Abstract
Fibrin is actively involved in platelet reactions essential for thrombus growth, in which von Willebrand factor (VWF) might be an important mediator. The aim of this study was to localize VWF domains that bind to fibrin and to determine their relevance in platelet adhesion. VWF binds specifically to fibrin with an apparent Kd of 2.2 μg/mL. Competition in the presence of 2 complementary fragments, SpIII (residues 1-1365) and SpII (residues 1366-2050), indicated that the high affinity binding site for fibrin is located in the C-terminal part, thus distinct from the A domains. Comparison of 2 deleted rVWF (ΔD4B-rVWF, ΔC1C2-rVWF) suggested that the C1C2 domains contained a fibrin binding site. This site is distinct from RGD, as shown by binding of D1746G-rVWF to fibrin. Perfusion studies at high shear rate demonstrated that C1C2 domains were required for optimal platelet adhesion to fibrin. With the use of a VWF-deficient mouse model, it was found that plasma VWF is critical for platelet tethering and adhesion to fibrin. These results suggest a dual role of fibrin-bound VWF in thrombus formation: first, fibrin-bound VWF is critical in the recruitment of platelets by way of glycoprotein (GP) Ib, and, second, it contributes to stationary platelet adhesion by way of binding to activated αIIbβ3.
Introduction
The process of hemostasis and pathologic thrombus formation at an injured vessel wall is initiated when platelets are captured from flowing blood by way of a rapid bond formation between their glycoprotein (GP) Ib receptor and von Willebrand factor (VWF) immobilized on collagen. Platelets subsequently roll over the damaged area prior to irreversible binding through integrin αIIb β3.1-3 GP Ib and αIIbβ3 binding sites on VWF are localized on the A1 and C1 domain, respectively.4-8 Immobilized VWF present on the platelet membranes then serves for further platelet recruitment and thrombus growth.9
In parallel with the platelet adhesion process, coagulation is initiated through release of tissue factor from the damaged vessel wall.10 Propagation of blood coagulation occurs by localized enzymatic complexes assembled on the plasma membrane of adherent platelets that expose negatively charged phospholipids (reviewed in Heemskerk et al11 ). The thrombin thus formed further activates platelets and stabilizes the growing thrombus by the formation of fibrin. Previous work from our laboratory12,13 and others14 suggests that in addition to its nonspecific trapping of platelets, fibrin could be actively involved in regulating thrombus growth. It is well accepted that under conditions of high shear stress VWF plays a critical role in the recruitment of circulating platelets to the fibrin network.3,15 The initial evidence indicating that VWF binds to fibrin from a plasma milieu was provided by Hada et al.16 Those investigators demonstrated that factor XIIIa mediated the cross-linking between fibrin and VWF. In contrast, Loscalzo et al17 demonstrated that VWF interacts noncovalently with immobilized fibrin monomers. Other investigators extended this finding by demonstrating that VWF interaction with fibrin depends on the presence of high-molecular-weight VWF multimers.18 More recently, it has been shown that VWF binds to fibrin only in a purified protein system and not from plasma.15 It was postulated that other plasma proteins compete with VWF for binding to fibrin. According to these investigators, interactions between VWF and fibrin could only be detected when platelets were present. Altogether, these conflicting observations initiated the present study in which we addressed the following questions: Does VWF contain a specific fibrin-binding site and does plasma VWF contribute in tethering platelets to fibrin under physiologically relevant conditions? Our results imply that the C-domain of VWF is a critical determinant of platelet adhesion to fibrin under conditions of high shear stress.
Materials and methods
Proteins
High-purity VWF concentrate was a kind gift from Dr C. Mazurier (LFB, Lille, France). Digestion of purified VWF by Staphylococcus aureus V-8 protease and purification of 2 dimeric complementary fragments, the N-terminal SpIII (residues 1-1365) and the C-terminal SpII (residues 1366-2050) were performed as described.6 Human fibrinogen, devoid of VWF, fibronectin, and plasminogen was obtained from Kordia (Leiden, The Netherlands). Human α-thrombin was purified as described.19 D-Phe-Pro-Arg chloromethylketone (PPACK) came from Calbiochem (San Diego, CA). Synthetic peptide Arg-Gly-Asp-Ser (RGDS) and bovine serum albumin (BSA) were from Sigma (St Louis, MO). Factor VIII–depleted plasma came from Stago Diagnostica (Asnieres-sur-Seine, France) and contained less than 1% VWF:Ag. Recombinant VWF (rVWF) and its mutants rVWF-ΔC1C2 and rVWF-D1746G were purified by immunoaffinity chromatography by using antibody RU8.20 rVWF-ΔD4B was purified by conventional chromatographic methods by using heparin-Sepharose and monoQ-Sepharose. The disintegrin kistrin, an RGD-containing cysteinerich peptide, was purified from the venom of the viper Agkistrodon rhodostoma as described.21
Animals
The wild-type and VWF-deficient mice22 were on a C57BL/6J background and were used between 10 and 16 weeks of age. Housing and experiments were done as recommended by French regulations and experimental guidelines of the European Union.
Antibodies
A polyclonal antibody against VWF was from Dako (Carpinteria, CA). Polyclonal antibodies to human SpII or SpIII fragments were raised in rabbits by immunization using the purified fragments. The immunoglobulin G (IgG) fractions were rendered monospecific by adsorption onto VWF-deficient plasma coupled to Sepharose 2B,23 then the pass-through was immunoadsorbed onto purified VWF coupled to Sepharose 2B.24 Specific antibodies were eluted with 0.1 M glycine, 0.5 M NaCl, pH 2.4.
SDS-agarose gel electrophoresis
Multimeric composition of 125I-VWF added to fibrin and that of the radioactive fibrin-bound material, eluted from 15 filters with a 2% sodium dodecyl sulfate (SDS) solution, was analyzed by 0.1% SDS-1% agarose (Amersham, Uppsala, Sweden) gel electrophoresis under nonreducing conditions as described earlier.25
125I-VWF binding to fibrin
VWF was labeled with Na125I (Amersham) and Iodo-gen.26 Specific radioactivity varied from 1 to 4 μCi/μg (0.037-0.148 MBq μg). Labeled protein was stored at 4°C and used within 1 week. Fibrin was formed in spin-X filters (2-mL, cellulose acetate, 0.45 μm; Corning BV, Schiphol-Rijk, The Netherlands) according to Hall et al.27 Briefly, thrombin (10 nM) was added to fibrinogen in 30 mM Tris (tris(hydroxymethyl)aminomethane)–HCl, pH 7.4, 150 mM NaCl, 0.1% BSA (TBS) containing 3 mM CaCl2 to a final volume of 50 μL. The clot was allowed to form for 15 minutes at 37°C and washed with 500 μL TBS by spinning the filter cup at 14 000 rpm during 3 minutes at room temperature. Thrombin bound to the fibrin was neutralized with 5 μM PPACK during a 5-minute incubation, and the fibrin clot was then washed as described earlier. To reduce nonspecific binding, the filter with fibrin was incubated for 30 minutes with TBS containing 3% BSA. VWF binding was determined by incubating the fibrin clot on the filter with 125I-VWF in TBS (50 μL) for 2 hours at 37°C. The filter was rinsed 3 times with 500 μL TBS containing 0.1% Tween-20 as described earlier. The amount of bound VWF was calculated from the radioactivity associated with the filter measured in a gamma-counter (Multigamma II counter; LKB Instruments SA, Bromma, Sweden). Specific binding was obtained by subtracting the amount of VWF bound to the filter in the absence of fibrin. Nonspecific filter binding ranged in all experiments from 3% to 5% of protein added. Free 125I-VWF was calculated by subtracting the amount of 125I-VWF bound to fibrin from the total amount of 125I-VWF added. Mean values ± SEM were calculated for 6 independent experiments.
Preparation of plasma-free human blood cells
Preparation of washed blood cells was adapted from Savage et al.3 Briefly, blood was taken from healthy volunteers who had not taken antiplatelet medication for the preceding 2 weeks. Five volumes of blood were taken into 1 volume of acid-citrate dextrose (ACD). Blood was then spun down at 2100g for 15 minutes at 23°C. The supernatant plasma was discarded and replaced by a double volume of HEPES buffer (10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 136 mM NaCl, 2.7 mM KCl, 2 mM MgCl2, 0.1% glucose, 0.1% BSA, pH 6.5) containing 5 mM EDTA (ethylenediaminetetraacetic acid). The cell suspension was spun down, and the cell-free supernatant was removed. This procedure was repeated twice. Finally, washed blood cells were resuspended into HEPES buffer, pH 7.45. The blood cell counts were about 95% of the original whole blood values.
Preparation of mouse fibrin–coated and human fibrin–coated coverslips
Nine volumes of blood were obtained by way of retro-orbital venous plexus sampling into 1 volume of 108 mM sodium citrate, and plasma was prepared by centrifugation of the blood at 2500g for 15 minutes. One volume of citrated mouse plasma was mixed with 7 volumes of human thrombin and CaCl2 to final concentrations of 20 nM and 8 mM, respectively. Subsequently, 0.6 mL of that mixture was placed between 2 glass coverslips (24 × 60 mm). After 10 minutes, the coverslips were separated, and the presence of fibrin was confirmed by observation under the microscope. Coverslips containing fibrin layers were mounted in a flow chamber.28 Fibrin was incubated for 30 minutes with 40 μM PPACK in TBS containing 3% BSA and then rinsed with TBS. Incubation of these fibrin layers with S2238 (0.2 mM) in HEPES buffer for 30 minutes did not result in a change in optical density measured at 405 nm, indicating that virtually all thrombin activity was neutralized.
For human fibrin formation, purified human fibrinogen and thrombin were mixed to final concentrations of 0.5 mg/mL and 10 nM, respectively, in TBS containing 3 mM CaCl2 (final volume of 0.2 mL) and immediately transferred onto a glass coverslip (24 × 60 mm). All subsequent steps were as described for the preparation of mouse fibrin–coated coverslips. All procedures were performed at ambient temperature (20°C-22°C).
Flow experiments with mouse blood
Blood (1 mL) was collected in 20 μL heparin (1000 U/mL; unfractionated, porcine intestinal mucosa; Sigma). Prior to perfusion, 0.25 mL anticoagulated blood was mixed with 0.75 mL HEPES buffer (pH 7.45) containing 3 mM CaCl2 and 20 U/mL heparin. Perfusion of wild-type (WT) or VWF-deficient blood over WT or VWF-deficient fibrin layers was performed at a flow rate of 125 μL/min (wall shear rate of 1000 s–1) for 7 to 10 minutes at room temperature (20°C-22°C) and visualized by using video microscopy.29 During the perfusion one field was recorded on videotape. At the end of the perfusion, the flow chamber was perfused with buffer at the same shear rate, and 10 fields were recorded for 2 minutes. Videotapes were analyzed off line to determine the number of stationary platelets and the number of translocating platelets on the fibrin surface.30 Platelets forming transient adhesion contacts (< 30 seconds) with fibrin surface are scored as translocating platelets. Stationary adhesion was defined as cells not moving more than a single cell diameter over a 30-second period. The translocation velocity was calculated as the average distance traveled per unit time of 10 platelets/field. Data are expressed as mean values ± SEM of 3 independent experiments.
Perfusion experiments with plasma-free human blood cells
Human fibrin layers were incubated for 2 hours at room temperature with plasma-derived VWF, VWF fragments, mutated rVWF, or combinations of these proteins. Perfusion was then performed with plasma-free blood at a shear rate of 1500 s–1 for 3 minutes. Surface was rinsed with HEPES buffer (pH 7.45), and the number of adherent platelets per field (14 400 μm2) was quantified as described for mouse platelets in “Flow experiments with mouse blood.” Data are expressed as mean values ± SEM of 3 independent experiments.
Statistical analysis
To determine the statistical significance of differences, P values were obtained with a nonparametric test for 2 independent variables (Mann-Whitney test). Error bars indicate SEM.
Results
Characterization of 125I-VWF binding to fibrin
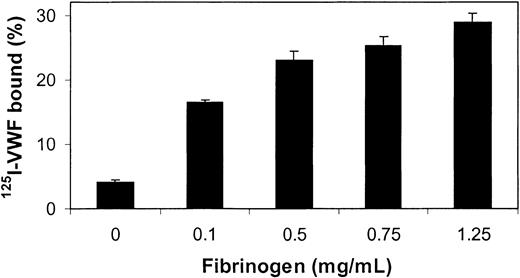
In an initial experiment, a fixed amount of purified human 125I-VWF (0.25 μg/mL) was added to varying amounts of thrombin-clotted fibrinogen. Figure 1 shows that relative to total VWF, the percentage of bound 125I-VWF increased nonlinearly with increasing fibrinogen concentrations. It was observed that when fibrinogen concentrations higher than 1.5 mg/mL were used, some retention of buffer occurred on the filter membrane, leading to ineffective washing of the fibrin. Therefore, all subsequent experiments were performed with clots prepared from incubations of 0.75 mg/mL fibrinogen with thrombin.
VWF binding to fibrin. Increasing concentrations of fibrinogen were mixed with 10 nM thrombin for 15 minutes at 37°C on the membrane of spin-X tubes. The fibrin strands were washed, and thrombin bound to fibrin was neutralized with PPACK. 125I-VWF (250 ng/mL) was then added to the filter cups and incubated for 2 hours at 37°C. After washing, binding percentages were determined by gamma counting of the filters and the supernatants. Error bars indicate SEM.
VWF binding to fibrin. Increasing concentrations of fibrinogen were mixed with 10 nM thrombin for 15 minutes at 37°C on the membrane of spin-X tubes. The fibrin strands were washed, and thrombin bound to fibrin was neutralized with PPACK. 125I-VWF (250 ng/mL) was then added to the filter cups and incubated for 2 hours at 37°C. After washing, binding percentages were determined by gamma counting of the filters and the supernatants. Error bars indicate SEM.
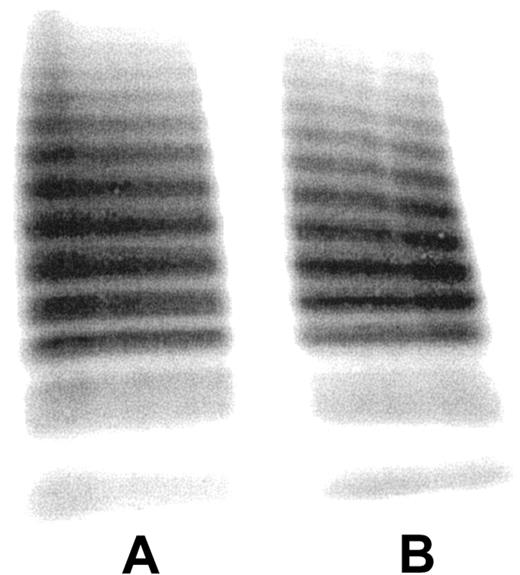
Figure 2 reveals no difference in the multimeric composition of purified human VWF (lane A) and that of VWF eluted from the fibrin clot (lane B). Thus, both high-molecular-weight and low-molecular-weight multimers are capable of binding to fibrin under the present experimental conditions, using a VWF concentration that is about 40-fold below the VWF plasma concentration.
Multimeric composition of fibrin-bound VWF.125I-VWF (lane A) and SDS-eluted fibrin-bound 125I-VWF (lane B) from 15 spin-X tubes were subjected to SDS-agarose electrophoresis. Gels were loaded with approximately 1 μg protein.
Multimeric composition of fibrin-bound VWF.125I-VWF (lane A) and SDS-eluted fibrin-bound 125I-VWF (lane B) from 15 spin-X tubes were subjected to SDS-agarose electrophoresis. Gels were loaded with approximately 1 μg protein.
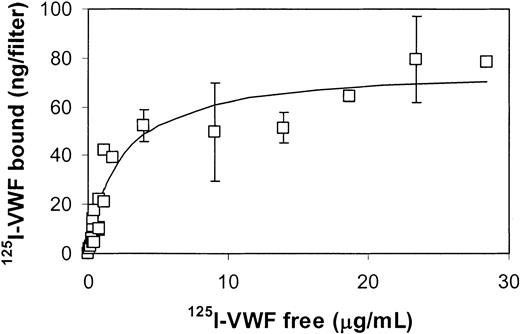
Figure 3 shows the amount of fibrin-bound 125I-VWF as a function of the concentration of free 125I-VWF. It is apparent that the binding data fits the Langmuir model with an apparent dissociation constant of 2.2 μg/mL. The maximal binding capacity of fibrin (37.5 μg) on the filter was 76 ng VWF. Thus, under saturating conditions about 2 ng VWF binds per 1 μg thrombinformed fibrin.
VWF dose dependency. Increasing amounts of 125I-VWF were added to fibrin strands formed from 0.75 mg/mL fibrinogen (37 μg fibrinogen on filter) and 10 nM thrombin. Specific binding, calculated from total binding and nonspecific binding, eg, in the absence of fibrin, is plotted versus the free VWF concentration. The solid line is the result of a nonlinear regression analysis that fits the data points of 3 independent experiments to a Langmuir binding model. Error bars indicate SEM.
VWF dose dependency. Increasing amounts of 125I-VWF were added to fibrin strands formed from 0.75 mg/mL fibrinogen (37 μg fibrinogen on filter) and 10 nM thrombin. Specific binding, calculated from total binding and nonspecific binding, eg, in the absence of fibrin, is plotted versus the free VWF concentration. The solid line is the result of a nonlinear regression analysis that fits the data points of 3 independent experiments to a Langmuir binding model. Error bars indicate SEM.
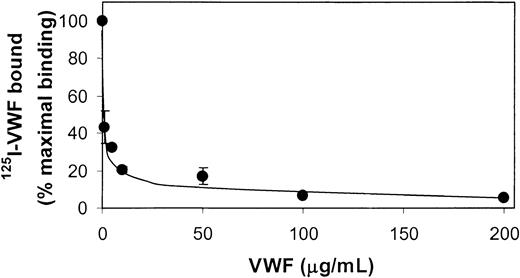
To examine the specificity of the 125I-VWF binding to fibrin, a competition experiment was performed with nonlabeled VWF. 125I-VWF (0.25 μg/mL) was added to amounts of nonlabeled VWF that varied from 0 to 0.2 mg/mL and incubated with fibrin present on the filter membrane. It is clearly shown in Figure 4 that nonlabeled VWF can displace nearly all fibrin-bound 125I-VWF.
Competition of nonlabeled VWF with 125I-VWF.125I-VWF (250 ng/mL) and increasing concentrations of unlabeled VWF were incubated in filters without and with fibrin (37 μg) for 2 hours at 37°C. Nonspecific binding in the absence of fibrin was subtracted from the total binding to obtain the specific binding. Results are expressed as percentages of specific binding of 125I-VWF in the absence of nonlabeled VWF. Error bars indicate SEM.
Competition of nonlabeled VWF with 125I-VWF.125I-VWF (250 ng/mL) and increasing concentrations of unlabeled VWF were incubated in filters without and with fibrin (37 μg) for 2 hours at 37°C. Nonspecific binding in the absence of fibrin was subtracted from the total binding to obtain the specific binding. Results are expressed as percentages of specific binding of 125I-VWF in the absence of nonlabeled VWF. Error bars indicate SEM.
To investigate whether plasma proteins compete with VWF for binding to fibrin, we performed serial dilutions of VWF-deficient plasma in HEPES buffer and determined the specific binding of 125I-VWF (0.25 μg/mL). Compared with a specific binding of 125I-VWF of 26% in the absence of VWF-deficient plasma, it was found that binding percentages varied around this value when the plasma protein concentration increased from 1% to 100%. It is concluded that other plasma proteins do not interfere with the binding of VWF to fibrin.
Identification of the fibrin binding sites on VWF
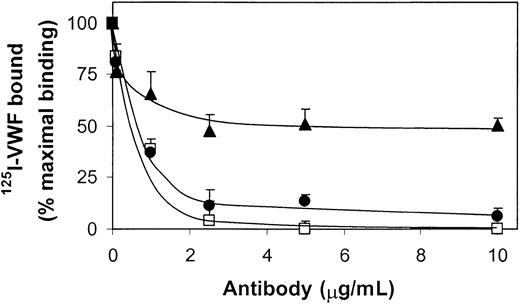
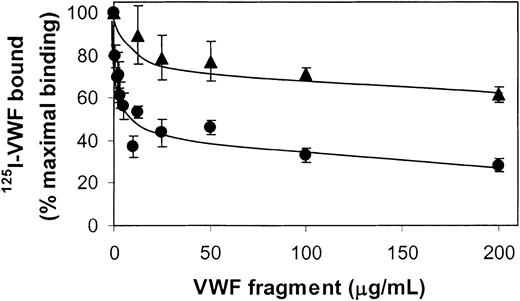
Figure 5 shows that the binding of 125I-VWF (0.25 μg/mL) to fibrin (0.75 mg/mL) is completely abrogated in the presence of a polyclonal antibody against VWF (2.5 μg/mL), whereas polyclonal antibodies against the SpII fragment inhibited the binding of VWF dose dependently, down to 5% of the amount obtained in the absence of antibody. In contrast, in the presence of anti-SpIII (5 μg/mL), binding reached 50% of that in the absence of antibody. Next, purified VWF fragments SpII and SpIII were examined for their ability to compete with 125I-VWF for binding to fibrin. Low concentrations of SpII effectively inhibited binding of VWF to fibrin (Figure 6). In contrast, SpIII did not appear to inhibit 125I-VWF binding to fibrin in an efficient way. Collectively, these results imply that SpII contains VWF's major binding site for fibrin.
Inhibition of 125I-VWF binding to fibrin by polyclonal antibodies against VWF and its proteolytic fragments SpII and SpIII. Polyclonal antibodies to VWF(□), SpII (•), or SpIII (▴) were preincubated with 250 ng/mL 125I-VWF before addition to filters without and with fibrin (37 μg) for 2 hours at 37°C. For each point nonspecific binding (< 3% of total binding) was subtracted from the total binding to obtain the specific binding. Results are expressed as percentages of specific binding of 125I-VWF measured in the absence of antibody. Error bars indicate SEM.
Inhibition of 125I-VWF binding to fibrin by polyclonal antibodies against VWF and its proteolytic fragments SpII and SpIII. Polyclonal antibodies to VWF(□), SpII (•), or SpIII (▴) were preincubated with 250 ng/mL 125I-VWF before addition to filters without and with fibrin (37 μg) for 2 hours at 37°C. For each point nonspecific binding (< 3% of total binding) was subtracted from the total binding to obtain the specific binding. Results are expressed as percentages of specific binding of 125I-VWF measured in the absence of antibody. Error bars indicate SEM.
Inhibition of 125I-VWF binding to fibrin by SpII and SpIII.125I-VWF (250 ng/mL) and increasing concentrations of unlabeled SpII (•) and SpIII (▴) were incubated on filters without and with fibrin (37 μg) for 2 hours at 37°C. For each point nonspecific binding (< 3% of total binding) was subtracted from the total binding to obtain the specific binding. Results are expressed as percentages of specific binding of 125I-VWF in the absence of competitor. Error bars indicate SEM.
Inhibition of 125I-VWF binding to fibrin by SpII and SpIII.125I-VWF (250 ng/mL) and increasing concentrations of unlabeled SpII (•) and SpIII (▴) were incubated on filters without and with fibrin (37 μg) for 2 hours at 37°C. For each point nonspecific binding (< 3% of total binding) was subtracted from the total binding to obtain the specific binding. Results are expressed as percentages of specific binding of 125I-VWF in the absence of competitor. Error bars indicate SEM.
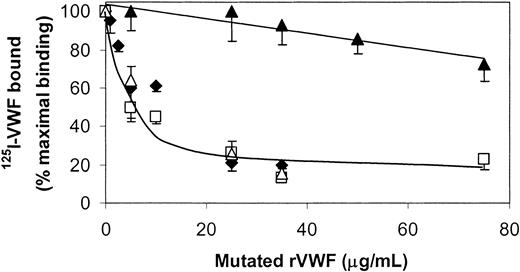
Competition experiments performed with 125I-rVWF in the presence of mutated rVWF lacking the domains D4 and B (ΔD4B-rVWF) or C1 and C2 (ΔC1C2-rVWF) revealed that both rVWF and the ΔD4B-rVWF were capable of competing with 125I-rVWF for interaction with fibrin (Figure 7). However, the ΔC1C2-rVWF hardly affected the binding of 125I-rVWF, indicating that this mutant lacks the fibrin binding site. In addition, Figure 7 also shows that D1746G-rVWF could compete with rVWF for binding to fibrin, showing that the primary binding site in the C domains does not require an intact RGD sequence. Moreover, neither the RGDS peptide nor the disintegrin kistrin competed with VWF for binding to fibrin (data not shown).
Effect of VWF mutants on the binding of 125I-VWF to fibrin.125I-WT-rVWF (250 ng/mL) and increasing concentrations of WT-rVWF (♦), ΔC1C2-rVWF (▴), ΔD4B-rVWF (□), and D1746G-rVWF (▵) were incubated on filters without and with fibrin (37 μg) for 2 hours at 37°C. For each point nonspecific binding (< 3% of total binding) was subtracted from the total binding to obtain the specific binding. Results are expressed as percentages of specific binding of 125I-VWF in the absence of competitor. Error bars indicate SEM.
Effect of VWF mutants on the binding of 125I-VWF to fibrin.125I-WT-rVWF (250 ng/mL) and increasing concentrations of WT-rVWF (♦), ΔC1C2-rVWF (▴), ΔD4B-rVWF (□), and D1746G-rVWF (▵) were incubated on filters without and with fibrin (37 μg) for 2 hours at 37°C. For each point nonspecific binding (< 3% of total binding) was subtracted from the total binding to obtain the specific binding. Results are expressed as percentages of specific binding of 125I-VWF in the absence of competitor. Error bars indicate SEM.
Platelet adhesion on fibrin layers incubated with human VWF, VWF fragments, or mutated rVWF under high shear rate conditions
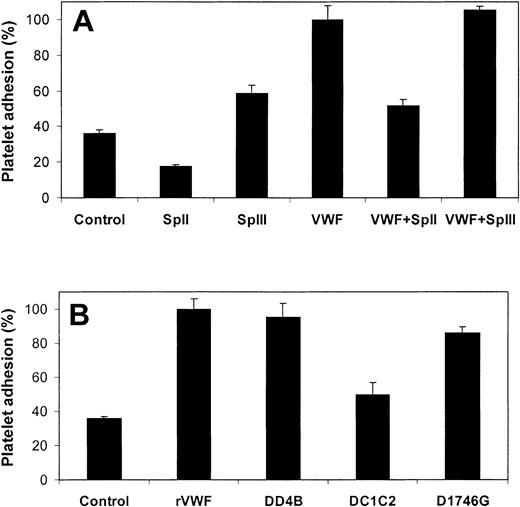
Incubation of fibrin layers with VWF was also evaluated for its relevance in platelet adhesion at high shear rate. Three minutes after the start of a high shear rate (1500 s–1) perfusion of washed blood cells over a fibrin surface, preincubated with purified plasma-derived VWF (5 μg/mL), 379 ± 62 stationary platelets/field were measured. This is 2.6-fold higher than the control fibrin layers that were not preincubated with VWF (Figure 8A). These results confirm the role of VWF in platelet adhesion to fibrin under high shear rate conditions.
Effect of VWF, VWF fragments, and VWF mutants on platelet adhesion to fibrin. (A) Human fibrin surfaces were incubated with SpII (200 μg/mL), SpIII (200 μg/mL), plasma-purified VWF (5 μg/mL), and plasma-purified VWF (5 μg/mL) in the presence of 200 μg/mL VWF fragments SpII or SpIII. (B) Human fibrin surfaces were incubated with WT-rVWF (5 μg/mL), ΔD4B-rVWF (5 μg/mL), and ΔC1C2-rVWF (5 μg/mL) and D1746G-rVWF (5 μg/mL) for 2 hours at ambient temperature. After removing nonbound material, plasma-free blood was perfused at a shear rate of 1500 s–1 over the fibrin surfaces for 3 minutes. Platelet adhesion data are calculated relative to the mean value obtained with fibrin that was incubated with plasma-purified VWF (A) or WT-rVWF (B). Controls are nontreated fibrin layers. Error bars indicate SEM.
Effect of VWF, VWF fragments, and VWF mutants on platelet adhesion to fibrin. (A) Human fibrin surfaces were incubated with SpII (200 μg/mL), SpIII (200 μg/mL), plasma-purified VWF (5 μg/mL), and plasma-purified VWF (5 μg/mL) in the presence of 200 μg/mL VWF fragments SpII or SpIII. (B) Human fibrin surfaces were incubated with WT-rVWF (5 μg/mL), ΔD4B-rVWF (5 μg/mL), and ΔC1C2-rVWF (5 μg/mL) and D1746G-rVWF (5 μg/mL) for 2 hours at ambient temperature. After removing nonbound material, plasma-free blood was perfused at a shear rate of 1500 s–1 over the fibrin surfaces for 3 minutes. Platelet adhesion data are calculated relative to the mean value obtained with fibrin that was incubated with plasma-purified VWF (A) or WT-rVWF (B). Controls are nontreated fibrin layers. Error bars indicate SEM.
The platelet adhesion data from the perfusion experiments with SpII (0.2 mg/mL) and SpIII (0.2 mg/mL) in the presence of VWF (5 μg/mL) are in accordance with their competing potencies as established in the direct binding experiments. That is, SpIII did not affect (P = .53) the VWF-mediated platelet adhesion to fibrin, whereas SpII significantly (P < .002) reduced the number of adherent platelets to the value found in the absence of VWF (Figure 8A). Furthermore, the results of platelet adhesion experiments with fibrin layers that were incubated with SpII or SpIII in the absence of VWF are compatible with those obtained with the competition experiments. That is, fibrin layers incubated with SpII showed even a lower number of adherent platelets when compared with the control, whereas fibrin incubated with SpIII showed a partly reduced platelet adhesion when compared with fibrin incubated with VWF. The effect of SpII can be explained by assuming that SpII prevents the interaction between fibrin and platelet- or plasma-derived VWF that apparently contaminates the plasma-free blood preparation. Because SpII lacks the GP Ib binding site, SpII cannot support platelet adhesion under conditions of high shear stress. SpIII, however, does contain the GP Ib binding site but apparently binds to fibrin with a lower affinity when compared with VWF.
As expected from the competition binding experiments (cf Figure 7), the mutant ΔD4B-rVWF (5 μg/mL) supported platelet adhesion, whereas the mutant ΔC1C2-rVWF (5 μg/mL) did not (Figure 8B). This finding strongly supports the notion of a functional fibrin-binding site on the C-domains of VWF. To examine whether VWF is also necessary for stationary platelet adhesion to fibrin by way of the platelet integrin αIIbβ3, fibrin surface was incubated with the mutant D1746G-rVWF. This VWF mutant is capable of interacting with fibrin but cannot interact with integrin αIIbβ3 because it lacks the RGD sequence. Platelet adhesion to fibrin incubated with D1746G-rVWF (Figure 8B) did not differ from that observed with WT-rVWF (P = .63).
Contribution of plasma-derived VWF to platelet adhesion on fibrin
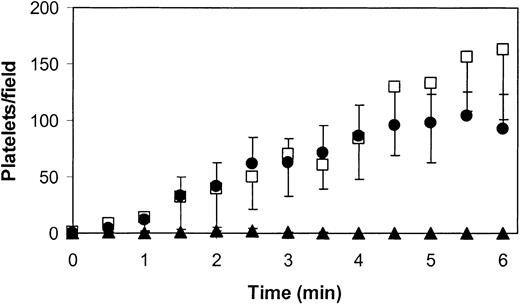
We have previously reported that fibrin layers formed from recalcified, platelet-free plasma perfused at high shear rate over a surface of phospholipid and tissue factor contain sufficient amounts of VWF to support platelet adhesion.13 To find support for the notion that VWF adsorbs to fibrin from circulating plasma rather than is being trapped during clot formation, perfusion experiments were performed with blood and fibrin layers from wild-type and VWF-deficient mice at a shear rate of 1000 s–1. During the perfusion of wild-type blood over wild-type fibrin, the number of platelets that made contact with the fibrin surface increased in time (Figure 9). The majority of these platelets showed stop-and-go movements with an average translocation velocity of 21 ± 9 μm/s. A few adherent platelets (about 5%-10% of total) attained a stationary adhesion. The rather slow initial phase of platelet adhesion suggests that optimal platelet adhesion requires the uptake of VWF from flowing blood. To confirm this notion, wild-type fibrin was perfused with VWF-deficient blood. Under these conditions, platelets did not contact the surface, suggesting that no functional VWF remained attached to the fibrin during its preparation. To further confirm this notion, the complementary experiment was performed in which VWF-deficient fibrin was perfused with healthy blood. Like platelet adhesion on healthy fibrin, a slow increase in the number of translocating platelets (mean translocation velocity of 24 ± 13 μm/s) was observed during the initial phase of the experiment, followed by a more rapid and linear increase in platelets that translocated on the fibrin surface (Figure 9). In addition, a few firmly adherent platelets were observed at the end of the perfusion.
Dependency of platelet adhesion to mouse fibrin on plasma VWF. Platelet-surface contacts are depicted as a function of perfusion time for wild-type mouse blood perfused over wild-type mouse fibrin (•), VWF-deficient mouse blood perfused over wild-type mouse fibrin (▴), and wild-type mouse blood perfused over VWF-deficient mouse fibrin (□). Perfusions were performed at a shear rate of 1000 s–1. Values are mean ± SEM of 3 independent experiments. Error bars indicate SEM.
Dependency of platelet adhesion to mouse fibrin on plasma VWF. Platelet-surface contacts are depicted as a function of perfusion time for wild-type mouse blood perfused over wild-type mouse fibrin (•), VWF-deficient mouse blood perfused over wild-type mouse fibrin (▴), and wild-type mouse blood perfused over VWF-deficient mouse fibrin (□). Perfusions were performed at a shear rate of 1000 s–1. Values are mean ± SEM of 3 independent experiments. Error bars indicate SEM.
Discussion
The main findings of this study are the following: (1) VWF binds specifically and in a saturable manner to fibrin, (2) the fibrin high-affinity binding site on VWF is located on the C-domain, and (3) plasma VWF binding to fibrin by way of its C-domain is essential for platelet adhesion under high shear rate conditions.
We found that VWF binds to polymerized, noncross-linked fibrin with an apparent Kd of 2.2 μg/mL. This value is lower than the Kd of 15 μg/mL reported previously.17 The difference may be attributed to the fact that the present study examined the interaction of VWF with polymerized fibrin, whereas Loscalzo et al17 used soluble fibrin monomer covalently linked to acrylonitrile beads. Specificity of VWF binding to fibrin was confirmed in competition experiments, showing that nonlabeled VWF could displace 125I-VWF bound to fibrin.
Some of the characteristics of the VWF-fibrin interaction reported here seem to contradict earlier findings. First, in contrast to a previous report18 we did not find any indication that fibrin binds preferentially the high-molecular-weight multimers of VWF. The explanation of this difference is probably related to differences in the source of VWF (released from endothelial cells versus plasma derived) and binding conditions (plasma clot versus preformed fibrin). Second, whereas we found that binding of radio-labeled VWF in a plasma environment was not different from that in a buffers system, others15 reported that no binding of VWF could be detected when plasma was perfused over a fibrin surface or when immobilized fibrinogen was incubated with control plasma in an enzyme-linked immunosorbent assay. We note, however, that the conditions of our binding assay differed markedly from that used by others,15 which could explain our apparent conflicting observation.
To establish the localization of the fibrin binding site on the VWF subunit (1-2050), competition experiments of 125I-VWF binding were performed in the presence of unlabeled complementary SpIII and SpII fragments, with mutated rVWF containing deletions or single residue substitution, as well as inhibition studies in the presence of polyclonal antibodies prepared against each of these fragments. Our results point at the C domain of VWF as the major binding site for fibrin, because (1) SpII (residues 1366-2050) was a better competitor of VWF binding to fibrin than SpIII (residues 1-1365), because binding was reduced by 50% in the presence of approximately 6 μg/mL SpII, whereas SpIII at this concentration hardly prevented the binding of VWF; (2) preincubation of VWF with a polyclonal antibody against SpII inhibited the binding of VWF to fibrin by 95%, whereas anti-SpIII had very little effect; and (3) unlike ΔD4B-rVWF, ΔC1C2-rVWF did not compete with r-VWF for binding to fibrin. Because the RGD sequence is present in the C domain, we verified using the D1746G-rVWF that binding did not involve RGD, a finding confirmed by the absence of inhibition of VWF binding to fibrin by an RGDS peptide or the disintegrin kistrin.
We have further substantiated the role of VWF binding to fibrin by way of its C-domain by showing its importance in platelet adhesion at a high shear rate. A 2- to 3-fold increase in platelet adhesion was measured when fibrin was preincubated with full-length rVWF or ΔD4B-rVWF, whereas a preincubation with ΔC1C2-rVWF did not result in a significant increase (P = .21). In separate experiments, we verified that ΔC1C2-rVWF contained a functional GP Ib binding site, because both WT-rVWF and ΔC1C2-rVWF competed equally well with 125I-VWF for binding to GP Ib (data not shown). The fact that after a 3-minute perfusion with plasma-free blood cells a substantial number of adherent platelets were observed on the control fibrin surface suggests that the plasma-free blood preparation contains either plasma VWF or platelet VWF that is released during the washing procedure.
It has been reported that VWF competes with fibrin for occupancy of activated integrin αIIb β3.31 To re-investigate the role of VWF interaction with the integrin αIIbβ3 in platelet adhesion to fibrin at high shear rate, the VWF mutant D1746G-VWF was used. D1746G-VWF was as effective as rVWF in platelet adhesion on fibrin under conditions of high shear. This finding is compatible with the notion that the fibrin binding site on VWF does not overlap with its integrin αIIbβ3 binding site and, therefore, allows fibrin-bound VWF to compete with fibrin for binding to platelet integrin αIIbβ3.
To establish the role of plasma VWF in platelet adhesion to fibrin at high shear rates, experiments were performed with blood and fibrin from wild-type and VWF-deficient mice. Indeed, when VWF-deficient blood was perfused at high shear rate over a fibrin layer prepared from VWF-deficient plasma, no translocating or firmly adherent platelets were detected on fibrin. In contrast, numerous translocating platelets were observed when wild-type blood was perfused over wild-type fibrin. Surprisingly, the number of surface-contacting platelets increased relatively slowly during the first minutes of the perfusion. It appeared that fibrin prepared from wild-type mouse plasma does not contain functional VWF, because no platelets adhered to this fibrin when perfused with blood from VWF-deficient mouse. As expected, a similar delay in platelet adhesion was observed when fibrin prepared from VWF-deficient mouse plasma was perfused with wild-type mouse blood. It is apparent that under high shear rate conditions optimal platelet adhesion is achieved when sufficient amounts of plasma VWF are adsorbed on fibrin.
The perfusion experiments with mouse blood and mouse fibrin also disclose that, besides increasing numbers of translocating platelets in time, the number of stationary platelets increased, as well. The finding that the majority of platelets show translocation, suggests that under the conditions of this experiment, integrin αIIbβ3 is not activated and, thus, refers to an earlier observation that integrin αIIbβ3 needs to be activated before platelets can irreversibly bind to fibrin.32
In conclusion, this study demonstrates that the major binding site on VWF for fibrin resides in the C-domains between amino acids 1637 and 1899 at a site that does not overlap the RGD sequence. The relevance of the VWF-fibrin interaction in platelet adhesion at a high shear rate was demonstrated in perfusion experiments with human and mouse blood over fibrin layers in the presence and absence of VWF. Collectively, our results point at a dual role of fibrin-bound VWF during thrombus growth, which might add to a better understanding of the contribution of VWF to the formation of fibrin-rich thrombi at a high shear rate.33 We suggest that fibrin-bound VWF present at an injured vessel wall or disrupted atherosclerotic plaque may critically contribute to thrombus growth by tethering (nonactivated) platelets from fast flowing blood. Subsequently, VWF bound to fibrin would stabilize the fibrin-rich thrombus by bridging more platelets through activated integrin αIIbβ3. Therefore, drugs that hamper fibrin-VWF interaction could be effective antithrombotic agents, not causing hemorrhagic tendency because platelet interactions with extracellular matrix proteins would be preserved.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-07-2267.
Supported by INSERM and the Dutch Research Organization ZonMw-NWO (grant 910-408-027) (D.B., T.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs P. G. de Groot and J. W. M. Heemskerk for helpful discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal