Abstract
The Edmonston vaccine strain of measles virus (MV-Edm) propagates efficiently in a broad range of human tumor cells, killing them selectively. However, the oncolytic potency of MV-Edm in different human tumor xenograft therapy models is highly variable and there is no convenient way to map the distribution of virus-infected tissues in vivo. To enhance the oncolytic potency of MV-Edm against radiosensitive malignancies and to facilitate noninvasive imaging of infected tissues, we generated a recombinant MV-Edm encoding the human thyroidal iodide symporter (NIS). MV-NIS replicated almost as efficiently as unmodified MV-Edm, and human tumor cells efficiently concentrated radioiodine when infected with MV-NIS. Intratumoral spread of MV-NIS was noninvasively demonstrated by serial gamma-camera imaging of iodine-123 (123I) uptake both in MV-sensitive KAS-6/1 myeloma xenografts, which regressed completely after a single intravenous dose of MV-NIS, and in MM1 myeloma xenografts, which were unresponsive to MVNIS therapy. However, MV-resistant MM1 tumors regressed completely when 131I was administered 9 days after a single intravenous injection of MV-NIS (radiovirotherapy). 131I alone had no effect on MM1 tumor growth. While the potential hematopoietic toxicity of this new therapy requires further evaluation, image-guided radiovirotherapy is a promising new approach to the treatment of multiple myeloma, an incurable but highly radiosensitive plasma cell malignancy. Testing in other radiosensitive cancers is warranted.
Introduction
Multiple myeloma is a disseminated malignancy of antibody-secreting plasma cells that reside in active bone marrow. Clinical features of the disease include bone pain, lytic lesions, pathologic fractures, hypercalcemia, anemia, suppression of humoral immunity, and renal dysfunction caused by the tumor-derived monoclonal immunoglobulin.1 In most patients, the fraction of proliferating cells is less than 1% until late in the disease.2 Standard therapy is with alkylating agents (melphalan, cyclophosphamide) plus prednisone or combination chemotherapy (vincristine, doxorubicin, and dexamethasone) followed by high-dose melphalan with stem cell rescue.1,3 At relapse, patients can be offered thalidomide or investigational drugs such as PS-341.3-5 The disease, however, remains incurable and new therapeutic approaches are required.
Myeloma cells are highly radiosensitive, and local radiotherapy provides effective palliation for painful bone lesions.6 However, the disseminated nature of myeloma precludes curative external beam radiation therapy due to unacceptable end organ toxicity.7 Bone-seeking radioisotopes that bind to bone mineral are being tested in multiple myeloma,8 but their appeal and efficacy are limited by their inability to penetrate into the centers of myelomatous bone marrow deposits.
Replicating viruses have considerable potential as cytoreductive agents for cancer.9,10 Of the oncolytic viruses currently under investigation, measles virus (MV) is naturally lymphotrophic11 and is therefore an appealing candidate for myeloma virotherapy. We previously reported that the attenuated Edmonston vaccine strain of measles (MV-Edm) has considerable oncolytic activity against both lymphoid and nonlymphoid malignancies,12-14 and this activity is retained when the virus is engineered to express additional genes.14,15 MV-Edm could selectively infect and destroy myeloma cell lines as well as primary myeloma cells, while sparing nontransformed lymphocytes, fibroblasts, and epithelial cells. In vivo, the virus was potently oncolytic against ARH-77 tumor xenografts in immunosuppressed mice, although repeated intravenous injections were usually required for complete tumor eradication.13 Also, tumors derived from some myeloma cell lines (eg, RPMI 8226 and MM1) persisted despite repeated virus injections. Thus, an engineered MV with increased oncolytic potency would be desirable.
A second issue associated with the use of MV-Edm as an oncolytic agent is the need for a convenient noninvasive strategy to monitor virus spread in the treated patient.16 Without reliable information about the number and location of virally infected cells it will be difficult to conduct meaningful pharmacokinetic, pharmacodynamic, dose-determining, and scheduling studies in human subjects. We previously generated oncolytic measles viruses expressing soluble marker peptides that allow noninvasive monitoring of viral gene expression, and one of these will soon be tested in the clinic.15 However, soluble marker peptides do not enhance the oncolytic effect of the virus and do not provide any information about the anatomic location of virally infected cells.
Radioiodine is efficiently trapped by experimental tumors transduced with a gene coding for the thyroidal iodide symporter (NIS), a membrane ion channel expressed on thyroid follicular cells.17-19 Tumor ablation can be achieved by administration of 131I as a source of ionizing radiation, and noninvasive assessment of NIS gene expression can be achieved through gamma-camera imaging of iodine-123 (123I) uptake. Local bystander killing is considerable because the average tissue-path length of the beta particles emitted by 131I is approximately 0.4 mm.20 NIS expression in thyroid tissue has been exploited for more than 50 years in clinical practice for thyroid imaging (with 123I or Technetium 99m[Tc99m] or ablation (with 131I) and for systemic therapy of well-differentiated thyroid malignancies.21 We recently reported that myeloma xenografts stably expressing NIS regressed completely after a single dose of 131I, even when half of the tumor cells did not express the gene.22
Here we report the generation of a replication competent MV engineered to express NIS in infected cells (MV-NIS). The virus retains the oncolytic potential of the parent virus and induces NIS expression in myeloma cell lines and primary myeloma cells. The distribution and expression of the virus in vivo can be monitored noninvasively over time, and the virus has an enhanced therapeutic effect whereby a relatively small dose of virus followed by 131I can lead to the eradication of resistant tumors.
Materials and methods
Cells
The rescue cells 293-3-4623 were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and geneticin (1.2 mg/mL).
Vero cells were maintained in DMEM with 5% FBS. ARH-77 and MM1 cells were maintained in RPMI 1640 with 10% FBS, while KAS-6/1 cells (D. F. Jelinek, Mayo Clinic, Rochester, MN) were maintained in RPMI with 10% FBS and supplemented with human interleukin-6 (IL-6, 1 ng/mL). Primary myeloma cells (CD138 enriched) were isolated from bone marrow aspirates of patients with established myeloma seen at our institution. Red blood cells in the aspirate were lysed and the nucleated cells washed. After a 15-minute incubation with an anti-CD138 antibody, the cells are washed again and separated using an immunomagnetic separator (AutoMACS; Miltenyi, Auburn, CA) according to the manufacturer's recommendations. The isolated cells had a purity of 95%. The use of bone marrow samples from patients with myeloma was approved by the institutional review board (IRB) of the Mayo Clinic and Foundation in compliance with federal regulations.
All growth media and serum were from Gibco BRL (Grand Island, NY), and human IL-6 was from R&D Systems (Minneapolis, MN).
Viruses
Human NIS (hNIS) was amplified by the polymerase chain reaction (PCR) using the following primers: 5′-CCCATCAACGCGTACGTAGCGCGCATGGAGGCCGTGGAGACCGGGGAA-3′ (sense) and 5′-GGGGTCGCGAATCGATCGCGAGACGTCAGCGCTGCGCGCATCAGAGGTTTGTCTCCTGCTGGT-3′ (antisense). The restriction sites for Mlu1 and AatII used for cloning are underlined. The reaction mixture was initially denatured at 94°C for 4 minutes followed by 25 cycles of denaturation at 94°C for 45 seconds, annealing at 58°C for 45 seconds, and elongation at 72°C for 2 minutes with a 5-minute extension after the last cycle. A proofreading polymerase (Pwo; Roche Diagnostics, Mannheim, Germany) was used for DNA amplification. The purified PCR product was cloned instead of the gene for green fluorescent protein in plasmid p (+) MHIrGFPV after digestion with Mlu1 and AatII to generate plasmid p (+) MV-NIS.
MV-NIS was rescued as previously described.23 Briefly, 293-3-46 cells were transfected with plasmids p (+) MV-NIS (5 μg) and pEMCLa (50 ng) using calcium phosphate precipitation, and, 3 days later, the producer cells were overlaid on Vero cells. Rescue was inferred from the observation of syncytium formation. Individual syncytia were isolated, lysed by vortexing, and used to infect new Vero cells. Cell-associated virus was released by freeze thawing the cells twice in liquid nitrogen and the cell lysates were cleared by centrifugation. For titration of virus stocks, serial logarithmic dilutions of the virus were used to infect Vero cells in 96-well plates, and the 50% tissue-culture infective dose (TCID50/mL) was determined 4 days later as described.23
One-step growth curves were determined by infecting Vero cells with MV-NIS or MV-Edm at a multiplicity of infection (MOI) of 3. Vero cells were seeded into 6-well plates at a density of 4 × 105 cells per well and allowed to attach at 37°C for 4 hours. The medium was aspirated and the cells were incubated with the recombinant viruses in Opti-MEM (1 mL) for 2 hours. After infection, the culture medium was changed to DMEM with 5% FBS and the cells kept at 32°C. Every 12 hours, cells were collected in Opti-MEM (Gibco BRL) and stored at –80°C. Virus titers were established as previously described.
In vitro iodide uptake studies
ARH-77 cells (1 × 106/mL) were infected with MV-Edm or MV-NIS at an MOI of 0.02 for 2 hours in Opti-MEM and resuspended in their media. In vitro iodide uptake studies were performed starting 48 hours after infection as previously described.24 Briefly, the cells were washed twice in Hanks balanced salt solution (HBSS) and resuspended in 0.8 or 0.9 mL HBSS with HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.3). Potassium perchlorate (100 μL of 10 μM solution) was added to the tubes with the 800 μL buffer and 100 μL NaI (125I), with an activity of 1 × 105 cpm/0.1 mL was added to each tube. After a 50-minute incubation at 37°C, the cells were washed with cold HBSS twice, and the activity in the cells was determined in a gamma counter. Primary myeloma cells were infected with MV-NIS at an MOI of 2, and iodide uptake studies were performed after 72 hours.
In vivo studies
All in vivo studies were performed with 6-week-old female CB17 severe combined immunodeficient (SCID) mice (Harlan Sprague Dawley, Madison, WI). At 24 hours after total body irradiation (250 cGy), mice were implanted in their right flank with 1 × 107 washed cells (ARH-77, MM1, or KAS-6/1) in 200 μL PBS. Tumor growth was determined by caliper measurements in 2 dimensions and the volume estimated using the formula a2b/2, where a is the smaller diameter. The mice were fed levo-thyroxine (5 mg/mL) in their drinking water to suppress thyroidal NIS expression.25 When the tumors reached a mean diameter of 0.6 cm, the mice were injected via the tail vein intravenously either with MV-Edm or MV-NIS (2 × 106 plaque forming units [pfu]). Tumor imaging was performed after the mice were injected with 123I (500 μCi[18.5 MBq]) intraperitoneally and imaged serially using a gamma camera (Helix System; Elscint, Haifa, Israel).
Tumor dosimetry was performed by serial imaging of mice at 1, 3, 5, 7, 15, and 24 hours after a single injection of 123I. Regions of interest were drawn around the mice and tumors, and the respective activities were corrected for gamma-camera background activity, the physical half-life of the isotope (13.2 hours), and image capture time with reference to the one-hour image. The absorbed dose was calculated by determining the area under the activity time curve for the tumors, corrected for the half-life of 131I (8 days), and multiplied with established “S” values. “S” is the absorbed dose per μCi (MBq) per hour of cumulative activity in the tumor.
For tumor eradication, 131I (1000 μCi[37 MBq]) was injected intraperitoneally in tumor-bearing mice 9 days after intravenous injection of virus (MV-Edm or MV-NIS). Tumor responses were determined by serial measurements of tumor growth. The mice were killed if the tumors grew to more than 10% of the weight of the mouse, if tumors ulcerated, or if the mice were unable to eat or drink. All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) and performed along Assessment and Accreditation of Laboratory Animal Care (AAALAC)–approved guidelines.
Statistics
Analysis of variance was performed by repeated measures analysis testing for differences in tumor volume among the treatment groups with time as a variable using SAS version 8 (SAS Institute, Triangle Park, NC).
Results
Generation and characterization of MV-NIS
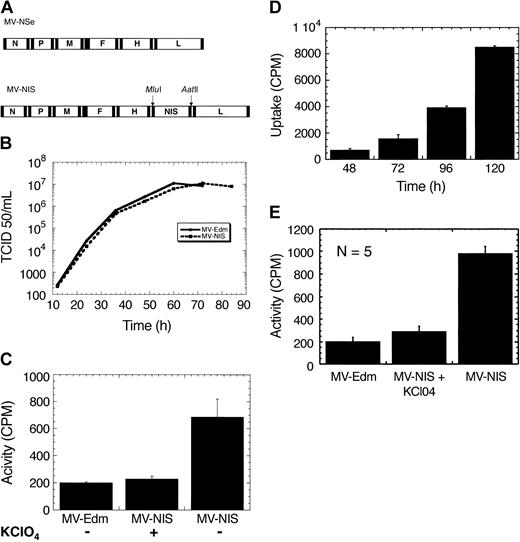
The cDNA for human NIS was inserted as an additional transcription unit downstream of the viral hemagglutinin gene in a full-length molecular clone of MV-Edm (Figure 1A) and the virus rescued.23 Parallel one-step growth curves for MV-Edm and MV-NIS showed that the addition of hNIS does not interfere with virus replication (Figure 1B).
Engineering, rescue, and characterization of MV-NIS. (A) Schematic representation of the genomes of MV-Edm and MV-NIS. hNIS was cloned as an additional transcription unit downstream of the hemagglutinin gene, H, using Mlu1 and Aat II. (B) One-step growth curves for MV-Edm and MV-NIS. Cloning of hNIS does not interfere with virus replication. (C) Myeloma cells infected with MV-NIS at an MOI of 0.02 express NIS and concentrate radioiodine. The data were obtained 48 hours after infection. Iodide uptake is blocked by perchlorate, a specific inhibitor of NIS. Cells infected with MV-Edm do not concentrate iodide. (D) MV-NIS replicates in myeloma cells with increasing NIS expression leading to higher iodide uptake with time. (E) Infection of primary myeloma cells with MV-NIS (MOI, 2) leads to significant iodide uptake 72 hours after infection. The control cells were infected with MV-Edm. There were 5 different primary myeloma cell samples studied with similar results. Each experiment was performed in triplicate. Error bars indicate SD.
Engineering, rescue, and characterization of MV-NIS. (A) Schematic representation of the genomes of MV-Edm and MV-NIS. hNIS was cloned as an additional transcription unit downstream of the hemagglutinin gene, H, using Mlu1 and Aat II. (B) One-step growth curves for MV-Edm and MV-NIS. Cloning of hNIS does not interfere with virus replication. (C) Myeloma cells infected with MV-NIS at an MOI of 0.02 express NIS and concentrate radioiodine. The data were obtained 48 hours after infection. Iodide uptake is blocked by perchlorate, a specific inhibitor of NIS. Cells infected with MV-Edm do not concentrate iodide. (D) MV-NIS replicates in myeloma cells with increasing NIS expression leading to higher iodide uptake with time. (E) Infection of primary myeloma cells with MV-NIS (MOI, 2) leads to significant iodide uptake 72 hours after infection. The control cells were infected with MV-Edm. There were 5 different primary myeloma cell samples studied with similar results. Each experiment was performed in triplicate. Error bars indicate SD.
Myeloma cell lines were infected with MV-NIS and incubated with 125I, which led to significant iodide uptake increasing with time due to virus replication and increasing NIS expression (Figure 1C). Iodide uptake was blocked by perchlorate, a specific inhibitor of NIS. Cells infected with the parent virus, MV-Edm, do not express NIS and do not concentrate radioiodine (Figure 1D). Primary CD138+ myeloma cells were isolated from 5 patient bone marrow aspirates and infected with MV-NIS at an MOI of 2, which led to NIS expression and efficient iodide uptake in vitro (Figure 1E). We estimated the intracellular iodide concentration in the infected primary cells to be 50-fold higher than that of the incubation medium.
In vivo imaging of MV-NIS
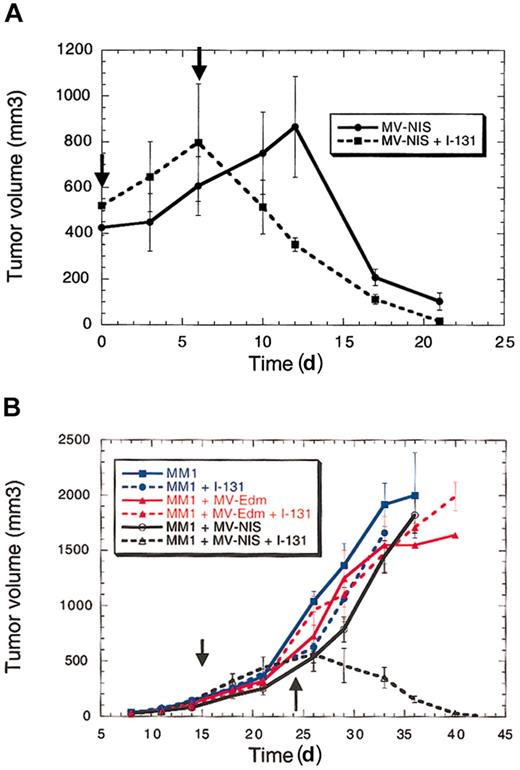
There were 3 different myeloma cell lines (ARH-77, KAS-6/1, and MM1) implanted subcutaneously in irradiated female SCID mice. When the tumors reached a mean diameter of 0.6 cm, the mice were injected intravenously with either MV-Edm or MV-NIS (2 × 106 pfu). After 3 days, the mice were injected with 123I (500 μCi [18.5 MBq]) and imaged one hour later using a gamma camera. The imaging procedure was repeated on days 9 and 17 following virus administration. As expected, there was no gamma photon signal from the myeloma xenografts in mice injected with MV-Edm due to lack of iodide uptake (Figure 2A). However, all MV-NIS–treated tumors were able to concentrate radioiodine and were readily visualized by gamma-camera imaging (Figure 2B-D). Viral gene expression and viral replication are tightly coupled. The mice were injected with a single dose of virus and imaged serially, each serving as their own control. All images were acquired one hour after injection of the same dose of isotope (500 μCi [18.5 MBq]) and adjusted for the same image intensity. Thus serial iodide uptake by the tumors should reflect increasing NIS expression and therefore serve as a surrogate for MV-NIS replication. Indeed, serial imaging of the mice over a period of 17 days shows that iodide uptake changes with time, reflecting MV-NIS replication and NIS expression in the tumors (Figure 2D-F). We calculated iodide uptake by the tumors one hour after isotope injection for all time points studied and established that NIS expression peaked approximately 9 days after virus injection. ARH-77 and MM1 tumors did not regress after a single dose of MV-NIS, but KAS-6/1 tumors regressed completely over a period of 3 weeks, indicating that MV-NIS retains the oncolytic activity of the parent virus, MV-Edm (Figure 4A).
In vivo imaging of myeloma tumor xenografts after MV infection. In panel A, an ARH-77 tumor in a mouse injected intravenously with MV-Edm does not concentrate 123I and gives no signal on the gamma camera 9 days after virus administration. Injection of MV-NIS in mice with MM1 (B), ARH-77 (C), and KAS-6/1 (D-F) tumors led to NIS expression and 123I uptake by the tumors in vivo 9 days after virus administration. Serial imaging of mice with KAS-6/1 tumors on days 3 (D), 9 (E), and 17 (F) after a single injection of MV-NIS shows changes in iodide uptake due to viral and tumor cell replication. All images are adjusted for equal background and were acquired over a 5-minute exposure. The large arrows point to the myeloma tumor xenografts in the right flanks of the mice. Magnification is 0.55 mm per pixel.
In vivo imaging of myeloma tumor xenografts after MV infection. In panel A, an ARH-77 tumor in a mouse injected intravenously with MV-Edm does not concentrate 123I and gives no signal on the gamma camera 9 days after virus administration. Injection of MV-NIS in mice with MM1 (B), ARH-77 (C), and KAS-6/1 (D-F) tumors led to NIS expression and 123I uptake by the tumors in vivo 9 days after virus administration. Serial imaging of mice with KAS-6/1 tumors on days 3 (D), 9 (E), and 17 (F) after a single injection of MV-NIS shows changes in iodide uptake due to viral and tumor cell replication. All images are adjusted for equal background and were acquired over a 5-minute exposure. The large arrows point to the myeloma tumor xenografts in the right flanks of the mice. Magnification is 0.55 mm per pixel.
Therapeutic efficacy of MV-NIS with or without radioiodine for multiple myeloma. (A) Radiovirotherapy results in faster tumor eradication compared with the virus alone in the susceptible tumor cell line KAS-6/1. (B) Radiovirotherapy for MM1 cell line–derived tumors that are not eradicated by MV-Edm. 131I by itself or MV-Edm with or without 131I does not eradicate the tumors. MV-NIS by itself does not slow tumor growth but in combination with 131I results in tumor eradication in all mice. There were 5 mice treated per arm of the study (30 mice total). The vertical arrows represent the time of vector and isotope injection. Radiovirotherapy is associated with prolonged survival in the treated mice. Error bars indicate SD.
Therapeutic efficacy of MV-NIS with or without radioiodine for multiple myeloma. (A) Radiovirotherapy results in faster tumor eradication compared with the virus alone in the susceptible tumor cell line KAS-6/1. (B) Radiovirotherapy for MM1 cell line–derived tumors that are not eradicated by MV-Edm. 131I by itself or MV-Edm with or without 131I does not eradicate the tumors. MV-NIS by itself does not slow tumor growth but in combination with 131I results in tumor eradication in all mice. There were 5 mice treated per arm of the study (30 mice total). The vertical arrows represent the time of vector and isotope injection. Radiovirotherapy is associated with prolonged survival in the treated mice. Error bars indicate SD.
Radiovirotherapy of myeloma tumor xenografts
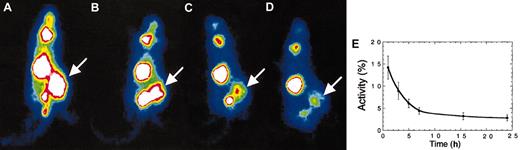
Radiation destroys cells by energy deposition in tissues. The total energy deposited is a function of the number of disintegrations that occur in the tumor and therefore depends on how long the radioisotope is retained by the tumor. Thus we evaluated the in vivo isotope retention time for MM1 tumor xenografts after a single dose of MV-NIS (intravenously). At 9 days after virus injection (2 × 106 pfu), mice were injected with 123I (500 μCi [18.5 MBq]) and were imaged serially under anesthesia (Figure 3A-D). The tumors absorbed 12% to 17% of the isotope injected and released it slowly with time, but some activity persisted even at 24 hours after injection (Figure 4E). Dosimetric calculations from the area under the activity time curve showed that these tumors would absorb at least 400 cGy from a dose of 1000 μCi (37 MBq) 131I.
In vivo iodide retention by tumor cells infected with MV-NIS. (A) MM1 tumors infected with MV-NIS concentrate iodide and retain it for at least 24 hours. At 9 days after intravenous injection of MV-NIS, mice were imaged serially at 1 (A), 3 (B), 5 (C), 7 (D), 15, and 24 hours after 123I injection. Images A-D were all acquired over 5 minutes and are adjusted for the same image intensity. (E) Quantification of iodide uptake and retention by MM1 tumors after infection with MV-NIS. MM1 tumors take up 12% to 17% of the isotope injected and retain it for at least 24 hours. Error bars indicate SD. Magnification is 0.55 mm per pixel.
In vivo iodide retention by tumor cells infected with MV-NIS. (A) MM1 tumors infected with MV-NIS concentrate iodide and retain it for at least 24 hours. At 9 days after intravenous injection of MV-NIS, mice were imaged serially at 1 (A), 3 (B), 5 (C), 7 (D), 15, and 24 hours after 123I injection. Images A-D were all acquired over 5 minutes and are adjusted for the same image intensity. (E) Quantification of iodide uptake and retention by MM1 tumors after infection with MV-NIS. MM1 tumors take up 12% to 17% of the isotope injected and retain it for at least 24 hours. Error bars indicate SD. Magnification is 0.55 mm per pixel.
Based on these dosimetric calculations, we proceeded to attempt myeloma xenograft eradication by combining the oncolytic effect of MV-NIS with high-energy electron therapy from 131I decay. Initially, we sought to determine whether combined viral and radioiodine therapy (radiovirotherapy) would impact the response of KAS-6/1. This cell line forms tumor xenografts in SCID mice that are eradicated slowly by a single intravenous dose of MV-NIS. In their right flank, 10 mice were implanted with washed KAS-6/1 cells. Tumor growth was measured in 2 dimensions using calipers, and when the tumors reached a mean diameter of 10 mm the mice were injected intravenously with MV-NIS (2 × 106 pfu). At 6 days after MV-NIS administration, 5 mice were injected with a single dose of 131I (1000 μCi [37 MBq], intraperitoneally). All tumors regressed but the combination of MV-NIS and radioiodine resulted in faster tumor eradication (Figure 4A). The difference in response rates was statistically significant with respect to both tumor volumes and time (P < .05). Thus, the combination of 131I with MV-NIS does not diminish the potency of the virus and may accelerate tumor regression in this myeloma xenograft model.
We next sought to determine whether radiovirotherapy is effective against MM1 myeloma xenografts, which are completely resistant to MV-Edm. There were 30 female SCID mice implanted with MM1 cells in their right flank; 10 mice were left untreated (controls), 10 were injected intravenously with MV-Edm (2 × 106 pfu), and another 10 with MV-NIS (2 × 106 pfu). Then, 9 days later, 5 mice from each group were injected with 131I (1000 μCi [37 MBq], intraperitoneally). Tumor growth was measured in 2 dimensions by calipers and overall survival recorded. As expected, radioiodine had no effect on uninfected MM1 tumors or tumors infected with MV-Edm (Figure 4B). MV-NIS by itself did not control the tumors, but the virus in combination with radioiodine led to complete tumor regression in 4 mice (Figure 4B). It is interesting to note that 1 of the 5 KAS-6/1 mice and 1 of the 5 MM1 mice that responded to radiovirotherapy died. Both mice were experiencing very rapid tumor regression, and we suspect that death was due to tumor lysis syndrome. The remaining mice have been observed for more than 2 months and the tumors have not recurred.
Discussion
In the last few years there has been renewed interest in the use of replicating viruses for cancer therapy.9,10,26,27 For a virus to be effective as an oncolytic agent for treatment of multiple myeloma, it should infect malignant plasma cells with high efficiency. Many viruses can be excluded on this basis, particularly adenoviruses, which have low infectivity on lymphoid cells.28 Measles virus, by contrast, is notable for its strong lymphotropism, and occasionally patients with lymphomas who acquired wild-type measles have experienced complete remissions.29,30 Attenuated strains of MV such as MV-Edm have been used to vaccinate millions of people with remarkable safety.31 MV-Edm has powerful oncolytic activity against a variety of tumor cells including myeloma, lymphomas, and ovarian carcinoma.12-14 Often, however, repeated doses of the virus are necessary for tumor control and some tumors do not respond.13 Thus, a more potent virus is desirable. We therefore generated a recombinant MV that expresses NIS. NIS expression allows noninvasive imaging of viral gene expression and enhances the oncolytic effect of the virus against resistant tumors when combined with radioiodine. A single and relatively small dose of the virus combined with 131I led to complete eradication of tumors that were resistant to MV-Edm alone.
Multiple myeloma is one of the most radiosensitive tumors known,6 and MV-NIS provides us with an opportunity to combine targeted radiation to myeloma deposits with a naturally oncolytic attenuated measles virus. Magnetic resonance imaging of patients with myeloma shows that the distribution of the disease in the bone marrow is frequently multifocal, comprising multiple discrete plasmacytomas.32 Plasmacytomas may provide favorable tumor geometry that maximizes particle cross fire within the tumor exposing noninfected myeloma cells to sufficient radiation to mediate their destruction. This favorable tumor geometry also increases the therapeutic effect of radioiodine by enhancing isotope reuptake and therefore retention time in the tumor (D.D. et al, manuscript in preparation, June 2003). Efficient infection of plasma cells by MV-NIS in vivo requires access of the virus to the tumor. Myeloma is associated with significant angiogenesis in the bone marrow, which may provide access of the virus to infect the cells.33
One likely toxicity of radiovirotherapy for multiple myeloma is that the accumulation of 131I in the bone marrow might suppress or eliminate normal hematopoiesis. This is more likely to be a significant concern where the marrow is diffusely infiltrated with malignant plasma cells, as opposed to the more classical focal disease distribution. It is difficult to study the potential impact of radiovirotherapy on hematopoiesis using our current myeloma xenograft model, but we plan to evaluate this problem experimentally in orthotopic models of multiple myeloma that recapitulate either focal or diffuse disease. However, it seems likely that stem cell transplantation will be necessary for some myeloma patients after radiovirotherapy. Our virus is not targeted to infect only myeloma cells, although our in vitro data suggest that the virus preferentially infects and replicates in tumor cells compared with nonmalignant cells.13,14 Measles virus infects primarily lymphoid cells and thus we expect minimal toxicity to additional organs from the virus and/or isotope, since nonlymphoid tissues are not infected significantly by the virus and thus should not express NIS and concentrate the isotope. However, we are working to develop viruses that do not bind to either of the known receptors for MV (CD46 and CD150) and should therefore infect cells with high selectivity and specificity via targeted receptors such as CD38. This should enhance the safety of these vectors for clinical studies and minimize toxicity.
Most adults are immune to MV infection either due to prior natural MV infection or vaccination. Preformed antibodies may neutralize a virus that is administered into the bloodstream, thereby blocking access of the virus to the tumor cells. However, patients with myeloma have a profound defect in the humoral arm of the immune system that progressively worsens with advancing disease.34 We studied a group of patients with advanced myeloma and found that many of them have low (nonprotective) levels of antimeasles antibodies (D.D., R. A. Kyle, and S.J.R., unpublished observations, April 2003). Thus, rapid neutralization of MV-NIS by circulating antibodies is less likely in myeloma patients than in patients with other malignancies.
The mechanistic basis for the striking oncolytic specificity of MV-Edm has not yet been determined but is the subject of ongoing investigations. One intriguing property of MV-Edm, acquired during tissue culture adaptation, that distinguishes it from wild-type measles virus is its ability to enter cells efficiently through CD46.35,36 CD46 is a regulator of complement activation that protects cells from complement-mediated lysis and is expressed at higher levels on human tumors (including myeloma) than on nontransformed cells.14,37,38 This may, in part, explain the observed tropism of the virus for malignant plasma cells compared with nontransformed peripheral blood lymphocytes.13,14 Also, in an effort to enhance the myeloma specificity of measles virus, we recently demonstrated that MV-Edm could be engineered to enter cells through the myeloma cell surface antigen CD38 by genetically fusing a single-chain antibody to the C-terminus of its H attachment protein.39
In summary, to enhance the oncolytic potency of MV-Edm against radiosensitive malignancies and to facilitate noninvasive imaging of infected tissues, we generated a recombinant MV-Edm encoding the human thyroidal iodide symporter (NIS). MV-NIS retains the oncolytic activity of the parent virus (MV-Edm), and its spread can be followed noninvasively in vivo using 123I. When combined with 131I (radiovirotherapy) MV-NIS can eliminate tumors resistant to the virus alone. Image-guided radiovirotherapy is a promising new approach to the treatment of multiple myeloma, and testing in other malignancies is currently under way.
Prepublished online as Blood First Edition Paper, November 6, 2003; DOI 10.1182/blood-2003-07-2233.
Supported by grants from the National Institutes of Health (NIH; CA100634 and CA62242), the Harold W. Siebens Foundation, and the Multiple Myeloma Research Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank R. Fonseca and D. F. Jelinek for the MM1 and KAS-6/1 cell lines, respectively; S. Vongpunsawad, Royce Ruter, Greg Ahmann, and Christy Finke for excellent technical support; A. Gabriela Rosales for help with the statistical analysis; and M. Craft for secretarial assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal