Abstract
Although both P- and E-selectin are constitutively expressed on bone marrow endothelial cells, their role in the regulation of hematopoiesis has only recently been investigated. We have previously shown that P-selectin glycoprotein ligand-l (PSGL-1/CD162) is expressed by primitive human bone marrow CD34+ cells, mediates their adhesion to P-selectin, and, more importantly, inhibits their proliferation. We now demonstrate that adhesion to E-selectin inhibits the proliferation of human CD34+ cells isolated either from human umbilical cord blood, adult mobilized blood, or steady-state bone marrow. Furthermore, a subpopulation, which does not contain the most primitive hematopoietic progenitor cells, undergoes apoptosis following E-selectin–mediated adhesion. The same phenomenon was observed in cells isolated from mouse bone marrow. Using lineage-negative Sca-1+ c-KIT+ bone marrow cells from PSGL-1–/– and wild-type mice, we establish that PSGL-1 is not the ligand involved in E-selectin–mediated growth inhibition and apoptosis. Moreover, stable transfection of the human myeloid cell line K562 (which does not express PSGL-1) with α(1,3) fucosyltransferase VII alone was sufficient to recapitulate the E-selectin–mediated growth inhibition and apoptosis observed in hematopoietic progenitor cells. These data demonstrate that an E-selectin ligand(s) other than PSGL-1 transduces growth inhibitory and proapoptotic signals and requires posttranslational fucosylation to be functional.
Introduction
The hematopoietic compartment of bone marrow contains a heterogenous population of cells at various stages of differentiation, including hematopoietic stem cells and their more committed progeny, in close contact with bone marrow (BM) stromal cells.1 Adhesive interactions are critical to the localization of these cells within the BM hematopoietic microenvironment and can transduce regulatory signals involved in proliferation, differentiation, and survival.2,3 The selectin family of adhesion molecules (CD62) plays a major role in leukocyte trafficking to lymph nodes and the rolling and recruitment of leukocytes to sites of tissue damage and inflammation.4 Selectins are type I transmembrane proteins with an N-terminal C-type lectin domain capable of interacting with a number of different glycoprotein ligands. Leukocyte selectin or L-selectin (CD62L) is constitutively expressed by the majority of circulating leukocytes and is critical for homing of lymphocytes to lymph nodes.5,6 Endothelial selectin or E-selectin (CD62E) is transcriptionally regulated and is surface expressed several hours after exposure to specific inflammatory mediators.7 Platelet selectin or P-selectin (CD62P) is stored in secretory storage granules of both platelets and endothelial cells and is translocated to the surface within minutes of activation.8 Interestingly, both E- and P-selectin are permanently expressed on BM endothelial cells9,10 where they mediate rolling of hematopoietic stem and progenitor cells (HPCs) on BM endothelium,11,12 a critical step in HPC homing and engraftment into the BM.11,13,14
P-selectin glycoprotein ligand-1 (PSGL-1/CD162) is the sole P-selectin ligand on human HPCs15 and our group has shown that PSGL-1–mediated adhesion to P-selectin results in growth inhibition and apoptosis of human BM–derived primitive CD34+ HPCs.15 We now demonstrate that both human and murine HPCs adhere to immobilized recombinant E-selectin and that E-selectin–mediated adhesion results in growth inhibition and apoptosis of a subpopulation of HPCs through a novel receptor that is not PSGL-1.
Materials and methods
Mice
C57BL6/J mice were purchased from the Animal Resource Centre (Perth, Australia). PSGL-1–/– mice (backcrossed 5 times into C57BL6/J) and their wild-type littermates were kindly provided by Drs Barbara Furie and Bruce Furie (Beth Israel Hospital, Boston, MA).
Isolation and purification of HPCs
Human CD34+ cells were isolated from normal adult BM, mobilized peripheral blood following 5 days granulocyte colony-stimulating factor (G-CSF) administration (10 μg/kg/d), or umbilical cord blood. CD34+ cells were selected using CD34-conjugated Dynabeads (Dynal, Oslo, Norway) as previously described.15 Purity (> 98%) was assessed by flow cytometry with fluorescein isothiocyanate (FITC)–conjugated mouse antihuman CD34 (HPCA-2; Becton Dickinson, San Jose, CA).
Mouse BM cells were collected from the femurs of 10-week-old female C57BL6/J mice. Lineage-positive cells were removed using magnetic bead depletion with biotinylated lineage monoclonal antibodies (mAbs) as previously described.16 Lineage-depleted cells were stained with FITC-conjugated rat anti–Sca-1 (Ly-6A, clone E13-161.7), phycoerythrin (PE)–conjugated rat antimouse c-KIT (CD117, clone 2B8), and Red670-conjugated streptavidin and sorted for the lineage-negative (lineageneg) Sca-1+ c-KIT+ subpopulation.16
K562 cells stably expressing human PSGL-1 or FucT-VII
The generation of K562 cells stably transfected with human fucosyltransferase VII (FucT-VII), human PSGL-1, or both has been described.17 Briefly, cDNAs for PSGL-1 and FucT-VII were subcloned into a modified SRα mammalian expression vector containing a neomycin resistance (neoR) or hygromycin resistance (hygroR) cassette and used to transfect K562 cells by electroporation. Following selection with either G418 (Geneticin; Invitrogen, Carlsbad, CA) or Hygromycin B (Calbiochem, La Jolla, CA), cells expressing both PSGL-1 and FucT-VII were sorted by 2-color flow cytometry using KPL1 (a mouse antihuman PSGL-1 immunoglobulin G1 (IgG1) monoclonal antibody)18 and the rat anticutaneous lymphocyte antigen HECA-452 (IgM isotype), which recognizes an epitope associated with FucT-VII activity.19 Neither KPL1 nor HECA-452 binds to untransfected K562 cells.
Plate coating and adhesion assays
Recombinant human and murine E-selectin–IgG1 Fc fusion protein (rhu and rmu E-selectin–Fc), recombinant human and murine P-selectin–IgG1 Fc fusion protein (rhu and rmu P-selectin–Fc), and recombinant human CD14-IgG1 Fc fusion protein (CD14-Fc) were purchased from R&D Systems (Minneapolis, MN). Native human P-selectin purified from platelets20 was kindly donated by Dr Michael C. Berndt (Monash University, Melbourne, Australia). Tissue culture wells (96-well plates) were coated with various concentrations of selectins or controls (0-10 μg/mL) for 16 hours at 4°C in 20 mM Tris-buffered saline (pH 7.4) with 1 mM CaCl2. Unbound selectin was removed and wells were blocked for 1 hour at room temperature and washed 4 times with Iscove modified Dulbecco medium with 1% bovine serum albumin (BSA).
Site density of plastic-bound recombinant selectin–IgG1 fusion proteins was determined by saturation binding using 125I–protein G (Amersham, Little Chalfont, United Kingdom). Selectin-coated and blocked wells were incubated for 1 hour with 1 μg/mL 125I–protein G of known specific activity in phosphate-buffered saline (PBS) with 1% BSA. Following extensive washing, wells were treated with 0.1 M NaOH, 1% sodium dodecyl sulfate (SDS) for 10 minutes, and the selectin densities per μm2 were calculated from the radioactivity of the harvested wells.
Adhesion of human CD34+ cells, mouse lineageneg Sca-1+ c-KIT+ cells, or K562 transfectants to P- or E-selectin was performed as previously described.15 Briefly, cells were labeled for 40 minutes at 37°C with 10 μM calcein-AM (Molecular Probes, Eugene, OR) and 20 000 labeled cells were added to selectin-coated wells. Following sedimentation by centrifugation, cells were incubated for 30 minutes at 4°C, washed 4 times, and attached cells were lysed in 1% SDS. The fluorescence of each well was quantified using a fluorescence plate reader (Molecular Imager FX; Biorad, Hercules, CA). In some experiments, cells were incubated with either 10 μg/mL mouse antihuman PSGL-1 antibody KPL1,18 10 μg/mL mouse IgG1 isotype control, 200 μg/mL O-sialoglycoprotein endopeptidase (Cedarlane Laboratories, Hornby, ON, Canada), or 10 μg/mL human neutrophil elastase and 20 μg/mL human cathepsin G (Elastin Products, Owensville, MO) for 60 minutes at either 0°C (for antibodies) or 37°C (for proteases) prior to the adhesion assay.
Proliferation assay, TUNEL, and pre–colony-forming cell assays
Plates (96 well) were coated with selectin, CD14-Fc, or BSA as described in “Plate coating and adhesion assays.” Sorted human cells were resuspended in serum-deprived medium21 supplemented with cytokines (10 ng/mL rhu interleukin 3 [rhuIL-3], 10 ng/mL rhuIL-6, 100 ng/mL rhuG-CSF, 100 ng/mL rhu stem cell factor [rhuSCF]) and cultured at 1000 cells/well in triplicate. Following centrifugation for 5 minutes at 200g to bring cells in contact with the precoated surface, plates were cultured at 37°Cin5%CO2. At day 7, cells were counted and an aliquot taken from each well for TUNEL (terminal deoxynucleotidyl transferase [TdT]–mediated deoxyuridine triphosphate [dUTP] nick end labeling) assay as previously described.15 For pre–colony-forming cell (pre-CFC) assays, cells were subcultured every 7 days, and after 21 days, methylcellulose clonogenic assays were performed in the presence of IL-3, IL-6, granulocytemacrophage colony-stimulating factor (GM-CSF), G-CSF, and SCF as previously described.15
Mouse lineageneg Sca-1+ c-KIT+ cells were resuspended in serum-deprived medium supplemented with various cytokine combinations: 36GS (rmuIL-3 10 ng/mL, rhuIL-6 10 ng/mL, rhuG-CSF 100 ng/mL, recombinant rat SCF [rrSCF] 100 ng/mL), 11FTS (rhuIL-11 10 ng/mL, rhuFlt-3L 100 ng/mL, rhu thrombopoietin 100 ng/mL, rrSCF 100 ng/mL), or 3611FTS (rmuIL-3, rhuIL-6, rhuIL-11, rhuFlt-3L, rhu thrombopoietin, rrSCF as described above). Cells were cultured in precoated wells at 500 cells/well and counted and subcultured every 5 to 7 days. Clonogenic assays were performed in methylcellulose with IL-3, G-CSF, GM-CSF, SCF, IL-6, and erythropoietin (EPO), and total colonies were determined after culturing for 12 days.22 The clonogenic efficiency is calculated as the number of colonies divided by the number of cells put in methylcellulose following 14 days culture (expressed as a percentage).
Statistical analyses
Statistical analyses were performed with a paired Student t test using the BSA controls for each treatment group. P values less than .05 were considered statistically significant.
Results
Human CD34+ HPCs adhere to E-selectin in a dose-dependent manner
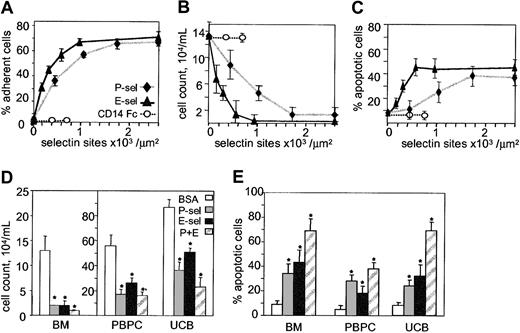
To determine whether primitive hematopoietic cells adhere to E-selectin, CD34+ cells purified from mobilized peripheral blood were incubated for 30 minutes on microwells coated with increasing concentrations of selectin or controls. Wells coated with CD14-Fc control at 5 and 10 μg/mL resulted in CD14-Fc site densities of 400 and 650 sites/μm2. Wells coated with recombinant E-selectin–Fc at 1, 3, 5, or 10 μg/mL resulted in E-selectin site densities of 175, 280, 550, and 900/μm2, respectively. Interestingly, P-selectin absorbed more strongly to wells, coating with 1, 3, 5, and 10 μg/mL resulting in P-selectin site densities of 400, 1100, 1800, and 2600/μm2. Adhesion of CD34+ cells to selectins was found to be dose-dependent with 50% adhesion occurring at 400 E-selectin sites/μm2 and to P-selectin at 800 sites/μm2 (Figure 1A).
Human CD34+ HPCs adhere to P- and E-selectins resulting in growth inhibition and apoptosis. (A) Dose-dependent adhesion of CD34+ HPCs to microwells coated with increasing concentrations of CD14-Fc control or E- and P-selectin–Fc. (B) BM CD34+ proliferation at day 7 of culture in the presence of 36GS on microwells coated with increasing concentrations of CD14-Fc (○), E-selectin-Fc (▴) or P-selectin-Fc (♦). Cells were seeded at 104/mL. (C) Apoptosis of BM CD34+ cells at day 7 of culture in the presence of 36GS on microwells coated with increasing concentrations of CD14-Fc, E-selectin–Fc, or P-selectin–Fc. (D) Proliferation of CD34+ cells isolated from steady-state BM, mobilized peripheral blood (PBPC), or umbilical cord blood (UCB) on microwells coated with BSA (10 μg/mL), P-selectin (1800 sites/μm2), or E-selectin (550 sites/μm2) alone or in combination (P+E). Cells (104/mL) were seeded at day 0 in the presence of 36GS and counted on day 7. *indicates statistically different (P < .05) from BSA-treated control group. (E) Apoptosis of CD34+ cells isolated from steady-state BM, mobilized peripheral blood, or umbilical cord blood on microwells coated as described for panel D with BSA, P- or E-selectin alone, or in combination. Cells were grown in the presence of 36GS and TUNEL assays performed on day 7. (A-C) Data are expressed as a mean ± SD of one representative experiment. (D-E) Data are expressed as mean ± SEM for 3 independent experiments on BM, peripheral blood, or umbilical cord blood CD34+ HPCs. * indicates statistically different (P < .05) from BSA control groups.
Human CD34+ HPCs adhere to P- and E-selectins resulting in growth inhibition and apoptosis. (A) Dose-dependent adhesion of CD34+ HPCs to microwells coated with increasing concentrations of CD14-Fc control or E- and P-selectin–Fc. (B) BM CD34+ proliferation at day 7 of culture in the presence of 36GS on microwells coated with increasing concentrations of CD14-Fc (○), E-selectin-Fc (▴) or P-selectin-Fc (♦). Cells were seeded at 104/mL. (C) Apoptosis of BM CD34+ cells at day 7 of culture in the presence of 36GS on microwells coated with increasing concentrations of CD14-Fc, E-selectin–Fc, or P-selectin–Fc. (D) Proliferation of CD34+ cells isolated from steady-state BM, mobilized peripheral blood (PBPC), or umbilical cord blood (UCB) on microwells coated with BSA (10 μg/mL), P-selectin (1800 sites/μm2), or E-selectin (550 sites/μm2) alone or in combination (P+E). Cells (104/mL) were seeded at day 0 in the presence of 36GS and counted on day 7. *indicates statistically different (P < .05) from BSA-treated control group. (E) Apoptosis of CD34+ cells isolated from steady-state BM, mobilized peripheral blood, or umbilical cord blood on microwells coated as described for panel D with BSA, P- or E-selectin alone, or in combination. Cells were grown in the presence of 36GS and TUNEL assays performed on day 7. (A-C) Data are expressed as a mean ± SD of one representative experiment. (D-E) Data are expressed as mean ± SEM for 3 independent experiments on BM, peripheral blood, or umbilical cord blood CD34+ HPCs. * indicates statistically different (P < .05) from BSA control groups.
Inhibition of proliferation following adhesion of CD34+ HPCs to E-selectin
Bone marrow CD34+ cells were cultured for 7 days on microwells coated with BSA or increasing concentrations of CD14-Fc, P- or E-selectin–Fc in the presence of IL-3, IL-6, G-CSF, and SCF (36GS), a cocktail of cytokines providing efficient stimulus for CD34+ proliferation in stroma-free suspension culture.23 A dose-dependent inhibition of proliferation was observed, with maximum inhibition in wells coated with 550 E-selectin sites/μm2 or 1800 P-selectin sites/μm2 (Figure 1B). The effects of immobilized selectin were specific as no inhibition of growth was observed in wells coated with BSA alone or with CD14-Fc control. Human CD34+ HPCs grown on BSA control wells expanded 13-fold after 7 days incubation, whereas those grown on either E-selectin– or P-selectin–coated wells only doubled in cell number during this time (Figure 1D).
To determine whether P- and E-selectin growth inhibition occurred in primitive hematopoietic cells from other sources, we examined the proliferation of CD34+ cells isolated from umbilical cord blood and mobilized peripheral blood. Cells were cultured in microtiter wells coated with 1800 P-selectin sites/μm2, 550 E-selectin sites/μm2, both P- and E-selectin, or BSA alone (Figure 1D). Although selectin-mediated growth inhibition was observed in CD34+ cells from all 3 sources (P < .01; n = 12), maximum inhibition was noted for CD34+ HPCs purified from steady-state BM.
Importantly, the proliferation of BM CD34+ cells incubated in noncoated wells containing 5 μg/mL soluble E-selectin–Fc was not inhibited (data not shown), demonstrating that the E-selectin–mediated growth inhibition is not due to toxicity of the recombinant E-selectin–Fc protein and that clustering of E-selectin by immobilization is necessary for the growth inhibitory effects of this molecule.
Induction of apoptosis following adhesion of CD34+ HPCs to E-selectin
The inhibition of CD34+ HPC proliferation by P- or E-selectin may result from the initiation of programmed cell death, inhibition of cell division, or a combination of both. To test whether selectin-mediated adhesion induced apoptosis, cells were incubated for 7 days on either immobilized P-selectin, E-selectin, BSA, or CD14-Fc and the number of apoptotic cells was measured using TUNEL assay. A dose-dependent increase in apoptosis was observed in cells incubated on either P- or E-selectin (Figure 1C), with a 4-fold increase in apoptosis observed when wells were coated with E-selectin (550 sites/μm2) or P-selectin (1800 sites/μm2) (P < .01; n = 6). Although selectin-mediated apoptosis was observed in CD34+ HPCs purified from all sources tested, the effect was most pronounced in cells purified from steady-state BM and the least pronounced in cells from umbilical cord blood (Figure 1E). The percentage of apoptotic cells was markedly increased when cells were incubated in wells coated with both selectins together, demonstrating an additive effect of P- and E-selectin. No increase in apoptosis was observed when cells were cultured in uncoated wells in the presence of 5 μg/mL soluble E-selectin–Fc (data not shown).
Adhesion to E-selectin does not induce apoptosis of primitive human HPCs
Although inducing apoptosis in 30% to 40% of CD34+ HPCs (Figure 1C-E), 60% to 70% of the cells survived culture on immobilized P- or E-selectin. Surviving cells were transferred to noncoated wells, cultured in 36GS culture media for an additional 14 days, and assayed in clonogenic assays.23,24 No significant difference in pre-CFCs was evident between cells cultured on P-selectin, E-selectin, or BSA after 21 days liquid culture (BSA 30.7 ± 6.6 pre-CFCs per original cell, P-selectin 27.8 ± 2.9, and E-selectin 26.4 ± 4.2; mean ± SEM of 3 independent experiments), indicating that the more primitive HPCs (pre-CFCs) did not undergo apoptosis after being cultured for one week in the presence of E- or P-selectin. These data show that a subpopulation of CD34+ HPCs undergoes apoptosis when cultured on immobilized P- or E-selectin but the hierarchically more primitive pre-CFCs are spared.
E-selectin–induced apoptosis is mediated by fucosylated receptors in the absence of PSGL-1
To further characterize the nature of E-selectin receptors expressed by human HPCs, CD34+ cells were preincubated with either the function-blocking antihuman PSGL-1 mAb KPL1,18 O-sialoglycoprotein endopeptidase (an endoprotease that specifically cleaves the protein core of sialomucins downstream of their sialylated mucin attachments), or a combination of 2 neutrophil serine proteases, neutrophil elastase and cathepsin G. Adhesion to P-selectin was abolished by pretreatment with either KPL1, O-sialoglycoprotein endopeptidase, or neutrophil elastase together with cathepsin G, demonstrating that this adhesion is solely mediated by PSGL-1, a sialomucin cleaved by O-sialoglycoprotein endopeptidase,25 as well as neutrophil elastase and cathepsin G26 (Figure 2). In contrast, adhesion to E-selectin (although partially sensitive to the serine proteases, neutrophil elastase, and cathepsin G) was resistant to KPL1 or O-sialoglycoprotein endopeptidase pretreatment, suggesting that E-selectin binds to CD34+ cells through glycoproteins either distinct from PSGL-1 or on a region of PSGL-1 more proximal to the transmembrane domain (Figure 2).
Differing adhesion of human CD34+ cells to P- and E-selectin in the presence of function-blocking antibody to PSGL-1 or the proteases O-sialoglycoprotein endopeptidase, neutrophil elastase, and cathepsin G. CD34+ HPCs were preincubated with either isotype control mAb, KPL1 mAb, O-sialoglycoprotein endopeptidase (OSG), or a combination of neutrophil elastase and cathepsin G (NE+CG) and added to microwells coated with 10 μg/mL BSA, P-selectin (1800 sites/μm2), or E-selectin (550 sites/μm2). Results are expressed as mean ± SD of one representative experiment from 3 made in triplicate. *indicates statistically different (P < .05) from isotype-treated control groups.
Differing adhesion of human CD34+ cells to P- and E-selectin in the presence of function-blocking antibody to PSGL-1 or the proteases O-sialoglycoprotein endopeptidase, neutrophil elastase, and cathepsin G. CD34+ HPCs were preincubated with either isotype control mAb, KPL1 mAb, O-sialoglycoprotein endopeptidase (OSG), or a combination of neutrophil elastase and cathepsin G (NE+CG) and added to microwells coated with 10 μg/mL BSA, P-selectin (1800 sites/μm2), or E-selectin (550 sites/μm2). Results are expressed as mean ± SD of one representative experiment from 3 made in triplicate. *indicates statistically different (P < .05) from isotype-treated control groups.
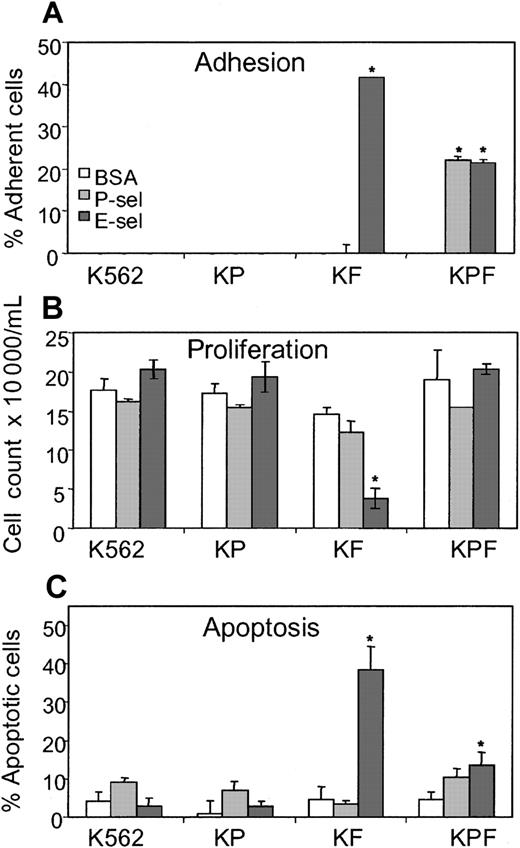
To test the possibility that glycoproteins other than PSGL-1 could be E-selectin ligands on human HPCs, we used the K562 human myeloid cell line that expresses neither PSGL-1 nor FucT-VII, an α(1,3) fucosyltransferase responsible for posttranslational modifications necessary to the generation of functional P- and E-selectin ligands.17,27 K562 cells were transfected with either FucT-VII (KF cells), PSGL-1 (KP cells), or both PSGL-1 and FucT-VII (KPF cells). Adhesion assays confirmed that KF transfectants adhered to E-selectin and that both PSGL-1 and FucT-VII (KPF cells) were required for adhesion to P-selectin (Figure 3A and Snapp et al17 ). As expected, K562 and KP cells did not adhere to selectin nor did they exhibit any selectin-mediated growth inhibition or apoptosis (Figure 3B-C). Importantly, the transfection of FucT-VII alone (KF cells) was sufficient to recapitulate the growth inhibition and apoptosis following adhesion to E-selectin as observed with normal human CD34+ HPCs. Interestingly KPF cells did not exhibit marked growth inhibition or apoptosis when incubated on either P- or E-selectin, although adhesion did occur (Figure 3A-C). This lack of inhibition is possibly due to lower levels of FucT-VII in the KPF cells as determined by semiquantitative reverse transcriptase–polymerase chain reaction (RT-PCR),17 which would explain why decreased adhesion to E-selectin is observed in the KPF cells compared with the KF cells (Figure 3A). Taken together, these data show that a common cell surface protein(s) present on human myeloid cells can become an E-selectin ligand(s) mediating adhesion, growth inhibition, and apoptosis, following fucosylation by FucT-VII in the absence of PSGL-1.
K562 cells stably transfected with FucT-VII exhibit E-selectin-mediated growth inhibition and apoptosis in the absence of PSGL-1. (A) Adhesion of K562 alone or transfected with PSGL-1 (KP cells), FucT-VII (KF cells), or both (KPF cells) to microwells coated with P-selectin (1800 sites/μm2) or E-selectin (550 sites/μm2). (B) Proliferation of K562, KP, KF, and KPF cells grown in serum-deprived medium on microwells coated with BSA, P-selectin, or E-selectin. Cells were seeded at 104/mL and counted after 5 days incubation. (C) Apoptosis of K562, KP, KF, and KPF cells grown in serum-deprived medium on microwells coated with 10μg/mL BSA, P-selectin, or E-selectin. TUNEL assay was performed after 5 days incubation. *indicates statistically different (P < .05) from BSA control groups. Results are expressed as mean ± SEM of 3 independent experiments made in triplicate.
K562 cells stably transfected with FucT-VII exhibit E-selectin-mediated growth inhibition and apoptosis in the absence of PSGL-1. (A) Adhesion of K562 alone or transfected with PSGL-1 (KP cells), FucT-VII (KF cells), or both (KPF cells) to microwells coated with P-selectin (1800 sites/μm2) or E-selectin (550 sites/μm2). (B) Proliferation of K562, KP, KF, and KPF cells grown in serum-deprived medium on microwells coated with BSA, P-selectin, or E-selectin. Cells were seeded at 104/mL and counted after 5 days incubation. (C) Apoptosis of K562, KP, KF, and KPF cells grown in serum-deprived medium on microwells coated with 10μg/mL BSA, P-selectin, or E-selectin. TUNEL assay was performed after 5 days incubation. *indicates statistically different (P < .05) from BSA control groups. Results are expressed as mean ± SEM of 3 independent experiments made in triplicate.
Murine HPCs adhere to E-selectin, resulting in inhibition of proliferation and increased apoptosis
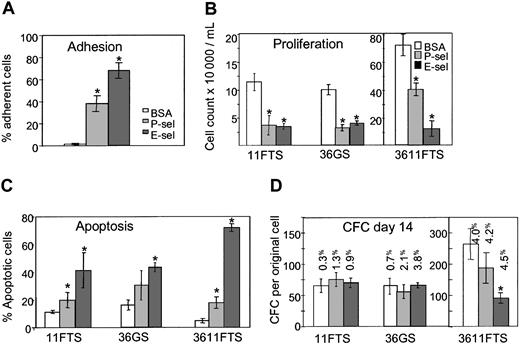
Similar experiments were performed using murine HPCs. Lineageneg Sca-1+ c-KIT+ cells, which contain all long-term reconstituting cells,28 were sorted from steady-state BM of C57BL6/J mice and incubated on immobilized rmu E-selectin–Fc, rmu P-selectin–Fc, or BSA. Around 72% of murine lineageneg Sca-1+ c-KIT+ cells adhered to the E-selectin–coated wells (Figure 4A). Following 7 days incubation on immobilized E-selectin, a decrease in cell proliferation with a corresponding increase in the proportion of apoptotic cells was observed, irrespective of the stimulating cytokine combination used (36GS, 11FTS, or 3611FTS; Figure 4B-C). However, 3611FTS was the most potent cytokine combination to promote E-selectin–mediated apoptosis, with 75% of cells grown on E-selectin–coated wells dying compared with only 5% in BSA-coated wells (Figure 4C). Surviving cells were removed from immobilized selectins or BSA at day 7, cultured in suspension for an additional 7 days without selectins, and plated in clonogenic assays to determine the number of pre–colony-forming cells derived from one original lineageneg Sca-1+ c-KIT+ cell (Figure 4D). Cultures on immobilized P- or E-selectin did not alter the number of pre-CFCs in the presence of either 11FTS or 36GS, demonstrating that these primitive cells were not among the 40% to 45% that went through apoptosis. However, culture on E-selectin, in the presence of the 3611FTS cytokine combination, resulted in greater apoptosis and, although the cloning efficiency of each well was similar (4.0% for BSA, 4.2% for E-selectin, 4.5% for P-selectin), the number of pre-CFCs derived from one original lineageneg Sca-1+ c-KIT+ cell was reduced when compared with cultures grown on BSA. These results suggest that the presence of the 6 stimulatory cytokines (3611FTS), which enhances cell cycling, leads to greater expansion of pre-CFCs and renders them more susceptible to the growth inhibitory and proapoptotic effects of E-selectin.
E-selectin inhibits proliferation and induces apoptosis of mouse HPCs. (A) Adhesion of murine BM lineageneg Sca-1+ c-KIT+ (LSK) cells to immobilized BSA, rmu P-selectin (1800 sites/μm2), or rmu E-selectin (550 sites/μm2). (B) Proliferation of murine BM LSK cells grown in the presence of cytokine combinations 11FTS, 36GS, or 3611FTS on microwells coated with BSA, P-selectin, or E-selectin. Cells were seeded at 104/mL and counted after 7 days incubation in serum-deprived medium. (C) Apoptosis of murine BM LSK cells cultured 7 days in the presence of 11FTS, 36GS, or 3611FTS on microwells coated with BSA, P-selectin, or E-selectin. (D) Pre–colony-forming cell assay. Bone marrow LSK cells were cultured in the presence of 11FTS, 36GS, or 3611FTS on microwells coated with BSA, P-selectin, or E-selectin. After 7 days incubation, cells were transferred to fresh noncoated wells and cultured an additional 7 days before colony assays were performed. The results are expressed as the number of colonies derived from a single original LSK cell. The clonogenic efficiencies (expressed as a percentage) of the cells put into methylcellulose are indicated above each column. *Statistically different (P < .05) from BSA treated control groups. Results are expressed as mean ± SEM of 3 (D) or 5 (A-C) independent experiments made in triplicate.
E-selectin inhibits proliferation and induces apoptosis of mouse HPCs. (A) Adhesion of murine BM lineageneg Sca-1+ c-KIT+ (LSK) cells to immobilized BSA, rmu P-selectin (1800 sites/μm2), or rmu E-selectin (550 sites/μm2). (B) Proliferation of murine BM LSK cells grown in the presence of cytokine combinations 11FTS, 36GS, or 3611FTS on microwells coated with BSA, P-selectin, or E-selectin. Cells were seeded at 104/mL and counted after 7 days incubation in serum-deprived medium. (C) Apoptosis of murine BM LSK cells cultured 7 days in the presence of 11FTS, 36GS, or 3611FTS on microwells coated with BSA, P-selectin, or E-selectin. (D) Pre–colony-forming cell assay. Bone marrow LSK cells were cultured in the presence of 11FTS, 36GS, or 3611FTS on microwells coated with BSA, P-selectin, or E-selectin. After 7 days incubation, cells were transferred to fresh noncoated wells and cultured an additional 7 days before colony assays were performed. The results are expressed as the number of colonies derived from a single original LSK cell. The clonogenic efficiencies (expressed as a percentage) of the cells put into methylcellulose are indicated above each column. *Statistically different (P < .05) from BSA treated control groups. Results are expressed as mean ± SEM of 3 (D) or 5 (A-C) independent experiments made in triplicate.
HPCs from PSGL-1–/– mice are still sensitive to E-selectin–mediated growth inhibition and apoptosis
PSGL-1/CD162 is the sole P-selectin ligand on human CD34+ HPCs.15 PSGL-1 has been reported to bind both P- and E-selectins,29,30 therefore it is possible that the signaling events initiated by adhesion to either P- or E-selectin are mediated through the same cell surface receptor, PSGL-1. To test this possibility, lineageneg Sca-1+ c-KIT+ cells were purified from the BM of PSGL-1–/– mice and from control wild-type mice and cultured for 5 days in serum-deprived medium with 3611FTS in the presence of immobilized rmu P-selectin–Fc, rmu E-selectin–Fc, or BSA. As anticipated, PSGL-1–/– lineageneg Sca-1+ c-KIT+ cells did not attach to P-selectin (data not shown), their proliferation was not inhibited, and they did not undergo apoptosis when cultured on immobilized P-selectin, demonstrating that as in humans, PSGL-1 is the sole P-selectin receptor expressed by mouse HPCs (Figure 5). In sharp contrast, PSGL-1–/– lineageneg Sca-1+ c-KIT+ cells still attached normally to E-selectin (data not shown) with growth inhibition and induction of apoptosis in 70% ± 8% of the cells, similar to lineageneg Sca-1+ c-KIT+ cells from control wild-type mice (Figure 5). These data conclusively demonstrate that both the induction of apoptosis and the inhibition of proliferation observed in mouse HPCs following adhesion to E-selectin is mediated by receptor(s) that are not PSGL-1.
E-selectin inhibits proliferation and induces apoptosis of HPCs from PSGL-1–/– mice. Bone marrow lineageneg Sca-1+ c-KIT+ (LSK) sorted cells from PSGL-1–/– and wild-type control mice were cultured for 5 days in the presence of 6 cytokines (3611FTS) on wells coated with either BSA (□), rmu P-selectin–Fc at 1800 sites/μm2 (▦), or rmu E-selectin–Fc at 550 sites/μm2 (▪). Concentrations of viable cells and percentage of apoptotic cells are indicated in panels A and B, respectively. Mean values ± SD of 2 experiments made in triplicate. *Statistically different (P < .05) from BSA control groups.
E-selectin inhibits proliferation and induces apoptosis of HPCs from PSGL-1–/– mice. Bone marrow lineageneg Sca-1+ c-KIT+ (LSK) sorted cells from PSGL-1–/– and wild-type control mice were cultured for 5 days in the presence of 6 cytokines (3611FTS) on wells coated with either BSA (□), rmu P-selectin–Fc at 1800 sites/μm2 (▦), or rmu E-selectin–Fc at 550 sites/μm2 (▪). Concentrations of viable cells and percentage of apoptotic cells are indicated in panels A and B, respectively. Mean values ± SD of 2 experiments made in triplicate. *Statistically different (P < .05) from BSA control groups.
Discussion
Although both P- and E-selectin are constitutively expressed on BM endothelial cells,9,10 their role in the regulation of hematopoiesis has only recently been investigated. Previous studies have demonstrated that primitive HPCs in humans and rodents are capable of rolling on and adhering to E- and P-selectin in vivo and in vitro12,31-33 and that both E- and P-selectin–mediated rolling, together with integrin α4β1-mediated adhesive interactions, are critical in HPCs homing into the BM.11,13,14 Our group was the first to demonstrate that PSGL-1 on CD34+ human HPCs functions as both an adhesion molecule and an inhibitor of HPC proliferation following engagement with P-selectin.15 Since mice deficient in a single endothelial selectin exhibit a very mild phenotype, whereas mice deficient in both P- and E-selectin exhibit severe leukocytosis and enhanced hematopoiesis in the BM and spleen,34,35 we examined whether E-selectin could independently mediate a similar inhibition of HPC proliferation.
In this study, we demonstrate that both human CD34+ and mouse lineageneg Sca-1+ c-KIT+ HPCs adhere to immobilized recombinant E-selectin. Furthermore, adhesion of human or mouse HPCs to E-selectin results in profound inhibition of proliferation as well as apoptosis of a proportion of primitive human CD34+ cells and mouse lineageneg Sca-1+ c-KIT+ cells. Interestingly, among human HPCs, the effect was more pronounced with adult BM cells than with mobilized peripheral blood or umbilical cord blood HPCs. This difference may be due to the lower adhesiveness of umbilical cord blood cells to P- and E-selectin compared with adult cells as reported by Hidalgo et al.13 It is also possible that accelerated cell cycling enhances E-selectin–mediated growth inhibition and apoptosis. This would explain why mobilized peripheral blood CD34+ cells, which are deeply quiescent compared with steady-state BM CD34+ HPCs,36,37 are less susceptible to the effects of E-selectin (Figure 1D).
E-selectin–mediated growth inhibition and apoptosis were not observed when soluble divalent E-selectin–Fc chimera was substituted for the immobilized molecule, indicating that growth inhibition and apoptosis were not due to toxicity of the recombinant E-selectin–Fc protein. Similar results were found using divalent P-selectin–Fc chimera (data not shown). We have previously reported that P-selectin purified from human platelets triggers growth inhibition and apoptosis either in solution or following immobilization to plastic.15 Since P-selectin purified from human platelets forms large multimolecular aggregates,61 it appears that both P- and E-selectins require clustering to bind to their cognate receptors and transduce a signal.
The identity of E-selectin receptors on human and murine HPCs remains to be established. Selectin receptors require a protein or lipid core with multiple O- or N-linked oligosaccharide chains that contain the tetrasaccharide Neu NAc α2-3 Gal β1-4 (Fuc α1-3) Glc N Ac (sialyl-Lewisx) or a related structure.38 Several glycoproteins have been proposed as E-selectin ligands including PSGL-1,29,30,39-44 a glycoform of CD44H expressed on human HPCs,45 L-selectin,46 and in the mouse, E-selectin ligand-1 (ESL-1).47,48 We have found that the enforced expression of FucT-VII on K562, a myeloid human cell line that does not express PSGL-1 and does not adhere to either P- or E-selectin, results in adhesion to E-selectin but not to P-selectin as well as E-selectin–mediated growth inhibition and apoptosis. Although we have not identified the exact molecular nature of the HPC E-selectin receptor(s) responsible for this growth inhibition and apoptosis, relatively common cell surface glycoproteins (such as those expressed on K562 cells) can become E-selectin ligands transducing growth inhibition and apoptosis when fucosylated by FucT-VII. Interestingly, unlike PSGL-1, these E-selectin ligand(s) on human HPCs are not sensitive to O-sialoglycoprotein endopeptidase, but at least some do appear to be glycoproteins, as adhesion to E-selectin is halved following cleavage by neutrophil serine proteases.
HPCs isolated from PSGL-1–/– mice do not adhere to P-selectin43,49 ; however, we show that these cells still adhere to E-selectin resulting in growth inhibition and apoptosis similar to wild-type lineageneg Sca-1+ c-KIT+ cells. Together, these results unambiguously demonstrate that HPCs express E-selectin receptors signaling growth inhibition and apoptosis that are independent from PSGL-1.
Deletion of both P- and E-selectin genes in the mouse induces severe leukocytosis and enhanced hematopoiesis in the BM and spleen.34,35 Interestingly, enhanced hematopoiesis is also observed in mice deficient in either FucT-VII27 or deficient in guanosine 5′-diphosphate (GDP)–4-keto-6-deoxymannose 3,5-epimerase-4 reductase (FX), an enzyme necessary for the synthesis of the fucosyl transferase substrate GDP-fucose.50 Our finding that either P- or E-selectin induce HPC growth inhibition and apoptosis in vitro (and that the effect is additive) is consistent with the observation that enhanced hematopoiesis is not as pronounced in mice deficient for P-selectin alone51 and not observed in mice lacking only E-selectin,34 as the disruption of only one of these 2 negative regulations would not be sufficient to release HPCs from this proliferation block in vivo. Our results are also consistent with the fact that increased hematopoiesis is not observed in mice deficient in PSGL-149 but is observed in FucT-VII– or FX-deficient mice in which ligands for both P- and E-selectin are nonfunctional.27,50 These latter 2 observations indeed suggest that in addition to PSGL-1, the deletion of other selectin ligands requiring FucT-VII–mediated fucosylations is necessary to recapitulate the phenotype of mice deficient in both P- and E-selectin.
In regards to the physiologic relevance of the growth inhibition mediated by P- and E-selectins, several aspects should be considered. The site density of P-selectin on cultured human umbilical vein endothelial cells stimulated with IL-4 is fewer than 10 sites/μm2,52 whereas transfected Chinese hamster ovary (CHO) cells express selectins at densities from 10 to 1000 sites per μm2.53 There is no data regarding selectin densities at the surface of freshly purified primary BM endothelial cells that, unlike cultured human umbilical vein endothelial cells or endothelial cells from any other tissue, express both P- and E-selectin in a constitutive manner without prior stimulation.9,12,13 That murine HPCs do spontaneously roll on BM venules and sinusoids in vivo as observed by intravital microscopy12,13 strongly implies that the constitutive expression of P- and E-selectin is of a sufficiently high density. Both P- and E-selectin are present in the bone marrow stroma in 2 forms: a cell attached transmembrane form and a soluble shed form in the fluid that bathes the stroma. Within the BM, P-selectin is expressed by endothelial cells, megakaryocytic precursors,54,55 and possibly some macrophages,56 and it is therefore likely to be available in both soluble and cell attached forms at the hematopoietic niche as well as on the bone marrow endothelium. Moreover, P-selectin is released by platelets and present in plasma in a soluble form that binds to PSGL-157,58 at levels sufficient to inhibit HPC proliferation.15 In contrast, E-selectin expression in vivo appears to be restricted to endothelial cells9 ; however, it is also shed and present in the general circulation at levels between 10 and 100 ng/mL,59 despite very low expression in the noninflamed vasculature outside of the BM. Thus, it is likely that concentrations of soluble E-selectin could be much higher locally within the BM microenvironment at levels resulting in growth inhibition of HPCs.
In conclusion, in addition to previous experiments showing that P-selectin together with E-selectin are critically important for the homing of HPCs into the BM,11,13,14 our study demonstrates that both human and mouse HPCs express receptors for E-selectin, independent from PSGL-1, that also requires fucosylation by FucT-VII for function and are signaling molecules transducing growth inhibition and apoptosis. Importantly, this regulatory function of E-selectin seems to be altered in leukemia cells, particularly T-lineage acute lymphoblastic leukemia and chronic myeloid leukemia. For instance, function-blocking anti–E-selectin monoclonal antibodies have been reported to reduce in vitro survival of T-acute lymphoblastic leukemia cells cultured on a monolayer of pre-established human BM cells, suggesting that E-selectin may be a survival factor for these cells.60 On the other hand, we have found that adhesion to E-selectin of CD34+ cells isolated from the BM and peripheral blood of 3 chronic myeloid leukemia patients in blast crisis is reduced by 70% compared with normal CD34+ cells and, more importantly, that immobilized E-selectin has no significant effect on the proliferation of these leukemic cells in response to 36GS (I.G.W. and J.-P.L. unpublished data, March 2002). These data suggest that the mutations involved in the genesis of both T-acute lymphoblastic leukemia and chronic myeloid leukemia allow leukemic cells to escape E-selectin–mediated negative signaling.
Prepublished online as Blood First Edition Paper, October 30, 2003; DOI 10.1182/blood-2003-06-1921.
Supported by a Translational Research Grant from the Leukemia and Lymphoma Society of America (6589-01) and partly by the National Health and Medical Research Council of Australia (9937158) to J.-P.L. and P.J.S.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Dr David N. Haylock and Dr Susie Nilsson for the provision of human samples, Mr Andrew Fryga and Ralph Rossi for sorting lineageneg Sca-1+ c-KIT+ cells, Dr Michael C. Berndt (Monash University, Melbourne, Australia) for purified human P-selectin, and Drs Barbara Furie and Bruce Furie for kindly providing BM cells from PSGL-1–/– mice and their control littermates.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal