Abstract
Hematopoietic defects in HOXA9–/– mice demonstrate a key role for this homeoprotein in blood cell development. Conversely, enforced HOXA9 expression is leukemogenic in mice, and HOXA9 is frequently activated in human acute myeloid leukemia (AML). Although HOXA9 is thought to function as a transcription factor, few downstream targets have been identified. We searched for early HOXA9 target genes by using a transient overexpression strategy in 3 hematopoietic cell lines (2 myeloid, 1 lymphoid). cDNA microarray analyses identified 220 genes whose expression was modulated at least 2-fold. Expression signatures in myeloid and lymphoid cells demonstrated that HOXA9 functions as both an activator and repressor of a variety of genes in cell-specific patterns suggesting that the transcriptional effects of HOXA9 are largely dependent on the cell context. Transient transcription assays and target gene expression patterns in HOXA9–/– marrow cells imply that we have identified direct physiologic targets. Many target genes are expressed in CD34+ stem cells or are members of gene families involved in proliferation or myeloid differentiation. Expression of 14 HOXA9 target genes correlated with high-level HOXA9 expression in primary AML. These data suggest that many genes identified in this survey may mediate the biologic effects of HOXA9 in normal and leukemic hematopoiesis.
Introduction
HOX homeodomain proteins exert important effects during embryogenesis and blood cell development.1-3 As a member of this large family, HOXA9 is expressed in normal human CD34+ blood cells and is down-regulated during differentiation. HOXA9-deficient mice display a variety of myeloid and lymphoid defects.4,5 HOXA9 overexpression in bone marrow cells induces marked stem cell expansion, and HOXA9–/– hematopoietic stem cells appear to have a major proliferative defect6 (H.J.L. et al, in preparation).
Several studies have established a role for HOXA9 overexpression in myeloid leukemogenesis. Acute myeloid leukemia (AML) arising in leukemia-prone BXH2 mice shows frequent retroviral activation of HOXA9.7 Retroviral overexpression of HOXA9 immortalizes murine marrow cells in vitro and readily leads to leukemic transformation in vivo.8,9 HOXA9 is also important in human AML because this gene is activated in most patients with acute AML, and a t(7;11) chromosomal translocation resulting in a NUP98-HOXA9 gene fusion is found in some patients.7,10 In a previous gene expression study in human leukemia, HOXA9 emerged as one of the top 20 genes that distinguished AML from acute lymphoid leukemia (ALL), and high levels of HOXA9 expression correlated with poor prognosis.11,12 Thus, HOXA9 expression is part of the gene expression signature for AML and may also be a prognostic factor.
Several HOX genes, including HOXA9, are direct targets of the mixed lineage leukemia gene (MLL), and leukemias associated with MLL translocations show uniform activation of HOXA9.13-16 The ability of an MLL fusion gene to induce myeloid leukemia in murine marrow cells requires the presence of HOXA9, suggesting that HOXA9 is a mediator of the transforming effects of MLL fusion proteins.17 Thus, altered HOXA9 gene expression may be a common pathway that unifies diverse initiating events in many myeloid leukemias.18
Because HOXA9 exerts major biologic effects on normal and leukemic hematopoiesis, and because very little is known about the molecular pathways regulated by this homeoprotein, we completed a comprehensive gene expression profile of hematopoietic cells in which HOXA9 was transiently overexpressed. This study reveals that HOXA9 rapidly modulates the expression of a large number of genes that are implicated in normal hematopoiesis and in oncogenesis.
Materials and methods
Cell lines
Tetracycline (tet)–inducible Jurkat cells were purchased from Clontech (Palo Alto, CA). Tet-inducible U937 cells were provided by Dr Gerald Grosveld (St Jude Children's Research Hospital, Memphis, TN). Tet-inducible K562 cells were supplied by Dr Dan Rosson (Lankenau Medical Research Center, Wynnewood, PA). All Tet-inducible cell lines were maintained in RPMI with 10% tet-free serum. NIH3T3 fibroblasts were transduced with MIG-GFP or MIG-HOXA9/GFP containing murine stem cell virus (MSCV)–based retroviruses and maintained in Dulbecco modified Eagle medium (DMEM) with 10% serum.
DNA transfections
A Flag epitope-tagged HOXA9 cDNA encoding a 246–amino acid protein (accession number NM_010456) was cloned into the tet-inducible vector, pBI-eGFP (Clontech), yielding pBI-FlagHOXA9-eGFP. pBI-eGFP contains a tet-activator responsive bidirectional promoter region. In the absence of tetracycline, pBI-FlagHOXA9-eGFP expresses both Flag-HOXA9 and eGFP proteins. pBI-eGFP (reference control) or pBI-FlagHOXA9-eGFP was electroporated into tet-activator expressing U937, K562, and Jurkat cells. All studies were performed using transient transfections in the absence of tetracycline, coupled with fluorescence-activated cell sorting (FACS) of eGFP+ cells 24 hours after transfection. Two independent eGFP+ transfections and sorts were performed for each cell line to yield biologic replicates. HOXA9 protein expression was verified by Western blot analysis with an anti-HOXA9 antibody.
RNA amplification
Total RNA from sorted cells was isolated with the RNeasy Mini kit (Qiagen, Valencia, CA). mRNA in 1 μg total RNA was amplified 5- to 20-fold as previously described.19 Briefly, mRNA was reverse transcribed into cDNA using an oligo-dT-T7 primer and was subsequently converted to double-stranded cDNA. T7 RNA polymerase was used to generate a pool of amplified RNA.
cDNA microarrays
A 44 022 human cDNA clone set, purchased from Research Genetics (Invitrogen, Carlsbad, CA), was printed onto 2 poly-l-lysine–coated glass slides (approximately 20 000 genes/slide) and postprocessed following protocols listed at http://www.microarrays.org/pdfs/PostProcessing2001.pdf. To control for variability between the 2 samples, HOXA9– (reference) samples were cultured, transfected, and sorted simultaneously with the HOXA9+ samples with which they were hybridized. Then, 2 μg amplified mRNA from HOXA9– and HOXA9+ samples from the same transfection/sorting experiment were labeled with Cyanine 3 (Cy3) or Cy5, respectively, and combined for hybridization to microarrays.20 In addition to performing biologic replicate hybridizations from 2 independent sorting experiments, the third hybridization represented a technical replicate, which consisted of independently labeled RNA samples from one of the eGFP+ sorting experiments. Arrays were hybridized overnight in a 63°C water bath and washed twice with 2 × sodium chloride/sodium citrate (SSC)/0.01% sodium dodecyl sulfate (SDS) and once with 0.5 × SSC. The slides were scanned with a dual-laser Axon Instruments 4000B Scanner using GenePix 4.0 software (Axon Instruments, Union City, CA). Images for the Cy5 and Cy3 channels were acquired separately. Spots that were covered by array or hybridization artifacts were manually flagged and were not used in subsequent analyses. GenePix results files containing ratio of medians measurements, calculated as Cy5/Cy3, were uploaded to the NOMAD microarray database for storage and normalization. NOMAD is a database that is used to archive microarray GenePix results (.gpr) files. This “warehouse” is an open source system for storing and querying microarray experiments. For further details go to http://sourceforge.net/projects/ucsf-nomad/.
Microarray analysis
When microarray .gpr files were uploaded to NOMAD, normalized fluorescence ratios were calculated. Signal intensities between the Cy5 and Cy3 images were normalized by applying a scaling factor to all of the Cy5 channel values so that the mean of ratios = 1.21 Spot quality was ensured by inclusion of data points where R2 is greater than 0.75. Next, a table of microarray data containing rows of genes and columns of data points from individual experiments was created in NOMAD. This file was opened in the CLUSTER program, and data points were log2 transformed and filtered. After average linkage hierarchical clustering was performed, values in the resulting .cdt file were visualized by TREEVIEW software.22 Statistical significance of the microarray data was assessed by Statistical Analysis for Microarrays (SAM), a t test permutation algorithm.23 The input parameters used for this analysis were: row average imputer and one class response.
RT-PCR validation
EPS8, EST AA789015, MYB, TCN1, LYN, and GAPDH transcript levels were detected in amplified RNA from K562 transfected cells by standard reverse transcription-polymerase chain reaction (RT-PCR). PCR products were visualized on ethidium bromide–stained agarose gels. Fold difference was calculated from densitometric values of PCR product intensities from PCR cycles within the linear range. ALDH1 and JUNB transcripts were detected by a one-step quantitative real-time RT-PCR method with TaqMan fluorogenic probes.24
VHL, ID2, MBNL, SFRS11, and CD36 expression levels were measured in amplified RNA from each cell line, and expression was quantified using real-time PCR with SYBR Green. Annealing and acquisition temperatures were determined empirically from dissociation curves.25 Fold differences for all real-time PCR experiments were calculated using the 2-ΔΔCT method.26
Luciferase reporter vector cloning and assays
A 1-kb fragment of the human ALDH1 promoter was PCR amplified from a 2.5-kb fragment in an ALDH1 promoter-containing vector provided by Dr Fred Gonzalez (National Cancer Institute, Bethesda, MD). A 1-kb region of the human CD36 promoter was PCR amplified from genomic DNA (gDNA). PCR products were cloned into the PCR2.1 TOPO T/A cloning vector and then into the pGL3-enhancer vector.
Reporter and expression plasmids were transfected with Lipofectamine 2000 (LF2000; Invitrogen). Two micrograms of plasmid DNA (1 μg pBI-GFP or pBI-HOXA9/GFP and 1 μg reporter vector) were added to 4 μL LF2000 and incubated with 6 × 105 K562 or U937 cells, or 7.5 × 105 Jurkat cells in 24-well plates. Cells were harvested 48 hours later and lysed in Luciferase Assay Reagent (Promega, Madison, WI). Reporter light units were measured in a Monolight 2010 Luminometer (BD Biosciences, San Jose, CA).
Mouse bone marrow
Marrow cells from the tibias and femurs of HOXA9–/– mice and wild-type controls were enriched for Lin– Sca+ immature progenitors using the Spin-Sep technique (StemCell Technologies, Vancouver, BC, Canada).5 RNA was isolated, and 1 μg was amplified following the protocol described in “RNA amplification.” Real-time PCR was performed for JUNB and EPS8 using SYBR Green.
Patient samples
Total RNA was isolated from 11 cryopreserved bone marrow samples of de novo AML, provided by the UCSF Leukemia Cell Bank. Relative expression levels of HOXA9 and JUNB were compared to GAPDH, as measured by real-time PCR.
Results
Gene expression profiling of early HOXA9 gene targets
To identify primary HOXA9 target genes in hematopoietic cells we used a transient, FACS-selectable expression system coupled with cDNA microarrays. Three human leukemic cell lines, 2 myeloid (U937, myelomonocytic, and K562, erythroid/megakaryocytic) and one lymphoid (Jurkat, T cell), were analyzed. Whereas U937 cells express measurable levels of HOXA9 mRNA, K562 and Jurkat cells do not10 (and C.M.F., unpublished data, January 2002).
Plasmids overexpressing eGFP alone or eGFP with an internal ribosomal entry site (IRES)–-driven HOXA9 cDNA were transiently transfected into each cell line. Cells were harvested 24 hours later and sorted for eGFP positivity. High levels of HOXA9 mRNA and protein in cells transfected with the HOXA9 vector were confirmed by real-time PCR and Western blot analyses (data not shown; Figure 1A). mRNA from the HOXA9– and HOXA9+ samples was amplified by in vitro transcription (IVT) and labeled with Cy3 and Cy5, respectively. The 2 samples were combined and hybridized to large-scale cDNA microarrays. IVT amplification fidelity, comparing unamplified to amplified RNA samples, and array hybridization-to-hybridization reproducibility of independently amplified samples were 75% and 85%, respectively, as measured by the R2 correlation coefficient. To decrease the percentage of misclassifications, triplicate microarray hybridizations were performed for each cell line.27
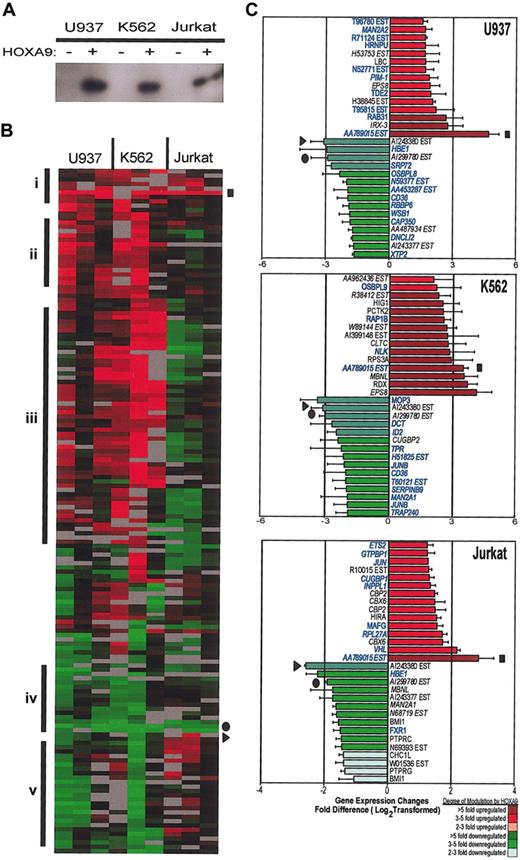
Gene expression profiling of early HOXA9 gene targets. (A) Western blot analysis shows HOXA9 protein expression in transfected U937, K562, and Jurkat cell lines 24 hours after transfection with GFP (HOXA9–) or GFP/HOXA9 (HOXA9+) expression vectors. (B) Comparison of HOXA9-modulated transcript expression patterns between cell lines using the Eisen hierarchical cluster analysis.22 The cluster contains 141 known genes with a minimum of 3 data points that were at least 2.5-fold up- or down-regulated. Red indicates induction, green indicates repression, gray indicates excluded data, and black indicates no change. Genes were grouped into smaller clusters showing similar expression patterns: i, up-regulated in all 3 cell lines; ii, up-regulated in myeloid, no change in lymphoid; iii, up-regulated in myeloid, down-regulated in lymphoid; iv, down-regulated in all 3 cell lines; and v, down-regulated in myeloid, up-regulated in lymphoid. ▪ = AA789015, ▸ = AI234380, • = AI299780. (C) Genes exhibiting the greatest magnitude of change by HOXA9 in each cell line. Red indicates gene activation; green indicates repression in at least 2 of 3 experiments. Gene names that are highlighted in blue were detected in CD34+ stem cells.63 Names of genes or ESTs that were identified as being statistically significant by the SAM algorithm are italicized. For U937 and K562 cell lines, the false discovery rate (FDR) was set at 10%. For the Jurkat cell line, the FDR was 46%. ▪ = AA789015; ▸ = AI234380; • = AI299780. Error bars indicate SE of mean fold change for at least 2 experiments.
Gene expression profiling of early HOXA9 gene targets. (A) Western blot analysis shows HOXA9 protein expression in transfected U937, K562, and Jurkat cell lines 24 hours after transfection with GFP (HOXA9–) or GFP/HOXA9 (HOXA9+) expression vectors. (B) Comparison of HOXA9-modulated transcript expression patterns between cell lines using the Eisen hierarchical cluster analysis.22 The cluster contains 141 known genes with a minimum of 3 data points that were at least 2.5-fold up- or down-regulated. Red indicates induction, green indicates repression, gray indicates excluded data, and black indicates no change. Genes were grouped into smaller clusters showing similar expression patterns: i, up-regulated in all 3 cell lines; ii, up-regulated in myeloid, no change in lymphoid; iii, up-regulated in myeloid, down-regulated in lymphoid; iv, down-regulated in all 3 cell lines; and v, down-regulated in myeloid, up-regulated in lymphoid. ▪ = AA789015, ▸ = AI234380, • = AI299780. (C) Genes exhibiting the greatest magnitude of change by HOXA9 in each cell line. Red indicates gene activation; green indicates repression in at least 2 of 3 experiments. Gene names that are highlighted in blue were detected in CD34+ stem cells.63 Names of genes or ESTs that were identified as being statistically significant by the SAM algorithm are italicized. For U937 and K562 cell lines, the false discovery rate (FDR) was set at 10%. For the Jurkat cell line, the FDR was 46%. ▪ = AA789015; ▸ = AI234380; • = AI299780. Error bars indicate SE of mean fold change for at least 2 experiments.
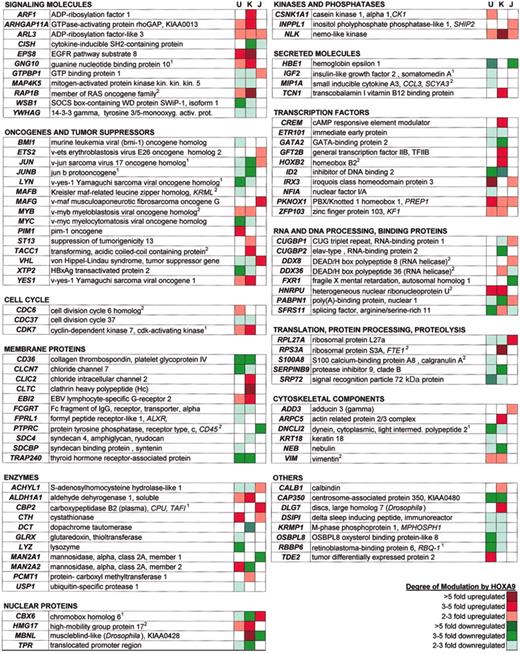
CLUSTER software was used to filter the microarray data for each cell line. This analysis created a list of 220 genes that were modulated at least 2-fold. Selected named genes enumerated by these criteria are arranged in functional groups in Figure 2. The classes of genes represented included signaling molecules, oncogenes, kinases, cell surface molecules, enzymes, and other transcription factors. Ninety expressed sequence tags (ESTs) were also identified (data not shown). More genes were modulated in the U937 and K562 cells (132 and 106 genes) than in the Jurkat cells (41 genes). In the 2 cell lines that lack detectable endogenous HOXA9, K562 and Jurkat, HOXA9 up-regulated more genes than it down-regulated (68 genes up versus 38 genes down in K562; 23 genes up versus 18 genes down in Jurkat). However, in U937, HOXA9 overexpression induced the up-regulation of 36 genes, but down-regulated 96 genes. Thus, HOXA9 appears to modulate many genes, acting both as an activator and a repressor.
Functional classification of selected HOXA9 target genes. The superscripted 1 indicates genes that were represented by more than one spot/accession number; superscripted 2, genes that were modulated by HOXA9 as determined by the CLUSTER analysis, but were not classified as statistically significant as determined by the parameters set in the SAM analysis.
Functional classification of selected HOXA9 target genes. The superscripted 1 indicates genes that were represented by more than one spot/accession number; superscripted 2, genes that were modulated by HOXA9 as determined by the CLUSTER analysis, but were not classified as statistically significant as determined by the parameters set in the SAM analysis.
Next, we performed another analysis with the CLUSTER software using microarray data from all 3 cell lines to compare expression patterns between the different cell lines. This pattern was then manually divided into smaller groups based on differential target regulation in myeloid versus lymphoid backgrounds (Figure 1B groups i-v). Many genes showed up-regulation in myeloid, and either no expression or down-regulation in lymphoid cells, suggesting that the transcriptional effects of HOXA9 depend largely on cellular context (Figure 1B groups ii-iv). A few genes were regulated in the same manner in all 3 lines, indicating that transcription of some targets is modulated in a cell context-independent fashion (Figure 1B groups i and iv). The ESTs AA789015 and PKNOX1 were up-regulated and 3 ESTs (AI243377, AI299780, and AI243380) were down-regulated in all 3 lines. Figure 1C presents genes that showed the greatest degree of change in each cell line.
We also used the Significance Analysis of Microarrays algorithm, SAM,23 to analyze the microarray data for each cell line. Most of the genes previously highlighted by the CLUSTER analysis, 174(79%) of 220, were shown to be significantly changed by HOXA9 overexpression and are italicized in Figure 1C. All of the genes listed in Figure 2 were significant as determined by SAM analysis, except where indicated.
Validation of HOXA9 targets identified by microarray analysis
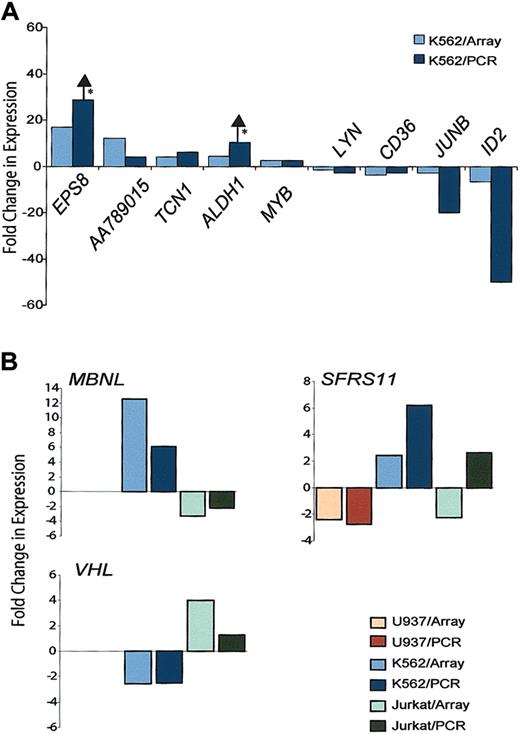
Of the 48 genes that were expressed in a similar manner in more than one cell line, 9 were chosen for validation by RT-PCR in K562 cells. We selected genes involved in malignant transformation, such as JUNB and EPS8, the stem cell–specific gene ALDH1, which also plays a role in drug resistance,28 and genes, such as transcobalamin 1 (TCN1), CD36, MYB, ID2, and LYN, that have been previously detected in myeloid cells. The direction of each gene's change in expression as determined by microarray was confirmed by RT-PCR (Figure 3A). The array data also suggested that HOXA9 can modulate the same gene in opposite directions in different cell lines. Of the 12 genes that were differentially regulated between cell lines, 3 were chosen for further analysis. Figure 3B shows real-time PCR data confirming this phenomenon for MBNL, SFRS11, and VHL. In 15 (94%) of 16 comparisons of gene expression levels by the 2 methods, the direction of change seen by RT-PCR was consistent with changes observed on the microarrays.
RT-PCR validation of selected HOXA9 gene targets. (A) Confirmation of HOXA9-regulated genes in K562 cells. Gene expression levels were calculated by normalizing PCR data from HOXA9– and HOXA9+ samples to control genes as described in “Materials and methods.” Fold up- or down-regulation was calculated using the ratio of HOXA9+/HOXA9– normalized PCR values and the mean of ratios of HOXA9+/HOXA9– signals from the microarray data. *No expression was detected in one of the HOXA9 conditions such that the ratio could not be calculated, and a lower limit of differential expression is reported. Arrows indicate that the expression of these genes may be higher than what is reported because the HOXA9– sample value was 0. (B) Quantitative verification of differential expression of HOXA9 target genes in different cell lines. Expression of 3 genes was analyzed by real-time RT-PCR, and fold changes in expression are expressed as the ratio of GAPDH-normalized HOXA9+/HOXA9– values. Fold changes from the arrays represent the mean values from at least 2 hybridizations.
RT-PCR validation of selected HOXA9 gene targets. (A) Confirmation of HOXA9-regulated genes in K562 cells. Gene expression levels were calculated by normalizing PCR data from HOXA9– and HOXA9+ samples to control genes as described in “Materials and methods.” Fold up- or down-regulation was calculated using the ratio of HOXA9+/HOXA9– normalized PCR values and the mean of ratios of HOXA9+/HOXA9– signals from the microarray data. *No expression was detected in one of the HOXA9 conditions such that the ratio could not be calculated, and a lower limit of differential expression is reported. Arrows indicate that the expression of these genes may be higher than what is reported because the HOXA9– sample value was 0. (B) Quantitative verification of differential expression of HOXA9 target genes in different cell lines. Expression of 3 genes was analyzed by real-time RT-PCR, and fold changes in expression are expressed as the ratio of GAPDH-normalized HOXA9+/HOXA9– values. Fold changes from the arrays represent the mean values from at least 2 hybridizations.
To determine if genes identified in this survey were direct targets of HOXA9, we performed luciferase reporter gene analysis of the ALDH1 and CD36 promoters (Figure 4).29 For ALDH1, we observed that HOXA9 overexpression increased transcription from this promoter 1.6- and 2.4-fold in U937 and K562 cells, respectively, but down-regulated transcription 2.4-fold in Jurkat cells. Analysis of the HOXA9 responsiveness of the CD36 promoter showed down-regulation by HOXA9 by 2.3-, 1.9-, and 4.4-fold in U937, K562, and Jurkat cells, respectively. For both genes, the effect of HOXA9 on the promoter-driven reporter activity paralleled the changes in mRNA levels detected by microarray and RT-PCR.
Reporter gene analysis of HOXA9 target promoters in all 3 cell lines. Luciferase reporter plasmids contain the following promoter regions: (A) ALDH1 promoter (–980 to +23); (B) CD36 promoter (–1129 to +36).
Reporter gene analysis of HOXA9 target promoters in all 3 cell lines. Luciferase reporter plasmids contain the following promoter regions: (A) ALDH1 promoter (–980 to +23); (B) CD36 promoter (–1129 to +36).
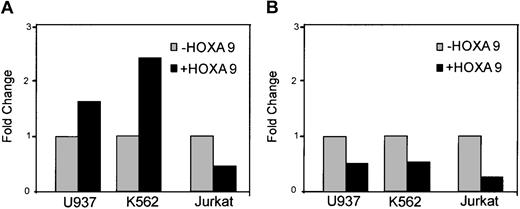
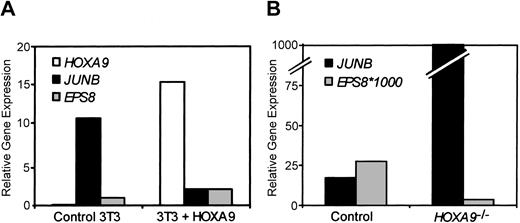
To analyze whether HOXA9 gene targets identified in myeloid cell lines were also targets in other cell types, JUNB and EPS8 mRNA levels were assessed in NIH3T3 fibroblasts stably transduced with a HOXA9 expression vector. Figure 5A shows HOXA9 expression is increased over 100-fold in the transduced cells as compared with parental cells. Consistent with the changes seen in K562 cells, JUNB expression is decreased and EPS8 expression is increased in HOXA9-overexpressing cells. If these 2 genes are authentic targets of HOXA9 in hematopoietic cells, we reasoned that their expression levels would be altered in marrow cells from HOXA9-deficient mice. Figure 5B shows JUNB and EPS8 expression levels in immature precursor-enriched bone marrow cells of control and HOXA9–/– mice. JUNB expression is increased by 60-fold, whereas EPS8 expression is decreased 8-fold, thus exhibiting the converse of the changes observed in HOXA9-overexpressing cell lines.
Putative HOXA9 gene target expression in other cell types. (A) JUNB and EPS8 expression levels are modulated by HOXA9 in NIH3T3 fibroblasts. HOXA9, JUNB, and EPS8 expression levels were measured in RNA from parental cells and stably transduced HOXA9 overexpressing cells by real-time PCR. (B) Confirmation of converse changes of EPS8 and JUNB in HOXA9-deficient murine marrow cells. Marrow cells were isolated from adult HOXA9–/– mice and wild-type (control) littermates and enriched for Lin– Sca+ primitive cells. HOXA9, JUNB, and EPS8 levels were assessed by real-time PCR. EPS8 levels are multiplied by 1000 for scaling purposes.
Putative HOXA9 gene target expression in other cell types. (A) JUNB and EPS8 expression levels are modulated by HOXA9 in NIH3T3 fibroblasts. HOXA9, JUNB, and EPS8 expression levels were measured in RNA from parental cells and stably transduced HOXA9 overexpressing cells by real-time PCR. (B) Confirmation of converse changes of EPS8 and JUNB in HOXA9-deficient murine marrow cells. Marrow cells were isolated from adult HOXA9–/– mice and wild-type (control) littermates and enriched for Lin– Sca+ primitive cells. HOXA9, JUNB, and EPS8 levels were assessed by real-time PCR. EPS8 levels are multiplied by 1000 for scaling purposes.
HOXA9 target expression in CD34+ stem cells
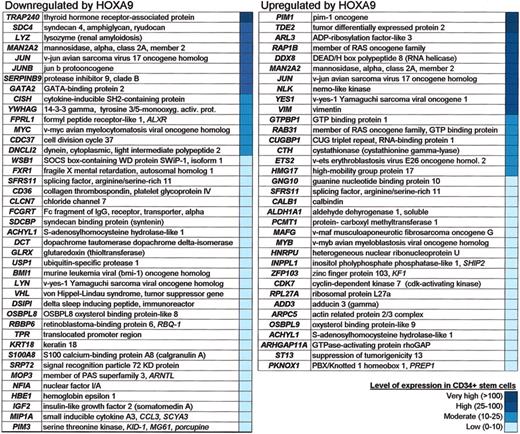
To discern which HOXA9 target genes identified in this microarray analysis were involved in normal stem cell biology, we searched the most comprehensive, current database of CD34+ human bone marrow cells, The Human Stem Cell Transcriptome Database (http://westsun.hema.uic.edu/cd34.html).30 The expression of 36 genes that are activated by HOXA9 and 42 genes that are repressed by this homeodomain protein were detected in CD34+ cells from low to very high expression levels (Figure 6). In addition to confirming HOXA9 expression, this database reported that several uncharacterized HOXA9 targets, including EST AA789015, are expressed in CD34+ stem cells (highlighted in blue in Figure 1C). These data indicate that HOXA9 can modulate a number of characterized and uncharacterized genes involved in normal stem cell function.
HOXA9 target genes that are expressed in CD34+ stem cells. All genes listed are significant as determined by SAM.
HOXA9 target genes that are expressed in CD34+ stem cells. All genes listed are significant as determined by SAM.
Analysis of HOXA9 targets in AML
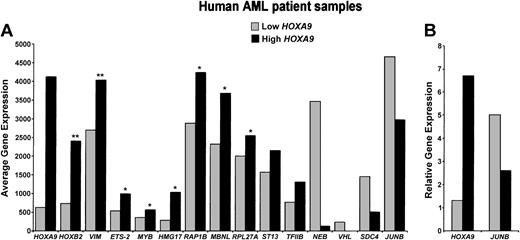
AML is a heterogeneous disease that can result from different chromosomal abnormalities, and HOXA9 mRNA levels, although elevated in most cases, vary within this disease. Therefore, to determine if any candidate HOXA9 gene targets were similarly altered in primary AML samples with high HOXA9 levels, we performed an in silico analysis of the Affymetrix GeneChip dataset published by Armstrong et al.16 These AML patient samples were divided into high and low HOXA9-expressing groups based on their HOXA9 mRNA expression levels (Figure 7A). Average gene expression levels were calculated for all genes and these values were compared between the 2 groups. Ten HOXA9 targets are up-regulated and 4 are down-regulated in a manner that correlates with HOXA9 expression. In addition, JUNB expression was confirmed by real-time PCR in a separate set of AML patient samples and was inversely related to HOXA9 expression with a 2-fold lower level of expression in the high HOXA9 group as compared to the low HOXA9 group (Figure 7B). Thus, 14 (10%) of the named genes that were up- or down-regulated by HOXA9 in our transient overexpression study are similarly modulated in primary AML samples. Whereas many of the other target genes were either not represented on the GeneChip or were not differentially regulated, a few were regulated in the opposite direction. However, it was not our expectation that the transcriptome of HOXA9-transfected cell lines would necessarily completely overlap with the transcriptome of primary AML, given the array of genetic mutations in this disease. Rather, we posit that areas of overlap represent genes worthy of further study.
Expression levels of HOXA9 targets in primary samples of AML. (A) Gene expression profiles for 28 human primary AML patient samples were downloaded from the www.genome.wi.mit.edu/MPR website.16 HOXA9 expression levels were used to divide the samples into 2 groups. High HOXA9 samples had pixel intensities ranging from 2000 to 6000 (n = 17) and low HOXA9-expressing samples had pixel intensity values of 2000 or below (n = 11). A Student t test was performed to determine which gene expression differences were statistically significant. *P < .05; **P < .001. Fourteen genes whose expression was significantly different between the HOXA9-high and HOXA9-low groups of primary AML showed the same direction of change of expression as seen in the microarray studies. (B) JUNB mRNA levels in high and low HOXA9-expressing AML patient samples, as measured by quantitative real-time PCR. Samples were segregated into one of 2 groups, based on relative expression of HOXA9, and normalized to GAPDH. High HOXA9-expressing samples had HOXA9/GAPDH ratios of more than 0.05 (n = 4), and low HOXA9 samples had ratios less than 0.05 (n = 7). Values for JUNB represent the mean relative expression level for each HOXA9 expression group.
Expression levels of HOXA9 targets in primary samples of AML. (A) Gene expression profiles for 28 human primary AML patient samples were downloaded from the www.genome.wi.mit.edu/MPR website.16 HOXA9 expression levels were used to divide the samples into 2 groups. High HOXA9 samples had pixel intensities ranging from 2000 to 6000 (n = 17) and low HOXA9-expressing samples had pixel intensity values of 2000 or below (n = 11). A Student t test was performed to determine which gene expression differences were statistically significant. *P < .05; **P < .001. Fourteen genes whose expression was significantly different between the HOXA9-high and HOXA9-low groups of primary AML showed the same direction of change of expression as seen in the microarray studies. (B) JUNB mRNA levels in high and low HOXA9-expressing AML patient samples, as measured by quantitative real-time PCR. Samples were segregated into one of 2 groups, based on relative expression of HOXA9, and normalized to GAPDH. High HOXA9-expressing samples had HOXA9/GAPDH ratios of more than 0.05 (n = 4), and low HOXA9 samples had ratios less than 0.05 (n = 7). Values for JUNB represent the mean relative expression level for each HOXA9 expression group.
Discussion
A major finding of this study is that activation of HOXA9 can rapidly modulate the expression of a large group of genes, representing a wide variety of functional categories not previously known to be targets of any HOX gene. Real-time PCR or SAM analysis confirmed a large percentage of these changes, and transient transcription assays suggest that at least some of these genes are direct targets of HOXA9. This study also provides insights concerning the general transcriptional function of HOX genes by confirming that they can act as activators and repressors of gene transcription.31,32 We also demonstrate that a human HOX gene can positively or negatively regulate a given gene, depending on cellular context, as previously recognized in Drosophila.33 Importantly, we also report that many of the HOXA9 targets we identified are expressed in CD34+ stem cells, where HOXA9 is expressed during normal blood cell development. We also showed that some HOXA9 target genes are dysregulated in primary samples of human AML and correlate with HOXA9 expression in this disease.
In addition, we found some HOXA9 targets that undergo expression changes during myeloid differentiation as demonstrated by gene expression profiling analyses of U937 and NB4 cells with or without retinoic acid.34,35 These experiments revealed changes in a variety of gene families, including transcription factors and cytoskeletal and transcription/translation-associated proteins. Because HOXA9 is expressed in CD34+ cells,4 and its expression decreases as cells mature, we surmised that the expression pattern of some of its targets may be reversed during differentiation. Indeed, examination of these published datasets showed that VIM, MYB, GTFIIB, and ribosomal genes, which are up-regulated by HOXA9 in our overexpression study, are down-regulated on differentiation, and ID2, WSB-1, and S100A8, which are down-regulated by HOXA9 in our study, are up-regulated as differentiation progresses. In the HL60 cell line, vitamin D, retinoic acid, and 12-O-tetradecanoylphorbol-13-acetate (TPA)–induced differentiation showed changes for small inducible cytokines, including MIP1A, which is up-regulated in differentiation, yet repressed by HOXA9 in our experiments.36-38 Lastly, MafB induction is critical for monocytic differentiation, and HOXA9 may aid in keeping cells in an immature state by repressing this gene in CD34+ cells.39
Other candidate HOXA9 targets play roles in embryonic development such as NLK, IRX-3, and NFIA. NLK, the vertebrate homolog of the Drosophila gene, nemo, has been shown to have diverse roles in signaling during development, and plays a role in organism patterning.40 Expression of IRX-3, a member of the Iroquois homeodomain family, is also expressed during embryonic development, and has recently been shown to be a putative target of HOXA3.41,42 The expression pattern of nuclear factor I/A during mouse embryogenesis points to its potential role for this gene in development.43
Among other targets identified, several emerge as potential mediators of the leukemogenic effects of HOXA9, including genes involved in cell cycle regulation and proliferation. For example, Cdc6 and Cdk7 were up-regulated in myeloid cells overexpressing HOXA9. Cdc6 has been reported to bind to chromatin and aid in the initiation of DNA replication and cell proliferation.44 Cdk7 mediates several processes including cell cycle control and transcriptional regulation.45 In addition, increased expression of ribosomal proteins, elongation factors, RNA-splicing factors, and RNA helicases was identified in a proliferation cluster of genes that was associated with increased doubling time in cell lines analyzed by Ross et al.21 Members of these gene families were also up-regulated by HOXA9, such as RPL27a, RPS3a, HNRPU, DDX8, DDX36, ADD3, and SFRS11.
Some genes that are repressed by HOXA9 have tumor suppressor functions. JUNB and LYN were both down-regulated by HOXA9 in the myeloid cells tested. Loss of JUNB in mice results in a myeloproliferative syndrome that resembles chronic myeloid leukemia (CML) and is associated with up-regulation of antiapoptotic bcl-2 and bcl-xl proteins.46 LYN-deficient mice display increased numbers of myeloid progenitors and develop monocyte/macrophage tumors.47 Although the VHL gene was also shown to be down-regulated by HOXA9 in myeloid cells, a connection between this gene has been associated with other cancers, and we report that its expression is not detected in HOXA9 high-expressing AML samples.
Conversely, several oncogenes, including MYB, and 2 Ras family members, EPS8 and RAP1B, were up-regulated in the myeloid cell lines analyzed. MYB overexpression transforms cells in culture and results in acute leukemia in animals.48 EPS8 and RAP1B have transforming potential when overexpressed in NIH3T3 fibroblasts.49,50 Other HOXA9 target genes that were grouped into a leukemia cluster by Ross et al are PTPRC and ETS2.21 ETS2 has been previously shown to participate in t(8;21) translocations in AML-M2.51 In addition, several other HOXA9 targets have been previously associated with leukemia such as HOXB2, MBNL (KIAA0428), PIM-1, HMG17, and RPS3A (FTE1).14,16,52-54 Thus, several potentially oncogenic pathways appear to be modulated by HOXA9 and may therefore mediate its leukemogenic effects. Further support for the roles of HOXA9 targets, such as EPS8, RPS3A, or TACC1, as oncogenes lies in the fact that their expression was not detected in CD34+ stem cells, which may mean that their expression is the result of aberrant activation of HOXA9 in primitive myeloid cells. However, other oncogenes that have been previously shown to be dysregulated in leukemia are also expressed in stem cells, such as ETS2, HMG17, and PIM1, suggesting that further research on the expression of these genes is required.
Although HOXA9 appears to regulate a variety of genes, the same phenomenon has been observed for other transcription factors and oncogenes such as Myc, Myb, and Ras.55-57 Overexpression strategies of these proteins has shown that they also modulate many genes with vastly different functions. Some shared targets between HOXA9 and RAS include YES, RAP1B, MAN2A1, SDC4, and VIM. RPL27a, JUNB, ID2, ETS2, VHL, and JUN are targets that are shared between HOXA9 and MYC. Targets in common between MYB and HOXA9 are transcription/translation-associated proteins, small inducible cytokines, and cytoskeletal genes.
Lastly, some of the HOXA9 targets identified have been shown to be targets or family members of targets of other HOX genes in other cell systems. For example, in the instances of RAP1B and IRX-3, HOXB4 has been shown to regulate RAP1 and IRX-5.58,59 A study of MLL target genes showed that IRX-3 was downstream of this protein, presumably through a HOX gene.60 Overexpression of HOXD3 showed that it also regulates VIM, as well as syndecan 1, RAB13, and several keratins.31 HOXC13 overexpression in murine skin also regulates several keratin-associated proteins and 14-3-3β.61 Comparison of HOXA9 and HOXA13 gene targets revealed that they both regulate small inducible cytokines and 14-3-3 proteins.62 Nonetheless, the genes showing the largest and most consistent changes in expression following HOXA9 activation are ESTs of unknown function, suggesting that several important HOXA9 target genes are as yet uncharacterized.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-07-2202.
Supported by grants from the Research Service of the Department of Veterans Affairs, and the National Institutes of Health, R01DK48642 and R21 CA91245 (H.J.L.), and R01CA80029 (C.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sarah Elmes and Jane Gordon for assistance with cell sorting and Stephen Fong for his aid with HOXA9–/– bone marrow isolation.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal