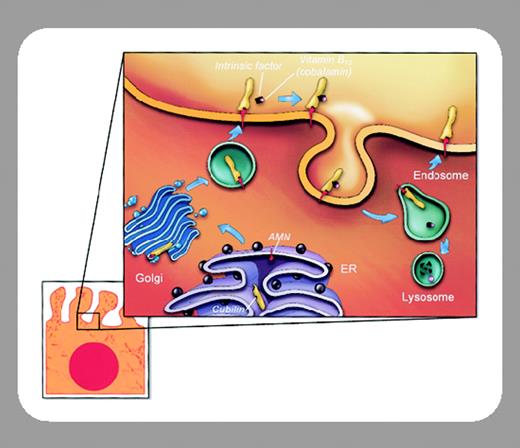
Patients with Imerslund-Gräsbeck syndrome (I-GS, megaloblastic anemia 1 [MGA1], hereditary megaloblastic anemia, Online Mendelian Inheritance in Man [OMIM] no. 2611001 ) usually present with megaloblastic anemia between 1 and 5 years of age. They have decreased levels of serum vitamin B12 (cobalamin) in the presence of normal levels of intrinsic factor (IF), and many patients have proteinuria of the tubular type. The Schilling test result is characteristic of the inability of enterocytes to absorb the intrinsic factor–cobalamin complex. Patients in the original studies were described as being Finnish or Norwegian. Currently, more than 250 patients have been identified, many of whom are of Middle Eastern descent. I-GS has locus heterogeneity; in most Finnish families, the disease is caused by mutations in the cubilin (CUBN) gene on chromosome 10p12.1.2 However, mutations in CUBN were not found in Norwegian patients showing the same phenotype. Through linkage studies, a candidate gene was located on the long arm of chromosome 14q32, and mutations were found in the AMN gene in the Norwegian patients.3 This gene emerged as a candidate because of an expression pattern similar to that of CUBN, with high levels of expression in both small intestine and kidney.FIG1
Ever since the unexpected discovery of mutations in either the CUBN or the AMN gene in patients with I-GS, it has become essential to address the interaction of the 2 gene products: cubilin and amnionless. Fyfe and colleagues (page 1573) show colocalization of cubilin and amnionless proteins in the apical membranes and endocytic apparatus of renal proximal tubule cells. They also demonstrate physical interaction between these 2 proteins following sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) affinity purification. Cotransfection of Chinese hamster ovary (CHO) cells with CUBN and AMN constructs alters the exclusively intracellular locations seen with transfection of CUBN alone, to include the plasma membrane, and allows the endocytosis of IF-cobalamin. These data reinforce findings in the canine model of I-GS in which cubilin is not expressed on the surface of intestinal and renal cells and is retained in an early biosynthetic compartment. Canine I-GS maps to a region orthologous to human chromosome 14 and presumably mutations in AMN will soon be found in affected dogs.
A number of questions are still unanswered. Megalin, a member of the low-density lipoprotein (LDL) receptor family, has been postulated to be involved in cubilin function. Previous work has shown the colocalization of cubilin with megalin, and megalin-deficient mice have decreased cubilin expression and uptake of cubilin ligands. The case for amnionless has been made much stronger than that for megalin because of the finding of mutations in AMN in I-GS and by the studies of Fyfe and colleagues. What, if anything, then is the physiologic role of megalin in cobalamin absorption? Also, both cubilin and amnionless are implicated in early embryonic development in rodents. Little is known about the mechanisms involved; the only known phenotype resulting from mutations in the human CUBN and AMN genes is I-GS. Finally, which ligands other than cobalamin–intrinsic factor interact with the new complex of cubilin and amnionless for which Fyfe and colleague have coined the name “cubam”?

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal