Abstract
Heat shock proteins (HSPs) are reported to act as effective adjuvants to elicit anti-tumor and anti-infection immunity. Here, we report that Hsp70-like protein 1 (Hsp70L1), a novel HSP derived from human dendritic cells (DCs), has potent adjuvant effects that polarize responses toward Th1. With a calculated molecular weight of 54.8 kDa, Hsp70L1 is smaller in size than Hsp70 but resembles it both structurally and functionally. Hsp70L1 shares common receptors on DCs with Hsp70 and can interact with DCs, promoting DC maturation and stimulating secretion of the proinflammatory cytokines interleukin 12p70 (IL-12p70), IL-1β, tumor necrosis factor-α (TNF-α), and the chemokines IP-10, macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and normal T cell expressed and secreted (RANTES). The induction of interferon-γ–inducible protein 10 (IP-10) secretion by Hsp70L1 is not shared by Hsp70, and other functional differences include more potent stimulation of DC IL-12p70, CC-chemokine, and CCR7 and CXCR4 expression by Hsp70L1. Immunization of mice with the hybrid peptide Hsp70L1-ovalbumin(OVA)257-264 induces an OVA257-264-specific Th1 response and cytotoxic T lymphocyte (CTL) that results in significant inhibition of E.G7-OVA tumor growth. The ability of Hsp70L1 to activate DCs indicates its potential as a novel adjuvant for use with peptide immunizations; the Hsp70L1 antigen peptide hybrid may serve as a more effective vaccine for the control of cancer and infectious diseases.
Introduction
The Th1 cellular immune response is crucial for antitumor and antimicrobial immunity,1,2 and powerful immunomodulatory adjuvants that induce Th1 polarization are an important facet of vaccination strategies.3,4 While traditional adjuvants, such as aluminum salts and oil emulsions, mainly evoke Th2 responses, characterized by production of antibodies specific for conformational antigenic determinants,5 new generation adjuvants, including CpG-rich motifs, monophosphoryl lipid A (MPA), and quil a saponin (QS21) promote Th1 polarization.6 These effects are thought to result from the activation of antigen-presenting cells (APCs), in particular, dendritic cells (DCs).7,8 In recent years the molecular chaperone heat shock protein (HSP) has been revealed to interact with APCs, and its considerable capacity to induce antigen-specific CD8+ cytotoxic T lymphocyte (CTL) and Th1 responses has attracted much attention.9,10 Extracellular HSPs can interact with APCs, exhibiting potent adjuvant functions in stimulating the host immune response.10,11 This interaction triggers a cascade of events, including re-presentation of the chaperoned peptides by the major histocompatibility complex (MHC), secretion of proinflammatory cytokines, and maturation of DCs.12-14 These properties combine to make HSPs a potent adjuvant, eliciting significant immune responses against associated antigens.
The Hsp70 subfamily is one of the most important HSP subfamilies. Hsp70 prepared from tumor cells or virus-infected cells are capable of eliciting potent antigen-specific CD8+ CTL responses10,15 ; these responses are CD4+ T-cell–independent and have been attributed to antigenic peptides bound to the HSP.16 It also has been shown that Hsp70 complexed with ovalbumin (OVA) antigen-specific epitopes can activate DCs and induce OVA-specific CTL responses.17 Hsp70 can bind to CD91, CD14, and TLR2/4 receptors on the surface of APCs, activating APCs and facilitating the re-presentation of HSP-associated antigen via a TAP-dependent or TAP-independent route.13,14,18,19 These findings demonstrate the adjuvant effects of Hsp70 and encourage the potential application of Hsp70 in vaccination.
We report here a new member of the Hsp70 subfamily cloned from a human DC cDNA library, Hsp70-like protein 1 (Hsp70L1). Recombinant Hsp70L1 can bind DCs, resulting in DC maturation and activation. While Hsp70L1 shares common receptors (CD91, TLR2, TLR4) on DCs with Hsp70, there are functional differences between Hsp70L1 and Hsp70. Hsp70L1 can induce DCs to secrete IP-10, a cytokine vital for Th1 polarization, but Hsp70 cannot. Moreover, Hsp70L1 is more potent than Hsp70 in the stimulation of IL-12p70, MIP-1α, MIP-1β, RANTES, CCR7, and CXCR4 expression by DCs. Hsp70L1 may therefore polarize Th1 responses more effectively than Hsp70. We used OVA as a model antigen to evaluate the adjuvant effects of Hsp70L1 in vivo, finding that Hsp70L1-OVA257-264 hybrid peptide immunization could strongly induce OVA257-264–specific Th1 responses, CTL, and potent antitumor immunity. Together these results demonstrate that Hsp70L1 has enhanced Th1 polarizing and adjuvant properties, which appear to be mediated via activation of DCs.
Materials and methods
Cell lines
E.G7-OVA, an OVA-expressing clone derived from the EL4 thymoma cell line (C57BL/6 origin), was kindly provided by Prof E. Gilboa (Duke University, Durham, NC). E.G7-OVA was grown in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Hyclone, Logan, UT) and 400 μg/mL G418. EL4, HeLa, Lovo, PC-3, HT-29, MCF-7, A172, Caov-3, A549, K562, and Molt-4 cell lines were obtained from ATCC (American Type Culture Collection, Manassas, VA) and grown according to ATCC instructions.
Generation of DCs
Human peripheral monocyte–derived DCs were generated as described.20
Isolation of Hsp70L1 cDNA
A full-length cDNA clone, SBBI126, was isolated from a human DC cDNA library.20 The cDNA was identified as sharing homology with Hsp70 family members and thus designated as Hsp70L1 cDNA.
Northern blot analysis
Expression of Hsp70L1 mRNA in human tissues was analyzed as described21 by human adult multiple-tissue Northern blots (Clontech, Palo Alto, CA), using the full-length encoding sequence of Hsp70L1 cDNA labeled with 32P-dCTP as a probe.
Inducible expression test of Hsp70L1
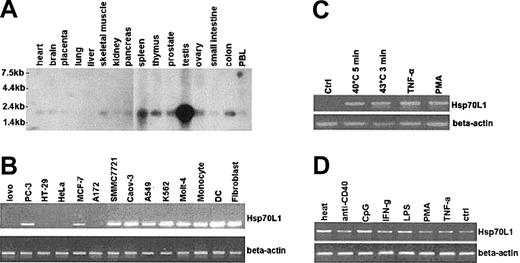
HeLa cells were heat stressed (40°C for 5 minutes or 43°C for 3 minutes) or treated with 50 U/mL TNF-α (Sigma Chemical, St Louis, MO) or 1 μg/mL phorbol myristate acetate (PMA) (Sigma) for 30 minutes. To assess Hsp70L1 expression in DCs, 6-day DCs generated from human peripheral monocytes were stimulated at 5 × 105 cells/mL for 8 hours with 1 μg/mL lipopolysaccharide (LPS) (Sigma), 30 μg/mL CpG oligonucleotides (5′-TCGTCGTTCCCCCCCCCC-CC-3′; SBS Genetech, Beijing, China), 100 U/mL interferon-γ (IFN-γ) (R&D Systems, Minneapolis, MN), 5 μg/mL anti–CD40 mAb (PharMingen, San Diego, CA), 50 ng/mL PMA or 100 ng/mL TNF-α, or for 5 minutes with heat (40°C). Cells were then harvested and Hsp70L1 expression assessed by semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR) as described,22 using the primers 5′-GCAATGTGTCCAGAGCAAG-3′ and 5′-GTCTTCCACCAACAGGTTTTC-3′.
Proteins
Recombinant Hsp70L1 protein was obtained by inserting the full-length encoding region of Hsp70L1 cDNA into the eukaryotic expression vector pcDNA3.1-Myc/His(–)B, to give pcDNA-Hsp70L1, encoding production of recombinant Hsp70L1 protein with a 6xHis tag at the N-terminus. HeLa cells were transfected with pcDNA-Hsp70L1 using LipofectAMINE reagent (Life Technologies, Rockville, MD) and resulting His-tagged recombinant Hsp70L1 purified using His-Trap metal chelation chromatography (Amersham Biosciences, Uppsala, Sweden) and an adenosine triphosphate (ATP)–agarose column23 (Sigma). Recombinant human mitochondrial creatine kinase with a 6xHis tag at the N-terminus (His-CK) was obtained by us from Escherichia coli. Recombinant human Hsp70 was purchased from Stressgen Biotechnologies (Cedar Creek, TX). The purity of Hsp70L1, Hsp70, and His-CK proteins was more than 97%, as confirmed by silver stained sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). LPS contamination was less than 0.03 pg/μg protein for Hsp70L1 fusion protein and 0.03 to 3 pg/μg protein for Hsp70 and His-CK as determined by Limulus amebocyte lysate assay (BioWhittaker, Walkersville, MD).
Cell surface binding with FITC-labeled Hsp70L1
Hsp70L1, Hsp70, and His-CK proteins were conjugated to fluorescein isothiocyanate (FITC) using FluoroTag FITC conjugation kits (Sigma), with between 3 and 5 FITC molecules binding each protein molecule. Cell surface binding was performed as described.14 Briefly, 5-day DCs were fixed in 2% paraformaldehyde then incubated with 20 μg/mL FITC-Hsp70L1, FITC-His-CK, or FITC-Hsp70 in phosphate-buffered saline (PBS) containing 1% nonfat milk powder for 20 minutes at 4°C. Cells were washed twice and visualized using a Zeiss LSM confocal microscope or analyzed using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). The competitive binding assay was performed by mixing labeled FITC-Hsp70L1 together with increasing doses of unlabeled competitor proteins prior to incubation with fixed DCs. DCs were washed extensively to remove unbound proteins and mean fluorescence intensities measured by fluorescence-activated cell-sorter scanner (FACS). For endocytosis studies, unfixed DCs were labeled with FITC-Hsp70L1 and incubated at 37°C during confocal microscope observation.
Hsp70L1 TLR2/4 binding assay
For lysate preparation and binding, Lysis Buffer (Cell Signaling Technology, Beverly, MA) supplemented with 0.1% Triton-X-100 and 20 mM imidazole was used. Different amounts of purified recombinant His-Hsp70L1 fusion proteins were diluted in 200 μL lysis buffer containing 20 μL Ni-beads and incubated for 4 hours at 4°C with gentle agitation. Ni-beads were collected by centrifugation and washed extensively in lysis buffer. 1 × 107 6-day DCs were lysed, briefly sonicated, and supernatants collected for centrifugation at 13 000g for 10 minutes. Added to the Ni-beads and incubated for another 4 hours with gentle agitation were 250 μL total cell lysate containing 200 μg protein. Beads were washed thoroughly and samples subjected to SDS-PAGE and Western blot assay for detection of TLR2 and TLR4 in Ni-precipitated complexes.
Transient transfection of HEK293 cells and measurement of luciferase activity
HEK293 cells were cotransfected with pGL3.5XκB-luciferase reporter (gift from Seamus J. Martin), pRL-TK-renilla-luciferase, and TLR2/CD14 or TLR4/CD14/MD2 plasmids (gifts from Dr K. Miyake), using Superfect transfection reagent (Qiagen, Valencia, CA) and cultured for 24 hours. Transfected cells were then treated with 10 μg/mL Hsp70, Hsp70L1, His-CK, or 1 μg/mL LPS for 6 hours, lysed, and NF-κB luciferase activity measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI), following manufacturer's instructions.
FACS analysis and mixed lymphocyte reaction (MLR)
Five-day human DCs were stimulated with 10 μg/mL Hsp70L1, Hsp70, or His-CK, or 1 μg/mL LPS for 48 hours, then collected and washed. For phenotypic analysis, DCs were stained with phycoerythrin (PE)–conjugated mAbs to CD40 and CD80, and FITC-conjugated mAbs to CD83, CD86s, and HLA-DR (PharMingen) for 30 minutes at 4°C, prior to analysis by flow cytometry. Allogeneic T-cell stimulating capacity assessment was performed as described.24 Irradiated DCs (30 Gy [3000 rad]) were used as stimulator cells. T lymphocytes enriched from blood of a different donor using anti-CD3 magnetic beads (Miltenyi Biotec, Auburn, CA) were incubated with the irradiated DCs for 5 days at different responder/stimulator ratios. On day 4, 1 μCi (0.037 MBq) [3H] thymidine was added to each well; cells were harvested 18 hours later and incorporated [3H] thymidine counted by liquid scintillation spectroscopy.
DC cytokine and chemokine production
Human DCs were cultured for 7 days and adjusted to 5 × 105 cells/mL for Hsp70L1 stimulation. For comparative experiments, DCs were stimulated with 10 μg/mL Hsp70L1, boiled Hsp70L1, Hsp70, or His-CK or 1 μg/mL LPS for 72 hours. For time-dependent experiments, DCs were incubated with 10 μg/mL Hsp70L1 for 4, 24, or 72 hours. Supernatants were collected for quantification of IP-10, MIP-1α, MIP-1β, or RANTES using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems). To assay cytokine secretion, day-5 human DCs were stimulated at 5 × 105 cells/mL with 10 μg/mL Hsp70L1, Hsp70, His-CK, or 1 μg/mL LPS for 24 hours. Each protein was tested under 3 conditions: native protein, native protein in the presence of 50 μg/mL polymyxin B (PMB) (an LPS inhibitor), and denatured protein (100°C, 20 minutes). IL-1β, TNF-α, and IL-12p70 in supernatants were measured by ELISA (Endogen, Cambridge, MA).
Assessment of CCR7 and CXCR4 expression
Five-day DCs were stimulated with 10 μg/mL Hsp70L1, boiled Hsp70L1, Hsp70, boiled Hsp70 or His-CK, or 1 μg/mL LPS for 40 hours. DCs were then harvested and expression of CCR7 and CXCR4 in DCs assessed by semiquantitative RT-PCR and flow cytometric analysis. Primers used were 5′-GATTACATCGGAGACAACACC-3′ and 5′-TAGTCCAGGCAGAAGAGTCG-3′ for CCR7, 5′-GGGCAATGGATTGGTCATCCTGGTC-3′ and 5′-GTGGTCTTGAGGGCCTTGCGCTTCT-3′ for CXCR4. Flow cytometric analysis was performed using FITC-conjugated mAbs to CCR7 and CXCR4 (R&D Systems).
Preparation of HSP-peptide hybrid and immunization protocols
Mice and immunization
C57BL/6 (H-2b) male mice, 6 to 8 weeks old, were obtained from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences and housed in specific pathogen-free conditions. Immunization of mice was performed according to Blachere method.17 Mice were subcutaneously immunized twice, one week apart, with 25 μg Hsp70L1, Hsp70, Hsp70L1-OVA257-264, or Hsp70-OVA257-264 hybrid, 75 nmol OVA257-264 mixed with 25 μg Hsp70L1, or 25 μg OVA (Sigma) in PBS. Each group contained 10 mice.
ELISPOT assay for IFN-γ and ELISA for IL-2
Seven days following the final immunization splenocytes were isolated and restimulated at 1 × 106/mL with 10 μg/mL OVA, OVA257-264, or VSV324-332 (control peptide, corresponding to vesicular stomatitis virus nucleoprotein 324-332aa, RGYVYQGL, GL Biochem), or 5 μg/mL Con A (Sigma). For ELISPOT assay, splenocytes were restimulated for 72 hours, transferred to ELISPOT plates and IFN-γ–secreting spots, developed, and counted using a IFN-γ ELISPOT kit (R&D Systems) as described.26 For ELISA, splenocytes were restimulated for 24 hours then supernatants collected for IL-2 measurement (Endogen).
CTL assay
Splenocytes from immunized mice were cultured at 2 × 106 cells/mL and restimulated with 4 × 105 irradiated (150 Gy [15 000 rad]) E.G7-OVA cells in RPMI 1640 medium containing 10% FBS and 20 U/mL IL-2 (Sigma) for 7 days. Splenocytes were then purified by Ficoll-Paque (Pharmacia, Uppsala, Sweden) density centrifugation and used as CTL effector cells. OVA-specific lysis was measured in a standard 4-hour 51Cr-release assay as described.27 EL4 pulsed with OVA257-264 or E.G7-OVA cells were used as OVA-specific target cells, while control target cells were parental EL4 cells.
Tumor challenge studies
Five days after the final immunization C57BL/6 mice were challenged subcutaneously with 3 × 106 E.G7-OVA tumor cells in the flank area. Tumor growth was monitored by measuring the diameter of the tumor with a caliper every 2 days and recorded as the average of 2 perpendicular diameter measurements. Survival following tumor challenge also was recorded.
Results
Cloning and characterization of Hsp70L1
By large-scale random sequencing of a human DC cDNA library, we identified a full-length cDNA encoding Hsp70L1, a Hsp70-like molecule of 509 amino acids. The putative amino acid sequence has highest identity (90.5%) to the sequence of mouse Hsp70 (GenBank accession no. BC002056). Containing an Hsp70 signature sequence and 2 potential N-linked glycosylation sites, Hsp70L1 is approximately 33.7% to 35.7% homologous to human Hsp70 subfamily molecules, primarily in the N-terminal ATPase domain fragment (1-394 aa) (Figure 1).
Putative amino acid sequence of Hsp70L1 and alignment with human Hsp70s. Identical residues are in black and similar residues are in gray. Hsp70 family signature sequence is boxed, and potential N-linked glycosylation sites marked with an asterisk (*). Hsp70L1 sequences are available from GenBank under accession number AF143723. Other human Hsp70 family members are Hsp72 (HS72_human), Hsp76 (HS76_human), Hsp71 (HS71_human), Hsp70-HOM (HS7H_human), and Hsc70 (HS7C_human).
Putative amino acid sequence of Hsp70L1 and alignment with human Hsp70s. Identical residues are in black and similar residues are in gray. Hsp70 family signature sequence is boxed, and potential N-linked glycosylation sites marked with an asterisk (*). Hsp70L1 sequences are available from GenBank under accession number AF143723. Other human Hsp70 family members are Hsp72 (HS72_human), Hsp76 (HS76_human), Hsp71 (HS71_human), Hsp70-HOM (HS7H_human), and Hsc70 (HS7C_human).
The calculated molecular weight and isoelectric point of Hsp70L1 protein is 54.8 kDa and 5.48, respectively. The Hsp70L1 gene is located at 10p12.33 and does not encode either a transmembrane region or a signal peptide, indicating that Hsp70L1 is a cytosolic protein.
Expression pattern and inducibility of Hsp70L1
A major mRNA band of 2.1 kb was detected by Northern blot analysis, with highest expression of Hsp70L1 in testis, spleen, and thymus. Hsp70L1 mRNA also was observed in all other tissues tested (Figure 2A). RT-PCR showed that Hsp70L1 also was expressed in various cell lines (Figure 2B). Hsp70L1 mRNA expression thus appears to be ubiquitous, with high expression in immune organs (spleen, thymus) and immune cells, indicating that Hsp70L1 may be an immune-related molecule. Interestingly, no expression was detected in HeLa cells (Figure 2B), which were used in the following experiments to investigate the inducible expression of Hsp70L1 by heat and other danger signals.
Expression of Hsp70L1. (A) Northern blot analysis of Hsp70L1 tissue distribution, using full-length Hsp70L1 cDNA as a probe. Molecular size markers are indicated. (B) Expression levels of Hsp70L1 mRNA in various cell lines, as determined by semiquantitative RT-PCR. (C) Inducible expression of Hsp70L1 mRNA in HeLa cells. HeLa cells were pretreated with heat, TNF-α, or PMA, then analyzed by RT-PCR for Hsp70L1 mRNA expression. (D) Expression of Hsp70L1 mRNA in DCs. DCs cultured for 6 days were stimulated with the indicated stimuli, then RT-PCR analysis of Hsp70L1 expression performed.
Expression of Hsp70L1. (A) Northern blot analysis of Hsp70L1 tissue distribution, using full-length Hsp70L1 cDNA as a probe. Molecular size markers are indicated. (B) Expression levels of Hsp70L1 mRNA in various cell lines, as determined by semiquantitative RT-PCR. (C) Inducible expression of Hsp70L1 mRNA in HeLa cells. HeLa cells were pretreated with heat, TNF-α, or PMA, then analyzed by RT-PCR for Hsp70L1 mRNA expression. (D) Expression of Hsp70L1 mRNA in DCs. DCs cultured for 6 days were stimulated with the indicated stimuli, then RT-PCR analysis of Hsp70L1 expression performed.
HeLa cells were treated by heat stress, or by TNF-α or PMA stimulation. Significant expression of Hsp70L1 was elicited by these stimuli (Figure 2C), confirming that Hsp70L1 shares the stress-inducible characteristics of other members of the HSP family. Hsp70L1 mRNA expression in DCs was markedly up-regulated by heat shock, CpG, and LPS, whereas IFN-γ, anti–CD40 mAb, and TNF-α induced Hsp70L1 to a lesser extent (Figure 2D).
Specific binding of Hsp70L1 by DCs
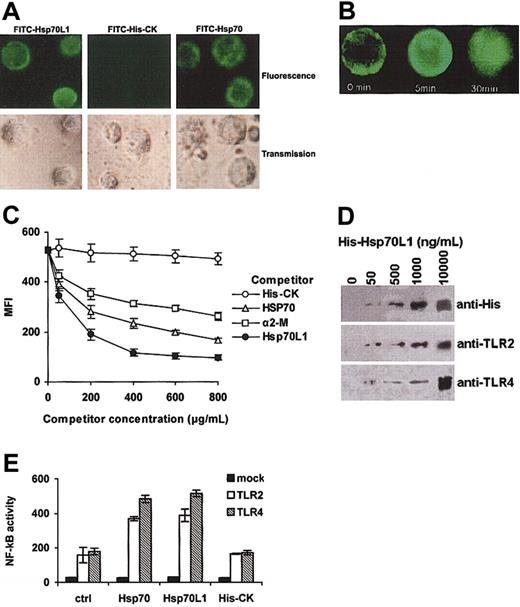
FITC-conjugated Hsp70L1 (FITC-Hsp70L1) could bind to the surface of DCs (Figure 3A). We also observed surface binding and endocytosis of fluoresceinated Hsp70L1 by DCs using confocal microscopy (Figure 3B). This binding was specific to Hsp70L1, as the His-tagged control protein His-CK could not bind, and binding of FITC-Hsp70L1 to DCs could be markedly inhibited by the presence of unlabeled Hsp70L1 (Figure 3C).
DC binding site or receptor for Hsp70L1. (A) Confocal microscopy. DCs, fixed with paraformaldehyde, were stained with FITC-Hsp70L1, FITC-His-CK, or FITC-Hsp70 at 4°C, then observed by confocal microscope. (B) Internalization of FITC-Hsp70L1 by DCs. Unfixed, viable DCs were allowed to bind FITC-Hsp70L1 at 4°C, then incubated at 37°C with concurrent visualization by confocal microscopy at the indicated times. (C) Competitive binding. Paraformaldehyde-fixed DCs were incubated with FITC-Hsp70L1 and competitor mixtures. FITC-Hsp70L1 was used at a final and fixed concentration of 20 μg/mL. Competitor proteins were used at increasing doses (50-800 μg/mL final concentration), as indicated. Cells were washed extensively to remove unbound protein and analyzed by flow cytometry. Mean fluorescence intensities (MFIs) are plotted as a function of increasing quantities of competitor proteins. Error bars represent 3 independent experiments. (D) Binding assay of Hsp70L1 with TLR2/4. Ni-beads were pre-incubated with the indicated concentrations of purified recombinant His-Hsp70L1 fusion protein, washed, and then incubated with DC cell lysates. Proteins precipitated by Ni-beads were detected by Western blot assay using anti-His, anti-TLR2, or anti-TLR4 antibody. (E) Activation of NF-κB pathway by recombinant Hsp70L1 protein in TLR2- or TLR4-expressing HEK293 cells. HEK293 cells transiently transfected with luciferase reporter (mock, TLR2–TLR4–), TLR2/CD14, and luciferase reporter (TLR2), or TLR4/CD14/MD2 and luciferase reporter (TLR4) plasmids were cultured for 24 hours and then treated with 10 μg/mL Hsp70L1, Hsp70, or His-CK for 6 hours. Cells were lysed and NF-κB luciferase activity measured using the Dual-Luciferase Reporter Assay System. Data were normalized by Renilla-luciferase activity and shown as means ± SEM of 3 independent experiments.
DC binding site or receptor for Hsp70L1. (A) Confocal microscopy. DCs, fixed with paraformaldehyde, were stained with FITC-Hsp70L1, FITC-His-CK, or FITC-Hsp70 at 4°C, then observed by confocal microscope. (B) Internalization of FITC-Hsp70L1 by DCs. Unfixed, viable DCs were allowed to bind FITC-Hsp70L1 at 4°C, then incubated at 37°C with concurrent visualization by confocal microscopy at the indicated times. (C) Competitive binding. Paraformaldehyde-fixed DCs were incubated with FITC-Hsp70L1 and competitor mixtures. FITC-Hsp70L1 was used at a final and fixed concentration of 20 μg/mL. Competitor proteins were used at increasing doses (50-800 μg/mL final concentration), as indicated. Cells were washed extensively to remove unbound protein and analyzed by flow cytometry. Mean fluorescence intensities (MFIs) are plotted as a function of increasing quantities of competitor proteins. Error bars represent 3 independent experiments. (D) Binding assay of Hsp70L1 with TLR2/4. Ni-beads were pre-incubated with the indicated concentrations of purified recombinant His-Hsp70L1 fusion protein, washed, and then incubated with DC cell lysates. Proteins precipitated by Ni-beads were detected by Western blot assay using anti-His, anti-TLR2, or anti-TLR4 antibody. (E) Activation of NF-κB pathway by recombinant Hsp70L1 protein in TLR2- or TLR4-expressing HEK293 cells. HEK293 cells transiently transfected with luciferase reporter (mock, TLR2–TLR4–), TLR2/CD14, and luciferase reporter (TLR2), or TLR4/CD14/MD2 and luciferase reporter (TLR4) plasmids were cultured for 24 hours and then treated with 10 μg/mL Hsp70L1, Hsp70, or His-CK for 6 hours. Cells were lysed and NF-κB luciferase activity measured using the Dual-Luciferase Reporter Assay System. Data were normalized by Renilla-luciferase activity and shown as means ± SEM of 3 independent experiments.
In order to observe whether Hsp70L1 binds DCs via the same receptors as Hsp70, we performed a competitive binding test using increasing quantities (50-800 μg/mL final concentration) of unlabeled competitor proteins including Hsp70, α2-macroglobulin (α2-M (Biodesign, Saco, ME), a known ligand for the CD91 receptor), Hsp70L1, and the control protein His-CK to compete with FITC-Hsp70L1 (final concentration 20 μg/mL) to bind DCs. The dose-response curves (Figure 3C) show that Hsp70, α2-M, and Hsp70L1 all can inhibit binding of FITC-Hsp70L1 to DCs, whereas the control protein His-CK cannot, suggesting that Hsp70L1 shares a common receptor or receptors with Hsp70 and α2-M. Notably, Hsp70, Hsp70L1, and α2-M differ in their effectiveness as competitors. Unlabeled Hsp70L1 appears to be the most potent competitor for FITC-Hsp70L1 binding DCs; even at the highest concentration (unlabeled-to-labeled ratio of 40:1), Hsp70 and α2-M did not inhibit binding of FITC-Hsp70L1 to the degree that unlabeled Hsp70L1 did, suggesting that Hsp70L1 either has higher affinity than Hsp70 and α2-M for their receptors on DCs or that some receptors react with Hsp70L1 but not Hsp70 or α2-M.
Biochemical and functional demonstration of interaction between Hsp70L1 and TLR2/4
TLR2 and TLR4 have been reported to recognize Hsp70 in the context of DC activation and maturation.18 To study whether Hsp70L1 also was recognized by TLR2 and TLR4, we used recombinant Hsp70L1 (His-tagged at the N-terminus)–coated Ni-beads to precipitate proteins from the DC slurry, then analyzed whether TLR2 and TLR4 could be trapped by Hsp70L1 by Western blot. As shown in Figure 3D, Hsp70L1 could bind TLR2 and TLR4. This interaction was further confirmed by incubating TLR2 and TLR4 reconstituted HEK293 cells with Hsp70L1, Hsp70, His-CK, or medium. As shown in Figure 3E, both Hsp70L1 and Hsp70 activate the NF-κB pathway in TLR2 or TLR4 reconstituted HEK293 cells, demonstrating the functional relevance of the Hsp70L1-TLR2/4 interaction.
Phenotypic and functional maturation of DCs induced by recombinant Hsp70L1
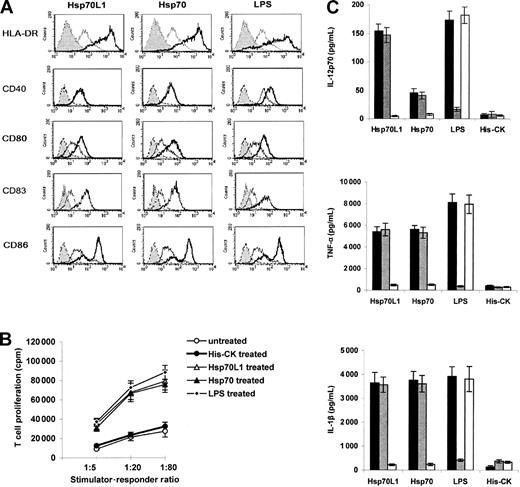
Hsp70L1, Hsp70, and LPS all promoted up-regulation of HLA-DR, CD80, CD83, and CD86 expression in DCs, but only LPS could significantly induce CD40 expression (Figure 4A). His-tagged control protein His-CK did not affect maturation marker expression. In MLR, Hsp70L1, Hsp70, and LPS-treated DCs induced T-cell proliferation significantly (P < .05 compared to His-CK–treated or nontreated DCs, Figure 4B), indicating that Hsp70L1 can induce DCs to become functionally mature.
Induction of DC maturation and cytokines secretion by recombinant Hsp70L1 protein. (A) Phenotypic maturation of DCs induced by recombinant Hsp70L1 protein. DCs were treated with Hsp70L1, Hsp70, LPS, or His-CK for 48 hours then collected for FACS analysis of CD40, CD80, CD83, CD86, and HLA-DR expression. Gray histograms indicate autofluorescence; open thin line histograms, His-CK–treated DCs; and open thick line histograms, HSP- or LPS-treated DCs. (B) Functional DC maturation, assessed by MLR. DCs pretreated with the indicated stimuli, as described for panel A, were irradiated and used as stimulators, with T cells from a different donor as responders. T-cell proliferation was measured by thymidine incorporation. Experiments were repeated 3 times, and data are displayed as means ± SEM. (C) Cytokine production of DCs stimulated with recombinant Hsp70L1. DCs were cultured with Hsp70L1, Hsp70, His-CK, or LPS proteins in 3 formats; native protein (▪), native protein in the presence of 50 μg/mL PMB (▪), or denatured protein (100°C, 20 minutes) for 24 hours (□). The levels of IL-12p70, TNF-α, and IL-1β in supernatants were measured by ELISA. Data are displayed as mean cytokine concentration (pg/mL) ± SEM. P < .05 for IL-12p70 production between Hsp70L1 and human Hsp70.
Induction of DC maturation and cytokines secretion by recombinant Hsp70L1 protein. (A) Phenotypic maturation of DCs induced by recombinant Hsp70L1 protein. DCs were treated with Hsp70L1, Hsp70, LPS, or His-CK for 48 hours then collected for FACS analysis of CD40, CD80, CD83, CD86, and HLA-DR expression. Gray histograms indicate autofluorescence; open thin line histograms, His-CK–treated DCs; and open thick line histograms, HSP- or LPS-treated DCs. (B) Functional DC maturation, assessed by MLR. DCs pretreated with the indicated stimuli, as described for panel A, were irradiated and used as stimulators, with T cells from a different donor as responders. T-cell proliferation was measured by thymidine incorporation. Experiments were repeated 3 times, and data are displayed as means ± SEM. (C) Cytokine production of DCs stimulated with recombinant Hsp70L1. DCs were cultured with Hsp70L1, Hsp70, His-CK, or LPS proteins in 3 formats; native protein (▪), native protein in the presence of 50 μg/mL PMB (▪), or denatured protein (100°C, 20 minutes) for 24 hours (□). The levels of IL-12p70, TNF-α, and IL-1β in supernatants were measured by ELISA. Data are displayed as mean cytokine concentration (pg/mL) ± SEM. P < .05 for IL-12p70 production between Hsp70L1 and human Hsp70.
Cytokine production of DCs stimulated by recombinant Hsp70L1
Supernatants of 5-day DCs stimulated with Hsp70L1 were analyzed for IL-1β, TNF-α, and IL-12p70 secretion (Figure 4C). Interestingly, we found that DCs induced by Hsp70L1 secreted significantly higher amounts of IL-12p70 than those stimulated with human Hsp70 (Figure 4C). The possibility of endotoxin contamination could be excluded as the LPS inhibitor PMB had no effect on Hsp70L1-induced cytokine release, whereas heat denaturation of Hsp70L1 completely blocked its effects. The observed cytokine profile suggests a Th1 bias, as IL-12 typically induces Th1 polarization.4,28
Expression of chemokines and chemokine receptors by DCs stimulated with Hsp70L1
Seven-day human DCs incubated with recombinant Hsp70L1 exhibited a dose- and time-dependent increase in IP-10, MIP-1α, MIP-1β, and RANTES secretion (Figure 5A). Strikingly, IP-10 secretion was not induced by human Hsp70 but was triggered strongly by Hsp70L1 (Figure 5B). The levels of MIP-1α, MIP-1β, and RANTES induced by Hsp70L1 also were significantly higher than those induced by human Hsp70. Boiled Hsp70L1 elicited significantly lower chemokine production (P < .05), as did the His-tagged control protein His-CK (Figure 5B), indicating that it is Hsp70L1 and not endotoxin contamination or the His-tag that induces chemokine production.
Chemokine production and chemokine receptor expression of DCs stimulated with recombinant Hsp70L1. (A) Dose- and time-dependent secretion of chemokines by human DCs stimulated with recombinant Hsp70L1. For the dose-dependent assay DCs were stimulated with a 2-fold titration series of Hsp70L1, while for the time-dependent experiment, DCs were stimulated with 10 μg/mL Hsp70L1 and supernatants harvested at different times for ELISA assay. Data are mean chemokine concentration (pg/mL) ± SEM of 3 independent experiments. In comparative experiments (B), DCs were cultured with the stimuli shown in the key. Supernatants were collected and assayed for IP-10, MIP-1α, MIP-1β, and RANTES by ELISA. The data are presented as mean ± SEM of 6 independent experiments. For RANTES, MIP-1α, and MIP-1β, P < .05 between Hsp70L1 and human Hsp70 stimulation; for IP-10, P < .01 between Hsp70L1 and human Hsp70 stimulation. (C) Expression of the chemokine receptors CCR7 and CXCR4. DCs were stimulated with LPS, His-CK, Hsp70L1, or boiled Hsp70L1, Hsp70, or boiled Hsp70 for 40 hours, then cells' total RNA was extracted and used for analysis of CCR7 and CXCR4 expression by semiquantitative RT-PCR, or cells were stained with FITC-conjugated mAbs to CCR7 and CXCR4 for FACS analysis.
Chemokine production and chemokine receptor expression of DCs stimulated with recombinant Hsp70L1. (A) Dose- and time-dependent secretion of chemokines by human DCs stimulated with recombinant Hsp70L1. For the dose-dependent assay DCs were stimulated with a 2-fold titration series of Hsp70L1, while for the time-dependent experiment, DCs were stimulated with 10 μg/mL Hsp70L1 and supernatants harvested at different times for ELISA assay. Data are mean chemokine concentration (pg/mL) ± SEM of 3 independent experiments. In comparative experiments (B), DCs were cultured with the stimuli shown in the key. Supernatants were collected and assayed for IP-10, MIP-1α, MIP-1β, and RANTES by ELISA. The data are presented as mean ± SEM of 6 independent experiments. For RANTES, MIP-1α, and MIP-1β, P < .05 between Hsp70L1 and human Hsp70 stimulation; for IP-10, P < .01 between Hsp70L1 and human Hsp70 stimulation. (C) Expression of the chemokine receptors CCR7 and CXCR4. DCs were stimulated with LPS, His-CK, Hsp70L1, or boiled Hsp70L1, Hsp70, or boiled Hsp70 for 40 hours, then cells' total RNA was extracted and used for analysis of CCR7 and CXCR4 expression by semiquantitative RT-PCR, or cells were stained with FITC-conjugated mAbs to CCR7 and CXCR4 for FACS analysis.
Immature DCs stimulated with Hsp70L1, Hsp70, or LPS up-regulated expression of mRNA for the chemokine receptors CXCR4 and CCR7, whereas heat degeneration of Hsp70L1 or Hsp70 abrogated the effect (Figure 5C). Hsp70L1 stimulated CCR7 and CXCR4 expression more strongly than human Hsp70; this was further confirmed by flow cytometric analysis of surface CCR7 and CXCR4 levels (Figure 5C), suggesting that Hsp70L1 may be a more potent promoter of immune response initiated by DCs.
Induction of antigen-specific Th1 response and CTL by Hsp70L1-OVA257-264 hybrid immunization
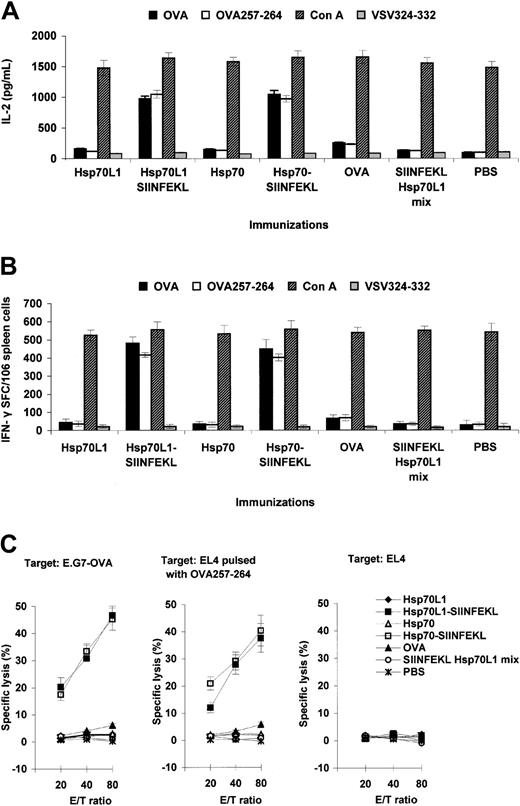
Supporting the notion that Hsp70L1 can act as a Th1 polarizing adjuvant, we observed higher levels of IL-2 secretion (Figure 6A) and generation of more IFN-γ–secreting cells (Figure 6B) when splenocytes from Hsp70L1-OVA257-264 or Hsp70-OVA257-264 hybrid immunized mice were restimulated with OVA257-264 peptide, as compared to controls (P < .05).
Immunization with Hsp70L1-OVA257-264 hybrid induces OVA peptide-specific Th1 responses and CTL. Splenocytes from immunized mice were used to assess OVA257-264 peptide-specific Th1 responses by IL-2 ELISA (A), IFN-γ ELISPOT (B), and CTL assay (C). To induce OVA257-264 peptide-specific IFN-γ–producing cells and IL-2 secretion, the splenocytes were restimulated with OVA, OVA257-264 peptide, Con A, or unrelated control peptide VSV324-332. For CTL assay, the splenocytes were restimulated with irradiated E.G7-OVA cells and then used as effector cells (E) in an OVA-specific lysis test by standard 4-hour 51Cr-release assay. EL4 cells pulsed with OVA257-264 peptide and E.G7-OVA cells were used as OVA-specific targets (T); control targets were EL4 cells. Various E/T ratios were tested as indicated. IL-2 concentration is expressed in pg/mL, with ELISPOT results expressed as the number of IFN-γ–positive spot-forming cells (SFCs)/106 splenocytes. Data are mean ± SEM of 3 independent experiments.
Immunization with Hsp70L1-OVA257-264 hybrid induces OVA peptide-specific Th1 responses and CTL. Splenocytes from immunized mice were used to assess OVA257-264 peptide-specific Th1 responses by IL-2 ELISA (A), IFN-γ ELISPOT (B), and CTL assay (C). To induce OVA257-264 peptide-specific IFN-γ–producing cells and IL-2 secretion, the splenocytes were restimulated with OVA, OVA257-264 peptide, Con A, or unrelated control peptide VSV324-332. For CTL assay, the splenocytes were restimulated with irradiated E.G7-OVA cells and then used as effector cells (E) in an OVA-specific lysis test by standard 4-hour 51Cr-release assay. EL4 cells pulsed with OVA257-264 peptide and E.G7-OVA cells were used as OVA-specific targets (T); control targets were EL4 cells. Various E/T ratios were tested as indicated. IL-2 concentration is expressed in pg/mL, with ELISPOT results expressed as the number of IFN-γ–positive spot-forming cells (SFCs)/106 splenocytes. Data are mean ± SEM of 3 independent experiments.
OVA257-264–specific CTL also could be induced by the Hsp70L1-OVA257-264 hybrid in vivo. CTL from both Hsp70L1-OVA257-264 and Hsp70-OVA257-264 hybrid immunized mice were able to lyse E.G7-OVA target cells and EL4 cells pulsed with OVA257-264 peptide but were unable to lyse parental EL4 cells. In contrast, effector cells from mice immunized with Hsp70L1 or OVA257-264 Hsp70L1 mix showed almost no target cell killing (Figure 6C), indicating that the induction of a potent CTL response requires binding of OVA257-264 to the Hsp70L1 adjuvant.
Growth inhibition of OVA-expressing tumor in mice immunized with Hsp70L1-OVA257-264 hybrid
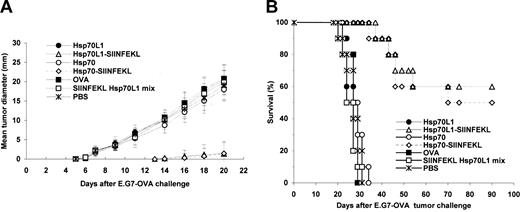
The induction of in vivo antitumor immunity by Hsp70L1-OVA257-264 hybrid immunization was assessed in a murine model with E.G7-OVA tumor challenge. Mice immunized with Hsp70L1-OVA257-264 or Hsp70-OVA257-264 hybrid showed significant tumor growth inhibition (P < .01, generalized Wilcoxon test), while no significant protection nor improvement in survival was observed in other control groups (Figure 7). All control mice developed palpable tumors 6 days after tumor challenge, but no tumors were observed in Hsp70L1-OVA257-264 hybrid immunized mice until 14 days after E.G7 tumor challenge. Notably, 60% of mice in the Hsp70L1-OVA257-264 hybrid immunized group survived for longer than 90 days following E.G7 tumor challenge, remaining free of detectable tumor for the duration. In contrast, all mice in control groups died between day 20 and day 34 after tumor challenge. We also challenged Hsp70L1-OVA257-264 hybrid immunized mice with parental EL4 cells (do not present OVA257-264) and failed to observe any sign of tumor growth inhibition or improvement in survival (data not shown), indicating that the antitumor effect obtained through Hsp70L1-OVA257-264 hybrid immunization was OVA257-264 specific. These experiments demonstrate that immunization of mice with the Hsp70L1-OVA257-264 hybrid, but not with OVA protein or OVA257-264 peptide mixed with Hsp70L1, induces a potent and protective immune response against OVA257-264–expressing tumor cells, suggesting that Hsp70L1 is an effective adjuvant for peptide immunization.
Immunization with Hsp70L1-OVA257-264 hybrid peptide protects mice against E.G7-OVA tumor challenge. C57BL/6 mice were immunized subcutaneously on the thigh with reconstituted Hsp70L1-OVA257-264, Hsp70-OVA257-264 peptide hybrid, SIINFEKL Hsp70L1 mix, Hsp70L1, Hsp70, or OVA alone. Five days after the final immunization, mice were challenged subcutaneously with 3 × 106 E.G7-OVA tumor cells to the flank area. (A) Tumor growth curves. Following E.G7-OVA tumor challenge, tumor growth was monitored by measuring the diameter of the tumor every 2 days and recorded as the average tumor diameter. (B) Survival of immunized mice after E.G7-OVA tumor challenge. Each group contained 10 mice.
Immunization with Hsp70L1-OVA257-264 hybrid peptide protects mice against E.G7-OVA tumor challenge. C57BL/6 mice were immunized subcutaneously on the thigh with reconstituted Hsp70L1-OVA257-264, Hsp70-OVA257-264 peptide hybrid, SIINFEKL Hsp70L1 mix, Hsp70L1, Hsp70, or OVA alone. Five days after the final immunization, mice were challenged subcutaneously with 3 × 106 E.G7-OVA tumor cells to the flank area. (A) Tumor growth curves. Following E.G7-OVA tumor challenge, tumor growth was monitored by measuring the diameter of the tumor every 2 days and recorded as the average tumor diameter. (B) Survival of immunized mice after E.G7-OVA tumor challenge. Each group contained 10 mice.
Discussion
In this study we have identified a new HSP molecule, Hsp70L1, and demonstrated its adjuvant effects for peptide immunization and its ability to activate DCs. Interaction of recombinant Hsp70L1 with DCs promotes maturation and stimulates DCs to secrete IL-1β, IL-12p70, TNF-α, MIP-1α, MIP-1β, RANTES, and IP-10, as well as up-regulate the expression of the chemokine receptors CXCR4 and CCR7. Although Hsp70L1 shares common receptors on DCs with Hsp70, it appears to be functionally different from typical Hsp70 in that Hsp70L1 can stimulate DCs to secrete IP-10 but Hsp70 cannot. These Hsp70L1 functions may provide insight into DC-related immune responses and have implications in immunotherapy of tumors or infectious diseases.
Hsp70L1 appears to be a member of the Hsp70 subfamily, possessing a number of definitive characteristics. Although it lacks strong primary sequence homology, Hsp70L1 is heat inducible, as demonstrated in heat-stressed HeLa cells and DCs. Furthermore, Hsp70L1 has an ATPase-binding domain and functional similarities to human Hsp70 in that it binds the same receptors (TLR2, TLR4, CD91) and stimulates DCs to secrete cytokines and chemokines and become mature.
An important finding is that Hsp70L1 can activate DCs. As professional APCs, with a potent capacity to present antigen and activate naive T cells, DCs can both initiate and modulate immune responses.29,30 We demonstrate that the interaction of Hsp70L1 with DCs enhances the expression of CD80, CD83, CD86, HLA-DR, CCR7, and CXCR4 and that these DCs are able to induce primary allogeneic T-cell proliferation more efficiently. This phenotypic and functional maturation is critical for DCs to effectively activate immune responses. Hsp70L1-DC interaction also led to increased secretion of the cytokines IL-12p70, IL-1β, and TNF-α, and the chemokines IP-10, MIP-1α, MIP-1β, and RANTES. IL-12 and IP-10 are essential for inducing Th1 polarization,4,31,32 thus the cytokine profile seen here suggests bias toward a Th1 response. The chemokines secreted from DCs are expected to chemoattract T cells, monocytes, and DCs, resulting in enhanced induction of immune responses. In addition, enhanced CCR7 and CXCR4 expression following Hsp70L1 stimulation is important for homing of mature DCs from the periphery into T-cell–rich areas of secondary lymphoid organs for T-cell activation.29,33,34 The above consequences suggest that Hsp70L1 has multiple effects on DC functions; the discovery of Hsp70L1 may provide fundamental insights into the functioning of the immune system.
Another important finding of this study is that Hsp70L1 is a potent adjuvant for peptide immunization. We used the standard Hsp70-peptide binding method to prepare Hsp70L1-OVA257-264 hybrid peptide and tested the induction of antigen-specific Th1 responses and CTL following Hsp70L1-OVA257-264 immunization. Hsp70L1-OVA257-264 hybrid strongly induced OVA257-264–specific IFN-γ– and IL-2–secreting Th1 cells, as well as OVA257-264–specific CTLs. Induction of IFN-γ –secreting cells, in particular, suggests a polarization toward the Th1 response.35 This was further supported by our observation of potent OVA257-264–specific antitumor immunity following Hsp70L1-OVA257-264 immunization in a murine tumor challenge model. Our demonstration that Hsp70L1 can induce DCs to produce the essential Th1-polarizing cytokines IP-10 and IL-12 leads us to presume that the adjuvant effects of Hsp70L1 are mediated via DC activation. Besides inducing DC maturation, IL-12–dominant cytokines, and CC-chemokine production, Hsp70L1-DC interaction also may enhance ability of DCs to capture and present antigens, facilitating presentation of antigenic peptides carried by Hsp70L1. However, to be effectively presented by DCs, the antigenic peptides must be associated with Hsp70L1, as Hsp70L1 alone or OVA257-264 peptide simply mixed with Hsp70L1 failed to elicit OVA257-264–specific CTL or tumor growth inhibition. Together these findings demonstrate the potent Th1-polarizing adjuvant effect of Hsp70L1 for peptide immunization through activation of DCs. Although we used OVA peptide as a model antigen to demonstrate the adjuvant effects of Hsp70L1 in this study, it is reasonable to assume that as a member of the Hsp70 subfamily, Hsp70L1 may bind a variety of viral antigenic peptides or tumor-specific antigens, conferring adjuvant properties to them. Thus, Hsp70L1, as a novel and potent adjuvant of mammalian origin, may be used widely to induce and enhance immune responses against cancer, as well as infectious diseases such as HIV, human papillomavirus (HPV), and hepatitis B virus (HBV) infection.
To reveal the functional novelty of Hsp70L1, we compared it with human Hsp70, a prototypical member of the Hsp70 subfamily. Structurally, except for the N-terminal ATPase domain, the amino acid coding sequence of Hsp70L1 has many differences from that of Hsp70, which suggests that this novel HSP may possess other functions. Hsp70L1 is indeed more potent than human Hsp70 in stimulating DCs to secrete IL-12p70, MIP-1α, MIP-1β, and RANTES and up-regulate chemokine receptor CCR7 and CXCR4 expression. Most importantly, we found that Hsp70L1 could induce DCs to secrete IP-10, but Hsp70 could not. The functional differences between Hsp70L1 and Hsp70 should not be ascribed to the His-tag of recombinant Hsp70L1, as the control protein His-CK never elicited any functional effects. IP-10 is indispensable for effector T-cell generation and trafficking and is critical for retaining Th1 lymphocytes within T-cell areas of draining lymphoid nodes and optimizing Th1-mediated immune responses.31,32 IL-12 is also a Th1 polarizing cytokine, leading us to propose that Hsp70L1 has a stronger tendency than Hsp70 to polarize toward Th1 responses. This unique property of Hsp70L1 suggests that it may help to enhance immune responses or to break immune tolerance status in diseases such as chronic HBV infection or spontaneous tumors. As Hsp70L1 is a molecule of human origin, its advantages and effects may not be fully exhibited in a mouse model. The results of the DC binding study also suggest that Hsp70L1 has its own receptor or binding site, different from the known receptors of Hsp70 on DCs (including TLR2, TLR4, and CD91 receptor). These findings on the functional novelty of Hsp70L1 are very interesting, and its mechanisms of action deserve to be further explored.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-08-2828.
Supported by grants from the National High Technology Research and Development Program of China (2001AA217021 and 2002AA2Z3307 [T.W.]), the National Natural Science Foundation of China (30170865 [T.W.], 30070854 [G.C.], 30121002 [X.C.]), and the National Key Basic Research Program of China (2001CB510002 [X.C.]).
T.W. and X.Z. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Frances Gotch of the Imperial College of Science, Technology and Medicine, London; and Dr Jane Rayner for critically reading the manuscript. We thank Dr Long He, Dr Wenya Wang, and Dr Yizhi Yu for helpful discussions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal