Abstract
Chronic hepatitis C is often asymptomatic, at least during the first decade following hematopoietic stem cell transplantation. Progression to advanced liver disease or cirrhosis in patients surviving more than 10 years is currently thought to be rare. Among 1078 patients who underwent an allogeneic transplantation between January 1973 and January 1995, 96 patients infected by hepatitis C virus (HCV) during the transplantation period were studied. Cumulative incidence and analysis of risk factors for cirrhosis were analyzed, and the rate and risk of cirrhosis in transplant recipients were compared with those of 158 HCV-infected controls who did not receive transplants. At a median follow-up of 15.7 years, 15 patients developed biopsy-proven cirrhosis, leading to a cumulative incidence of cirrhosis of 11% and 24% at 15 and 20 years, respectively. By multivariate analysis, extrahepatic HCV manifestations and HCV genotype 3 were associated with risk of cirrhosis. The median time to cirrhosis in transplant recipients was 18 years as compared with 40 years in the control population. The risk of cirrhosis in transplant recipients relative to controls was significantly higher by multivariate analysis (P = .0008). Roughly a quarter of long-term HCV-infected survivors with transplants progressed to cirrhosis that is much more rapid than in patients without transplants. Systematic detection of HCV infection, liver biopsy, and therapeutic intervention are therefore warranted in long-term marrow transplant recipients.
Introduction
Since its introduction nearly 3 decades ago, bone marrow transplantation has become a widely used therapeutic instrument to cure patients with malignant and nonmalignant hematologic disorders. Nearly 90% of the patients who survived more than 2 years after the procedure free of their original disease are expected to become long-term survivors, leading to thousands of cured patients worldwide.1 The late clinical effects of hematopoietic stem cell transplantation are thus of major concern in the 21st century.
Hepatitis C is the most common chronic blood-borne infection in the United States. The Centers for Disease Control estimated that during the 1980s, an average of 230 000 new infections occurred each year. Although the annual number of new infections has declined by more than 80% since the 1990s, population-based studies indicate that 40% of chronic liver diseases are hepatitis C virus (HCV) related. HCV is transmitted primarily through blood exposure. However, blood transfusion, which accounted for a substantial proportion of cases of HCV infection acquired more than 10 years ago, rarely accounts for recently acquired infections owing to systematic screening of blood products for HCV.2,3 Nevertheless, long-term survivors who received stem cell transplants from the late 1970s until the early 1990s, when efficient blood-screening procedures for HCV were introduced, were at high risk for HCV contamination. Indeed, prospective studies of transfusion recipients in the United States demonstrated that the rates of posttransfusion hepatitis in the 1970s exceeded 20%.2
A recent prospective study of the European Group for Blood and Marrow Transplantation, which included patients who received transfusions in the “postscreening” era, showed that the prevalence of HCV RNA–positive stem cell transplant recipients was 6.0%.4 Thus, chronic hepatitis C in long-term survivors remains an important clinical issue in this setting.5 Chronic hepatitis C is often asymptomatic, with fluctuating transaminase levels and no signs or symptoms of decompensated liver disease at least during the first decade following hematopoietic stem cell transplantation.5,6 Progression to cirrhosis in patients surviving more than 10 years is currently thought to be rare.5,6
In the present study, we report that a significant proportion of such long-term survivors did develop cirrhosis and hepatocellular carcinoma. We also aimed to describe risk factors of progression to cirrhosis in these patients and compared the rate of progression to cirrhosis in patients with transplants versus a cohort of HCV-infected individuals who have received transfusions but not transplants and in whom the date of infection was known (from age at onset of drug use or date of transfusion). It turned out that long-term HCV-infected survivors who had received transplants progressed to cirrhosis in roughly a quarter of the cases, but also seemed to progress to cirrhosis more rapidly than patients who had not received transplants. We thus would like to alert the medical community on this issue since treatment of hepatitis C with the combination of interferon and ribavirin has been proven to be effective in patients who have not had transplants.
Patients and methods
Study design and patient recruitment
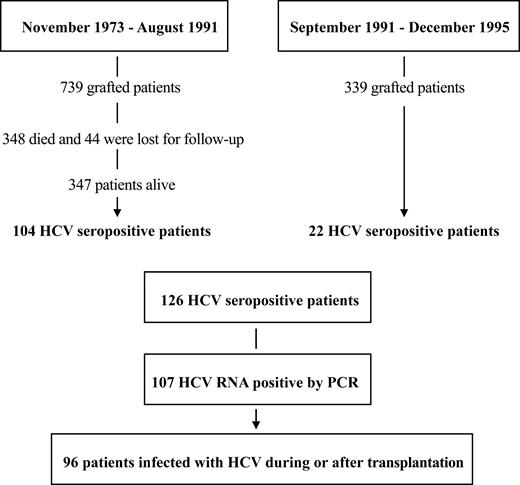
This retrospective single-center study was conducted in the Department of Bone Marrow Transplantation of the Hôpital Saint Louis (Paris, France). All patients who underwent an allogeneic transplantation between November 1973 and January 1995 were evaluated for HCV infection. For every subject included in the study, a data collection form was filled in. The long-term follow-up of these patients included at least a yearly visit for the first 10 years after the transplantation and every 2 years thereafter. At the long-term follow-up visit, HCV clinical manifestations were noted, liver function tests were routinely done, and liver biopsies were performed when indicated (see “Factors analyzed in the long-term follow-up”). Immunosuppressive therapy for graft-versus-host disease (GVHD) prophylaxis or treatment and HCV-specific treatment were also recorded (and their effects, if any). All patient, disease, and transplantation characteristics were prospectively recorded in a personal database. From November 1973 (date of first transplantation in our unit) until September 1991 (date of third-generation HCV serologic test systematic screening), 739 patients underwent allogeneic bone marrow transplantation at the Hôpital Saint Louis. From September 1991 to December 1995 (last patient included in this survey was enrolled on October 12, 1995), an additional 339 patients underwent transplantation. We found 107 patients to be HCV+ by reverse transcription–polymerase chain reaction (RT-PCR). Our main interest was to analyze the role, if any, of the transplantation procedure in the history of HCV infection after transplantation. We thus decided to retain and analyze data on 96 patients who were infected after the transplantation procedure (Figure 1). Finally, since reliable HCV testing was not available until September 1, 1991, and since patients who died before this date could not all be retrospectively tested for HCV infection, no attempt was made to calculate the cumulative incidence of HCV infection in this cohort.
Flow chart of the patient population. From November 1973 to December 1995, 1078 patients underwent bone marrow transplantation.
Flow chart of the patient population. From November 1973 to December 1995, 1078 patients underwent bone marrow transplantation.
Patients with HCV infection
Patient, disease, and transplantation characteristics of 96 patients who were infected during the transplantation period were retained for the analysis and are summarized in Table 1. The mean age of the patients at transplantation was 19.4 years (range, 3-53 years); 53 (55%) were female and 43 (45%) were male. Details concerning transplantation procedures and clinical care in our unit have been already described.7,8
Patient, donor, and transplantation characteristics, N = 96
Characteristic . | Value . |
|---|---|
| Age, y, median (SD) | 19.4 (12.6) |
| Sex, male/female, no. | 53/43 |
| Primary disease, no. | |
| Hematologic malignancy | 50 |
| Acute leukemia | 26 |
| Chronic myelogenous leukemia | 23 |
| Myeloma | 1 |
| Aplastic anemia | 40 |
| Other diagnoses* | 6 |
| Donor type, no. | |
| HLA-identical sibling | 82 |
| Unrelated | 14 |
| Conditioning regimen, no. | |
| TBI containing | 79 |
| Cyclophosphamide plus TBI with or without other drug | 77 |
| Other | 2 |
| Non-TBI containing | 17 |
| Cyclophosphamide alone | 11 |
| Busulfan plus cyclophosphamide | 6 |
| GVHD prophylaxis, no. | |
| Cyclosporine alone | 25 |
| Methotrexate alone | 14 |
| Cyclosporine plus methotrexate with or without other drug | 47 |
| Cyclosporine with or without prednisone | 3 |
| Other regimen | 3 |
| None† | 4 |
| HCV genotype, no. | |
| Type 1 | 35 |
| Type 2 | 14 |
| Type 3 | 12 |
| Type 4 | 4 |
Characteristic . | Value . |
|---|---|
| Age, y, median (SD) | 19.4 (12.6) |
| Sex, male/female, no. | 53/43 |
| Primary disease, no. | |
| Hematologic malignancy | 50 |
| Acute leukemia | 26 |
| Chronic myelogenous leukemia | 23 |
| Myeloma | 1 |
| Aplastic anemia | 40 |
| Other diagnoses* | 6 |
| Donor type, no. | |
| HLA-identical sibling | 82 |
| Unrelated | 14 |
| Conditioning regimen, no. | |
| TBI containing | 79 |
| Cyclophosphamide plus TBI with or without other drug | 77 |
| Other | 2 |
| Non-TBI containing | 17 |
| Cyclophosphamide alone | 11 |
| Busulfan plus cyclophosphamide | 6 |
| GVHD prophylaxis, no. | |
| Cyclosporine alone | 25 |
| Methotrexate alone | 14 |
| Cyclosporine plus methotrexate with or without other drug | 47 |
| Cyclosporine with or without prednisone | 3 |
| Other regimen | 3 |
| None† | 4 |
| HCV genotype, no. | |
| Type 1 | 35 |
| Type 2 | 14 |
| Type 3 | 12 |
| Type 4 | 4 |
SD indicates standard deviation; HLA, human leukocyte antigen; and TBI, total body irradiation.
Thalassemia (n = 2), Glanzmann thrombasthenia, Diamond-Blackfan syndrome (n = 2), dyskeratosis congenital.
Transplantation in which the donor and recipient were twins.
Virological studies
Serum samples from all patients were examined for the presence of HCV antibodies and for HCV RNA. Screening for HCV antibodies was performed by enzyme immunoassay with the AxSYM HCV test, version 3.0 (Abbott Laboratories, Rungis, France). Detection of serum HCV RNA was performed by RT-PCR assay by means of the Cobas Amplicor HCV assay, version 2.0 (Roche Diagnostic, Meylan, France), as recommended by the manufacturers, with a sensitivity limit of 50 IU/mL. Among 126 HCV-seropositive patients, 107 were HCV RNA positive. Since our aim was to study the influence of transplantation on the natural history of HCV infection, we analyzed only patients proven to be infected at the time of, or just after, the graft. For every patient, we indeed found the date of contamination. In fact, in 1990 when HCV infection became a significant recognized problem, one of us (R.T.) used frozen serum samples that were available for PCR analysis (in all but 4 patients) to ascertain that patients were contaminated after transplantation (n = 92). The 4 remaining patients for whom we did not have frozen pretransplantation samples did not receive transfusions before the graft but had positive PCR after transplantation. Thus, 96 patients eligible for the study were all HCV RNA positive, all but 4 being anti-HCV–. Two patients had HCV+ donors. HCV genotyping was performed by means of reverse hybridization with the line-probe assay by means of the INNO-LiPA HCV II kit (Bayer Diagnostic, Cergy Pontoise, France).
Factors analyzed in the long-term follow-up
HCV-related complications. Hepatic complications, as well as extrahepatic manifestation of HCV, were assessed by means of stringent criteria. For each patient, the following signs or symptoms were recorded: abnormal thyroid function, vasculitis purpura, Raynaud phenomenon, arthralgia, myalgia, sicca syndrome (ocular or oral),9 peripheral neuropathy,10 and renal dysfunction.11 Nonliver manifestations were considered to be due to HCV if no other transplantation-related cause could be found (particularly chronic graft-versus-host disease); these included clinical manifestations of cryoglobulinemia, neuropathy, renal manifestations (biopsy proven), Porphyria cutanea tarda,11 peripheral thrombocytopenia,12 and sicca syndrome.11 During the posttransplantation period, serum tests for levels of serum aspartate aminotransaminase (AST), serum aminotransaminase alanine (ALT), gamma glutamyl transferase (GGT), alkaline phosphatase, and bilirubin were carried out by standard methods. Levels were systematically determined and recorded before transplantation, at 6 months, and yearly during the follow-up for each patient. Patients with evidence of increased AST or ALT levels underwent liver biopsy according to standard procedure.13 A single experienced pathologist read all liver biopsies, and lesions were retrospectively graded (inflammation) and staged (fibrosis) according to the Knodell scoring system.14 Biopsies were also assessed for the following factors: veno-occlusive disease (VOD), GVHD, iron overload, nonviral infectious manifestations, and steatosis. Sixty-seven patients underwent liver biopsies. These results were then compared with a cohort of 158 HCV-infected patients without transplants but with known date of infection who underwent a liver biopsy from November 2000 to July 2001 in the Liver Unit of Hôpital Beaujon (Clichy, France).15 Controls were untreated consecutive adults. Liver biopsy was performed prior to any antiviral therapy. Among 290 consecutive patients followed in the liver unit with chronic hepatitis C, duration of infection was available for 158. This comparison group includes both intravenous drug use (IVDU)–acquired and transfusion-acquired HCV. For patients with IVDU-acquired hepatitis C, the date of contamination was estimated, since the majority of them acquired HCV infection the first year of drug abuse. These patients are more similar to the transplant patients in terms of age at infection (young age at infection) than patients with transfusion-acquired HCV.
Long-term use of immunosuppressive therapy. The following drugs used either alone or in combination were recorded: corticosteroids, methotrexate, azathioprine, cyclosporine, or antithymocyte globulin. For each drug, the number of months of therapy, from the end of the first year to last follow-up, was recorded.
Secondary cancers and late infections. All patients were carefully checked for the occurrence of any cancer. Bacterial, fungal, viral, and protozoan infections were listed when microbiologically documented (blood or tissue samples) and when these appeared after the first year of follow-up.
Statistical analysis
The reference date was January 1, 2003. Cumulative incidence of cirrhosis, cancer, and infection was estimated in a competing-risks setting, with death treated as the competing event. The prognostic value of the following variables was tested by means of the Gray test16 for qualitative variables and the Wald test in the subdistribution of Fine and Gray17 : age; sex; diagnoses (aplastic anemia versus other hematologic disorder); donor type (HLA-identical sibling donors versus others); conditioning regimen (irradiation based versus other); posttransplantation immunosuppressive therapy (methotrexate or methotrexate plus cyclosporine versus other regimen); VOD of the liver (yes/no); acute GVHD of grade II or greater; use of steroids more than 2 mg/kg; chronic GVHD; use of azathioprine; liver tests before transplantation (normal versus abnormal); HCV treatment; grade of inflammation and stage of fibrosis on liver biopsy; alcohol consumption; and HCV-related disorders. HCV genotypes (known in 65 patients) were analyzed as type 1, 2, or 3 and as type 3 versus other type. Multivariate analysis was performed by means of a stepwise procedure to construct a set of independent predictors of each end point, in which all variables achieving a P value of less than .15 were considered and were sequentially removed if, in the multiple model, the P value was more than .05. All tests were 2-sided, and the type 1 error was fixed at 0.05. Statistical analyses were performed by means of the Splus2000 software package (Math-Soft, Seattle, WA).
Results
Long-term clinical outcomes and non–HCV-related complications
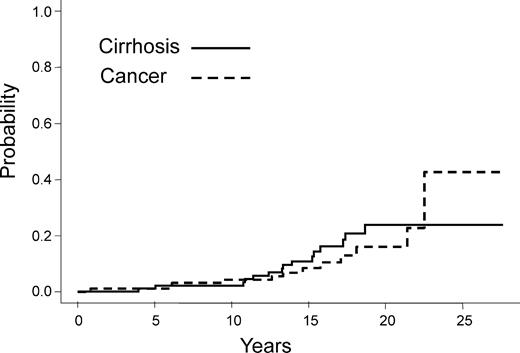
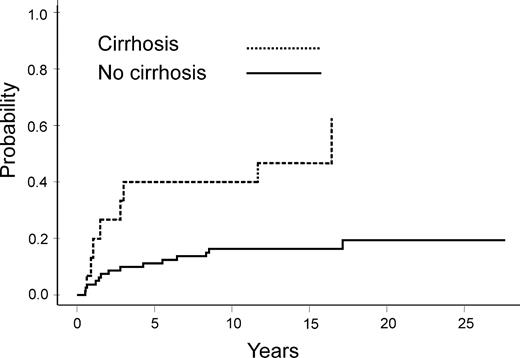
The median follow up was 15.8 years (range, 8-28.1 years). Thirty-eight patients (41%) died owing to infection (n = 9), GVHD (n = 9), end-stage liver disease related to HCV (n = 8, including 3 cases of hepatocellular carcinoma), secondary cancer (n = 3), relapse of the primary disease, and other causes (n = 9). As of January 1, 2003, 58 patients were alive. During the early posttransplantation period, 4 patients developed VOD disease (2 pathologically proven); 29 patients developed grade II or higher GVHD (20 developed grade II; 8, grade III; and 1, grade IV). Fifty-five patients presented with chronic GVHD, 22 with limited and 33 with extensive GVHD. At the date of analysis, 14 patients developed a secondary cancer, including 3 hepatocellular carcinomas. Other malignancies included squamous cell carcinoma in 6 cases, and 1 case each of papillary carcinoma of the thyroid, sarcoma, non-Hodgkin lymphoma, melanoma, and basal cell carcinoma. The cumulative incidence of secondary cancer reached 16% at 20 years after transplantation (95% confidence interval, 10%) (Figure 2). Twenty-five patients had at least one severe microbiologically proven bacterial infection beyond a year, leading to a 26% cumulative incidence rate of such infections at 20 years (95% confidence interval, 10%). Of note, patients who developed cirrhosis had a higher infection rate as compared with those who did not (P =. 001) (Figure 3). Other types of infection are described in Table 2.
Cumulative incidence of cirrhosis and cancer in patients who became HCV infected during or after transplantation.
Cumulative incidence of cirrhosis and cancer in patients who became HCV infected during or after transplantation.
Cumulative incidence of late (more than 1 year) bacterial infection in HCV-infected patients with and without cirrhosis.
Cumulative incidence of late (more than 1 year) bacterial infection in HCV-infected patients with and without cirrhosis.
Infections occurring after one year in transplant recipients infected with HCV
Infection . | No. (%) . |
|---|---|
| Viral cases | 44 (45.8) |
| Chronic hepatitis B virus | 3 |
| Varicella-zoster infection | 33 |
| Epstein-Barr virus | 1 |
| HIV | 1 |
| Measles | 1 |
| Cytomegalovirus disease | 5 |
| Bacterial cases | 25 (26) |
| Patients with at least one bacterial infection | 25 |
| Type of infection* | |
| Escherichia coli with or without other species | 11 |
| Pneumococcus with or without other species | 10 |
| Haemophilus with or without other species | 9 |
| Pseudomonas aeriginosa with or without other species | 2 |
| Klebsiella | 1 |
| Streptococcus plus Salmonella | 1 |
| Site of first bacterial infection | |
| Lung with or without other site | 15 |
| Kidney | 2 |
| Meninges | 3 |
| Other site† | 5 |
| Fungal/parasitic cases | 8 (8.3) |
| Aspergillosis | 3 |
| Pneumocytosis | 3 |
| Toxoplasmosis | 2 |
Infection . | No. (%) . |
|---|---|
| Viral cases | 44 (45.8) |
| Chronic hepatitis B virus | 3 |
| Varicella-zoster infection | 33 |
| Epstein-Barr virus | 1 |
| HIV | 1 |
| Measles | 1 |
| Cytomegalovirus disease | 5 |
| Bacterial cases | 25 (26) |
| Patients with at least one bacterial infection | 25 |
| Type of infection* | |
| Escherichia coli with or without other species | 11 |
| Pneumococcus with or without other species | 10 |
| Haemophilus with or without other species | 9 |
| Pseudomonas aeriginosa with or without other species | 2 |
| Klebsiella | 1 |
| Streptococcus plus Salmonella | 1 |
| Site of first bacterial infection | |
| Lung with or without other site | 15 |
| Kidney | 2 |
| Meninges | 3 |
| Other site† | 5 |
| Fungal/parasitic cases | 8 (8.3) |
| Aspergillosis | 3 |
| Pneumocytosis | 3 |
| Toxoplasmosis | 2 |
More than one species may be recovered from an infection site.
Each of the 5 cases involved a single organ: liver, sinus, skin, joint, or the digestive tract.
HCV-related complications
Fifteen patients were diagnosed with cirrhosis at a mean elapsed time of 13 years after transplantation (range, 4.0-18.7 years). In all cases, cirrhosis was biopsy proven. Table 3 lists the clinical features of these 15 patients with cirrhosis. Of these patients, 10 presented clinical features of portal hypertension or hepatic insufficiency: variceal hemorrhage (n = 5), ascitis (n = 5), hypersplenism (n = 4),and encephalopathy (n = 2). When deaths from other causes were considered as a competing event, the cumulative incidence of cirrhosis was 2%, 11%, and 24% at 10, 15, and 20 years, respectively (Figure 2). The evolution of liver enzymes (transaminases), as a function of time since transplantation was similar in patients who developed cirrhosis as compared with those who did not. Among patients with cirrhosis, 3 developed biopsy-proven hepatocellular carcinoma. The cumulative incidence of hepatocellular carcinoma was 5% at 20 years after transplantation (95% confidence interval, 4%).
Clinical features of the 15 patients with cirrhosis
Patient . | Age at BMT, y . | Sex . | Time from transplantation to cirrhosis diagnosis, y . | Immunosuppressive therapy after 1 year, mo . | Clinical manifestations . | HCV-related disorder . | Genotype . | Cause of death . |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | M | 5 | 0 | Ascitis, SBP, VH, encephalopathy | — | NA | Liver failure |
| 2 | 15 | M | 15.8 | 0 | Ascitis, EV | — | 1 | HCC |
| 3 | 40 | F | 10.8 | 1 | EV, VH | Cryoglobulinemia, neuropathy | 3 | — |
| 4 | 18 | M | 4 | 0 | Hypersplenism | Sicca syndrome | 2 | Liver failure |
| 5 | 5 | M | 18.7 | 0 | Hypersplenism, EV | — | NA | Liver failure |
| 6 | 35 | F | 12.5 | 0 | Ascitis, SBP, EV, VH | Glomerulonephritis,* PCT | 3 | Liver failure |
| 7 | 34 | M | 13.4 | 0 | EV, VH | Sicca syndrome | 3 | Liver failure |
| 8 | 19 | M | 15.3 | 27 | — | Cryoglobulinemia | NA | — |
| 9 | 22 | M | 17.2 | 0 | — | — | 1 | — |
| 10 | 12 | M | 11.4 | 0 | — | Thrombocytopenia | 3 | — |
| 11 | 24 | M | 11 | 106 | Hypersplenism | Glomerulonephritis* | 1 | HCC |
| 12 | 32 | M | 14 | 17 | — | — | 1 | HCC |
| 13 | 22 | M | 17.4 | 11 | — | — | 1 | — |
| 14 | 33 | M | 15.3 | 96 | Ascitis, SBP, EV, hypersplenism | Glomerulonephritis* | 3 | — |
| 15 | 46 | F | 13.3 | 29 | Ascitis, SBP, EV, VH, encephalopathy | Neuropathy | 3 | — |
Patient . | Age at BMT, y . | Sex . | Time from transplantation to cirrhosis diagnosis, y . | Immunosuppressive therapy after 1 year, mo . | Clinical manifestations . | HCV-related disorder . | Genotype . | Cause of death . |
|---|---|---|---|---|---|---|---|---|
| 1 | 10 | M | 5 | 0 | Ascitis, SBP, VH, encephalopathy | — | NA | Liver failure |
| 2 | 15 | M | 15.8 | 0 | Ascitis, EV | — | 1 | HCC |
| 3 | 40 | F | 10.8 | 1 | EV, VH | Cryoglobulinemia, neuropathy | 3 | — |
| 4 | 18 | M | 4 | 0 | Hypersplenism | Sicca syndrome | 2 | Liver failure |
| 5 | 5 | M | 18.7 | 0 | Hypersplenism, EV | — | NA | Liver failure |
| 6 | 35 | F | 12.5 | 0 | Ascitis, SBP, EV, VH | Glomerulonephritis,* PCT | 3 | Liver failure |
| 7 | 34 | M | 13.4 | 0 | EV, VH | Sicca syndrome | 3 | Liver failure |
| 8 | 19 | M | 15.3 | 27 | — | Cryoglobulinemia | NA | — |
| 9 | 22 | M | 17.2 | 0 | — | — | 1 | — |
| 10 | 12 | M | 11.4 | 0 | — | Thrombocytopenia | 3 | — |
| 11 | 24 | M | 11 | 106 | Hypersplenism | Glomerulonephritis* | 1 | HCC |
| 12 | 32 | M | 14 | 17 | — | — | 1 | HCC |
| 13 | 22 | M | 17.4 | 11 | — | — | 1 | — |
| 14 | 33 | M | 15.3 | 96 | Ascitis, SBP, EV, hypersplenism | Glomerulonephritis* | 3 | — |
| 15 | 46 | F | 13.3 | 29 | Ascitis, SBP, EV, VH, encephalopathy | Neuropathy | 3 | — |
BMT indicates bone marrow transplantation; SBP, spontaneous bacterial peritonitis; VH, variceal hemorrhage; —, none; NA, not available; EV, esophageal varices; HCC, hepatocellular carcinoma; and PCT, porphyria cutanea tarda.
Biopsy-proven glomerulonephritis with glomerular infiltration with monocytes and intraluminal thrombi, without associated cryoglobulinemia.
An HCV-related disorder was diagnosed in 11 patients (11%), including 9 patients with and 2 patients without cirrhosis. HCV-related disorders in patients with cirrhosis are summarized in Table 3. All cases of membranoproliferative glomerulonephritis and cutaneous manifestations were pathologically proven; the 2 peripheral neuropathies and the 2 sicca syndromes occurred in patients without other etiology than HCV (in particular, not linked to chronic GVHD or toxic drugs). Two cases of peripheral thrombocytopenia were considered to be the direct consequence of HCV, and 2 patients presented with clinical manifestations of cryoglobulinemia. In the 2 patients without cirrhosis, these manifestations included one case each of cryoglobulinemia and peripheral thrombocytopenia. While these 2 patients did not have evidence of cirrhosis on liver biopsy, they did have stage III fibrosis.
HCV genotypes could be determined in 65 patients (68%) (Table 1). Among the 15 patients with cirrhosis, HCV genotype was available in 12 patients: 5, genotype 1; 1, genotype 2; 6, genotype 3; and no genotype 4. Eight patients died as a consequence of HCV infection: 5 from end-stage liver disease and 3 from hepatocellular carcinoma.
Treatment of HCV infection
During the study, 22 patients were treated for high inflammation grade and/or high stage of fibrosis. Nineteen patients received recombinant alpha interferon at a dose of 3 million units 3 times per week for 1 year. Two patients received ribavirin alone because of interferon contraindication; 1 patient received a combination of interferon and ribavirin. Among these 22 patients, 9 achieved complete biochemical response with normal ALT levels; 5 patients had decreases in ALT levels of more than 50%; and 8 patients had no biochemical response. Four patients are not yet evaluable because they just started treatment. At the end of the study, HCV RNA remained undetectable by PCR in only one patient. We noted an exacerbation of chronic GVHD during interferon treatment in one patient.
Risk factors for cirrhosis
Four factors were significantly associated with cirrhosis in univariate analysis: male sex (P = .03), VOD of the liver (P < .001), high-grade inflammation (Knodell score higher than 6) (P = .02), and HCV-related disorders (P < .001). In multivariate analysis according to the Fine and Gray test, HCV-related disorders (hazard ratio, 1.70; standard error, 0.59 [P = .004]) remained statistically associated with the development of cirrhosis. When the analyses were restricted to the 65 patients in whom HCV genotypes were available, genotype 3 also emerged as a significant risk factor in multivariate analysis (hazard ratio, 1.89; standard error, 0.61 [P = .002]).
Progression to cirrhosis as compared with a population that did not receive transplants
Sixty-seven of the 96 patients had a total of 104 liver biopsies after BMT (Table 4); 40 of these patients had 1 biopsy, 17 had 2 biopsies, and 10 had 3 biopsies. The median elapsed time from transplantation to last biopsy was 8.7 years. The 29 patients who did not have liver biopsies shared the same initial clinical characteristics (age at infection, alcohol consumption, sex, underlying diagnoses) and HCV genotypes as those who had biopsies. However, they all had persistently normal transaminase levels and thus did not undergo biopsies. In patients who had received transplants, liver biopsy also showed evidence of transplantation-related complications, including pathologic evidence of GVHD in 16 patients, VOD in 2 patients, steatosis in 23 patients, iron overload in 24 patients, and other non–HCV-related lesions in 11 cases, with some patients having more than one type of histologic lesion. Importantly, iron overload was noted in 4 patients among the 15 with cirrhosis (27%) as compared with 20 among the 52 patients without cirrhosis who had a liver biopsy (38%) (P statistically nonsignificant).
Pathologic findings in liver biopsies
. | Cirrhosis, no. (%) . | No cirrhosis, no. (%) . | Total, no. (%) . |
|---|---|---|---|
| HCV-associated lesions only | 9 (60) | 25 (48) | 34 (51) |
| GVHD | 2 (14) | 14 (27) | 16 (24) |
| VOD | 1 (7) | 1 (2) | 2 (3) |
| Iron overload | 4 (27) | 20 (38) | 24 (36) |
| Steatosis | 13 (86) | 10 (19) | 23 (34) |
. | Cirrhosis, no. (%) . | No cirrhosis, no. (%) . | Total, no. (%) . |
|---|---|---|---|
| HCV-associated lesions only | 9 (60) | 25 (48) | 34 (51) |
| GVHD | 2 (14) | 14 (27) | 16 (24) |
| VOD | 1 (7) | 1 (2) | 2 (3) |
| Iron overload | 4 (27) | 20 (38) | 24 (36) |
| Steatosis | 13 (86) | 10 (19) | 23 (34) |
Patient populations are as follows: cirrhosis, n = 15; no cirrhosis, n = 52; and total, n = 67. Patients may have different lesions associated in the same biopsy. In patients with cirrhosis, these associations included GVHD plus VOD (1 patient) and GVHD plus steatosis (1 patient). In patients without cirrhosis, these included GVHD plus steatosis (5 patients) and VOD plus steatosis (1 patient).
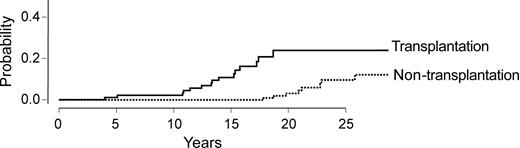
Then, 158 HCV-infected patients who had not received transplants and who had a known date of infection, clinical evaluation, and liver biopsy were compared with the marrow transplant recipients (Table 5). The cumulative incidences of cirrhosis in the 2 populations are represented Figure 4. Stem cell transplant recipients had an expected median time to cirrhosis of 18 years as compared with 40 years in the control population without transplants. We finally combined the 2 patient populations (with and without grafts) and analyzed the risk factors for developing cirrhosis, taking into account patient's age, sex, alcohol abuse, and whether or not the patient received a transplant. By multivariate analysis, only marrow transplantation emerged as a significant risk factor (hazard ratio, 1.47; standard error, 0.47 (P = .002]).
Characteristics of patients who did not receive a transplant (n = 158) compared with transplant recipients (n = 96)
. | Patients . | . | . | |
|---|---|---|---|---|
| Characteristic . | No transplant . | Transplant recipients . | P . | |
| Age, y (range) | 43 (24.5-71.5) | 19.4 (3.3-53.3) | < .0001 | |
| Male sex, no. (%) | 84 (53) | 53 (56) | Not significant | |
| Source of infection | ||||
| Transfusion, no. (%) | 68 (43) | Not applicable | Not applicable | |
| Intravenous drug user, no. (%) | 90 (57) | Not applicable | Not applicable | |
| Genotype* (1/2/3/4) | 74/4/38/15 | 35/14/12/4 | .001 | |
| Duration of HCV infection, y (range) | 20.6 (5-51) | 15.7 (13-19.5) | < .0001 | |
| Alcohol greater than 20 g/d, no. (%) | 36 (23) | 7 (7) | .001 | |
| Steatosis, no. (%) | 67 (42) | 23 (34) | NS | |
| Hemosiderosis, no. (%) | 22 (14) | 24 (36) | .0004 | |
. | Patients . | . | . | |
|---|---|---|---|---|
| Characteristic . | No transplant . | Transplant recipients . | P . | |
| Age, y (range) | 43 (24.5-71.5) | 19.4 (3.3-53.3) | < .0001 | |
| Male sex, no. (%) | 84 (53) | 53 (56) | Not significant | |
| Source of infection | ||||
| Transfusion, no. (%) | 68 (43) | Not applicable | Not applicable | |
| Intravenous drug user, no. (%) | 90 (57) | Not applicable | Not applicable | |
| Genotype* (1/2/3/4) | 74/4/38/15 | 35/14/12/4 | .001 | |
| Duration of HCV infection, y (range) | 20.6 (5-51) | 15.7 (13-19.5) | < .0001 | |
| Alcohol greater than 20 g/d, no. (%) | 36 (23) | 7 (7) | .001 | |
| Steatosis, no. (%) | 67 (42) | 23 (34) | NS | |
| Hemosiderosis, no. (%) | 22 (14) | 24 (36) | .0004 | |
Genotype was unknown in 27 transplant recipients, and genotype 2 is also more frequent in the transplant recipient group.
Cumulative incidence of cirrhosis in a comparison of HCV-infected patients who received a transplant with HCV-infected patients who had not undergone transplantation.
Cumulative incidence of cirrhosis in a comparison of HCV-infected patients who received a transplant with HCV-infected patients who had not undergone transplantation.
Discussion
In this study, we found that patients who were infected with HCV at the time of transplantation had an estimated cumulative incidence of cirrhosis of 24% at 20 years and that HCV infection ranked third, behind infection and GVHD, as a cause of late death. Extrahepatic manifestations and genotype 3 were found to be independently associated with cirrhosis. Moreover, we observed a more rapid rate of liver fibrosis progression in our population as compared with HCV-infected patients who did not receive a transplant. The expected median time to cirrhosis in allogeneic bone marrow transplant recipients was about 18 years as compared with 40 years in the control population, and by multivariate analysis only marrow transplantation emerged as a significant risk factor when the 2 populations were pooled.
While the risk of acquiring HCV infection is now extremely low, it is not uncommon for patients to come to transplantation already infected. Moreover, a large group of long-term stem cell survivors were infected by HCV during the 1990s before blood donors were routinely screened. For instance, in Seattle, WA, HCV was detected after transplantation in 113 (32%) of 355 patients who underwent hematopoietic cell transplantation (HCT) in 1987-1988.6,18 Whereas pre-existing HCV infection has clearly been associated with an increased risk of severe VOD,6,18-20 it is generally considered to run a benign course in the long term. In 1955, Ljungman et al21 concluded that HCV infection was not a major factor in morbidity and mortality during the first 5 to 10 years after allogeneic BMT. In another study, HCV infection did not affect the survival of long-term survivors over 10 years of follow-up.6,18 Therefore, the European Group for Blood and Marrow Transplantation (EBMT) group indicates that ongoing or previous infection with HCV in donor or recipient was not an absolute contraindication for hematopoietic stem cell transplantation.4 Furthermore, Tomas and coworkers,22 studying long-term liver dysfunction after marrow transplantation in 61 patients, did not observe an increased morbidity or mortality. Our results differ probably because the present cohort was larger and follow-up far longer than in these early studies. Indeed, cirrhosis was diagnosed beyond 10 years after transplantation in 13 of our 15 patients. The strength of these results is reinforced by the careful exclusion of patients who were infected with HCV before transplantation. Furthermore, 29 patients with normal liver enzymes did not have biopsies. It has been recently reported that asymptomatic patients with HCV may progress to cirrhosis.23 Thus, our data may indeed underestimate the cumulative incidence of cirrhosis in transplant recipients. However, HCV-related cirrhosis is a rare event. A previous publication by Strasser et al6 noted that the cumulative incidence of cirrhosis among all transplant patients was around 4% at 20 years; we found a 2.2% incidence, about the same as Strasser et al reported.
The rate of fibrosis progression in our population was higher than that of the control group, despite the fact that most transplant recipients were young at the onset of infection and did not consume alcohol. Age older than 40 years at the onset of infection, male sex, and alcohol consumption in excess of 50 g per day have repeatedly been associated with progression of fibrosis.24-27 The low rate of fibrosis progression in our control group is in accordance with previous studies.28,29 Among 376 women with HCV infection 17 years after being HCV-contaminated by anti-D immune globulin, 2% had probable or definite cirrhosis.28 In this study, the mean age at the onset of infection was 28 ± 6 years. As recently reviewed elsewhere, prospective cohort studies have shown that for persons who acquire HCV infection in young adulthood, fewer than 10% are estimated to develop cirrhosis within 20 years.24 A comparison of our data with these studies showed that our patients had all the characteristics usually associated with a low rate of fibrosis progression (young age at contamination, no alcohol consumption).
In multivariate analysis, we found that extrahepatic manifestations were associated with an increased risk of cirrhosis, as has been published in other settings.9,27,30 Inflammatory grade according to Knodell et al14 was not significantly associated with the risk of cirrhosis in our population. This may be due either to limited numbers that preclude any meaningful statistical analysis among subgroups or, as in patients with AIDS or recipients of organ transplants, to a more rapid onset of fibrosis without associated high-grade inflammatory activity in a setting of immune dysfunction (discussed later in this paragraph).31-36 Genotype 3 seemed to be associated with a higher risk of developing cirrhosis in our population. Although no association with genotype and disease progression in transplant recipients has been found, we earlier reported an association between HCV genotype 3 and progression of fibrosis.37 However, since only 65 patients had data on HCV genotype, this would clearly need confirmatory results. Although no clear association between genotype and disease progression has been found in patients who have received transplants, recent studies reported association between HCV genotype 3 and disease progression through steatosis.38 Hepatocyte steatosis has been recently considered to be a direct cytopathic effect of HCV genotype 3.39 Furthermore, we showed in a previous study that HCV genotype 3 was associated with higher quasi-species heterogeneity.40 Thus, our population differed from the control population only by the allogeneic immune setting, which may be involved in the genesis of liver fibrosis. Hepatic iron overload could have been an additional risk factor for fibrosis in this cohort.41 Indeed, in studying liver biopsies, we did not find any evidence for such an association. Furthermore, aplastic anemia patients, who were by far the group with the highest transfusion rate, did not show an increased risk of cirrhosis, as compared with other diagnoses. One striking possible explanation of the genesis of cirrhosis in our patients could be immune imbalance or impaired regulation between B and T cells. Indeed, we and others have found that, B- and T-cell cooperation is severely impaired in long-term stem cell transplant survivors.7,42 Evidence of this fine immune deficiency here came from patients with cirrhosis who developed late bacterial infection years before fibrosis at a higher rate as compared with those without cirrhosis. Evidence for an immune-mediated mechanism is further supported by data on HIV infection mechanisms and on solid organ transplant recipients, who developed cirrhosis more rapidly and earlier than other patient populations.31-35 Finally, extrahepatic, autoimmune-like manifestations in our patients may in fact represent allogeneic reaction of the donor immune system against the host.
Combination treatment with interferon and ribavirin has been available since 1998, and the combination of pegylated interferon plus ribavirin has recently been approved with higher efficacy in terms of sustained virological response.43,44 Only one of our patients has been treated with such an association, yet single therapy with standard interferon turned out to have low effectiveness in our experience. Thus, since hematopoietic stem cell transplant recipients seem to be at higher risk of earlier cirrhosis, it would probably be worthwhile to use the combination of pegylated interferon with ribavirin in selected long-term survivors following stem cell transplantation in the setting of multicenter trials. Recommendation of earlier combination therapy will depend on the results of these trials (in term of efficacy and tolerance).
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-06-2145.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank R. Porcher, PhD, for help with statistical analyses.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal