for all the experiments described herein.
Peripheral blood samples were obtained from 3 patients before Hu1D10 treatment and during the course of the initial intravenous Hu1D10 infusion as part of a recently completed phase 1 study.43 A lymphocyte-enriched fraction was isolated and immediately processed to obtain whole cell protein lysate.
Determination of Hu1D10 expression level on CLL cells
Mononuclear cells were isolated as described (see “CLL sample processing”). After washing cells twice in phosphate-buffered saline (PBS), cells were incubated with fluorescein isothiocyanate (FITC)–labeled anti-CD19 (monoclonal antibody [mAb] HIB19; BD Pharmingen, San Diego, CA) and either Hu1D10-phycoerythrin (PE) or MSL109-PE (both from Protein Design Labs, Fremont, CA). Cells were incubated with the antibody-fluorophore conjugates for 30 minutes at 4°C, rinsed twice in PBS, and analyzed by FACS. MSL-109, which is an anticytomegalovirus antibody, is an isotype control for Hu1D10 labeling of CLL cells.
In vitro anti–HLA-DR antibody and inhibitor treatment of cells prior to flow cytometry analysis
The anti–HLA-DR antibody Hu1D10 was produced by Protein Design Labs.33 The anti–HER2-receptor humanized monoclonal antibody trastuzumab, isotype IgG1 κ, (Genentech, San Francisco, CA) was used to control for nonspecific binding or Fc region-mediated events or both. Cross-linking antibody, goat antihuman Ig Fc γ fragment-specific (Jackson ImmunoResearch Laboratories, West Grove, PA), was added 5 minutes after the addition of Hu1D10. The concentration of Hu1D10, trastuzumab, and goat antihuman Fc antibody was 10 μg/mL in all experiments. Cytochalasin B (Sigma-Aldrich) was added 60 minutes prior to Hu1D10. B-cell membranes were depleted of cholesterol by incubation of the cells with 10 mM methyl-β-cyclodextran (Sigma-Aldrich) in RPMI 1640 for 30 minutes at 37°C, followed by washing 2 times in RPMI 1640. Stock solutions of piceatannol (trans-3,3′,4,5′-tetrahydroxystilbene) 10 mg/mL (Sigma-Aldrich) and LY294002 (2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one) 12.5 mM (Calbiochem, San Diego, CA) were prepared in dimethyl sulfoxide (DMSO). The inhibitors piceatannol or LY294002 were added 20 minutes prior to the addition of Hu1D10 to give final concentrations of 25 μg/mL and 25 μM, respectively. For the antioxidant experiments, stock solutions of N-acetyl-l-cysteine 500 mM or tiron 500 mM (4,5-dihydroxy-1,3-benzene-disulfonic acid; both from Sigma-Aldrich) were prepared just prior to use in PBS at pH 7.4. The antioxidants were diluted in supplemented RPMI 1640 media to yield final concentrations of 10 mM tiron or 25 mM N-acetyl-l-cysteine. Cells were incubated for 3 hours with the antioxidants prior to addition of Hu1D10. All experiments were performed in supplemented RPMI 1640 media at 106cells/mL, incubated at 37°C with 5% CO2.
Assessment of antibody-treated CLL cells by flow cytometry
Apoptotic cells were quantified using dual annexin V–FITC/propidium iodide (PI) staining with FACS analysis.44 Following incubation with the treatment drugs, cells were stained with annexin V–FITC (BD Pharmingen) and PI (BD Pharmingen) according to the manufacturer's directions and analyzed by flow cytometry. Cells staining with annexin V–FITC or PI were considered positive. Rhodamine 123 was used to monitor the integrity of mitochondria following drug treatment.45,46 Antibody-treated cells were washed once in RPMI 1640 media and then incubated in RPMI 1640 media containing 50 ng/mL rhodamine 123 (Molecular Probes, Eugene, OR) for 30 minutes at 37°C. Stained cells were washed once in RPMI 1640 media, placed on ice, and analyzed by flow cytometry. Experiments examining caspase dependence included addition of 100 μM Z-VAD-fmk (benzyloxycarbonyl-val-ala-asp [Ome] fluoromethylketone; Calbiochem) 30 minutes prior to addition of Hu1D10. DNA-laddering experiments for apoptosis were performed as described elsewhere.47
The intracellular formation of ROS following drug treatment was measured using the dye dihydroethidium (Molecular Probes).48 Treated cells were washed once in RPMI 1640, resuspended in RPMI 1640 containing 5 μM dihydroethidium, and then incubated for 30 minutes at 37°C. The cells were washed again in RPMI 1640 and immediately analyzed by flow cytometry.
For all flow cytometry experiments, FACS analysis was performed using a Beckman-Coulter model EPICS XL cytometer (Beckman-Coulter, Miami, FL). Quantitative FACS analysis was done as previously described.19 Fluorophores were excited at 488 nm. Fluorescence was measured using channel FL1 for annexin V–FITC and CD19-FITC, and channel FL3 for PI, rhodamine 123, oxidized dihydroethidium, and PE-labeled antibodies. Data were analyzed with the System II software package (Beckman-Coulter). At least 10 000 cells were counted for each treated sample. Each sample was run in duplicate. To avoid contamination of the data with fluorophore-stained necrotic or late apoptotic cells, only “intact cells” were analyzed in the dihydroethidium experiments. Gates for “intact cells” were determined based on the light-scattering properties of untreated cells and left unchanged for the analysis of the treated cells.
Western blot analysis
Whole cellular lysates were prepared as previously described by our group with the addition of the phosphatase inhibitors sodium vanadate (2 mM) and microcystin LR (0.004 μg/mL; both from Sigma-Aldrich) to the lysis buffer.49 Samples for ZAP-70 analysis were positively selected using MACS CD19 MicroBeads (Miltenyi Biotec, Auburn, CA) per the manufacturer's directions. Immunoprecipitation was performed by incubating 200 μg total protein in lysis buffer, diluted 1:1 with PBS, with the desired antibody for 1 hour. Then, 30 μL protein A/G Plus agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA) were added, followed by incubation at 4°C overnight. The agarose beads were washed with PBS plus enzyme inhibitors 3 times, then heated with sample loading buffer just prior to loading into a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel. Syk tyrosine phosphorylation (mAb 4G10; Upstate Biotechnology, Lake Placid, NY), Syk (mAb 4D10; Santa Cruz Biotechnology), ZAP-70 (mAb 2F3.2; Upstate Biotechnology), phospho-AKT (rabbit pcAb ser472/473/474; BD Pharmingen), AKT (rabbit pcAb; Cell Signaling Technology, Beverly, MA), caspase-3 (mAb Ab-2; Oncogene Research Products, San Diego, CA), caspase-8 (mAb 3-1-9; BD Pharmingen), caspase-9 (rabbit pAb Ab-1; Oncogene Research Products), poly(adenosine diphosphate ribose) polymerase (PARP; mAb Ab-2; Oncogene Research Products), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Chemicon International, Temecula, CA) were analyzed by Western blot SDS-PAGE. Equivalent protein samples (50 μg/lane) were separated on 10% polyacrylamide gels and transferred onto 0.2-μm nitrocellulose membranes (Schleicher & Schuell, Keene, NH). Following antibody incubation, the proteins were detected with chemiluminescent substrate (SuperSignal, Pierce, Rockford, IL). For the AKT immunoblots, the blots were developed with antiphospho-AKT, stripped, and reprobed with anti-AKT antibody. Protein bands were quantified by integration of the chemiluminescence signals on a ChemiDoc system with Quantity One software (Bio-Rad Laboratories, Hercules, CA) using the rectangular integration tool after background subtraction. Statistical analysis of the data was performed using Excel software (Microsoft, Redmond, WA) with standard methods.
Results
The 1D10 antigen is expressed on most CLL patient samples
To confirm the initial expression studies of the 1D10 antigen on tumor cells,33,37 we examined samples from 43 patients with CLL. A tumor cell sample was considered Hu1D10+ if more than 20% of the CD19+ cells had an Hu1D10-PE mean fluorescence intensity (MFI) more than twice the MFI of the MSL-109-PE isotype control-labeled cells, although virtually all patients expressing 1D10 had 1D10 expressed on 90% or more of their CD19+ cells. Of the 43 patients samples, 37 (86%) had positive expression of the 1D10 antigen by these criteria. There is considerable variability of Hu1D10 antigen expression among the different patient samples. For Hu1D10+ patients, the average MFI ratio (MFIR) is 11.5 (SD 7.2) with a maximum value of 31.7. Quantitative FACS analysis was performed on 4 different representative patient samples and revealed that 17 200 (SD 9500) Hu1D10-PE molecules were bound per CLL cell. The values of the Hu1D10 MFIR did not correlate with the donors' sex, age, stage of disease, or prior treatment for CLL.
Hu1D10 mAb induces apoptosis in CLL cells
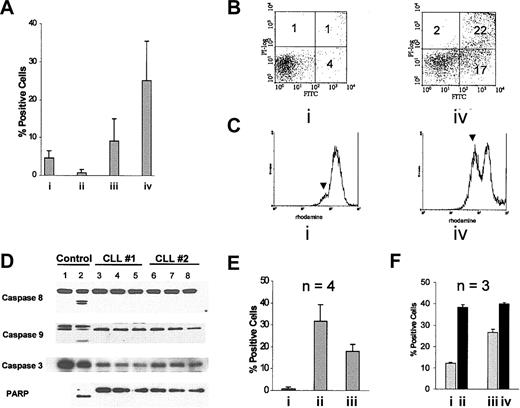
Studies have shown ligation of MHC class II antigens on normal lymphocytes and cell lines induces apoptosis.28-36 Two prior studies have shown that anti–HLA-DR antibodies lead to apoptosis in CLL cells.32,34 Here we show that the Hu1D10 anti–HLA-DR antibody induces apoptosis in CLL cells. In vitro exposure of human CLL cells to the Hu1D10 antibody (0.01-10 μg/mL) alone did not increase apoptosis significantly as measured by annexin V–FITC/PI staining with either short or long exposure (data not shown). However, when CLL cells from 8 Hu1D10+ patients (MFIR 13.5, SD 2.9) were incubated with the Hu1D10 (10 μg/mL) antibody in the presence of antihuman Fc antibody (10 μg/mL), apoptosis increased by 25.1% (SD 10.3%) above the media control following a 4-hour incubation (Figure 1A). Contrasting with this, incubation with unheated serum in the presence of Hu1D10 did not induce apoptosis as shown in Figure 1A, suggesting that complement activation does not contribute to or enhance apoptosis induced by Hu1D10. Data following treatment with Hu1D10 and antihuman Fc antibody treatment from a representative patient are shown in Figure 1B. Hu1D10 concentrations of less than 10 μg/mL produced less apoptosis; however, concentrations above 10 μg/mL of either Hu1D10 or antihuman Fc antibody did not augment in vitro response (data not shown). A significant increase in apoptosis was not observed after treatment with the negative control trastuzumab plus antihuman Fc antibody (Figure 1A). Although some samples treated with trastuzumab stained positive with annexin V–FITC, they did not stain positive with PI. Furthermore, patients' cells not expressing the 1D10 antigen did not have an increase in apoptosis (data not shown). It has recently been reported that activated murine lymphocytes may bind to annexin V even in the absence of apoptosis.50 Therefore, we sought to confirm the use of annexin V-FITC/PI+ staining as an accurate indicator of antibody-mediated apoptosis/necrosis in CLL cells using the mitochondrial dye rhodamine 123. Figure 1C shows a representative study where loss of mitochondrial potential is observed in approximately 43% of Hu1D10 plus antihuman Fc antibody-treated CLL cells. A correlative study showed 39% annexin–V/PI+ CLL cells. Forward and side light-scattering patterns of CLL cells indicate formation of a population of smaller more complex cells following Hu1D10 plus antihuman Fc antibody treatment, which is consistent with an apoptotic phenotype (data not shown). These experiments confirm that Hu1D10 plus antihuman Fc antibody is inducing rapid loss of mitochondrial membrane potential and apoptosis in CLL cells in vitro, which is dependent on the surface expression of the 1D10 antigen.
Cross-linked Hu1D10 induces apoptosis in CLL cells. (A) CLL samples were treated for 4 hours with either (i) Hu1D10 (10 μg/mL), (ii) Hu1D10 (10μg/mL) with media containing 30% autologous serum, (iii) trastuzumab plus antihuman Fc antibody (both 10 μg/mL), or (iv) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). Following annexin V–FITC/PI staining, samples were analyzed by flow cytometry. Values represent the percentage of positive cells above that measured for untreated cells. Error bars indicate the SD among the different samples and thus represent heterogeneity of the samples rather than measurement error. All samples strongly expressed the 1D10 antigen. (B) A representative annexin V–FITC/PI dot plot after media (i) or Hu1D10 plus antihuman Fc antibody (iv). The numbers in each quadrant indicate the percent of cells labeled with annexin V–FITC (lower right), PI (upper left), or annexin V–FITC and PI (upper right). (C) After a 4-hour incubation without (i) or with (iv) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL), cells were incubated with 50 ng/mL rhodamine 123 and then analyzed by flow cytometry. The rise in the lower intensity peak indicated by the arrow, with antibody treatment, indicates loss of the fluorescent dye rhodamine 123 from mitochondria. Histograms in panel C are representative of experiments performed on 4 different CLL patient samples. (D) Protein lysates from cells incubated for 4 hours in media (lanes 3 and 6), trastuzumab plus antihuman Fc antibody (both 10 μg/m; lanes 4 and 7) or Hu1D10 plus antihuman Fc antibody (both 10 μg/mL; lanes 5 and 8) were probed for PARP and caspases 3, 8, and 9. Unlike UV-irradiated Jurkat cell–positive control (lane 2), no processing of the caspases or PARP was observed despite apoptosis as depicted in panels A-C. (E) Prior to treating cells with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, cells were incubated for 30 minutes with (iii) or without (ii) cytochalasin B (20 μM). Cells treated with cytochalasin B only are indicated by I. Following treatment, cells were stained with annexin V–FITC/PI and analyzed by flow cytometry. Values shown are percent positive cells above untreated samples. Error bars indicate the SD among 4 different CLL samples tested. (F) Cells were incubated for 30 minutes in media (i-ii) or media plus 10 mM methyl-B-cyclodextran (iii-iv). Samples represented by ii and iv were then treated with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours. Cells were then stained with annexin V–FITC/PI and analyzed by flow cytometry. Note that the relative difference between the methyl-B-cyclodextran–treated samples (13.8%) is significantly less than the untreated samples (26.2%).
Cross-linked Hu1D10 induces apoptosis in CLL cells. (A) CLL samples were treated for 4 hours with either (i) Hu1D10 (10 μg/mL), (ii) Hu1D10 (10μg/mL) with media containing 30% autologous serum, (iii) trastuzumab plus antihuman Fc antibody (both 10 μg/mL), or (iv) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). Following annexin V–FITC/PI staining, samples were analyzed by flow cytometry. Values represent the percentage of positive cells above that measured for untreated cells. Error bars indicate the SD among the different samples and thus represent heterogeneity of the samples rather than measurement error. All samples strongly expressed the 1D10 antigen. (B) A representative annexin V–FITC/PI dot plot after media (i) or Hu1D10 plus antihuman Fc antibody (iv). The numbers in each quadrant indicate the percent of cells labeled with annexin V–FITC (lower right), PI (upper left), or annexin V–FITC and PI (upper right). (C) After a 4-hour incubation without (i) or with (iv) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL), cells were incubated with 50 ng/mL rhodamine 123 and then analyzed by flow cytometry. The rise in the lower intensity peak indicated by the arrow, with antibody treatment, indicates loss of the fluorescent dye rhodamine 123 from mitochondria. Histograms in panel C are representative of experiments performed on 4 different CLL patient samples. (D) Protein lysates from cells incubated for 4 hours in media (lanes 3 and 6), trastuzumab plus antihuman Fc antibody (both 10 μg/m; lanes 4 and 7) or Hu1D10 plus antihuman Fc antibody (both 10 μg/mL; lanes 5 and 8) were probed for PARP and caspases 3, 8, and 9. Unlike UV-irradiated Jurkat cell–positive control (lane 2), no processing of the caspases or PARP was observed despite apoptosis as depicted in panels A-C. (E) Prior to treating cells with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, cells were incubated for 30 minutes with (iii) or without (ii) cytochalasin B (20 μM). Cells treated with cytochalasin B only are indicated by I. Following treatment, cells were stained with annexin V–FITC/PI and analyzed by flow cytometry. Values shown are percent positive cells above untreated samples. Error bars indicate the SD among 4 different CLL samples tested. (F) Cells were incubated for 30 minutes in media (i-ii) or media plus 10 mM methyl-B-cyclodextran (iii-iv). Samples represented by ii and iv were then treated with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours. Cells were then stained with annexin V–FITC/PI and analyzed by flow cytometry. Note that the relative difference between the methyl-B-cyclodextran–treated samples (13.8%) is significantly less than the untreated samples (26.2%).
Previous studies in B-cell NHL cell lines, primary CLL cells, and in normal splenic B cells examined the mode of class II antibody-induced apoptosis have demonstrated varied pathways of cell death that involve both the extrinsic pathway of apoptosis31 and caspase-independent pathways of apoptosis.32 Immunoblot analysis in Figure 1D from 2 representative patients demonstrates no processing of the caspase-8, caspase-9, caspase-3 or cleavage of PARP, to the p85 fragment, which is typical of caspase-dependent apoptosis. This is despite cell death as demonstrated in Figure 1B-C. The observed Hu1D10-induced apoptosis was not inhibited by the broad caspase inhibitor Z-VAD-fmk (data not shown), which supports a caspase-independent mechanism of action for this antibody. Furthermore, consistent with a caspase-independent mechanism, we also did not observe small fragment DNA laddering following Hu1D10 plus antihuman Ig treatment of CLL cells from 3 patients (data not shown). These data suggest that Hu1D10 (and other HLA-DR–directed antibodies) mediates caspase-independent apoptosis through a pathway different from cytotoxic chemotherapy51,52 or rituximab26 in primary CLL cells.
Early cytoskeletal reorganization is required for Hu1D10-induced apoptosis
Cytoskeletal reorganization has been demonstrated to be necessary for CD47 antibody-mediated caspase-independent apoptosis in CLL cells.53 More recently, antibody-induced aggregation of HLA-DR within lymphocyte plasma membranes has been shown to be dependent on actin microfilament polymerization.54 Furthermore, as noted above, Hu1D10-induced apoptosis requires hyper–cross-linking. Therefore, we hypothesized that Hu1D10-induced apoptosis in CLL cells may require HLA-DR aggregation with concurrent cytoskeletal rearrangement. Figure 1E demonstrates that pretreatment of CLL cells with cytochalasin B, an agent that prevents actin polymerization, prior to Hu1D10 treatment, inhibits apoptosis in CLL cells. In contrast to Hu1D10, fludarabine nucleoside (2-F-ara-A)–induced apoptosis was not prevented by cytochalasin B (data not shown). Ligation of HLA-DR has been shown to induce aggregation of HLA-DR in lipid rafts with consequent intracellular signaling as measured by an increase in tyrosine phosphorylation of multiple proteins.55,56 As shown in Figure 1F, cholesterol depletion of CLL membranes with methyl-β-cyclodextran, with presumed disruption of lipid rafts as has been shown previously,56 significantly reduces Hu1D10-induced apoptosis. Our data suggest that large aggregates of HLA-DR with cytoskeletal rearrangement are, in part, necessary to trigger Hu1D10-induced apoptosis in CLL cells.
Increased formation of intracellular ROS accompanies Hu1D10-induced apoptosis and is antagonized by prevention of cytoskeletal rearrangements or lipid raft disruption
Formation of intracellular ROS plays an important role in complement-induced, caspase-independent apoptosis induced by rituximab in CLL cells.48 Furthermore, a prior study showed evidence for ROS formation following HLA-DR ligation of activated B cells.57 Thus, we hypothesized that Hu1D10 treatment would lead to generation of ROS in CLL cells. The membrane-permeable fluorescent dye dihydroethidium is oxidized in the presence of ROS, intercalates within dsDNA, and then fluoresces maximally at 600 nm. Increased cellular fluorescence, as measured by flow cytometry, indicates increased production of ROS. Figure 2 demonstrates that Hu1D10 plus antihuman Fc antibody causes a rapid increase in production of ROS in CLL cells as early as 1 hour prior to the 4-hour time point when apoptosis was noted. Selective gating using forward and side light-scattering properties to eliminate the later stage apoptotic cells and fragmented cells from analysis still demonstrated a significant increase of ROS, albeit slightly lower than that observed in apoptotic cells. These results suggest that the increase in ROS is not a phenomenon that is present only in late-stage apoptotic cells. The increased production of ROS was not observed at this time point with fludarabine (data not shown), suggesting that this rapid induction of ROS formation is relatively specific for Hu1D10 and other HLA-DR β-chain–directed antibodies. As expected, treatment of CLL samples with the ROS scavengers tiron or N-acetyl cysteine significantly diminished ROS as measured by the dihydroethidium dye (Figure 2A), thereby supporting the use of this technique to measure ROS formation in CLL cells.
Hu1D10 treatment of CLL cells leads to increased intracellular ROS formation, which is decreased by preincubation with antioxidants, cytochalasin B, or methyl-β-cyclodextran. (A) CLL cells were treated as follows: (i) media only, (ii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, (iii) pretreatment with 25 mM N-acetyl cysteine followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, or (iv) pretreatment with 10 mM tiron followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours. Following treatment, cells were incubated for 30 minutes at 37°C with 5 μM dihydroethidium and then analyzed by flow cytometry. These data represent one of 4 CLL samples tested. (B) CLL cells were treated as follows: (i) media only, (ii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, (iii) pretreatment with cytochalasin B (20 μM) followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours. Following treatment, cells were stained with dihydroethidium and then analyzed by flow cytometry. These data represent one of 3 CLL samples tested. (C) CLL cells were treated as follows: (i) media only, (ii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, (iii) pretreatment with methyl-β-cyclodextran followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours. Following treatment, cells were analyzed for ROS as noted. These data represent one of 3 CLL samples tested.
Hu1D10 treatment of CLL cells leads to increased intracellular ROS formation, which is decreased by preincubation with antioxidants, cytochalasin B, or methyl-β-cyclodextran. (A) CLL cells were treated as follows: (i) media only, (ii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, (iii) pretreatment with 25 mM N-acetyl cysteine followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, or (iv) pretreatment with 10 mM tiron followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours. Following treatment, cells were incubated for 30 minutes at 37°C with 5 μM dihydroethidium and then analyzed by flow cytometry. These data represent one of 4 CLL samples tested. (B) CLL cells were treated as follows: (i) media only, (ii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, (iii) pretreatment with cytochalasin B (20 μM) followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours. Following treatment, cells were stained with dihydroethidium and then analyzed by flow cytometry. These data represent one of 3 CLL samples tested. (C) CLL cells were treated as follows: (i) media only, (ii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours, (iii) pretreatment with methyl-β-cyclodextran followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for 4 hours. Following treatment, cells were analyzed for ROS as noted. These data represent one of 3 CLL samples tested.
We have shown that pretreatment of CLL cells with the actin polymerization inhibitor cytochalasin B, or depletion of membrane cholesterol with methyl-β-cyclodextran, reduces Hu1D10-induced apoptosis (Figure 1E-F). Pretreatment of CLL cells with either of these compounds markedly reduced Hu1D10-induced ROS formation (Figure 2B-C). These data suggest that formation of HLA-DR aggregates and cytoskeletal rearrangement are required for down-stream events that lead to ROS generation.
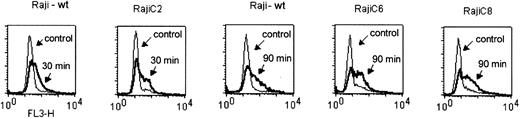
Potential sources of ROS include mitochondrial, cytoplasmic, and plasma membrane-associated oxidases.58,59 Because the mitochondrial respiration chain is a major site of cellular ROS generation due to electron leakage from respiratory complexes I and III, we tested if mitochondrial respiration might play a role in Hu1D10-induced increase of ROS. Several respiration-deficient cell subclones (ρ– cells) were derived from human B-lymphoblastic cells (Raji), which express the 1D10 antigen.33 The lack of mitochondrial respiration and inability to generate ROS through the respiration complexes in ρ– cells were confirmed by oxygen consumption and ROS generation assays as described previously.41 The ρ– cells were used to test the effect of Hu1D10 on ROS generation in comparison with the parental Raji cells. As shown in Figure 3, Hu1D10 treatment of wild-type Raji cells leads to production of intracellular ROS as measured by dihydroethidium staining with FACS analysis. Interestingly, Hu1D10 also caused the same increase of ROS production in all 3 ρ– cell clones tested (Figure 3) with similar kinetics. These data indicate that ligation of HLA-DR leads to formation of ROS, which appeared to be independent of mitochondrial respiration, suggesting that the source of ROS induced by Hu1D10 is likely extramitochondrial.
Induction of ROS generation in Raji cells and the respiration-deficient subclones RajiC2, RajiC6, and RajiC8. Cells in exponential growing phase were incubated with Hu1D10 (5 μg/mL) without the cross-linking antibody for the indicated time. Cellular superoxide radical content was determined by flow cytometry analysis using dihydroethidium dye. The results show that Hu1D10 was able to cause a significant increase of ROS in both the wild-type (wt) and respiration-deficient cells (C2, C6, and C8), suggesting that the drug-induced ROS generation was not from within the mitochondrial respiratory chain.
Induction of ROS generation in Raji cells and the respiration-deficient subclones RajiC2, RajiC6, and RajiC8. Cells in exponential growing phase were incubated with Hu1D10 (5 μg/mL) without the cross-linking antibody for the indicated time. Cellular superoxide radical content was determined by flow cytometry analysis using dihydroethidium dye. The results show that Hu1D10 was able to cause a significant increase of ROS in both the wild-type (wt) and respiration-deficient cells (C2, C6, and C8), suggesting that the drug-induced ROS generation was not from within the mitochondrial respiratory chain.
Hu1D10 promotes signaling through Syk and AKT
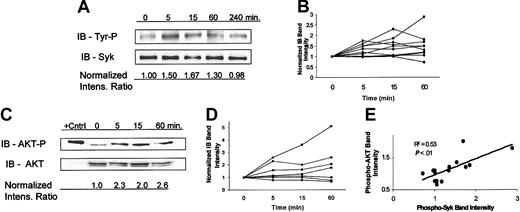
A previous study has shown that HLA-DR ligation on normal B cells promotes signaling via tyrosine phosphorylation of Syk.60 Furthermore, studies have shown that independent of HLA-DR ligation, the presence of ROS promotes tyrosine phosphorylation of multiple proteins and leads to the activation of Syk and the down-stream phosphatidylinositol 3-kinase (PI3 kinase)/AKT pathway in normal B cells61-63 and in a T-cell line.64 Given this background, we surmised the signaling events following ligation of HLA-DR on B cells may be, in part, a consequence of anti-HLA-DR–induced ROS formation as previously demonstrated (Figure 2). Figure 4A shows that Hu1D10 treatment of CLL cells leads to tyrosine phosphorylation of Syk. The relative increase in Syk phosphorylation following Hu1D10 treatment is variable among the different patient samples (Figure 4B). The variation in the maximum values of phospho-Syk/Syk level for each sample did not correlate (R2 = 0.17, P > .05) with the variable ZAP-70 levels among the different samples as determined by Western blot analysis (data not shown). An important downstream target of Syk is AKT, a kinase that promotes cell survival through phosphorylation of a variety of apoptosis-related proteins including caspase-9 and BAD.65,66 Figure 4C is a representative experiment demonstrating that Hu1D10 treatment promotes activation of AKT as measured by phosphorylation of the amino acid serine 473 (Ser473). As with Syk, phosphorylation of AKT following Hu1D10 treatment of CLL cells is variable among the different patient CLL samples (Figure 4D).
Ligation of HLA-DR by Hu1D10 promotes phosphorylation of both Syk and AKT. (A) CLL cells were treated with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for the indicated times. Cells were immediately lysed, immunoprecipated (IP) with anti-Syk/protein A-G agarose beads. Immunoblotting of the IP product was performed with antityrosine antibody (monoclonal antibody 4g10). Gels were stripped and then reprobed with anti-Syk. A representative patient is shown here demonstrating an increase in Syk tyrosine phosphorylation following Hu1D10 treatment. Panel B summarizes the results from 10 different patient samples. The intensity ratios are determined from the ratio of 4g10 band intensity to the Syk band intensity. Values are normalized to the value at time zero. (C) CLL cells were treated with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for the indicated times. Cells were immediately lysed and assessed for changes in AKT activity by determination of Ser473 phosphorylation through immunoblot analysis. A representative patient is shown here demonstrating an increase in phosphorylation of AKT following Hu1D10 treatment. The positive control is Jurkat cell lysate. Panel D summarizes the immunoblot data from 7 different patient samples. (E) Five samples were treated for 0, 5, 15, and 60 minutes with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). Using the techniques outlined, normalized ratios of Syk-P/Syk and AKT-P/AKT band intensities were determined for each sample at the noted times. For each sample at a given time point the value of the AKT-P and Syk-P ratio is plotted. By definition, the x, y values at time zero equals 1, so these values are not plotted. As indicated by the positive correlation coefficient (R2 = 0.53), phospho-AKT levels rise as phospho-Syk levels rise.
Ligation of HLA-DR by Hu1D10 promotes phosphorylation of both Syk and AKT. (A) CLL cells were treated with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for the indicated times. Cells were immediately lysed, immunoprecipated (IP) with anti-Syk/protein A-G agarose beads. Immunoblotting of the IP product was performed with antityrosine antibody (monoclonal antibody 4g10). Gels were stripped and then reprobed with anti-Syk. A representative patient is shown here demonstrating an increase in Syk tyrosine phosphorylation following Hu1D10 treatment. Panel B summarizes the results from 10 different patient samples. The intensity ratios are determined from the ratio of 4g10 band intensity to the Syk band intensity. Values are normalized to the value at time zero. (C) CLL cells were treated with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) for the indicated times. Cells were immediately lysed and assessed for changes in AKT activity by determination of Ser473 phosphorylation through immunoblot analysis. A representative patient is shown here demonstrating an increase in phosphorylation of AKT following Hu1D10 treatment. The positive control is Jurkat cell lysate. Panel D summarizes the immunoblot data from 7 different patient samples. (E) Five samples were treated for 0, 5, 15, and 60 minutes with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). Using the techniques outlined, normalized ratios of Syk-P/Syk and AKT-P/AKT band intensities were determined for each sample at the noted times. For each sample at a given time point the value of the AKT-P and Syk-P ratio is plotted. By definition, the x, y values at time zero equals 1, so these values are not plotted. As indicated by the positive correlation coefficient (R2 = 0.53), phospho-AKT levels rise as phospho-Syk levels rise.
If activation of Syk leads to activation AKT in these CLL samples, one would expect the relative degree of phosphorylation of Syk and phosphorylation of AKT to correlate among the multiple samples tested. For 5 different CLL samples both phospho-Syk (phospho-tyrosine) and phospho-AKT (Ser473) levels were determined at several time points following Hu1D10 treatment of the cells. The normalized ratios of phospho-Syk/Syk or phospho-AKT/AKT were determined by quantifying band intensities of immunoblots. Figure 4E shows a relatively good correlation (R2 = 0.53, P < 0.01) between phosphorylated AKT levels and phosphorylated Syk levels among the different samples at various time points, consistent with the hypothesis that the AKT survival signal is significantly associated with Syk activation.
Selective inhibition of PI3 kinase promotes synergy with Hu1D10 treatment
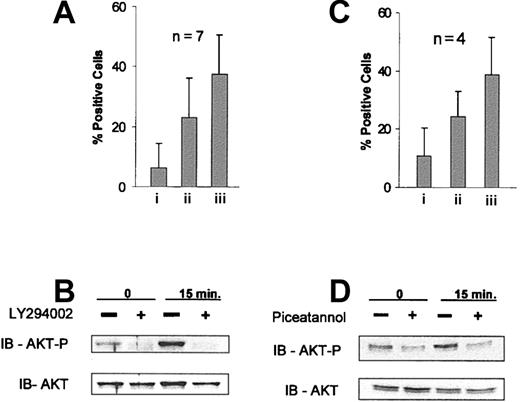
We next sought to determine if inhibition of AKT activation by the specific PI3 kinase inhibitor LY29400267 resulted in enhanced Hu1D10-mediated cell death. Figure 5A demonstrates that LY294002 alone promotes modest apoptosis at the 4-hour time point. However, when LY294002 is combined with Hu1D10 treatment, a positive synergistic effect in the frequency of apoptosis is observed (Figure 5A). Figure 5B shows that pretreatment of CLL cells with the LY294002 compound does indeed abolish Hu1D10-induced phosphorylation of AKT at Ser473.
Inhibition of PI3 kinase by LY294002 or Syk with piceatannol promotes synergistic apoptosis with Hu1D10 treatment and abrogates Hu1D10-induced phosphorylation of AKT. (A) CLL cells treated with (i) LY294002 25 μM, (ii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL), or (iii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) plus LY294002 25 μM for 4 hours and were assessed for apoptosis using annexin V/PI staining followed by FACS analysis. Error bars indicate the SD among 4 different CLL samples. (B) CLL cells were treated for the indicated time with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). + indicates samples that were pretreated with LY294002 25 μM for 20 minutes. Cells were immediately lysed following antibody treatment. Samples were immunoblotted using standard techniques. Blots were probed with anti–phospho-AKT (Ser473), stripped, and then reprobed with anti-AKT. (C) Samples were incubated with the Syk inhibitor piceatannol (25 μg/mL) for 20 minutes prior to Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) treatment. After 4 hours of antibody treatment, samples were stained with annexin V–FITC/PI and analyzed by FACS. Values indicate the percent apoptosis above the untreated sample. Error bars indicate the SD among 4 different CLL samples. (D) CLL cells were treated for the indicated time with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). If noted, samples were pretreated with piceatannol 25 μg/mL for 20 minutes. Blots were probed for phospho-AKT and AKT as noted.
Inhibition of PI3 kinase by LY294002 or Syk with piceatannol promotes synergistic apoptosis with Hu1D10 treatment and abrogates Hu1D10-induced phosphorylation of AKT. (A) CLL cells treated with (i) LY294002 25 μM, (ii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL), or (iii) Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) plus LY294002 25 μM for 4 hours and were assessed for apoptosis using annexin V/PI staining followed by FACS analysis. Error bars indicate the SD among 4 different CLL samples. (B) CLL cells were treated for the indicated time with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). + indicates samples that were pretreated with LY294002 25 μM for 20 minutes. Cells were immediately lysed following antibody treatment. Samples were immunoblotted using standard techniques. Blots were probed with anti–phospho-AKT (Ser473), stripped, and then reprobed with anti-AKT. (C) Samples were incubated with the Syk inhibitor piceatannol (25 μg/mL) for 20 minutes prior to Hu1D10 plus antihuman Fc antibody (both 10 μg/mL) treatment. After 4 hours of antibody treatment, samples were stained with annexin V–FITC/PI and analyzed by FACS. Values indicate the percent apoptosis above the untreated sample. Error bars indicate the SD among 4 different CLL samples. (D) CLL cells were treated for the indicated time with Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). If noted, samples were pretreated with piceatannol 25 μg/mL for 20 minutes. Blots were probed for phospho-AKT and AKT as noted.
Piceatannol is an inhibitor of Syk kinase activity.68 Thus if activation of Syk leads to AKT activation, pretreatment of CLL cells with piceatannol should decrease Hu1D10-induced phosphorylation of AKT and increase Hu1D10-induced apoptosis. As shown in Figure 5C, preincubation of CLL cells with piceatannol increases Hu1D10-induced apoptosis with some degree of synergism. Furthermore, piceatannol abrogates the Hu1D10-induced phosphorylation of AKT (Figure 5D). The correlation of Syk and AKT phosphorylation, the synergistic activity of the inhibitors with Hu1D10-induced apoptosis, and the immunoblots showing loss of AKT and Syk phosphorylation with inhibitor pretreatment provide strong evidence that Hu1D10 induces a survival signal in primary CLL cells through Syk by downstream activation of AKT.
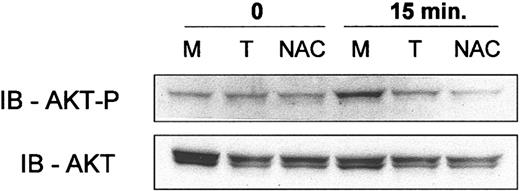
Antioxidants decrease phosphorylation of AKT
ROS, in the form of exogenous low-concentration hydrogen peroxide, leads to AKT activation in lymphocytic cell lines through a Syk-dependent pathway.63 Thus, the observed Hu1D10-induced ROS formation in CLL cells (Figure 2) may lead to the observed phosphorylation of AKT (Figure 4A). If Hu1D10 treatment of CLL cells leads to ROS formation with consequent AKT activation, preincubation of the CLL cells with antioxidants would be expected to decrease Hu1D10-induced phosphorylation of AKT. Figure 6 shows that preincubation of CLL cells with the free radical scavengers tiron or N-acetyl cysteine decreases the Hu1D10-induced phosphorylation of AKT (Ser473).
Antioxidants N-acetyl cysteine and tiron decrease Hu1D10 induced phosphorylation of AKT. CLL cells were treated as follows: (M) media only, (T) pretreatment with 10 mM tiron followed by Hu1D10 plus antihuman Fc antibody or (NAC) pretreatment with 25 mM N-acetyl cysteine followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). After a 15-minute treatment with cross-linked Hu1D10, the cells were lysed. Samples were immunoblotted using standard techniques. Blots were probed with anti–phospho-AKT (Ser473), stripped, and then reprobed with anti-AKT.
Antioxidants N-acetyl cysteine and tiron decrease Hu1D10 induced phosphorylation of AKT. CLL cells were treated as follows: (M) media only, (T) pretreatment with 10 mM tiron followed by Hu1D10 plus antihuman Fc antibody or (NAC) pretreatment with 25 mM N-acetyl cysteine followed by Hu1D10 plus antihuman Fc antibody (both 10 μg/mL). After a 15-minute treatment with cross-linked Hu1D10, the cells were lysed. Samples were immunoblotted using standard techniques. Blots were probed with anti–phospho-AKT (Ser473), stripped, and then reprobed with anti-AKT.
Phosphorylation of AKT in samples obtained from Hu1D10-treated patients
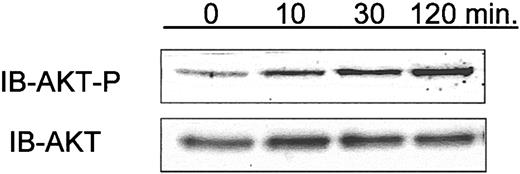
The clinical relevance of the mechanistic in vitro experiments with Hu1D10 described herein is uncertain. Hu1D10 is currently being evaluated for the treatment of CLL in an ongoing clinical trial. Having demonstrated that Hu1D10 treatment of primary CLL cells in vitro leads to phosphorylation of AKT, we sought to determine the relevance of this observation in vivo in patients receiving Hu1D10. Serial CLL cell isolates were obtained before and immediately after treatment with Hu1D10 in 3 consecutive patients. Using immunoblot analysis for phospho-AKT (Ser473), we found that for 2 of 3 patients tested, samples showed a sustained rise in the level of phospho-AKT (Ser473) following Hu1D10 treatment. A representative immunoblot is shown in Figure 7. This suggests that ligation of HLA-DR on CLL cells in vivo activates a survival pathway, as we have shown here with in vitro data, in addition to its previously noted in vivo cytotoxicity.43
Phosphorylation of AKT in CLL cells isolated from patients who have received intravenous Hu1D10 for the treatment of CLL. In conjunction with a clinical trial of Hu1D10, blood samples were obtained at pretreatment and 10, 30, and 120 minutes following the infusion of Hu1D10. Following density gradient isolation of lymphocytes, whole cell lysates were made and analyzed using immunoblots to determine relative changes in phosphorylated AKT (Ser473). Samples from 3 patients were tested. For 2 of 3 patients, samples showed a sustained rise in phospho-AKT following Hu1D10 treatment. Data shown here are representative of these 2.
Phosphorylation of AKT in CLL cells isolated from patients who have received intravenous Hu1D10 for the treatment of CLL. In conjunction with a clinical trial of Hu1D10, blood samples were obtained at pretreatment and 10, 30, and 120 minutes following the infusion of Hu1D10. Following density gradient isolation of lymphocytes, whole cell lysates were made and analyzed using immunoblots to determine relative changes in phosphorylated AKT (Ser473). Samples from 3 patients were tested. For 2 of 3 patients, samples showed a sustained rise in phospho-AKT following Hu1D10 treatment. Data shown here are representative of these 2.
Discussion
We have demonstrated that most patients with B-cell CLL express the antigen for the β-chain epitope of HLA-DR that the humanized antibody Hu1D10 targets. Unlike standard cytotoxic agents such as 2-F-ara-A or chlorambucil that require days to induce apoptosis in vitro, Hu1D10 induced significant caspase-independent apoptosis at 4 hours. Inhibition of actin cytoskeletal rearrangement or disruption of lipid rafts, which have been shown in previous studies to prevent antibody-induced HLA-DR aggregation,55,56 inhibited Hu1D10-induced apoptosis in CLL cells. Hu1D10 treatment of CLL cells led to the generation of intracellular ROS. The level of Hu1D10-induced ROS was markedly decreased by inhibition of actin cytoskeletal rearrangement or disruption of lipid rafts. However, disruption of the mitochondrial respiratory chain did not reduce Hu1D10-induced ROS formation. We next demonstrated in a subset of CLL patients, early transmembrane signaling induced by Hu1D10 resulted in phosphorylation of tyrosine residues on Syk and phosphorylation of Ser473 of AKT. In contrast to that observed with surface immunoglobulin ligation on CLL cells,69 overexpression of ZAP-70 was not predictive of enhanced Syk phosphorylation following Hu1D10 treatment. The specific PI3 kinase inhibitor LY294002 diminished Hu1D10-induced phosphorylation of AKT and greatly potentiated the Hu1D10-induced cell death. Furthermore, inhibition of Syk with piceatannol decreased Hu1D10-induced phosphorylation of AKT and increased Hu1D10-induced cell death. Hu1D10-induced phosphorylation of Ser473 of AKT was diminished by addition of the antioxidant agents tiron or N-acetyl cysteine. Finally, we have demonstrated the relevance of AKT activation in CLL samples taken from patients treated with Hu1D10. These in vitro and in vivo human data provide evidence that Hu1D10 promotes competing death and survival signals in CLL cells and provides a rationale for combination strategies using inhibitors of the PI3 kinase pathway with this novel antibody.
All of the mechanistic studies performed to date with HLA-DR antibodies have been performed in proliferating NHL cell lines with minimal description of the actual experiments performed with primary CLL cells. Our study is unique among those that have studied HLA-DR–mediated death in that all of our studies used freshly obtained CLL samples that are fully representative of the nonproliferating, apoptosis prone tumor cells on which eventual clinical trials with Hu1D10 or other alternative HLA-DR–directed antibodies would be performed. In addition, we have extended these observations to in vivo treated cellular samples derived from patients receiving Hu1D10.
We have demonstrated that ROS are generated early following treatment with Hu1D10. To our knowledge, no other studies have demonstrated that ROS generation occurs following anti–HLA-DR antibody treatment, or any antibody treatment, of primary CLL cells in the absence of complement. These changes occurred as early as 1 hour following ligation of the HLA-DR antigen. A study has demonstrated that rituximab-induced, complement-mediated cell lysis involves rapid generation of ROS, when cell death was also noted.48 Our study contrasts with this in vitro CLL rituximab study48 in that our culture system was complement free. Our data suggest a plasma membrane–associated or cytoplasmic oxidase as the source of the observed ROS. Of note, Epstein-Barr virus–transformed B-lymphocyte cell lines produce ROS on surface immunoglobulin cross-linking through a system analogous to the phagocytic reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.59 It is intriguing to consider a membrane-associated NAPDH oxidase as a source of anti–HLA-DR–induced ROS in CLL cells. Although many studies have focused on the cytotoxic role of ROS, we have examined the potential of ROS to activate a survival signal. Based on our observation that ROS scavengers decrease Hu1D10-induced phosphorylation of AKT, it appears that activation of Syk/AKT pathway reflects a cellular survival signaling in response to ROS generation induced by Hu1D10.
Several studies have shown that phosphorylation of AKT (Ser473) promotes ex vivo survival of CLL cells.70-73 Activation of AKT in CLL cells in vitro via human plasma71 or interleukin 4 (IL-4) and tetradecanoylphorbol72 decreased the sensitivity of CLL cells to chemotherapeutic agents. Of note, here we have shown evidence that a single agent can lead to both death and survival signals in CLL cells. The mechanism of this protective effect of AKT in B cells is not fully elucidated, although Ding et al provided evidence that the protective effect of AKT activation is, at least in part, through its known ability to diminish caspase-9 activation.63 Our data further support an important role of the PI3 kinase/AKT pathway in CLL survival.
Arsenic has recently been shown to activate AKT in a T-cell line.64 As with Hu1D10, arsenic was also shown to increase intracellular ROS, but likely involves a mitochondrial respiration mechanism.41 Furthermore, as we observed with Hu1D10, pretreatment with antioxidants prior to arsenic decreased AKT phosphorylation.64 These findings of ROS-induced activation of AKT in a T-cell line corroborate our findings in CLL cells.
Syk appears to play a central role in cells' response to oxidative stress, triggering both proapoptotic and survival pathways.74 A previous study showed that activation the PI3 kinase/AKT survival pathway in a B-cell line via low-dose hydrogen peroxide (< 100 μM) requires Syk kinase activity.63 Consistent with its role in the PI3 kinase/AKT pathway, we see a correlation between Syk phosphorylation and AKT phosphorylation. Furthermore, inhibition of Syk kinase activity with piceatannol reduced the observed AKT phosphorylation. The mechanism through which ROS activates Syk is not clear, although studies75,76 suggest that ROS-induced and membrane raft-dependent protein aggregation is the trigger.
The intracellular signaling induced by Hu1D10 with activation of Syk and downstream AKT provides potential strategies for combination with other therapeutics. The importance of AKT in this dual-signaling process is best substantiated by the synergy observed when Hu1D10 and the selective PI3 kinase inhibitor LY294002 are combined. Small molecule inhibitors of the AKT survival pathway, such as UCN-01 (7-hydroxystaurosporine), are currently under clinical investigation; thus, clinical combination studies could be initiated in the near future.77-79 Further characterization of the source of ROS generation following Hu1D10 treatment, evaluation of other upstream kinases that are activated by this process, and alterations of the caspase machinery following Hu1D10 treatment are required and under pursuit at this time. These data nonetheless demonstrate that HLA-DR antibody therapy for CLL may prove synergistic with current agents and possibly overcome resistance mechanisms associated with refractory disease. Based on the data herein, and promising results from a phase 1 trial, we have initiated a phase 2 trial of thrice weekly Hu1D10 in patients with relapsed and refractory CLL and plan to pursue combination trials of Hu1D10 and inhibitors of the AKT survival pathway in the future.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-08-2836.
Supported by the National Cancer Institute (P01 CA95426-01A1; CLL Research Consortium P01 CA81534-02, R01 CA102504, and R21 CA91564-02), the American Cancer Society, the Sidney Kimmel Cancer Research Foundation, the Leukemia and Lymphoma Society of America, and the D. Warren Brown Foundation. J.C.B. is a clinical scholar of the Leukemia and Lymphoma Society of America.
J.M.G. is employed by Protein Design Labs, whose potential product (Hu1D10) is studied in the present work.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Hong Wang of Protein Design Labs, Inc for providing Hu1D10-PE and MSL109-PE and Dr George Weiner for thoughtful discussions relative to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal