Abstract
It is well accepted that umbilical cord blood has been a source for hematopoietic stem cells. However, controversy exists as to whether cord blood can serve as a source of mesenchymal stem cells, which can differentiate into cells of different connective tissue lineages such as bone, cartilage, and fat, and little success has been reported in the literature about the isolation of such cells from cord blood. Here we report a novel method to obtain single cell-derived, clonally expanded mesenchymal stem cells that are of multilineage differentiation potential by negative immunoselection and limiting dilution. The immunophenotype of these clonally expanded cells is consistent with that reported for bone marrow mesenchymal stem cells. Under appropriate induction conditions, these cells can differentiate into bone, cartilage, and fat. Surprisingly, these cells were also able to differentiate into neuroglial- and hepatocyte-like cells under appropriate induction conditions and, thus, these cells may be more than mesenchymal stem cells as evidenced by their ability to differentiate into cell types of all 3 germ layers. In conclusion, umbilical cord blood does contain mesenchymal stem cells and should not be regarded as medical waste. It can serve as an alternative source of mesenchymal stem cells to bone marrow.
Introduction
It is well accepted that umbilical cord blood (UCB) is a source for hematopoietic stem cells, and transplantation of cord blood has been part of clinical practice for more than 10 years.1-3 However, controversy exists as to whether UCB contains mesenchymal stem cells (MSCs), which are capable of differentiating into cells of different connective tissue lineages such as bone, cartilage, and adipose tissues and are the best candidates for tissue engineering of musculoskeletal tissues.4 To date, the most common source of MSCs has been the bone marrow, but aspirating bone marrow from the patient is an invasive procedure and, in addition, it has been demonstrated that the number and the differentiating potential of bone marrow MSCs decreases with age.5 Therefore, the search for alternative sources of MSCs is of significant value. So far, little success has been reported in the literature about the isolation, characterization, and differentiation of MSCs from UCB. Erices et al6 reported that UCB-derived mononuclear cells gave rise to 2 adherent cell types, one of which expressed MSC-related surface antigens. Mareschi et al7 reported that in a given condition, it was possible to isolate MSCs from bone marrow but not UCB. Goodwin et al8 recently reported multilineage differentiation ability by cells isolated from UCB, which express bone, fat, and neural markers. In these 2 reports, neither Erices et al6 nor Goodwin et al8 provided sufficient evidence to fulfill the qualifying criteria for MSCs because relatively heterogeneous cells were reported by both groups.
Because the surface phenotype of MSCs has been clearly characterized and reported,9 which should be lineage-marker negative, it would be reasonable to deplete all these lineage-committed cells, such as CD3, CD7, CD19, CD38, and glycophorin A+ cells to get MSCs. Even with such cell enrichment procedures, the resulting cell population is still heterogeneous and it is imperative to obtain clonally expanded MSCs to demonstrate their multipotentiality. Most important of all, the proliferation and differentiation abilities of MSCs may decrease with the donor age. The cells contained in UCB can be considered as “very young” and, thus, UCB may be an excellent alternative source of MSCs. Unfortunately, most of the time UCB is still regarded as medical waste in the delivery rooms. Therefore, the aim of this study is to investigate the possibility of obtaining clonally expanded MSCs that have the potential for multilineage differentiation. We hypothesized that MSCs, which are multipotent, exist in UCB and can be isolated and expanded in culture.
Materials and methods
Cytokines
Nerve growth factor (NGF), epidermal growth factor (EGF), vitronectin, and transforming growth factor β (TGF-β1) were from Becton Dickinson (Flanders, NJ). Acidic and basic fibroblast growth factor (aFGF, bFGF), sonic hedgehog (SHH), brain-derived growth factor (BDNF), hepatocyte growth factor (HGF), and oncostatin M (OSM) were from R&D Systems (Minneapolis, MN).
Antibodies
Antibodies against human osteopontin, human microtubule-associated protein 2 (MAP-2), human glial fibrillary acidic protein (GFAP), and human type II collagen were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against human antigens CD3, CD7, CD19, CD29, CD31, CD33, CD34, CD44, CD45, CD49b, CD49d, CD51, CD58, CD61, CD62L, CD62P, CD90, CD105, CD117, CD135, HLA-ABC, and HLA-DR were purchased from Becton Dickinson. Antibodies against human antigen CD133 were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Antibodies against human antigens SH-2, SH-3, and SH-4 were purified from SH-2, SH-3, and SH-4 hybridoma cell lines (American Type Culture Collection, Rockville, MD). Antibodies against human albumin and secondary goat antimouse antibodies were from Dako (Carpinteria, CA).
MSC isolation and culture
Isolation. UCB was collected on delivery with informed consent (n = 11). UCB mononuclear cells were obtained by negative immunodepletion of CD3+, CD14+, CD19+, CD38+, CD66b+, and glycophorin A+ cells using a commercially available kit (RosetteSep, StemCell Technologies, Vancouver, BC, Canada), as per the manufacturer's instructions, followed by Ficoll-Paque (Amersham-Pharmacia, Piscataway, NJ) density gradient centrifugation (1.077 g/cm3), and plated in noncoated tissue culture flasks (Becton Dickinson) in expansion medium. Cells were allowed to adhere overnight and nonadherent cells were washed out with medium changes. Medium changes were carried out twice weekly thereafter. Expansion medium consists of Iscove modified Dulbecco medium (IMDM; Gibco, Grand Island, NY) and 20% fetal bovine serum (FBS; Hyclone, Logan, UT) supplemented with 10 ng/mL bFGF, 100 U penicillin, 1000 U streptomycin, and 2 mM l-glutamine (Gibco).
Limiting dilution. To obtain single cell-derived, clonally expanded MSCs, the isolated plate-adhering second-passage cells were serially diluted and plated on to 96-well plates (Becton Dickinson), in expansion medium, at a final density of 30 cells/96-well plate. Colonies that grew were culture expanded and tested for their differentiation potential.
Maintenance and culture expansion. Once adherent cells reached approximately 50% to 60% confluence, they were detached with 0.25% trypsin-EDTA (ethylenediaminetetraacetic acid; Gibco), washed twice with phosphate-buffered saline (PBS; Gibco), with centrifugation, 1000 rpm for 5 minutes, and replated at 1:3 under the same culture conditions.
In vitro differentiation
Osteogenic differentiation. To induce osteogenic differentiation, fifth- to seventh-passage cells were treated with osteogenic medium for 3 weeks with medium changes twice weekly. Osteogenesis was assessed at weekly intervals. Osteogenic medium consists of IMDM supplemented with 0.1 μM dexamethasone (Sigma-Aldrich, St Louis, MO), 10 mM β-glycerol phosphate (Sigma-Aldrich), and 0.2 mM ascorbic acid (AsA; Sigma-Aldrich).
Chondrogenic differentiation. To induce chondrogenic differentiation, fifth- to seventh-passage cells were transferred into 15-mL polypropylene tube and centrifuged at 1000 rpm for 5 minutes, to form a pelleted micromass at the bottom of the tube and then treated with chondrogenic medium for 3 weeks. Medium changes were carried out twice weekly and chondrogenesis was assessed at weekly intervals. Chondrogenic medium consists of high-glucose DMEM (Bio-fluid, Rockville, MD) supplemented with 0.1 μM dexamethasone, 50 μg/mL AsA, 100 μg/mL sodium pyruvate (Sigma-Aldrich), 40 μg/mL proline (Sigma-Aldrich), 10 ng/mL TGF-β1, and 50 mg/mL ITS+ premix (Becton Dickinson; 6.25 μg/mL insulin, 6.25 μg/mL transferrin, 6.25 ng/mL selenius acid, 1.25 mg/mL bovine serum albumin (BSA), and 5.35 mg/mL linoleic acid).
Adipogenic differentiation. To induce adipogenic differentiation, fifth- to seventh-passage cells were treated with adipogenic medium for 3 weeks. Medium changes were carried out twice weekly and adipogenesis was assessed at weekly intervals. Adipogenic medium consists of IMDM supplemented with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX; Sigma-Aldrich), 1 μM hydrocortisone (Sigma-Aldrich), 0.1 mM indomethacin (Sigma-Aldrich), and 10% rabbit serum (Sigma-Aldrich).
Neurogenic differentiation. To induce neurogenic differentiation a multistep protocol was used. Fifth- to seventh-passage cells, seeded at a density of 3000 cells/cm2, were treated with step 1 medium for 3 days, step 2 medium for 3 days, step 3 medium for 3 days, followed by step 4 medium for 3 days. Step 1 medium consists of IMDM supplemented with 5 ng/mL bFGF, 0.5 μM retinoic acid (Sigma-Aldrich), and 1 mM 2-mercaptoethanol (Sigma-Aldrich). Step 2 medium consists of IMDM supplemented with 1 mM cyclic adenosine monophosphate (cAMP; Sigma-Aldrich) and 100 μM AsA. Step 3 medium consists of IMDM supplemented with 10 μM hydrocortisone and 1 mM cAMP. Step 4 medium consists of IMDM supplemented with 20 ng/mL aFGF, 10 ng/mL SHH, 10 ng/mL BDNF, 10 ng/mL NGF, 25 ng/mL vitronectin, 100 μM AsA, 0.1 mM IBMX, 10 μM forskolin (Sigma-Aldrich), and 20 nM phorbol myristate acetate (PMA; Sigma-Aldrich). Neurogenesis was assessed on day 6 and day 10, and calcium imaging was performed on day 12.
Hepatogenic differentiation. To induce hepatogenic differentiation, fifth- to seventh-passage cells, at approximately 60% confluence, were treated with differentiation medium, which consists of IMDM supplemented with 20 ng/mL HGF, 0.5 μM dexamethasone, and 50 mg/mL ITS+ premix, for 14 days followed by maturation medium thereafter. Maturation medium consists of IMDM supplemented with 20 ng/mL OSM, 0.5 μM dexamethasone, and 50 mg/mL ITS+ premix. Medium changes were carried out twice weekly and hepatogenesis was assessed at time points as indicated by reverse transcription-polymerase chain reaction (RT-PCR).
Histologic, cytochemical, and immunocytochemical analysis
Cytochemical staining. For evaluation of mineralized matrix, cells were fixed with 4% formaldehyde and stained with 1% Alizarin-red S (Sigma-Aldrich) solution in water for 10 minutes. In addition, mineralized matrix was also evaluated by von Kossa staining using 1% silver nitrate (Sigma-Aldrich) under UV light for 45 minutes, followed by 3% sodium thiosulfate (Sigma-Aldrich) for 5 minutes, and then counterstained with van Gieson (Sigma-Aldrich) for 5 minutes. For oil-red O staining, cells were fixed with 4% formaldehyde, stained with oil-red O (Sigma-Aldrich) for 10 minutes, and then counterstained with Mayer hematoxylin (Sigma-Aldrich) for 1 minute.
Histologic analysis. Chondrogenic differentiation was evaluated after pellets were fixed in 4% formaldehyde, dehydrated in serial ethanol dilutions, and embedded in paraffin blocks. Blocks were cut and sections stained with safranin-O (Sigma-Aldrich).
Immunofluorescence. For staining of intracellular proteins, cells were fixed overnight with 4% formaldehyde at 4°C and permeabilized with 0.1% Triton X-100 (Sigma-Aldrich) for 10 minutes. Slides and dishes were incubated with mouse primary antibodies against human osteopontin (1:50), human MAP-2 (1:50), human GFAP (1:50), or human albumin (1:50) for 1 hour, followed by fluorescein- or phycoerythrin-coupled goat antimouse IgG secondary antibody for 1 hour. Between incubations, slides and dishes were washed with PBS.
Flow cytometry. For cell surface antigen phenotyping, fifth- to seventh-passage cells were detached and stained with fluorescein- or phycoerythrin-coupled antibodies and analyzed with FACSCalibur (Becton Dickinson).
Total RNA isolation and RT-PCR
RNA was extracted from 3 to 30 × 105 MSCs, differentiating cells, or differentiated cells using RNEasy (Qiagen, Stanford, Valencia, CA) per the manufacturer's instructions. The mRNA was reverse transcribed to cDNA using Advantage RT-for-PCR (Clontech, Palo Alto, CA) per the manufacturer's instructions. cDNA was amplified using a ABI GeneAmp PCR System 2400 (Perkin Elmer Applied Biosystems, Boston, MA) at 94°C for 40 seconds, 56°C for 50 seconds, and 72°C for 60 seconds for 35 cycles, after initial denaturation at 94°C for 5 minutes. Primers used for amplification are listed in Table 1.
Primers used for RT-PCR
Primer . | Sequence . | Product . |
|---|---|---|
| Collagen type I | S: 5′-TGCTTGAATGTGCTGATGACAGGG-3′ | 414 bp |
| A: 5′-TCCCCTCACCCTCCCAGTAT-3′ | ||
| Osteocalcin | S: 5′-CGCAGCCACCGAGACACCAT-3′ | 405 bp |
| A: 5′-GGGCAAGGGCAAGGGGAAGA-3′ | ||
| Osteopontin | S: 5′-CTAGGCATCACCTGTGCCATACC-3′ | 330 bp |
| A: 5′-CTACTTAGACTACTTGACCAGTGAC-3′ | ||
| Syndecan | S: 5′-CCTTCACACTCCCCACAC-3′ | 409 bp |
| A: 5′-GGCATAGAATTCCTCCTGTTG-3′ | ||
| Perlecan | S: 5′-CATAGAGACCGTCACAGCAAG-3′ | 300 bp |
| A: 5′-ATGAACACCACACTGACAACC-3′ | ||
| Aggrecan | S: 5′-TCAGGAACTGAACTCAGTGG-3′ | 441 bp |
| A: 5′-GCCACTGAGTTCCACAGA-3′ | ||
| Collagen type II | S: 5′-ACGGCGAGAAGGGAGAAGTTG-3′ | 352 bp |
| A: 5′-GGGGGTCCAGGGTTGCCATTG-3′ | ||
| Glial fibrillary acidic protein | S: 5′-GCAGAGATGATGGAGCTCAATGACC-3′ | 266 bp |
| A: 5′-GTTTCATCCTGGAGCTTCTGCCTCA-3′ | ||
| Microtubule-associated protein | S: 5′-TGCCATCTTGGTGCCGA-3′ | 370 bp |
| A: 5′-CTTGACATTACCACCTCCAGGT-3′ | ||
| α-fetoprotein | S: 5′-TGCAGCCAAAGTGAAGAGGGAAGA-3′ | 216 bp |
| A: 5′-CATAGCGAGCAGCCCAAAGAAGAA-3′ | ||
| Tyrosine aminotransferase | S: 5′-TGAGCAGTCTGTCCACTGCCT-3′ | 358 bp |
| A: 5′-ATGTGAATGAGGAGGATCTGAG-3′ | ||
| Albumin | S: 5′-TGC TTG AATGTGCTGATGACAGGG-3′ | 161 bp |
| A: 5′-AAGGCAAGTCAGCAGGCATCTCATC-3′ | ||
| Cytokeratin 18 | S: 5′-TGGTACTCTCCTCAATCTGCTG-3′ | 148 bp |
| A: 5′-CTCTGGATTGACTGTGGAAGT-3′ |
Primer . | Sequence . | Product . |
|---|---|---|
| Collagen type I | S: 5′-TGCTTGAATGTGCTGATGACAGGG-3′ | 414 bp |
| A: 5′-TCCCCTCACCCTCCCAGTAT-3′ | ||
| Osteocalcin | S: 5′-CGCAGCCACCGAGACACCAT-3′ | 405 bp |
| A: 5′-GGGCAAGGGCAAGGGGAAGA-3′ | ||
| Osteopontin | S: 5′-CTAGGCATCACCTGTGCCATACC-3′ | 330 bp |
| A: 5′-CTACTTAGACTACTTGACCAGTGAC-3′ | ||
| Syndecan | S: 5′-CCTTCACACTCCCCACAC-3′ | 409 bp |
| A: 5′-GGCATAGAATTCCTCCTGTTG-3′ | ||
| Perlecan | S: 5′-CATAGAGACCGTCACAGCAAG-3′ | 300 bp |
| A: 5′-ATGAACACCACACTGACAACC-3′ | ||
| Aggrecan | S: 5′-TCAGGAACTGAACTCAGTGG-3′ | 441 bp |
| A: 5′-GCCACTGAGTTCCACAGA-3′ | ||
| Collagen type II | S: 5′-ACGGCGAGAAGGGAGAAGTTG-3′ | 352 bp |
| A: 5′-GGGGGTCCAGGGTTGCCATTG-3′ | ||
| Glial fibrillary acidic protein | S: 5′-GCAGAGATGATGGAGCTCAATGACC-3′ | 266 bp |
| A: 5′-GTTTCATCCTGGAGCTTCTGCCTCA-3′ | ||
| Microtubule-associated protein | S: 5′-TGCCATCTTGGTGCCGA-3′ | 370 bp |
| A: 5′-CTTGACATTACCACCTCCAGGT-3′ | ||
| α-fetoprotein | S: 5′-TGCAGCCAAAGTGAAGAGGGAAGA-3′ | 216 bp |
| A: 5′-CATAGCGAGCAGCCCAAAGAAGAA-3′ | ||
| Tyrosine aminotransferase | S: 5′-TGAGCAGTCTGTCCACTGCCT-3′ | 358 bp |
| A: 5′-ATGTGAATGAGGAGGATCTGAG-3′ | ||
| Albumin | S: 5′-TGC TTG AATGTGCTGATGACAGGG-3′ | 161 bp |
| A: 5′-AAGGCAAGTCAGCAGGCATCTCATC-3′ | ||
| Cytokeratin 18 | S: 5′-TGGTACTCTCCTCAATCTGCTG-3′ | 148 bp |
| A: 5′-CTCTGGATTGACTGTGGAAGT-3′ |
Uptake of low-density lipoprotein
The Dil-Ac-LDL staining kit was purchased from Biomedical Technologies (Stoughton, MA) and assay was performed per the manufacturer's instructions.
Calcium imaging
For measurement of [Ca2+]i, differentiated cells were incubated in serum-free differentiation medium with 5 μM fura-2 am for 1 hour at 37°C. Cells were then washed 3 times with the serum-free differentiation medium and used for measurements. For fura-2 excitation, a λ DG4 system (Sutter, Novato, CA) was used, which was controlled by Metafluor software from Universal Imaging (Downingtown, PA). Ratiometric calcium estimates were made by using 10-nm wide filters centered on 340 and 380 nm (Chroma Technology, Brattleboro, VT), capturing the emitted light (485-540 nm) at each excitation wavelength for 300 ms through a × 20 objective (Zeiss Axiovert 200 microscope) and directing it to a cooled CCD camera (CoolsnapFx; Roper Scientific, Tucson, AZ). The ratio within each cell was computed from images obtained at 340 and 380 nm excitation wavelengths and subtracting the appropriate background fluorescence at each wave-length. Ratios were computed every second.
To stimulate the cell, a 3-μm glass micropipette loaded with HighK buffer (5 mM NaCl, 5 mM glucose, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 1 mM MgCl2, 150 mM KCl, 2.2 mM CaCl2, pH 7.3) was positioned at 10 μm from the recorded cell. The HighK buffer was puffed onto the cell for 3 seconds at 5 psi under the control of Picospritzer III (Parker Instrument, Parker Hannifin, Fairfield, NJ).
Results
Immunophenotypic characterization of UCB-derived MSCs
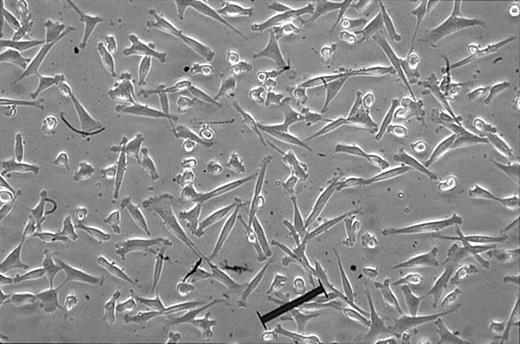
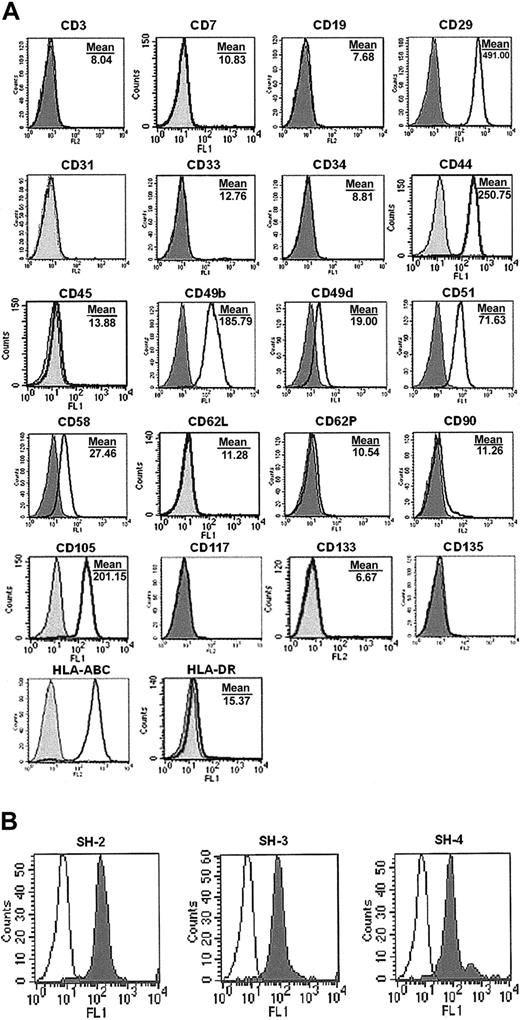
Fibroblast-like, rapidly diving cells (Figure 1) arising from limiting dilution were extensively expanded, and characterization by flow cytometry revealed that the cells isolated by the described method were negative for CD3 (T3), CD7 (gp40), CD19 (B4), CD34 (gp105-120), CD45 (leukocyte common antigen), CD117 (c-kit), CD133 (AC133), and CD135 (Flt-3), indicating these cells are not of hematopoietic origin (Figure 2A). Cells were also negative for matrix receptors CD31 (PECAM-1), CD62L (L-selectin), and CD62P (P-selectin), and negative for CD33 (P67), CD90 (Thy-1), and HLA-DR (Figure 2A). UCB-derived cells were found to be positive for integrins CD29 (β1-integrin), CD49b (α2-integrin), CD49d (α4-integrin), and CD51 (αv-integrin); positive for matrix receptors CD44 (hyaluronate receptor), CD58 (LFA-3), and CD105 (endoglin); and positive HLA-ABC (Figure 2A), which are consistent with the findings for bone marrow MSCs in the literature.9 In addition, the markers for human MSCs, SH-2, SH-3, and SH-4 (Figure 2B), were strongly positive.
Morphology of single cell-derived, clonally expanded UCB MSCs. The lineage-depleted mononuclear fraction was plated in culturing medium, culture expanded, and plated at a density of 30 cells/96-well plate. Single cell-derived clones were then culture expanded and maintained between 30% and 60% confluence. Original magnification, × 100. Scale bar indicates 100 μm.
Morphology of single cell-derived, clonally expanded UCB MSCs. The lineage-depleted mononuclear fraction was plated in culturing medium, culture expanded, and plated at a density of 30 cells/96-well plate. Single cell-derived clones were then culture expanded and maintained between 30% and 60% confluence. Original magnification, × 100. Scale bar indicates 100 μm.
Phenotype of UCB MSCs. Cells were cultured for 5 to 7 passages, harvested, and labeled with antibodies against human antigens (A) CD3, CD7, CD19, CD29, CD31, CD33, CD34, CD44, CD45, CD49b, CD49d, CD51, CD58, CD62L, CD62P, CD90, CD105, CD117, CD133, CD135, HLA-ABC, and HLA-DR, and with (B) SH-2, SH-3, SH-4, as indicated and analyzed by FACS. (A) Shaded histogram indicates background signal; open histogram, positive reactivity with the indicated antibody. (B) Open histogram indicates background signal; shaded histogram, positive reactivity.
Phenotype of UCB MSCs. Cells were cultured for 5 to 7 passages, harvested, and labeled with antibodies against human antigens (A) CD3, CD7, CD19, CD29, CD31, CD33, CD34, CD44, CD45, CD49b, CD49d, CD51, CD58, CD62L, CD62P, CD90, CD105, CD117, CD133, CD135, HLA-ABC, and HLA-DR, and with (B) SH-2, SH-3, SH-4, as indicated and analyzed by FACS. (A) Shaded histogram indicates background signal; open histogram, positive reactivity with the indicated antibody. (B) Open histogram indicates background signal; shaded histogram, positive reactivity.
In vitro differentiation of osteocytes, chondrocytes, and adipocytes from UCB-derived MSCs
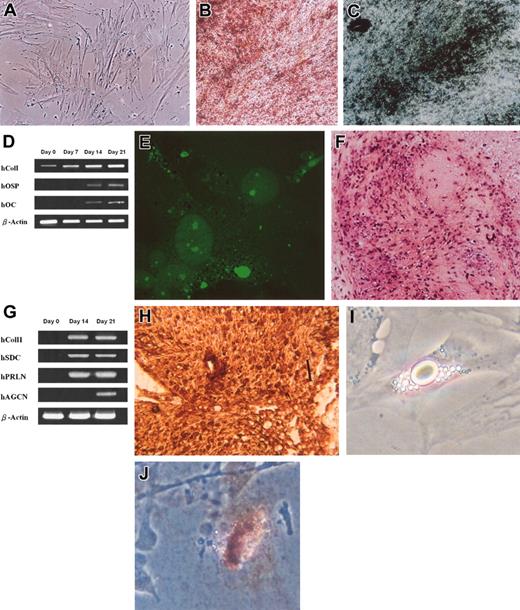
To investigate the osteogenic potential of the UCB-derived cells, fifth- to seventh-passage cells were plated at a density of 3 × 103 cells/cm2 and cultured under conditions appropriate for inducing differentiation for each lineage. When induced to differentiate under serum-free osteogenic conditions, the spindle shape of UCB-derived cells flattened and broadened with increasing time of induction (Figure 3A) and formed mineralized matrix as evidenced by Alizarin red staining (Figure 3B) and von Kossa staining (Figure 3C). The osteoblastic phenotype was also shown by the expression of marker genes osteopontin (hOSP) and osteocalcin (hOC), and by the gradual increase in type I collagen (hCol I) expression (Figure 3D). After 21 days of induction, cells were positive for immunofluorescence staining for osteopontin (Figure 3E). Immunofluorescence assays on undifferentiated cells stained negative for osteopontin (not shown).
Osteogenic, chondrogenic, and adipogenic differentiation from UCB MSCs. Osteogenic differentiation evidenced by morphology of cells after 9 days of induction (A), formation of mineralized matrix shown by Alizarin red (B), and von Kossa staining (C) and by the expression of bone-specific genes over 3 weeks of induction (D). Osteoblastic differentiation was further confirmed by immunofluorescence staining for osteopontin after 21 days of induction (E). Chondrogenic differentiation was evidenced by safranin-O staining (F), by the expression of chondrocyte-specific genes (G), and by immunohistochemical staining for type II collagen (H). Adipocytic differentiation was evidenced by the formation of lipid vacuoles in phase-contrast photograph (I) at original magnification × 200 and by oil-red O staining (J) shown at original magnification × 100. Original magnification, × 100 (A-C, E-F, H). Scale bar in panel H indicates 100 μm.
Osteogenic, chondrogenic, and adipogenic differentiation from UCB MSCs. Osteogenic differentiation evidenced by morphology of cells after 9 days of induction (A), formation of mineralized matrix shown by Alizarin red (B), and von Kossa staining (C) and by the expression of bone-specific genes over 3 weeks of induction (D). Osteoblastic differentiation was further confirmed by immunofluorescence staining for osteopontin after 21 days of induction (E). Chondrogenic differentiation was evidenced by safranin-O staining (F), by the expression of chondrocyte-specific genes (G), and by immunohistochemical staining for type II collagen (H). Adipocytic differentiation was evidenced by the formation of lipid vacuoles in phase-contrast photograph (I) at original magnification × 200 and by oil-red O staining (J) shown at original magnification × 100. Original magnification, × 100 (A-C, E-F, H). Scale bar in panel H indicates 100 μm.
The chondrogenic potential of UCB-derived cells was evaluated by culturing 1 × 106 cells under the pelleted micromass system in serum-free chondrogenic medium. After 3 weeks of differentiation the accumulation of sulfated proteoglycans was visualized by safranin-O staining (Figure 3F). Expression of mRNA of type II collagen (hColI I), syndecan (hSDC) as well as perlecan (hPRLN), marker genes for chondrocytes, was detected by RT-PCR after 14 and 21 days of induction, and the expression of aggrecan (hAGCN) detected at 21 days after induction (Figure 3G). Immunohistochemical analysis for human type II collagen 21 days after induction was also positive (Figure 3H). To assess the adipogenic potential, fifth- to seventh-passage cells were plated at a density of 3 × 103 cells/cm2 and cultured in adipogenic medium. Morphologic changes in cells as well as the formation of neutral lipid vacuoles were noticeable as early as 1 week after induction (Figure 3I) and visualized by staining with oil-red O (Figure 3J).
In vitro differentiation of neuroglial-like and hepatocyte-like cells from UCB-derived MSCs
Using the multistep induction method, fifth- to seventh-passage UCB-derived cells were seeded at a density of 3 × 103 cells/cm2 and tested for their neurogenic potential. After 10 days of differentiation, 69% of cells in the flask acquired the morphology of neuroglial cells exhibiting a refractile cell body with extended neuritelike structures, and in areas of higher cell density, differentiated cells arranged into a networklike structure (Figure 4A); 17% of cells had partially acquired the morphology of neuroglial cells, and 13% of cells exhibited morphology resembling those of restricted precursors of the neuroectodermal lineage. RT-PCR analysis revealed expression of GFAP, a marker gene of glial cells, by day 6 and at day 10, whereas the expression of neuronal marker gene, MAP-2, was barely detectable at day 6 and had increased by day 10 (Figure 4B). In addition, 2% of cells stained positive for immunofluorescence assays against MAP-2 (Figure 4C) at 6 days after differentiation and increased to approximately 63% by day 10, whereas 11% stained positive against GFAP (Figure 4D) at 6 days after differentiation and increased to approximately 26% by day 10. Immunofluorescence assays on undifferentiated cells stained negative for MAP-2 and GFAP (not shown). After 12 days of differentiation neuronlike cells showed acute and significant elevation in [Ca2+]i, as determined by the change in ratio of fura-2 fluorescence, when challenged with a high concentration of extracellular potassium, whereas undifferentiated cells did not show significant changes in [Ca2+]i (Figure 4E). The change in ratio of fura-2 fluorescence was 0.017 ± 0.002 for undifferentiated cells and 0.649 ± 0.066 for neuronlike cells (Figure 4F).
Ectodermal and endodermal differentiation. Neuroglial differentiation was evidenced by morphology after 10 days of induction (A), expression of neuroectodermal-specific genes (B), and by immunofluorescence assay for MAP-2 (C) and GFAP (D). Calcium imaging assays for the change in [Ca2+]i (E) and the ratio of change (F), as determined by fura-2 fluorescence, in the presence of high extracellular potassium. Hepatocytic differentiation was evidenced by morphology after 4 weeks of differentiation (G), expression of liver-specific genes (H), immunofluorescence assay for human albumin after 7 days of differentiation (I), and uptake of LDL after 6 weeks of differentiation (J). Original magnification, × 100 for all panels. Error bar in panel F indicates standard deviation.
Ectodermal and endodermal differentiation. Neuroglial differentiation was evidenced by morphology after 10 days of induction (A), expression of neuroectodermal-specific genes (B), and by immunofluorescence assay for MAP-2 (C) and GFAP (D). Calcium imaging assays for the change in [Ca2+]i (E) and the ratio of change (F), as determined by fura-2 fluorescence, in the presence of high extracellular potassium. Hepatocytic differentiation was evidenced by morphology after 4 weeks of differentiation (G), expression of liver-specific genes (H), immunofluorescence assay for human albumin after 7 days of differentiation (I), and uptake of LDL after 6 weeks of differentiation (J). Original magnification, × 100 for all panels. Error bar in panel F indicates standard deviation.
Schwartz et al10 reported that a higher cell density favored hepatogenesis, whereas densities less than 12.5 × 103 failed to give rise to hepatocyte-like cells. To determine whether UCB-derived cells can differentiate into hepatocyte-like cells in vitro, cells were allowed to grow to 60% confluence prior to induction. The cuboidal morphology of hepatocyte-like cells was observed as early as 21 days after culturing under hepatogenic conditions and further matured by day 28 (Figure 4G) in the presence of oncostatin M. The expression of liver-associated genes was detected at the indicated time points (Figure 4H). As early as 1 week after induction more than 70% of cells stained positive for immunofluorescence assays against human albumin (Figure 4I).
After culturing under hepatogenic conditions for 6 weeks, cells exhibited the ability to take up low-density lipoprotein (LDL; Figure 4J). Undifferentiated cells were negative for albumin and did not show the ability to take up LDL (not shown).
Discussion
The morphology of these cells from UCB resembles that of MSCs isolated from the bone marrow. The self-renewal capacity of these cells is remarkable and is expected of multipotent stem cells. Flow cytometric analysis showed that these cells were negative for various lineage markers but positive for human MSC markers SH-2, SH-3, and SH-4, as well as various other integrins and matrix receptors, and is consistent with that reported in the literature for the bone marrow counterpart, indicating the MSC nature of these UCB-derived cells.
The capacity of UCB-derived cells to differentiate into osteoblasts that produce mineralized matrices, chondrocytes that produce type II collagen, and adipocytes that accumulate lipid vacuoles under in vitro conditions is consistent with that reported for bone marrow MSCs and was demonstrated by morphology, RT-PCR analysis, and histochemical, cytochemical, and immunocytochemical evaluations.
In addition to the potential of MSCs to differentiate into multiple lineages of the mesoderm, recent reports in the literature have indicated that bone marrow MSCs are capable of cell fates crossing germ layer boundaries.11,12 The derivation of neuroglial cells of ectodermal origin13 and hepatocyte-like cells of endodermal origin14,15 from MSCs has been documented. However, skepticism has commonly been raised against such findings because these reports failed to show that differentiation into tissue lineages of mesodermal origin as well as nonmesodermal origin can be achieved from a single clonally expanded population of MSCs. Instead, a plate-adhering population obtained from plating total mononuclear cells was used, raising the possibility that such a heterogeneous population may contain progenitors of all 3 germ layers and that different inductive conditions simply favored the proliferation and differentiation of different progenitor cells, hence leading to misinterpretation. On the contrary, we further investigated the potential of UCB-derived cells to differentiate into neuroglial-like and hepatocyte-like cells using the same clonally expanded population.
Under neurogenic conditions, UCB-derived cells exhibited the morphology of and expressed genes specific of neuroglial cells, which was further confirmed by immunofluorescence assays for neuroglial-specific proteins. Undifferentiated cells did not express neuroglial marker genes and did not stain positive for neuroglial-specific proteins by immunofluorescence assays.
Voltage-sensitive ion channels are critical to the functioning of neuronal cells. When the concentration of extracellular potassium is elevated the membrane potential of neuronal cells is depolarized and the voltage-sensitive calcium channels open to allow an influx of calcium ions.16 When the differentiated cells were exposed to a high concentration of extracellular potassium a significant elevation in [Ca2+]i was observed, indicative of a resting membrane potential and the presence of voltage-sensitive calcium channels on the membrane. Undifferentiated cells did not show any significant change in [Ca2+]i under the same conditions.
When UCB-derived cells were cultured under hepatogenic conditions all cells acquired a cuboidal morphology as opposed to the fibroblast-like morphology of undifferentiated cells. Under hepatogenic conditions low-level expression of α-fetoprotein, an early developmental marker gene of hepatoblasts, was detectable by day 7 and remained detectable up to day 35. Expression of cytokeratin-18 and albumin was detected at all time points, and the expression of tyrosine aminotransferase, a late marker gene of hepatocytes, was detected by day 14 and increased with time of differentiation. Furthermore, by day 7, differentiated cells stained positive for albumin by immunofluorescence analysis. Undifferentiated cells did not express α-fetoprotein or tyrosine aminotransferase, but expressed low levels of albumin and cytokeratin-18. However, undifferentiated cells were negative for albumin by immunofluorescence analysis. After 6 weeks of differentiation the hepatocyte-like cells demonstrated the ability to take up LDL, a function characteristic of hepatocytes, whereas undifferentiated cells failed to take up LDL.
MSCs have been studied extensively over the past 3 decades and numerous independent research groups worldwide have successfully isolated MSCs from a variety of sources,17-19 most commonly, from the bone marrow. However, controversy exists over whether such stem cells are present in UCB and, to date, little evidence in the literature substantiates the existence of such cells in UCB. Erices et al6 and Goodwin et al8 independently published the successful isolation of progenitor cells of mesenchymal origin from UCB, whereas Mareschi et al7 concluded against such findings.
By definition, MSCs are fibroblast-like in morphology, self-renewable, and capable of differentiating into, at a minimum, 3 connective tissue lineages of the mesoderm, namely, bone, cartilage, and fat. We have shown in the present study that a plate-adhering population of fibroblast-like cells isolated from UCB, indeed, can be extensively clonally expanded in vitro while retaining the potential to differentiate, under in vitro conditions, into multiple lineages of the mesoderm.
Erices et al6 reported that UCB-derived mononuclear cells gave rise to 2 adherent cell types, one of which expressed MSC-related surface antigens. However, the multilineage differentiation ability of their cells was not shown in that study. Although Goodwin et al8 have shown that their UCB-derived progenitor cells can differentiate into osteoblasts and adipocytes, the ability to differentiate into chondrocytes was not shown. Further, the expression of MSC-specific antigens such as SH-2, SH-3, and SH-4 was not demonstrated. In addition, although Goodwin et al8 showed that their cells also expressed neural markers, a heterogeneous plate-adhering population from total mononuclear cells was used in their study, and the presence of neural progenitors in UCB has been documented.20 In this study, we have demonstrated that our relatively more homogeneous UCB-derived cells can differentiate into all 3 mesodermal lineages and, furthermore, the abilities to differentiate into neuroglial-like and hepatocyte-like cells were also evidenced. As a result of the broad differentiation potential of these cells the term MSC may seem somewhat inappropriate but, due to the similarities between these UCB-derived cells and the bone marrow MSCs, this nomenclature was adopted for the purpose of comparison, while research efforts are ongoing to fully characterize the differentiation potential and to determine a more appropriate terminology for these cells.
This report puts to rest the controversies and substantiates that UCB does contain MSCs. With the approach reported in this study, it is possible to obtain single cell-derived, clonally expanded MSCs from UCB with remarkable potential to differentiate into multiple lineages of mesodermal and nonmesodermal origin. With this technique, the application of UCB can be further extended and can be used as an alternative source of MSCs to bone marrow.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-05-1670.
Supported by an intramural grant from the Veterans General Hospital-Taipei (grant no. VGH 91-390-3).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 4. Ectodermal and endodermal differentiation. Neuroglial differentiation was evidenced by morphology after 10 days of induction (A), expression of neuroectodermal-specific genes (B), and by immunofluorescence assay for MAP-2 (C) and GFAP (D). Calcium imaging assays for the change in [Ca2+]i (E) and the ratio of change (F), as determined by fura-2 fluorescence, in the presence of high extracellular potassium. Hepatocytic differentiation was evidenced by morphology after 4 weeks of differentiation (G), expression of liver-specific genes (H), immunofluorescence assay for human albumin after 7 days of differentiation (I), and uptake of LDL after 6 weeks of differentiation (J). Original magnification, × 100 for all panels. Error bar in panel F indicates standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/5/10.1182_blood-2003-05-1670/6/m_zh80050457690004.jpeg?Expires=1763492796&Signature=1AmH7OP8-t5hDym2K~bSDCOkxh8D1dM53RHxEZA7eIFrtWL1RQ~G4tE-q2ZH~SzIeu7Qr2s2PsRZJznvbok~MVdIPyiLv~fG5xGhEacI1CxB3u5lUldt9dNUGSsBwA3j2xeLA3Tlun3zJvZzTfRnLvSxaWL8iBWBD9aWpDypSPnFO-q1Q6b4iHMM0WPkt1G73vHcTxeUCm6VkfFXer6PLXecASaUD6ofJKAocaMSvMVbewQMouvPWfkZdO8Pd8QCfj9SjTHxa5UrcjHnXCm5zV0nGRMId~VSadG1MeLKPQbJTSYccxMoIcEXSJtD9SUZqSesk6Vo-7tnIZJp6SyD3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal