Abstract
Prophylactic fluconazole prevents candidiasis; however, this drug has no activity against molds. We performed a randomized trial to determine whether prophylactic itraconazole prevents invasive mold infections (IMIs). A total of 304 patients receiving allogeneic stem cell transplants (SCT) were randomized to receive fluconazole (400 mg/d) or itraconazole (oral solution 2.5 mg/kg 3 times daily, or intravenous 200 mg daily) for 180 days after SC transplantation, or until 4 weeks after discontinuation of graft-versus-host disease (GVHD) therapy. Proven or probable invasive fungal infections (IFI) were evaluated by intent-to-treat and “on-treatment” analyses. More patients in the itraconazole arm developed hepatotoxicities, and more patients were discontinued from itraconazole because of toxicities or gastrointestinal (GI) intolerance (36% versus 16%, P < .001). Intent-to-treat analysis demonstrated no difference in the incidence of IFI during the intended study period (fluconazole 16% versus itraconazole 13%, P = .46); however, fewer patients in the itraconazole arm developed IFI on treatment (fluconazole 15% versus itraconazole 7%, P = .03). Itraconazole provided better protection against IMI (fluconazole 12% versus itraconazole 5%, P = .03), but similar protection against candidiasis (3% versus 2%, P = .69). There was no difference in overall or fungal-free survival. Itraconazole appears to prevent IMI in the subset of patients who tolerate the drug; however, toxicities and poor tolerability limit its success as prophylactic therapy.
Introduction
Invasive fungal infections are a frequent cause of transplantation-related mortality. Randomized studies performed during the early 1990s established the efficacy of fluconazole for preventing invasive infection caused by Candida albicans.1,2 In high-risk recipients of allogeneic stem cell transplants (SCTs), prevention of candidiasis improved overall survival.3 However, invasive mold infections (IMIs), especially those caused by Aspergillus species, have become increasingly important. Studies report that the incidence of invasive aspergillosis may be as high as 10% to 20% in patients receiving allogeneic SCTs.4-7 Mold infections now typically occur both early after SC transplantations, during the neutropenic period, and later, during graft-versus-host disease (GVHD) and its treatment.4,6,8
Itraconazole is an azole antifungal that has activity against both Candida species and molds. Availability of a well-absorbed oral solution and an intravenous formulation has made prophylactic administration feasible in patients with gastrointestinal (GI) tract mucositis. In patients with hematologic malignancies and neutropenia, prophylactic itraconazole successfully prevents candidiasis; however, efficacy in preventing Aspergillus infections is unclear.9-12 We performed the current randomized trial in patients who received allogeneic SCTs to determine whether itraconazole, administered long term during GVHD, prevents invasive fungal infections, particularly aspergillosis, better than fluconazole.
Patients, materials, and methods
Patients
Patients who underwent allogeneic SCT after receipt of myeloablative conditioning at the Fred Hutchinson Cancer Research Center (FHCRC) between March 1999 and July 2001 were approached for participation in this study. People aged 13 years or older who weighed 40 kg or more were considered for inclusion. Exclusion criteria included pre-existing moderate-to-severe hepatic abnormalities (transaminase, total bilirubin, or alkaline phosphatase levels > 5 times the upper limit of normal), a history of anaphylaxis attributed to azole antifungals, documented or suspected invasive fungal infection within 10 days prior to SC transplantation, presence of uncontrolled bacteremia at time of SC transplantation, and concomitant receipt of a contraindicated drug (eg, astemizole, terfenadine, and cisapride). Randomization was stratified by factors associated with the development of GVHD and invasive fungal infections, specifically, age (≤ 20 or > 20 years) and human leukocyte antigen (HLA) matching of the stem cell graft (HLA-matched related or HLA-mismatched/unrelated). This trial was approved by the FHCRC Institutional Review Board. All subjects signed an informed consent.
Study activities
Open-label study drugs were administered from the beginning of conditioning therapy for a minimum of 120 days. Patients who had GVHD requiring therapy with corticosteroids continued study drugs until 4 weeks after completion of corticosteroid therapy, for a maximum of 180 days. Fluconazole was administered at 400 mg daily, oral or intravenous, with doses adjusted on the basis of renal function. Itraconazole was administered as oral solution (2.5 mg/kg 3 times daily) or by way of intravenous infusion (200 mg daily). Temporary suspension (< 2 weeks) of study drugs was allowed in the setting of toxicities (such as nausea), provided no other antifungal drug was administered. Study drugs were permanently discontinued when infection was suspected or confirmed (possible, probable, or proven), according to the clinical criteria described in “Infection definitions.” In the case of persistent fever during neutropenia without other signs or symptoms of IFI, study drugs were temporarily held during administration of amphotericin B products but were not permanently discontinued.
Itraconazole levels were measured by high-performance liquid chromatography (HPLC) 10 days after start or adjustment in dosing of oral drug and 3 days after start of intravenous drug. Dosages were increased by 0.5 mg/kg/d if itraconazole levels were less than 0.5 μg/mL on repeated testing. Itraconazole doses were not routinely adjusted in patients who had adequate levels (> 0.5 μg/mL), unless patients had complaints of nausea or vomiting, in which case oral dosing was decreased to twice daily. In this case, levels were followed to assure they remained more than 0.5 μg/mL. Dosing was also decreased to twice daily in people who had levels that exceeded 2 μg/mL with 3 times daily administration.
Galactomannan antigenemia was included as a diagnostic variable for invasive aspergillosis, as per consensus definitions of disease.13 As this assay was not available for clinical use, tests were performed on sera that had been stored frozen (results were not available to the clinicians during patient care). Sera were obtained weekly from start of therapy until discharge from the FHCRC ambulatory clinic (approximately 100-120 days after SC transplantation) and daily when fungal infection was suspected. Specimens were stored frozen (–70°C) for subsequent analysis with the Bio-Rad Platelia galactomannan enzyme immunoassay (EIA; Bio-Rad Laboratories, Redmond, WA). Reactions were performed as directed by the manufacturer. One serum sample having an optical density (OD) index of 1.0 or greater on repeated testing was considered positive.14 Itraconazole levels were analyzed in patients who developed infections while receiving study drug (identified as part of the “on-treatment” analysis, described in “Statistical considerations”). Only levels that were obtained within 1 month of the date of onset of infection were analyzed. All organisms that caused proven or probable IFI in patients participating in this study were stored frozen in water stocks (–20°C), and antifungal susceptibilities were evaluated at the end of the study. Fluconazole susceptibilities were measured only among Candida species, and itraconazole susceptibilities were evaluated for both Candida species and molds. Susceptibilities were measured by using methods defined by the National Committee for Consensus in Laboratory Standards (NCCLS)15 ; organisms that had fluconazole minimum inhibitory concentrations (MICs) of 16 μg/mL or more and itraconazole MICs of 1 μg/mL or more were considered resistant.16,17
Infection definitions
Fungal infections were graded as possible, probable, or proven, according to the laboratory and clinical criteria described in standardized guidelines.13 To allow for blinded grading, patient identifiers and treatment information was masked from clinical records, and 2 investigators (M.B. and W.G.N.) reviewed the masked documents to determine grades (no infection, possible infection, probable infection, or proven infection) for all patients. Inconsistencies in coding were resolved by a third investigator who was also blinded to patient identity and treatment arm (K.A.M.).
Invasive mold infections. Invasive mold infections were considered proven if histopathologic examination or culture of sterile tissue revealed the organism. Invasive mold infections were considered probable in patients who had both clinical criteria (eg, pulmonary nodules, cavitated infiltrates) and at least one microbiologic criterion, defined as growth of an organism from respiratory secretions (sputum or bronchoalveolar lavage fluid) or a positive galactomannan EIA. Invasive mold infections were considered possible in patients who had either clinical criteria or microbiologic criteria. Hence, in the absence of clinical criteria (eg, symptoms or pulmonary abnormalities), a positive galactomannan EIA was considered evidence of possible aspergillosis.
Invasive Candida infections. Growth of any Candida species from blood culture in patients with signs and symptoms of infection was considered evidence of proven candidemia. A diagnosis of organ involvement required documentation of Candida by both histologic examination and culture of sterile tissue.
Statistical considerations
The primary efficacy endpoint for this study was the incidence of proven or probable fungal infection analyzed in the intent-to-treat (ITT) group of patients enrolled. Initial calculations of the trial sample size used invasive fungal infection incidence estimates that were derived from the early 1990s in our institution; it was estimated that 8% of patients in the fluconazole arm would develop a proven or probable invasive mold infection. By using these numbers, it was calculated that 578 patients would be required to detect a reduction in mold infections from 8% in the fluconazole arm to 2% in the itraconazole arm (> 85% power and an α of 0.05). The trial was discontinued early, after 50% of projected sample size enrollment and after review by the data and safety monitoring board. The reason for this discontinuation was that the frequency of proven and probable IFI during more recent years was actually higher (15%-22%)4,5,18 ; hence, only 299 patients would be required to detect a 67% reduction in IFI in the itraconazole arm (> 90% power, 2-sided α = 0.05). On the basis of these projections, the trial was stopped.
Secondary efficacy endpoints included the incidence of proven or probable IMI and proven Candida infections. We also separately evaluated all invasive fungal infections, IMIs, and proven Candida infections that occurred during receipt of study drug (“on-treatment” analysis). For the latter analysis, infections were considered to have developed on-treatment if the date of first clinical signs and symptoms occurred while receiving drug, or within 2 weeks of discontinuation of study drug.
Incidence estimates for IFIs, IMIs, and proven Candida infections were evaluated by using cumulative incidence methods, treating death and second transplantation as competing risk events for the ITT analysis. For the on-treatment analysis, the time point 2 weeks after discontinuation of study drug was also considered a competing risk event. Comparisons of infection endpoints were made by using cumulative incidence and standard error estimates at the 6-month time point.19
Other secondary endpoints included overall survival and survival without a proven or probable fungal infection (fungal-free survival); survival was examined early after SC transplantation (60 days) and after follow-up (250 days after SC transplantation). Overall survival and fungal-free survival were evaluated by using the method of Kaplan and Meier using time to death and death or fungal infection as endpoints, respectively. Comparisons between curves were made by using a log-rank test. Causes of death, coded by an investigator blinded to study arm (L.C.), were compared. Death in the presence of relapsed malignancy was considered to be because of relapse; however, because infection-related death was an important variable, death caused by infection in the presence of GVHD was considered because of infection, not GVHD.
A data and safety monitoring board (DSMB) was established at the onset of the study. Two reviews were planned; safety data were reviewed after 25% of planned enrollment, and safety and efficacy data were reviewed after 50% of the projected patient enrollment had reached 180 days of follow-up. Adverse events (AEs) examined during the first interim analysis included the incidence of acute GVHD (grades II-IV) and hepatic and renal toxicities that developed early (20 days) after transplantation. The incidence of invasive fungal infections and overall mortality were also reviewed during the second DSMB meeting.
Additional toxicity endpoints included the incidence of nephrotoxicity (tripling of baseline serum creatinine) and hepatotoxicities (tripling of baseline total bilirubin and transaminase levels). The frequency and severity of GVHD, graded according to published criteria,20,21 were also examined in patients enrolled in both study arms. The proportion of patients who were discontinued from study drugs because of suspected fungal infection or toxicity was compared. Continuously valued endpoints were compared between study arms by using the Wilcoxon rank sum test, and categorical factors were compared using chi-square or Fisher exact test, as appropriate. All reported P values are 2-sided.
Results
Patients
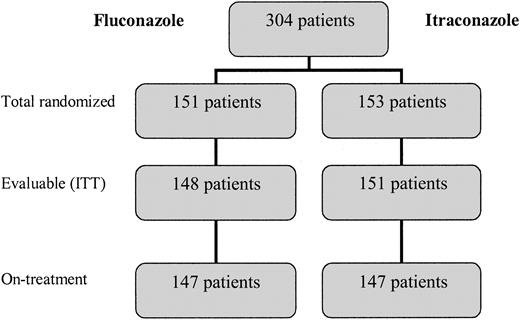
A total of 304 patients who received SCTs were randomly assigned to treatment arms between March 1999 and July 2001. Two patients in the fluconazole arm and 2 patients in the itraconazole arm never received an allogeneic SCT (3 received no transplant and one received an autologous SCT). One additional patient in the fluconazole arm was considered not evaluable because of receipt of other systemic antifungal therapy during the conditioning period, leaving 148 patients in the fluconazole arm and 151 patients in the itraconazole arm evaluable for ITT analysis (Figure 1). A modified ITT analysis was performed on-treatment, only among patients who received at least 1 day of study drug; there were 5 patients who were randomly assigned but did not receive either fluconazole or itraconazole because of evidence of pre-existing IFI, or because of protocol violation (Figure 1).
The demographic characteristics, risk categories according to underlying diseases, and transplant types were similar in the 2 treatment groups (Table 1). The median number of days until neutrophil engraftment and the severity of acute GVHD did not differ between itraconazole and fluconazole recipients. The median duration of follow-up among surviving fluconazole and itraconazole recipients was 23.3 and 23.6 months, respectively.
Patient demographic and transplant characteristics
Factor . | Fluconazole; n = 148 . | Itraconazole; n = 151 . |
|---|---|---|
| Age, n (%) | ||
| 20 y or younger | 3 (2) | 2 (1) |
| Older than 20 y | 145 (98) | 149 (99) |
| Sex, n (%) | ||
| Male | 81 (55) | 75 (50) |
| Female | 67 (45) | 76 (50) |
| Disease risk group (%)* | ||
| Low | 47 (32) | 43 (28) |
| Intermediate | 74 (50) | 74 (49) |
| High | 27 (18) | 34 (23) |
| Transplant type (%) | ||
| HLA MR | 82 (55) | 79 (52) |
| HLA MM/URD | 66 (45) | 72 (48) |
| Median neutrophil engraftment, d (range)† | 19 (10-35) | 19 (9-46) |
| Acute GVHD (%)‡ | ||
| Grades 0-I | 22 (18) | 30 (24) |
| Grades II-IV | 99 (82) | 93 (76) |
Factor . | Fluconazole; n = 148 . | Itraconazole; n = 151 . |
|---|---|---|
| Age, n (%) | ||
| 20 y or younger | 3 (2) | 2 (1) |
| Older than 20 y | 145 (98) | 149 (99) |
| Sex, n (%) | ||
| Male | 81 (55) | 75 (50) |
| Female | 67 (45) | 76 (50) |
| Disease risk group (%)* | ||
| Low | 47 (32) | 43 (28) |
| Intermediate | 74 (50) | 74 (49) |
| High | 27 (18) | 34 (23) |
| Transplant type (%) | ||
| HLA MR | 82 (55) | 79 (52) |
| HLA MM/URD | 66 (45) | 72 (48) |
| Median neutrophil engraftment, d (range)† | 19 (10-35) | 19 (9-46) |
| Acute GVHD (%)‡ | ||
| Grades 0-I | 22 (18) | 30 (24) |
| Grades II-IV | 99 (82) | 93 (76) |
HLA MR indicates human leukocyte antigen matched related; HLA MM/URD, human leukocyte antigen mismatched or unrelated donor.
Low-risk groups include chronic myelogenous leukemia (CML, chronic phase) and aplastic anemia; intermediate disease risk groups include CML in accelerated phase myelodysplastic syndromes (MDS) and hematologic malignancies in remission; and high-risk groups include CML in blast crisis, hematologic malignancies in other than 1st remission, multiple myeloma, and MDS-RAEBT (refractory anemia with excess blasts in transition).
Day of neutrophil engraftment was defined as third consecutive day on which the absolute neutrophil count exceeded 0.5 × 109/L. Median was calculated from 145 patients in each arm who achieved engraftment.
Calculated from 244 patients who had GVHD grades available.
Interim analyses
Findings of the first interim safety analysis suggested disproportionate hepatic and renal toxicities between itraconazole and fluconazole recipients early after SC transplantation. Analysis that was performed after enrollment of 197 patients (100 itraconazole and 97 fluconazole) noted a trend to higher average daily bilirubin levels (averaged over the first 20 days after SC transplantation) in patients in the itraconazole arm compared with patients in the fluconazole arm (median, itraconazole 1.47 versus fluconazole 1.37, P = .14). Also, relatively more patients who received itraconazole developed doubling of baseline serum creatinine during the first 20 days after SC transplantation (itraconazole 30 of 100, 30% versus fluconazole 18 of 97, 19%, P = .07). Hepatic toxicities appeared strongest in patients who received cyclophosphamide as part of their conditioning regimen (n = 176 patients); median of average daily bilirubin levels among itraconazole (n = 89) and fluconazole (n = 87) was 1.49 (range, 0.37-10.11) and 1.29 (range, 0.43-8.53), respectively (P = .07). Preliminary analysis of concurrently available data on cyclophosphamide pharmacokinetics suggested that toxicities were associated with study drug interactions with cyclophosphamide metabolism.22,23 With this concern, the protocol was amended such that drugs were initiated on the day of stem cell transfusion (day 0) rather than concurrent with conditioning therapy. The study resumed with these amendments and was discontinued after the second DSMB review.
Review of safety and efficacy after 50% of projected enrollment triggered discontinuation of the trial. At this time, there were concerns of persistent differences in safety parameters and overall survival, and the trial was considered to be sufficiently powered to evaluate relative efficacy of the drugs, given updated incidence estimates. In the following sections, efficacy analyses are presented for all patients evaluable (n = 299), and safety analyses are presented for patients who received both study drug schedules (209 patients who were enrolled prior to and 90 patients who were enrolled after the protocol amendment).
Study drug administration and safety
When considering all patients enrolled in the study, fluconazole was administered for a median of 120 days after transplantation (range, 1-183 days), and itraconazole was administered for a median of 89 days after transplantation (range, 1-189 days, P = .001). More patients were discontinued from itraconazole compared with fluconazole because of toxicities deemed related to the study drug (36% versus 16%, P < .001); the majority of itraconazole discontinuations were due to gastrointestinal complaints (eg, nausea, vomiting, or diarrhea, 23.8% versus 4.7%, P < .001).
Among all patients evaluable at the end of the study, there was a trend to more renal toxicities (doubling of baseline serum creatinine) in the itraconazole arm (11 [7%] of 151) compared with the fluconazole arm (4 [3%] of 148, P = .07). However, this difference was not apparent among patients who were enrolled after the protocol was amended to administer study drug after conditioning therapy; during the latter time period, 3 (7%) of 46 patients in the itraconazole arm and 4 (9%) of 44 patients in the fluconazole arm developed renal toxicities (P = .68).
Among all patients, there were also more patients in the itraconazole arm who had at least tripling of baseline total bilirubin level (143 [95%] of 151 itraconazole versus 128 [86%] of 143 fluconazole, P = .02). A trend to increased hepatic toxicities was still apparent among patients who received itraconazole after the protocol was amended (41 [89%] of 46 itraconazole versus 34 [77%] of 44 fluconazole, P = .13).
Efficacy of study drugs for prevention of fungal infections
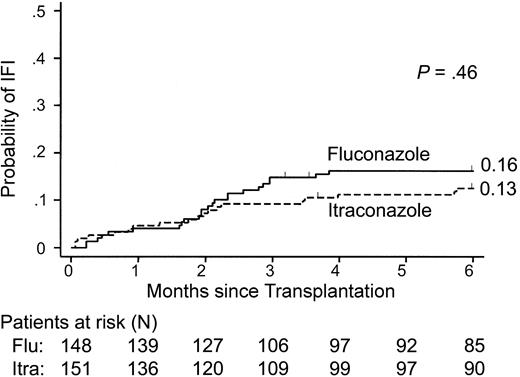
There was not a significant difference in the probability of proven or probable IFIs that occurred in patients who were randomly assigned to receive fluconazole or itraconazole (intent-to-treat analysis, P = .46; Figure 2; Table 2). However, significantly more patients developed proven or probable IFIs while receiving fluconazole compared with patients receiving itraconazole (on-treatment analysis, P = .03; Figure 3). This difference was due to fewer mold infections in itraconazole recipients (12% versus 5%, P = .03; Table 3). There was not a significant difference in the incidence of candidemia in patients receiving either fluconazole or itraconazole.
Cumulative incidence of proven or probable invasive fungal infections according to antifungal prophylaxis. The probability of infection among all patients is shown. The number of patients at risk (alive) at each interval in the fluconazole (flu) arm and itraconazole (itra) arm is indicated. The P value shown was calculated from tests comparing the incidences at 6 months.
Cumulative incidence of proven or probable invasive fungal infections according to antifungal prophylaxis. The probability of infection among all patients is shown. The number of patients at risk (alive) at each interval in the fluconazole (flu) arm and itraconazole (itra) arm is indicated. The P value shown was calculated from tests comparing the incidences at 6 months.
Invasive fungal infections diagnosed during the study period
Infection . | Fluconazole . | Itraconazole . |
|---|---|---|
| Invasive mold infections (%)* | 23 (16) | 23 (15) |
| Proven | 9 (6) | 11 (7) |
| Probable | 11 (7) | 5 (3) |
| Possible | 3 (2) | 7 (5) |
| Candidemia (%) | 5 (3) | 4 (3) |
Infection . | Fluconazole . | Itraconazole . |
|---|---|---|
| Invasive mold infections (%)* | 23 (16) | 23 (15) |
| Proven | 9 (6) | 11 (7) |
| Probable | 11 (7) | 5 (3) |
| Possible | 3 (2) | 7 (5) |
| Candidemia (%) | 5 (3) | 4 (3) |
Proven, probable, or possible infections were defined according to standardized criteria.13 Total percentages were calculated from 148 evaluable patients in the fluconazole arm and 151 patients in the itraconazole arm. One patient in the itraconazole arm developed both candidemia and probable invasive mold infection.
Cumulative incidence of proven or probable IFI while on-treatment. Probability of infections that occurred in patients while receiving or within 2 weeks of discontinuation of study drugs (SD) is shown. The number of patients at risk (alive and on SD) at each interval is indicated. The P value shown was calculated from tests comparing the incidences at 6 months.
Cumulative incidence of proven or probable IFI while on-treatment. Probability of infections that occurred in patients while receiving or within 2 weeks of discontinuation of study drugs (SD) is shown. The number of patients at risk (alive and on SD) at each interval is indicated. The P value shown was calculated from tests comparing the incidences at 6 months.
Organisms that caused IFIs that occurred while on-treatment
Infection* . | Fluconazole . | Itraconazole† . |
|---|---|---|
| Aspergillosis—total | 17 | 8 |
| A fumigatus | 11 | 5 |
| A species—NOS | 2 | 2 |
| A terreus | 2 | 0 |
| A niger | 1 | 0 |
| Multiple‡ | 1 | 1 |
| Other mold infections—total | 1 | 0 |
| Fusarium species | 1 | 0 |
| Candidemia—total§ | 4 | 3 |
| C glabrata | 1 | 2 |
| C krusei | 1 | 0 |
| C parapsilosis | 2 | 1 |
| C albicans | 0 | 0 |
Infection* . | Fluconazole . | Itraconazole† . |
|---|---|---|
| Aspergillosis—total | 17 | 8 |
| A fumigatus | 11 | 5 |
| A species—NOS | 2 | 2 |
| A terreus | 2 | 0 |
| A niger | 1 | 0 |
| Multiple‡ | 1 | 1 |
| Other mold infections—total | 1 | 0 |
| Fusarium species | 1 | 0 |
| Candidemia—total§ | 4 | 3 |
| C glabrata | 1 | 2 |
| C krusei | 1 | 0 |
| C parapsilosis | 2 | 1 |
| C albicans | 0 | 0 |
NOS indicates not otherwise specified (organism detected by histopathology only).
Only organisms that caused proven or probable IFIs are listed.
One patient developed both aspergillosis and candidemia.
One patient in the itraconazole arm developed a mixed infection with A niger and Cunninghamella, and one fluconazole recipient developed a mixed infection with A fumigatus and A niger.
All Candida species were resistant to fluconazole and itraconazole (data not shown).
Use of other antifungals for suspected fungal infection
The number of patients who received antifungal agents for suspected, but not confirmed, IFIs did not differ between treatment arms. During the posttransplantation period, 25 (16.9%) of 148 fluconazole recipients and 19 (12.6%) of 151 itraconazole recipients received alternative antifungals (amphotericin B formulations, caspofungin, and/or voriconazole) in the absence of a confirmed IFI. Of these patients, 10 fluconazole recipients (6.8%) and 8 itraconazole recipients (5.3%) were considered to have met clinical criteria for possible IFI according to signs or symptoms. The remaining patients were treated with alternative antifungals because of clinical suspicion, although they did not meet criteria for possible IFI. Retrospective measurement of circulating galactomannan in patients who had fever during neutropenia upgraded the diagnoses to possible in one patient in each treatment arm.
Itraconazole levels and antimicrobial susceptibilities
The majority (> 95%) of fluconazole and itraconazole recipients required intravenous study drug prior to engraftment. Itraconazole levels were examined both before engraftment, in patients receiving the intravenous formulation, and after engraftment, in patients receiving oral drug. Fifty-two patients who received 3 consecutive days of intravenous itraconazole had a median itraconazole level of 0.52 μg/mL (range, 0.16-2.1 μg/mL). In 27 patients (52%), levels were acceptable (> 0.5 μg/mL), 18 patients (35%) had levels in the intermediate range, (0.25-0.5 μg/mL), and 7 patients (13%) had low levels (< 0.25 μg/mL). During the postengraftment time period, the largest number of observations was from patients who were maintained on oral itraconazole given 3 times daily. During this administration schedule, 61 (90%) of 68 patients who received oral drug for at least 10 consecutive days had itraconazole levels more than 0.5 μg/mL, 3 patients (4%) had levels between 0.25 μg/mL and 0.5 μg/mL, and 4 patients (6%) had levels less than 0.25 μg/mL. Eleven patients had dosing decreased to twice daily because of persistent gastrointestinal complaints; 10 (91%) of these patients maintained itraconazole levels more than 0.5 μg/mL with twice daily dosing.
To determine whether breakthrough Aspergillus infections were associated with inadequate itraconazole levels, we examined drug levels that were obtained within 1 month of clinical onset of IFI. Five patients who developed invasive mold infections while receiving itraconazole had serum levels obtained within 1 month of diagnosis; of these, only one patient had less than 0.25 μg/mL itraconazole in serum. The remaining patients who developed mold infections while on-treatment had itraconazole levels more than 0.5 μg/mL (mean, 1.27 μg/mL; range, 0.53-2.4 μg/mL). There was no itraconazole resistance noted among Aspergillus isolates that caused breakthrough infection in patients enrolled in either arm. All isolates recovered from both itraconazole and fluconazole recipients had itraconazole MICs less than 1.0 μg/mL.
Survival
Among all patients evaluable, there was no difference in overall survival by 250 days after SC transplantation (fluconazole 69% versus itraconazole 61%, log-rank P = .11). Causes of death did not differ (Table 4).
Cause of death in all patients
Cause of death . | Fluconazole (%); n = 148 patients . | Itraconazole (%); n = 151 patients . |
|---|---|---|
| Relapsed malignancy | 9 (6) | 6 (4) |
| Idiopathic pneumonitis* | 8 (5) | 12 (8) |
| Bacterial sepsis or pneumonia | 5 (3) | 14 (9) |
| Fungal infections | 11 (7) | 12 (8) |
| Viral infections† | 4 (3) | 9 (6) |
| Other‡ | 7 (5) | 2 (1) |
Cause of death . | Fluconazole (%); n = 148 patients . | Itraconazole (%); n = 151 patients . |
|---|---|---|
| Relapsed malignancy | 9 (6) | 6 (4) |
| Idiopathic pneumonitis* | 8 (5) | 12 (8) |
| Bacterial sepsis or pneumonia | 5 (3) | 14 (9) |
| Fungal infections | 11 (7) | 12 (8) |
| Viral infections† | 4 (3) | 9 (6) |
| Other‡ | 7 (5) | 2 (1) |
Includes idiopathic pneumonitis, bronchiolitis obliterans—organizing pneumonia, and diffuse alveolar damage/hemorrhage.
Viral infections included diseases caused by cytomegalovirus (n = 7), human herpes virus 6 (n = 3), respiratory viruses (n = 2), and Epstein-Barr virus (n = 1).
Other causes of death included multiorgan failure (n = 3), regimen-related toxicity/venoocclusive disease (n = 2), pulmonary embolism (n = 1), encephalopathy (n = 1), meningitis of unknown etiology (n = 1), and trauma (n = 1).
Survival early after SC transplantation (at day 60) was also analyzed among patients enrolled before and after the protocol amendment. The probability of survival was lower among itraconazole recipients enrolled before the protocol was amended (Figure 4A). Although there was limited power to detect small differences in early survival in patients enrolled after the study amendment, there was no apparent survival difference in patients who received the altered study drug schedule (Figure 4B).
Survival early after transplantation. The probability of survival is shown among (A) 209 patients enrolled before the protocol amendment and (B) 90 patients enrolled after the protocol amendment. The P value shown was calculated from log-rank tests comparing the curves.
Survival early after transplantation. The probability of survival is shown among (A) 209 patients enrolled before the protocol amendment and (B) 90 patients enrolled after the protocol amendment. The P value shown was calculated from log-rank tests comparing the curves.
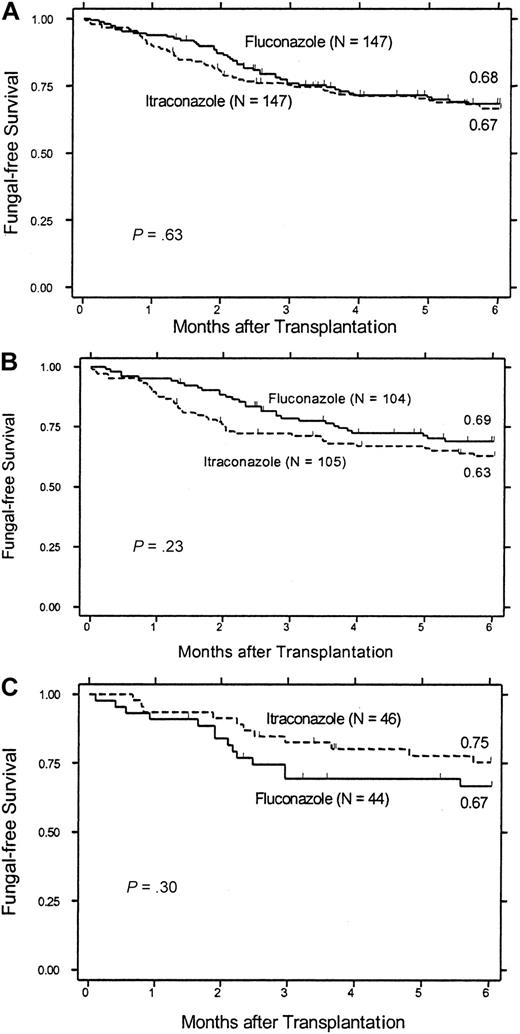
There were fewer itraconazole recipients who were alive and still receiving study drug 2 months after SC transplantation (83 itraconazole versus 123 fluconazole), the period during which patients were at highest risk of infection (Figures 3, 4). It is possible that an unequal distribution of deaths and withdrawal from study drug may have led to bias in intent-to-treat and on-treatment analyses of the probability of infections. We evaluated this possibility by analyzing features of the patients remaining in the 2 groups and compared fungal-free survival in patients enrolled before and after the protocol amendment was enacted. The latter composite endpoint minimizes bias by treating death because of any cause as an important efficacy endpoint. Patients remaining on each study drug at 2 months after SC transplantation did not differ with regard to age, underlying disease risk, transplant type, or severity of acute GVHD (data not shown). Among all patients enrolled in the study, there was no difference in overall fungal-free survival after 6 months (Figure 5A). Among patients who were enrolled during the early phase of the study (before the protocol amendment), there was a trend to superior fungal-free survival in the fluconazole arm (fluconazole 69% versus itraconazole 63%, P = .23; Figure 5B). However, after the protocol amendment, there was a trend to improved fungal-free survival among itraconazole recipients (fluconazole 67% versus itraconazole 75%, P = .30; Figure 5C). The primary difference between these groups was an improved fungal-free survival in itraconazole recipients enrolled after the protocol amendment (75%) relative to those patients who received itraconazole during conditioning therapy (63%, P = .11). These data suggest that overall fungal-free survival was at least in part affected by early toxicities that developed in patients who received concurrent itraconazole and conditioning therapy.
Fungal-free survival. Probability of survival without a proven or probable invasive fungal infection is shown for (A) all 294 people who received at least one dose of fluconazole or itraconazole during the course of the study, (B) 209 people who received study drug before the change in timing of drug administration, and (C) 90 people who received study drug after the change in study drug administration.
Fungal-free survival. Probability of survival without a proven or probable invasive fungal infection is shown for (A) all 294 people who received at least one dose of fluconazole or itraconazole during the course of the study, (B) 209 people who received study drug before the change in timing of drug administration, and (C) 90 people who received study drug after the change in study drug administration.
Discussion
Both fluconazole and itraconazole prevent candidal infections, resulting in fewer candidiasis-related deaths, and in the highest-risk patients, improved overall survival.1-3,9,10,12 In this large randomized trial of high-risk patients, we have shown that itraconazole prevented mold infections better than fluconazole; however, the overall success of this prophylaxis strategy was limited by poor tolerability of the drug and toxicities early after SC transplantation.
In this study, there was not a significant difference in fungal infections in the 2 study arms when incidence was compared by using intent-to-treat analysis. However, a 60% reduction in invasive mold infections was seen with on-treatment analysis. Similar results were reported in a recent study that compared invasive fungal infections in a small number of allograft recipients randomly assigned to receive itraconazole or fluconazole.24 Compared with that recent trial and prior studies that evaluated itraconazole prophylaxis in patients with hematologic malignancies,9,10,12 our trial enrolled a larger number of patients with very high risks for invasive mold infections; nearly 50% of patients had received an allogeneic SCT from an HLA-mismatched or unrelated donor. It is likely that the protective effect of itraconazole was apparent because of enrollment of a high-risk population, and possibly because prophylactic itraconazole was administered at a high dose for a long duration of time.
Prophylactic antifungal drugs targeted against invasive mold infections in patients who receive allogeneic SCTs require long-term administration, given the late onset of infection during GVHD.4-7,25,26 We found that itraconazole effectively prevented infection in patients who were tolerating the drug; however, almost one quarter of patients were withdrawn from this drug because of GI complaints. This frequency is high, but not outside the range reported in prior studies. Three large studies performed in patients with hematologic malignancies noted that GI intolerance occur frequently, with complaints of nausea, vomiting, and diarrhea in 15% to 57% of patients who receive itraconazole oral solution.9,10,12
The itraconazole dosing regimen (2.5 mg/kg twice daily) used in prior prophylaxis trials was based on the observation that twice daily dosing yields serum levels that typically exceed 0.25 μg/mL, the target cutoff for efficacy suggested in prior studies.9,27,28 Because the results of more recent analyses suggest a higher target cutoff for efficacy (0.5 μg/mL),29 we used a high-frequency dosing schedule (3 times daily). Our results confirm that 3 times daily dosing yields levels more than 0.5 μg/mL in most patients. Although these high levels may have increased the antimicrobial efficacy of the drug, the frequent dosing schedule may have also encouraged more GI complaints, because the hydroxyprophyl-β-cyclodextrin vehicle is known to cause diarrhea.10 The step-down strategy to twice daily dosing was effective, because most patients maintained target levels more than 0.5 μg/mL after decreased dosing. Breakthrough infections did not appear to be related to either inadequate drug levels or antimicrobial resistance, suggesting that future studies should use well-tolerated doses of drug.
Although this trial was not powered to measure differences in overall survival as an endpoint to define success, results emphasize the importance in comparing deaths to identify unexpected study drug toxicities. In this study, there was a trend to better survival in patients who received fluconazole, despite the efficacy of itraconazole in preventing invasive fungal infections. The timing of death (coincident with hepatic and renal toxicities) and improved outcomes after altering the study drug administration schedule suggest that the overall survival of itraconazole recipients was adversely affected by the differential toxicities encountered during conditioning therapy. We are currently investigating the hypothesis that hepatic and renal toxicities may be related to the effect of the study drugs on cytochrome P450–mediated metabolism of cyclophosphamide.30
The high frequency of patient withdrawal from itraconazole and disproportionate number of deaths can lead to bias in both intent-to-treat and on-treatment analyses of drug efficacy, because of a resultant imbalance in competing risk events (death and discontinuation of study drug). It has been suggested that composite variables such as fungal-free survival be used to minimize such bias, especially in the setting in which the cause of patient drop-out (drug-related toxicities or death) may be outcomes of interest.31,32 Analysis of fungal-free survival before and after the protocol was amended suggested improvement in outcomes among itraconazole recipients, likely related to fewer toxicities early after SC transplantation. In this study, successful prevention of fungal infections may well have had a more dramatic effect on overall survival had the drugs not been administered with conditioning therapy in the early phase of the study. Because safety is a major component of effectiveness, especially when measuring the effect of long-term administration of drugs that have complex and unpredictable toxicities, future antifungal prophylaxis studies could be constructed using endpoints that capture survival and infection within a composite variable.
The results of this trial demonstrate the importance of considering risk-benefit ratios in formulating preventative strategies, because the therapeutic benefits of antifungal coverage may be outweighed by negative outcomes associated with drug toxicities or interactions. These negative effects may be particularly apparent in patients who require long-term prophylaxis, especially in patients who have only small or moderate risks of infection. Future studies should determine whether “pre-emptive” antifungal therapy guided by early infection monitoring (eg, antigenemia, polymerase chain reaction [PCR]) would provide equivalent, or better overall outcomes.
In summary, the results of this large randomized trial suggest that antifungal prophylaxis might effectively prevent invasive mold infections when administered long term in patients who received allogeneic SCTs. For selected patients, itraconazole, initiated after completion of conditioning therapy, might be useful; however, tolerability and toxicities associated with the drug limit the overall success of the strategy. Future studies should be undertaken to identify an optimal strategy to prevent mold infections after allogeneic stem cell transplantation, using drugs with fewer toxicities and/or the use of early diagnostic tests.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-08-2644.
Supported in part by grants from the National Institutes of Health (grants CA18029, CA15704, and K08AI01571); partial funds for substudy analyses were provided by Ortho Biotech Products, LP.
One of the authors (K.A.M.) has served as a consultant for Ortho Biotech Products, LP, and for Pfizer. Several of the authors (M.B. and W.G.N.) have served as consultants for Pfizer.
K.A.M. and F.C. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the members of the Data and Safety Monitoring Board: Paul Martin, MD, George McDonald, MD, Richard Nash, MD, Barry Storer, PhD, and Anna Wald, MD, and thank Dr Jo Anne van Burik for her role in the initial design of this study. We also thank Nido Nguyen, Heather Hildebrandt, and Peter Choe for data entry, and the clinicians, microbiologists, and pathologists at the Fred Hutchinson Cancer Research Center, without whom this study would not have been possible.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal