Abstract
Anaplastic large-cell lymphomas (ALCLs) are lymphomas of T or null phenotype often associated with a chromosomal translocation, t(2;5)(p23;q35). This translocation leads to the expression of a hybrid protein consisting of the N-terminal portion of nucleophosmin (NPM) and the intracellular domain of the anaplastic lymphoma kinase (ALK). NPM-ALK possesses a constitutive tyrosine kinase activity responsible for its oncogenic property through activation of downstream effectors such as phospholipase Cγ (PLC-γ) and the type IA phosphoinositide 3-kinase. Here, we show that the Src-kinases, particularly pp60c-src, associate with and are activated by NPM-ALK expression in various cells, and in cell lines established from patients with ALCL. The kinase activity and the tyrosine 418 of NPM-ALK are required for its association with Src-kinases. Y418F mutation of NPM-ALK impaired its association with Src-kinases and strongly reduced the proliferation rate of Ba/F3 cells. In agreement, Src-kinase inhibitors or pp60c-src siRNA significantly decreased the proliferation rate of NPM-ALK–positive ALCL cell lines. Moreover, using active or inactive forms of pp60c-src and NPM-ALK, we provide evidence that NPM-ALK is a potential substrate of pp60c-src. Overall, our data place Src-kinases as new important downstream effectors of NPM-ALK and as attractive potential therapeutic targets for new ALCL treatment.
Introduction
Anaplastic large-cell lymphomas (ALCLs) are lymphoid tumors of T or null phenotype expressing the CD30 antigen.1,2 A characteristic feature of ALCLs is the presence of the t(2,5)(p23;q35) chromosomal translocation, associating the anaplastic lymphoma kinase (ALK) gene at 2p23 and the nucleophosmin gene at 5q35.3 This fusion leads to the expression of a chimeric nucleophosmin (NPM)–ALK protein, linking the N-terminal region of NPM, a ubiquitous nucleolar phosphoprotein, to the C-terminal region of ALK containing its kinase domain.3,4 Full-length ALK is a member of the insulin receptor family and is composed of an extracellular ligand binding domain, a transmembrane, and intracytoplasmic domain with tyrosine kinase activity.5 Expression of the full-length ALK protein is restricted to the central nervous system particularly during embryonic development.4,5 It is never expressed in cells of lymphoid origin except in very rare cases of diffuse large B-cell lymphoma.6 In ALCL cells, dimerization of NPM-ALK, through the oligomerization domain of NPM, leads to the constitutive activation and autophosphorylation of the kinase.7 Evidence is accumulating that NPM-ALK is a causative agent of ALCL and its transforming potential leads to oncogenicity, as now well established in vitro and in vivo.7-10 Several variant fusions such as TGF-ALK, TPM3-ALK, ATIC-ALK, or Clathrin-ALK have been recently described in approximately 20% of ALK-positive ALCLs.11 All of these variants are capable of dimerization leading to activation of the ALK–tyrosine kinase portion.11 In order to elucidate the still poorly known molecular basis of X-ALK–mediated oncogenesis, it is essential to determine the effectors recruited and/or activated by these oncogenes. Recently, phospholipase Cγ (PLC-γ) and p85, the regulatory subunit of type IA phosphoinositide 3-kinase (PI 3-kinase), have been shown to associate with NPM-ALK.12-14 Using the interleukin-3 (IL3)–dependent murine lymphoid Ba/F3 cell model, which exhibits a transformed phenotype and a loss of growth factor dependency upon expression of NPM-ALK,12 the residue Y664 of NPM-ALK was shown to support the association of PLC-γ. Interestingly, Ba/F3 cells expressing NPM-ALK (Y664F) no longer have a stably transformed phenotype.12 PLC-γ appears to be essentially required for the mitogenic properties of NPM-ALK. Although no direct association has been formally demonstrated in vivo, several studies have shown that NPM-ALK also associates with the p85 regulatory subunit of PI 3-kinase. In agreement, activation of Akt, a downstream effector of the PI 3-kinase pathway, has been shown in NPM-ALK–expressing cells including ALCL patient cell lines.13-15 Moreover, expression of dominant-negative p85 or Akt mutants induced apoptosis in NPM-ALK–expressing cell lines.13,14 However, this PI 3-kinase/Akt antiapoptotic pathway appears not to be involved in NPM-ALK inhibition of drug-induced apoptosis.15 Signal transducer and activator of transcription 5 (Stat5)16 and Stat317,18 have also recently been shown to be activated by NPM-ALK and implicated in the protection of ALK-positive tumors from cell death. Several other NPM-ALK interacting proteins have been identified, in particular the adaptor proteins Shc, growth factor receptor-bound protein 2 (Grb2), and insulin receptor substrate-1 (IRS-1).8 However, no biologic function has yet been attributed to these proteins in the process of NPM-ALK–mediated oncogenicity.
In this study we show that, although NPM-ALK is constitutively phosphorylated and activated, its level of tyrosine phosphorylation and kinase activity can be modulated by serum in Ba/F3 cells. This observation allowed us to establish optimal conditions for the identification of new potential substrates and downstream effectors of NPM-ALK. We demonstrate that Src-family kinases, particularly pp60c-src, interact with and are activated by NPM-ALK in various cell types including those established from patients with ALCL. The kinase activity of NPM-ALK as well as its tyrosine 418 are required for the constitutive association observed between these 2 tyrosine kinases. Loss of this interaction or direct inhibition of Src-kinases led to a sharp decrease in NPM-ALK–mediated cell proliferation. Overall, our results indicate a role for Src-kinases in NPM-ALK–mediated mitogenicity and suggest a role in the pathogenesis of ALCL.
Materials and methods
Reagents and antibodies
Geneticin (G418 sulfate) was purchased from (GIBCO BRL, Life Technologies, Grand Island, NY), murine recombinant IL3 (mrIL3) from R&D Systems Europe (Abingdon, United Kingdom), and γ-32P] adenosine triphosphate (ATP) from Amersham (Les Ulis, France). PP1 and SU6656 were from Tebu (Le Perray en Yvelines, France) and Calbiochem (San Diego, CA), respectively. All other chemicals were from Sigma Chemical (St Louis, MO) unless specified.
The ALK119 and ALKc20 monoclonal antibodies (mAbs), which recognize the intracellular kinase domain of ALK, have been previously described. The antiphosphotyrosine (clone 4G10) mAb was purchased from Upstate Biotechnology (Lake Placid, NY). C-Src (SRC 2) polyclonal antibody recognizing Src-kinases was from Santa Cruz Biotechnology (Santa Cruz, CA). SRC-2 was produced by immunizing rabbits against a C-terminal pp60c-src human peptide (identical to the murine sequence). However it can also recognize other members of the Src-family such as yes, fyn, and c-fgr. Src pY418 and Src pY215 polyclonal antibodies recognizing phosphorylated pp60c-src proteins were purchased from Biosource International (Camarillo, CA). Src siRNA, siRNA negative control, and pp60src antibody (clone GD11) were purchased from Dharmacon (Lafayette, CO).
Peroxidase-conjugated rabbit antimouse and pig antirabbit immunoglobulin (Ig) antisera were purchased from DakoCytomation (Glostrup, Denmark).
Cell lines and culture
Ba/F3 murine lymphoid cells were maintained in RPMI 1640 containing 10% fetal calf serum and 2 ng/mL mrIL3, whereas NPM-ALK–transfected Ba/F3 (wild type or mutants) were cultured only in RPMI 1640 containing 10% fetal calf serum. Jurkat cells were maintained in RPMI 1640 also with 10% fetal calf serum; COST (L. Lamant et al, manuscript in preparation, 2003), SUDHL-1,21 KARPAS 29922 (NPM-ALK positives), and FEPD23 (NPM-ALK negative) cells were maintained in Iscoves-modified Dulbecco medium (IMDM) supplemented with 10% certified fetal calf serum. RPMI 1640, IMDM, and fetal calf serum were purchased from Invitrogen (Cergy Pontoise, France). All culture media contained 2 mM glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mM sodium pyruvate (GIBCO BRL). Transfected Ba/F3 and Jurkat cells were cultured in the continuous presence of 1 mg/mL G418 in order to conserve stable transgene expression.
DNA transfection
PcDNA3 expression vector (Invitrogen, Groningen, the Netherlands), empty or containing wild-type NPM-ALK3 or mutants NPM-ALK (K210R), (Y664F), (Y418F), and (Y156F-Y418F) cDNA,12 was stably transfected into Jurkat cells as described previously.15
Ba/F3 cells transfected with pcDNA3 or pcDNA3 NPM-ALK (Ba/F3 neo or Ba/F3 N/A) were previously described.15 Ba/F3 cells were stably transfected with the pcDNA3 vector containing the NPM-ALK Y418F cDNA by electroporation with a Biorad (Ivry-sur-Seine, France) Gene Pulser apparatus at 250 V, 960 μF. After 48 hours of culture, transfected cells were selected with 1 mg/mL G418.
Jurkat cells expressing the NPM-ALK K210R mutant were transiently transfected with a pSGT vector, empty or containing the cDNA encoding the avian active form of pp60c-Src (SrcY527F). KARPAS cells were transiently transfected by electroporation with siRNA or negative control siRNA duplexes.
Immunoblot analysis
Total cellular proteins were extracted by sonication at 4°C in lysis buffer A containing 20 mM Tris (tris(hydroxymethyl)aminomethane) HCl, pH 7.5, 0.5 mM EDTA (ethylenediaminetetraacetic acid), 0.5 mM EGTA (ethylene glycol tetraacetic acid), 1 mM dithiothreitol (DTT), 10% vol/vol glycerol, 10 μg/mL leupeptin, 2 μg/mL aprotinin, 2 μg/mL pepstatin A, 1 mM 4-2 aminoethyl-benzenesulfonyl fluoride (AEBSF), 1 mM sodium orthovanadate, and 4 mM sodium fluoride.
Total cellular proteins (2 million cells) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and transferred onto nitrocellulose membranes. NPM-ALK and tyrosine phosphorylated proteins were detected using the ALKc (1/100) and 4G10 (1/1000) mAbs, respectively, followed by a horseradish peroxidase–conjugated rabbit antimouse Ig antiserum (1/3000). Src-kinases were revealed using the SRC2 antibody and activated pp60c-src with the Src pY418 and the Src pY215 polyclonal antibodies, followed by a horseradish peroxidase–conjugated pig antirabbit Ig antiserum (1/1000). Signal detection was performed with an enhanced chemoluminescence kit (ECL; Amersham).
Immunoprecipitation
Cell pellets were suspended in lysis buffer (80 mM Tris HCl, pH 7.5; 200 mM NaCl; 20 mM EDTA; 2% Triton X100) containing 10 μg/mL leupeptin, 2 μg/mL aprotinin, 2 μg/mL pepstatin A, 1 mM AEBSF, 1 mM sodium orthovanadate, and 4 mM sodium fluoride at 4°C for 30 minutes. Samples were precleared at 4°C for one hour using prewashed protein G–sepharose beads (ALK1 and 4G10 immunoprecipitation) or protein A–sepharose beads (Src immunoprecipitation). The supernatant was then incubated with ALK1-precoated sepharose beads at 4°C for one hour. For 4G10 and Src-2 immunoprecipitations, supernatants were first incubated with the appropriate antibodies for one hour prior to bead addition for an extra one hour at 4°C (protein G for 4G10 and protein A for Src-2). After 4 washes in lysis buffer, the beads were heated at 95°C for 4 minutes. Protein separation was performed by SDS-PAGE, followed by immunoblot analysis with appropriate antibodies.
In vitro kinase assay
Immunoprecipitation with ALK1 mAb was carried out as described above. Sepharose-bound immune complexes were washed 3 times in lysis buffer and twice in kinase buffer (20 mM HEPES, pH 7.4, 10 mM MnCl2, 10 mM sodium fluoride, 1 mM orthovanadate), before incubation with 5 μCi (0.185 MBq) [γ-32P]ATP (Redivue; Amersham) at 25°C for 15 minutes. Samples were boiled at 95°C for 4 minutes and separated on a 10% gel by SDS-PAGE prior to autoradiography.
Immunocytochemistry
Cytospins from FEPD, COST, and SUDHL-1 cell slides were fixed in acetone for 10 minutes after air drying. After blocking with 1% bovine serum albumin in phosphate-buffered saline (PBS-BSA) for 20 minutes, cells were treated for 10 minutes with hydrogen peroxide to inactivate endogenous peroxydase. Then, the slides were incubated or not (negative control) for 30 minutes with an unconjugated rabbit polyclonal antibody against the pY418-Src antigen. After 2 washing steps with PBS-BSA, the bound primary antibody was detected by sequential incubations with a biotinylated secondary pig antirabbit antibody (30 minutes, 1:200 dilution), a mix of streptavidin complexed with biotinylated peroxidase (DakoCytomation) for 30 minutes, and 3,3′-diaminobenzidine (DAB) for 3 minutes at room temperature. After hematoxylin counterstain, slides were dehydrated through graded alcohols and coverslips were applied using a histologic mounting medium (Eukitt; VWR International, Fontenay-sous-Bois, France).
Proliferation assay
Cells were washed once in RPMI or IMDM before being seeded into 96-well plates at a concentration of 2000 cells per well (BaF/3 wild type, BaF/3 NPM-ALK, BaF/3 clones 1 & 2, triplicate) or 10 000 cells per well (COST, KARPAS, SU-DHL1) in complete medium (10% FCS) with (BaF/3 wild type) or without IL3. The cells were treated with appropriate drugs. Before each absorbance reading, a volume of 10 μL of 15-mg/mL 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) dye was added to each well (one tenth of the original culture volume) and incubated at 37°C for 3 hours. Then the plates were centrifuged (1100 rpm, 5 minutes) and dimethyl sulfoxide (100 μL) was added and mixed. The absorbance of the converted dye is measured at a wavelength of 595 nm.
Results
NPM-ALK tyrosine phosphorylation and kinase activity are modulated by serum in Ba/F3 cells
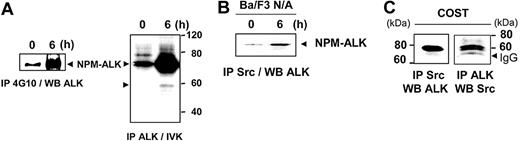
Ba/F3 murine lymphoid cells expressing the oncogenic tyrosine kinase NPM-ALK (Ba/F3 N/A) or control cells (Ba/F3 neo) were deprived of serum for 24 hours prior to serum readdition for 6 to 24 hours, and their tyrosine phosphorylation status was analyzed. Figure 1A indicates a dramatic difference in the whole pattern of phosphotyrosyl proteins between Ba/F3 cells expressing or not NPM-ALK after 6 hours of serum stimulation. This difference was independent of IL3 removal in Ba/F3 N/A since a similar profile was observed with or without IL3 (Figure 1B). In Ba/F3 N/A cells the level of tyrosine phosphorylation increased upon serum stimulation to reach a maximum at about 6 hours and slowly decreased between 12 and 24 hours (Figure 1A). The 80-kDa highly tyrosine-phosphorylated protein, which is absent in Ba/F3 neo cells, matched NPM-ALK as confirmed by an immunodepletion experiment performed with an NPM-ALK antibody followed by a Western blot with a 4G10 antibody (Figure 1C). The increase in tyrosine phosphorylation of NPM-ALK was associated with an increase in protein expression at 6 hours after stimulation (Figure 1A, lower panel).
NPM-ALK tyrosine phosphorylation in Ba/F3 cells and ALCL-derived cell lines. (A) Ba/F3 neo and Ba/F3 N/A cells were deprived of serum for 24 hours, prior to serum restimulation for various times before cell lysis and immunoblot analysis with an antiphosphotyrosine antibody 4G10. The nitrocellulose membrane was stripped and reprobed with anti-ALK and antiactin antibodies to assess NPM-ALK expression and protein loading, respectively (middle panel). Band intensity was semiquantified by densitometric scanning of the film (actin: white; NPM-ALK: black). Protein extracts from 5 × 106 cells were loaded in each lane. (B) As a control, Ba/F3 N/A cells cultured with or without IL3 were used for immunoblotting with the 4G10 antibody. (C) For NPM-ALK immunodepletion experiments, Ba/F3 N/A cells were harvested 6 hours after serum restimulation and subjected to immunoprecipitation with or without the ALK1 antibody (1: supernatant; 2: beads) followed by immunoblotting with the 4G10 antibody. (D) NPM-ALK–negative (FEPD) and –positive (COST, KARPAS, and SU-DHL1) ALCL cell lines were maintained in normal culture conditions as described in “Materials and methods” prior to cell lysis and immunoblot analysis with the 4G10 antibody (upper panel) or with the anti-ALKc antibody (middle panel). The nitrocellulose membrane was stripped and reprobed with antiactin antibody to assess protein loading (lower panel). From cell protein extracts, 60 μg was loaded in each lane. Data shown in this figure are representative of 3 to 4 independent experiments.
NPM-ALK tyrosine phosphorylation in Ba/F3 cells and ALCL-derived cell lines. (A) Ba/F3 neo and Ba/F3 N/A cells were deprived of serum for 24 hours, prior to serum restimulation for various times before cell lysis and immunoblot analysis with an antiphosphotyrosine antibody 4G10. The nitrocellulose membrane was stripped and reprobed with anti-ALK and antiactin antibodies to assess NPM-ALK expression and protein loading, respectively (middle panel). Band intensity was semiquantified by densitometric scanning of the film (actin: white; NPM-ALK: black). Protein extracts from 5 × 106 cells were loaded in each lane. (B) As a control, Ba/F3 N/A cells cultured with or without IL3 were used for immunoblotting with the 4G10 antibody. (C) For NPM-ALK immunodepletion experiments, Ba/F3 N/A cells were harvested 6 hours after serum restimulation and subjected to immunoprecipitation with or without the ALK1 antibody (1: supernatant; 2: beads) followed by immunoblotting with the 4G10 antibody. (D) NPM-ALK–negative (FEPD) and –positive (COST, KARPAS, and SU-DHL1) ALCL cell lines were maintained in normal culture conditions as described in “Materials and methods” prior to cell lysis and immunoblot analysis with the 4G10 antibody (upper panel) or with the anti-ALKc antibody (middle panel). The nitrocellulose membrane was stripped and reprobed with antiactin antibody to assess protein loading (lower panel). From cell protein extracts, 60 μg was loaded in each lane. Data shown in this figure are representative of 3 to 4 independent experiments.
Overall, these results allowed us to establish optimal conditions for the identification of new potential substrates and/or partners of the oncogenic tyrosine kinase NPM-ALK in Ba/F3 cells (ie, 6 hours after stimulation). One of the major tyrosine phosphorylated bands that increased in a time/serum-dependent manner identically to NPM-ALK phosphorylation was observed at approximately 60 kDa (Figure 1A). These variations could not be solely due to serum stimulation as the phosphorylation of a 60-kDa protein increased only slightly in Ba/F3 neo cell lysates after 6 hours of stimulation (Figure 1A, left panel). Moreover, analysis of the major tyrosine phosphorylated proteins of one NPM-ALK–negative (FEPD) and 3 NPM-ALK–positive (COST, KARPAS, and SU-DHL1) cell lines derived from patients with ALCL showed 2 major phosphotyrosyl proteins, NPM-ALK and again a 60-kDa protein, in COST, KARPAS, and SU-DHL1 (Figure 1D).
An Src-family kinase interacts with NPM-ALK
After 6 hours of stimulation, NPM-ALK was highly tyrosine phosphorylated and its tyrosine kinase activity strongly increased (Figure 2A). Interestingly, a 60-kDa phosphoprotein appeared associated with NPM-ALK as revealed in the in vitro kinase assay (Figure 2A) and the immunodepletion experiment (Figure 1C). We conjectured that a member of the Src non–receptor tyrosine kinase family could be a potential candidate for the 60-kDa phosphoprotein. Anti-Src immunoprecipitation in Ba/F3 cells expressing NPM-ALK was done using the Src-2 polyclonal antibody, which recognizes several members of the Src-family kinase (ie, pp60c-src, p62yes, p59fyn, and p55c-fgr). A coimmunoprecipitation with NPM-ALK was detected in Ba/F3 N/A cells, particularly after 6 hours of serum stimulation (Figure 2B). This interaction was also found in COST cells, a cell line established from a patient with ALCL. Indeed, anti-ALK antibody coprecipitated an Src-kinase and conversely an anti–Src-kinase antibody coprecipitated NPM-ALK (Figure 2C), suggesting a direct interaction between these 2 proteins. This interaction was also observed in a third cell line, Jurkat T cells expressing NPM-ALK (Figure 3).
Interaction of an Src-kinase with NPM-ALK. (A) Ba/F3 N/A cells (107 cells per point) were harvested at 0 and 6 hours after serum restimulation and subjected to immunoprecipitation with the 4G10 antibody followed by immunoblotting with the ALKc antibody (left panel). The same cells were used for immunoprecipitation with the ALK1 antibody followed by in vitro kinase (IVK) assay (right panel). (B) Ba/F3 N/A cells (107 cells per point), at 0 and 6 hours after serum restimulation, were subjected to anti-Src immunoprecipitation using the Src-2 antibody followed by immunoblot analysis with the ALKc antibody. (C) ALCL-derived COST cells (107 cells per point) were subjected to anti-Src immunoprecipitation followed by immunoblotting using the ALKc antibody (left panel) or the way inverse (right panel). The position of IgG heavy chain is indicated.
Interaction of an Src-kinase with NPM-ALK. (A) Ba/F3 N/A cells (107 cells per point) were harvested at 0 and 6 hours after serum restimulation and subjected to immunoprecipitation with the 4G10 antibody followed by immunoblotting with the ALKc antibody (left panel). The same cells were used for immunoprecipitation with the ALK1 antibody followed by in vitro kinase (IVK) assay (right panel). (B) Ba/F3 N/A cells (107 cells per point), at 0 and 6 hours after serum restimulation, were subjected to anti-Src immunoprecipitation using the Src-2 antibody followed by immunoblot analysis with the ALKc antibody. (C) ALCL-derived COST cells (107 cells per point) were subjected to anti-Src immunoprecipitation followed by immunoblotting using the ALKc antibody (left panel) or the way inverse (right panel). The position of IgG heavy chain is indicated.
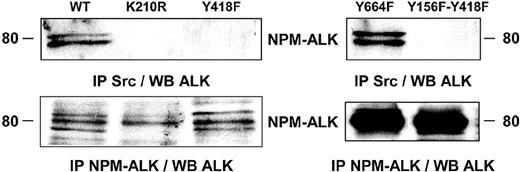
Implication of the kinase activity and the tyrosine 418 of NPM-ALK in its association with an Src-kinase. Jurkat cells stably expressing wild-type NPM-ALK or mutant forms of NPM-ALK were used to determine the tyrosine residue involved in NPM-ALK/Src-kinase association. Jurkat cells (107) expressing wild-type NPM-ALK (WT), kinase dead NPM-ALK (K210R), or Y418F, Y664F or the double Y156F-Y418F NPM-ALK mutants were subjected to anti-Src immunoprecipitation using the Src-2 antibody followed by immunoblotting using the ALKc antibody. The expression level of NPM-ALK WT, K210R, and Y418F mutants was comparable as well as the expression level of Y664F and Y156F-Y418F mutants (lower panels). Note that NPM-ALK is classically revealed as 2 to 3 distinct bands when expressed in Jurkat cells.15 Data shown are representative of 2 independent experiments with identical results.
Implication of the kinase activity and the tyrosine 418 of NPM-ALK in its association with an Src-kinase. Jurkat cells stably expressing wild-type NPM-ALK or mutant forms of NPM-ALK were used to determine the tyrosine residue involved in NPM-ALK/Src-kinase association. Jurkat cells (107) expressing wild-type NPM-ALK (WT), kinase dead NPM-ALK (K210R), or Y418F, Y664F or the double Y156F-Y418F NPM-ALK mutants were subjected to anti-Src immunoprecipitation using the Src-2 antibody followed by immunoblotting using the ALKc antibody. The expression level of NPM-ALK WT, K210R, and Y418F mutants was comparable as well as the expression level of Y664F and Y156F-Y418F mutants (lower panels). Note that NPM-ALK is classically revealed as 2 to 3 distinct bands when expressed in Jurkat cells.15 Data shown are representative of 2 independent experiments with identical results.
The tyrosine kinase activity and the tyrosine residue 418 of NPM-ALK are essential for the interaction with an Src-kinase
To determine which tyrosine residue on NPM-ALK was essential for the interaction with an Src-kinase we used Jurkat cells stably expressing wild-type and mutant forms of NPM-ALK. Src-kinase immunoprecipitation followed by immunoblot analysis with the anti-ALK antibody revealed that the association was abolished in Jurkat cells expressing the NPM-ALK kinase dead (K210R) mutant, indicating that a functional kinase domain is required for Src-kinases to bind to NPM-ALK. The Y418F single NPM-ALK mutant failed to interact with Src-kinases, indicating a critical role for the Y418 of NPM-ALK in the regulation of this association (Figure 3).
The NPM-ALK Y664F mutant, previously described as being the PLC-γ binding site,13 spared the association. Conversely, Src-kinase association was abolished in Jurkat cells expressing the Y156F-Y418F double NPM-ALK mutants (Figure 3). The loss of such an interaction was specific since single or double Y156F, Y418F mutations did not impair other NPM-ALK–mediated signaling pathways such as Akt activation.15 In this context, it is noteworthy that the tyrosine kinase activity of NPM-ALK was not affected by Y418F, Y664F, or Y156F-Y418F mutations.12
pp60c-src is activated in NPM-ALK–expressing cells
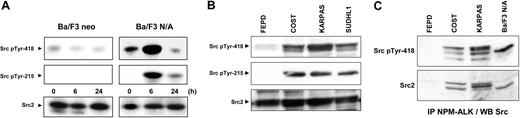
In order to check whether NPM-ALK could activate an Src-kinase, we took advantage of the Src pTyr-418 antibody that detects the activated form of Src-kinases24 and a pp60c-src-specific pTyr-215 antibody.25 Ba/F3 neo and Ba/F3 N/A cells were subjected to the serum deprivation/stimulation time course (as described in Figure 1), and immunoblot analysis of protein extracts was performed using the antiphospho–Src-kinases antibodies. Figure 4A shows that, at 6 hours after serum addition, NPM-ALK–expressing cells have a high level of phosphorylation on Src-kinase residues Y418 and Y215. Phosphorylation of Y418 reflects the autophosphorylation of Src-kinases. The phosphorylation of Y215 on pp60c-src has been shown to involve other kinases such as the platelet-derived growth factor (PDGF) receptor. No phosphorylation of tyrosine 215 could be seen in Ba/F3 neo cells at any time point tested, and a weak basal phosphorylation of Y418 was detected. Thus, this Src-kinase activation seems to be strictly correlated with NPM-ALK kinase activity (Figure 1D).
Activation of pp60c-src in NPM-ALK–expressing cells. (A) Ba/F3 neo and Ba/F3 N/A cells were subjected to the same serum deprivation/restimulation time course (24-hour serum withdrawal followed by 0-, 6-, and 24-hour serum readdition). Immunoblotting analysis of protein extracts (from 5 × 105 cells) was performed using anti-Src pTyr418 (signature of maximal activation of all Src-kinases), Src pTyr215 antibodies (specific for the activated form of pp60c-src), and anti-Src2 antibody (to visualize Src-family members). (B) Activation of Src-kinases was also revealed using the same antibodies in 4 ALCL-derived cell lines: FEPD (NPM-ALK negative), COST, KARPAS, and SU-DHL1 (NPM-ALK positive). Loaded per lane was 60 μg cell protein extracts. (C) NPM-ALK was immunoprecipitated (from 5 × 106 cells) with the ALK1 antibody followed by immunoblotting using the anti-Src pTyr418 antibody to analyze the activation state of the associated Src-kinases. The same nitrocellulose was stripped and reprobed using anti-Src2 antibody to visualize Src-family members. In panels A-B, proteins were separated on a 7.5% SDS-PAGE, whereas a 12% SDS-PAGE was used in panel C to achieve a higher resolution. Data shown are representative of 3 independent experiments with similar results.
Activation of pp60c-src in NPM-ALK–expressing cells. (A) Ba/F3 neo and Ba/F3 N/A cells were subjected to the same serum deprivation/restimulation time course (24-hour serum withdrawal followed by 0-, 6-, and 24-hour serum readdition). Immunoblotting analysis of protein extracts (from 5 × 105 cells) was performed using anti-Src pTyr418 (signature of maximal activation of all Src-kinases), Src pTyr215 antibodies (specific for the activated form of pp60c-src), and anti-Src2 antibody (to visualize Src-family members). (B) Activation of Src-kinases was also revealed using the same antibodies in 4 ALCL-derived cell lines: FEPD (NPM-ALK negative), COST, KARPAS, and SU-DHL1 (NPM-ALK positive). Loaded per lane was 60 μg cell protein extracts. (C) NPM-ALK was immunoprecipitated (from 5 × 106 cells) with the ALK1 antibody followed by immunoblotting using the anti-Src pTyr418 antibody to analyze the activation state of the associated Src-kinases. The same nitrocellulose was stripped and reprobed using anti-Src2 antibody to visualize Src-family members. In panels A-B, proteins were separated on a 7.5% SDS-PAGE, whereas a 12% SDS-PAGE was used in panel C to achieve a higher resolution. Data shown are representative of 3 independent experiments with similar results.
Although a weak increase in the level of pp60c-src expression was observed after 6 hours of serum addition in Ba/F3-expressing NPM-ALK (Figure 4, lower panel), this is not sufficient to support such activation. Interestingly, phosphorylations (pY418 and pY215) were strongly detected in ALCL patient–derived cell lines expressing NPM-ALK and hardly detectable in the FEPD ALCL cell line, which does not express the oncogene (Figure 4B).
As shown Figure 4C, the Src-kinases interacting with NPM-ALK were under an activated form (ie, phosphorylated on Y418). This figure also indicates that several members of the Src-family can interact with NPM-ALK, particularly in the human cell lines derived from patients with ALCL. Each hematopoietic cell lineage is known to express different members of the Src-family kinases. Both p56 Lck and p59 Fyn are found in normal resting T lymphocytes, but other members such as pp60c-src are expressed only upon activation.26,27
Immunocytologic studies using a streptavidin-biotin-peroxidase method and the anti-Src pTyr418 antibody (Figure 5) clearly confirmed the activation of Src-kinases in NPM-ALK–positive ALCL cell lines. This specific NPM-ALK–mediated activation of Src-kinases was also observed in immunohistologic studies of frozen sections of nude mice xenografts obtained after inoculation of ALCL cells (not shown).
Demonstration of Src-kinase activation in ALCL-derived cell lines expressing NPM-ALK by immunocytochemistry. Cytospins from FEPD (A,D), SU-DHL1 (B,E), and COST (C,F) cells were fixed in acetone and immunostained. Src-kinase activation was assessed using a streptavidin-biotin-peroxidase method with anti-Src pTyr418 polyclonal Ab and biotin-conjugated pig antirabbit IgG (A-C). The same protocol without anti-Src pTyr418 polyclonal Ab was performed in order to assess background nonspecific immunostaining (D-F). Original magnification, × 400.
Demonstration of Src-kinase activation in ALCL-derived cell lines expressing NPM-ALK by immunocytochemistry. Cytospins from FEPD (A,D), SU-DHL1 (B,E), and COST (C,F) cells were fixed in acetone and immunostained. Src-kinase activation was assessed using a streptavidin-biotin-peroxidase method with anti-Src pTyr418 polyclonal Ab and biotin-conjugated pig antirabbit IgG (A-C). The same protocol without anti-Src pTyr418 polyclonal Ab was performed in order to assess background nonspecific immunostaining (D-F). Original magnification, × 400.
Overall, these results indicate that Src-kinases, including pp60c-src, are downstream targets of NPM-ALK and are fully activated in cells expressing this chimeric oncogene.
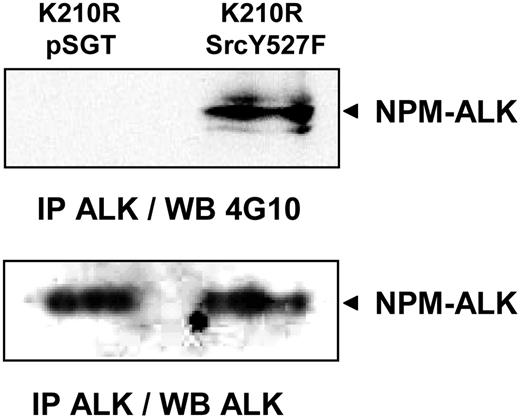
We then checked whether pp60c-src could phosphorylate NPM-ALK by using the Jurkat cell line stably expressing the kinase dead mutant of NPM-ALK (K210R). Transient transfection of these cells with the constitutively active Y527F mutant form of pp60c-src induced a tyrosine phosphorylation of the kinase inactive NPM-ALK (Figure 6). This result indicates that NPM-ALK is a potential substrate of pp60c-src in vivo.
Phosphorylation of NPM-ALK by pp60c-src. Jurkat cells stably expressing the K210R kinase dead NPM-ALK mutant were transiently transfected with pSGT empty vector (lane 1) or pSGT SrcY527F hyperactive form of pp60c-src. At 48 hours after transfection, cells were subjected to immunoprecipitation with the ALK1 antibody followed by immunoblot analysis with the antiphosphotyrosine 4G10 antibody. Data shown are representative of 2 independent experiments with identical results.
Phosphorylation of NPM-ALK by pp60c-src. Jurkat cells stably expressing the K210R kinase dead NPM-ALK mutant were transiently transfected with pSGT empty vector (lane 1) or pSGT SrcY527F hyperactive form of pp60c-src. At 48 hours after transfection, cells were subjected to immunoprecipitation with the ALK1 antibody followed by immunoblot analysis with the antiphosphotyrosine 4G10 antibody. Data shown are representative of 2 independent experiments with identical results.
Role of Src-kinases in NPM-ALK–mediated oncogenesis
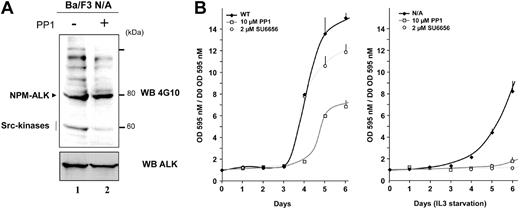
We analyzed the effect of 2 different Src-kinase inhibitors on Ba/F3 cells expressing NPM-ALK. The pyrolopyrimidine Src-kinase inhibitor PP1 strongly decreased the tyrosine phosphorylation of a 60-kDa protein matching pp60c-src and reduced the tyrosine phosphorylation of several other proteins (Figure 7A), suggesting a role for Src-kinases in this process. Under these conditions PP1 did not affect significantly the activity of NPM-ALK as shown by its autophosphorylation level.
Effect of Src-kinase inhibitors on NPM-ALK–mediated IL3-independent proliferation of the Ba/F3 cell line. (A) Ba/F3 N/A cells were deprived of serum for 24 hours followed by 6-hour serum readdition in the presence or absence of 10 μM PP1, as indicated. Cell lysates (5 × 105 cells) were subjected to immunoblot analysis with either antiphosphotyrosine 4G10 antibody (upper panel) or ALKc antibody (lower panel). (B) Wild-type Ba/F3 cells (WT) and Ba/F3 cells expressing NPM-ALK (Ba/F3 N/A) were seeded at 2 × 103 cells per well in a 96-well plate in medium enriched with (left) or deprived of (right) IL3. Cells were grown in the absence or in the presence of either 10 μM PP1 or 2 μM SU6656 as indicated. Cell proliferation was determined each day for 6 days using an MTT proliferation assay. Reported results are mean ± SEM of 3 independent experiments performed in triplicate.
Effect of Src-kinase inhibitors on NPM-ALK–mediated IL3-independent proliferation of the Ba/F3 cell line. (A) Ba/F3 N/A cells were deprived of serum for 24 hours followed by 6-hour serum readdition in the presence or absence of 10 μM PP1, as indicated. Cell lysates (5 × 105 cells) were subjected to immunoblot analysis with either antiphosphotyrosine 4G10 antibody (upper panel) or ALKc antibody (lower panel). (B) Wild-type Ba/F3 cells (WT) and Ba/F3 cells expressing NPM-ALK (Ba/F3 N/A) were seeded at 2 × 103 cells per well in a 96-well plate in medium enriched with (left) or deprived of (right) IL3. Cells were grown in the absence or in the presence of either 10 μM PP1 or 2 μM SU6656 as indicated. Cell proliferation was determined each day for 6 days using an MTT proliferation assay. Reported results are mean ± SEM of 3 independent experiments performed in triplicate.
The effects of the disruption of Src-mediated signaling on cell proliferation were then investigated. The Ba/F3 cell line is often used as a lymphoid model for transformation assays as it is normally dependent on IL3 for growth. Loss of IL3 dependence in this cell line as observed upon expression of NPM-ALK is a sign of cellular transformation.13 Treatment of Ba/F3 N/A cells with 10 μM PP1 or 2 μM of the newly described specific indolinone pp60c-src inhibitor SU665628 fully abolished the cell proliferation, whereas these compounds moderately affected the proliferation of wild-type Ba/F3 cells (Figure 7B). It is noteworthy that when Ba/F3 N/A cells were maintained in culture medium supplemented with IL3, the SU6656 compound was still able to strongly inhibit cell proliferation (within 2 days the number of cells increased by 3.4-fold in control conditions and 1.9-fold in the presence of 2 μM SU6656).
These results prompted us to evaluate the role of the interaction of Src-kinases with NPM-ALK in oncogenesis. The potential of 2 independent Ba/F3 clones expressing comparable amounts of Y418F mutant of NPM-ALK (Figure 8A) to proliferate in the absence of IL3 was evaluated. Immunoprecipitation with the anti-Src kinase antibody followed by immunoblot analysis with the anti-ALK antibody indicated that NPM-ALK Y418F no longer associated with Src-kinases in Ba/F3 cells (Figure 8B), as also shown in Jurkat cells (Figure 3).
Inhibition of NPM-ALK–mediated IL3-independent proliferation of Ba/F3 cells by disruption of NPM-ALK Src-kinase interaction. (A) The expression level of wild-type NPM-ALK (Ba/F3 N/A) or the NPM-ALK Y418F mutant Ba/F3 (clones 1, 2) was detected by immunoblotting with the ALKc antibody. (B) Of each clone, 10 million cells were subjected to immunoprecipitation with the Src-2 antibody followed by immunoblotting with ALKc. (C) Clones expressing wild-type NPM-ALK or the Y418F NPM-ALK mutant were plated at 103 cells per well in a 96-well plate in medium starved of IL3. Proliferation of the different clones was determined each day for 6 days using an MTT proliferation assay. Results are means of triplicates ± SEM of 1 experiment representative of 3.
Inhibition of NPM-ALK–mediated IL3-independent proliferation of Ba/F3 cells by disruption of NPM-ALK Src-kinase interaction. (A) The expression level of wild-type NPM-ALK (Ba/F3 N/A) or the NPM-ALK Y418F mutant Ba/F3 (clones 1, 2) was detected by immunoblotting with the ALKc antibody. (B) Of each clone, 10 million cells were subjected to immunoprecipitation with the Src-2 antibody followed by immunoblotting with ALKc. (C) Clones expressing wild-type NPM-ALK or the Y418F NPM-ALK mutant were plated at 103 cells per well in a 96-well plate in medium starved of IL3. Proliferation of the different clones was determined each day for 6 days using an MTT proliferation assay. Results are means of triplicates ± SEM of 1 experiment representative of 3.
After 5 days of IL3 starvation, Ba/F3 cells expressing the mutant form of NPM-ALK (Y418F) have a significantly slower proliferation rate compared with cells expressing wild-type NPM-ALK (Figure 8C). However, IL3 independence was not completely abolished as the cells were still capable to grow. These results indicate that association of the pY418 of NPM-ALK with Src-kinases, and possibly other partners, plays an important role in ALK-mediated cell proliferation.
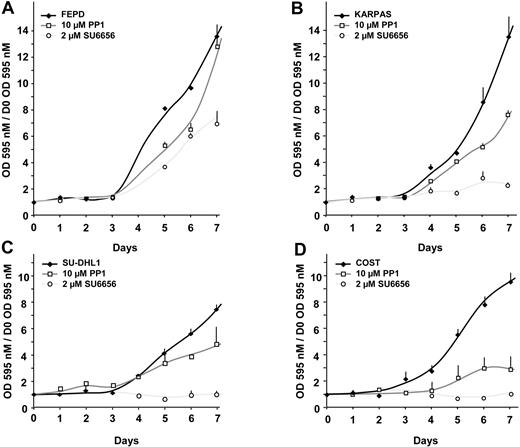
Finally, PP1 and SU6656 were used in proliferation assays with cell lines established from patients with ALCL (Figure 9).
Effect of the Src-kinase inhibitors on the proliferation of patient-derived ALCL cell lines. The ALCL cell lines FEPD (A), KARPAS (B), SU-DHL1 (C), and COST (D) were seeded at 104 cells per well in a 96-well plate. Cells were grown in the absence or in the presence of either 10 μM PP1 or 2 μM SU6656, as indicated. Cell proliferation was determined each day for 7 days using an MTT proliferation assay. Reported results are mean ± SEM of, at least, 3 different experiments performed in triplicate.
Effect of the Src-kinase inhibitors on the proliferation of patient-derived ALCL cell lines. The ALCL cell lines FEPD (A), KARPAS (B), SU-DHL1 (C), and COST (D) were seeded at 104 cells per well in a 96-well plate. Cells were grown in the absence or in the presence of either 10 μM PP1 or 2 μM SU6656, as indicated. Cell proliferation was determined each day for 7 days using an MTT proliferation assay. Reported results are mean ± SEM of, at least, 3 different experiments performed in triplicate.
After one week of treatment, PP1 had no significant effect on NPM-ALK–negative FEPD cell line growth, whereas NPM-ALK–positive cells (COST, KARPAS, and SU-DHL1) exhibited a marked sensitivity. The inhibition of proliferation by PP1 ranged from 35% (SU-DHL1) to 70% (COST) at day 7. Treatment with the SU6656 compound affected the growth of FEPD cells (mainly due to a delay and not an impairment of proliferation), but dramatically inhibited the proliferation of the 3 cell lines expressing NPM-ALK (Figure 9).
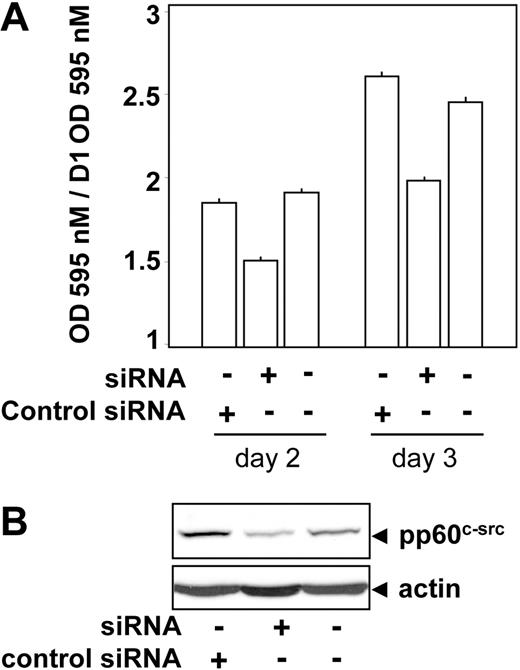
To directly assess the role of pp60c-src on proliferation of lymphoma cells we used an siRNA approach. As shown in Figure 10, a decrease in pp60c-src expression led to a significant reduction in cell proliferation measured after 48 and 72 hours. In agreement, in 2 independent series of experiments, FEPD (NPM-ALK negative) cells stably expressing a dominant-negative form of pp60c-src (double mutant K295A and Y527F)29 were viable, whereas we failed to establish stable COST or KARPAS (NPM-ALK positive) cell lines.
Involvement of pp60c-src on the proliferation of a patient-derived ALCL cell line. The ALCL cell line KARPAS was transfected or not by a specific pp60c-src siRNA or a control siRNA and seeded at 3 × 104 cells per well in a 96-well plate. At 24 hours after transfection, cell proliferation was determined each day for 2 days using an MTT proliferation assay. Reported results are mean ± SEM of at least 4 experiments. The decrease in proliferation rate observed in the presence of pp60c-src siRNA compared with control siRNA or untransfected cells was significant according to Student t test (P < .01). The expression level of pp60c-src and actin was assessed by immunoblotting 48 hours after transfection.
Involvement of pp60c-src on the proliferation of a patient-derived ALCL cell line. The ALCL cell line KARPAS was transfected or not by a specific pp60c-src siRNA or a control siRNA and seeded at 3 × 104 cells per well in a 96-well plate. At 24 hours after transfection, cell proliferation was determined each day for 2 days using an MTT proliferation assay. Reported results are mean ± SEM of at least 4 experiments. The decrease in proliferation rate observed in the presence of pp60c-src siRNA compared with control siRNA or untransfected cells was significant according to Student t test (P < .01). The expression level of pp60c-src and actin was assessed by immunoblotting 48 hours after transfection.
Discussion
The recruitment of specific signaling molecules by the NPM-ALK oncoprotein is thought to play an important role in its transforming activity. However, the effectors and/or interacting proteins of the constitutively active NPM-ALK tyrosine kinase are still poorly documented. Recent results have highlighted the critical role of PLC-γ, PI 3-kinase, and STAT3 in the process of NPM-ALK–mediated cell transformation.12-14,17,18 PLC-γ is involved in the regulation of growth,12 whereas PI 3-kinase and STAT3 seem to be more important for the antiapoptotic pathway initiated by NPM-ALK.13,14,17,18 Given the 21 tyrosine residues of NPM-ALK and the numerous adaptor molecules that may link tyrosine kinases to downstream effectors, several signaling pathways are likely activated by this oncogenic protein.8,16 We first determined the optimal culture conditions in NPM-ALK–transfected Ba/F3 lymphoid cells for the identification of potential substrates and/or partners of NPM-ALK. We observed that 6 to 24 hours of serum deprivation prior to 6 hours of serum readdition induced maximal tyrosine phosphorylation of NPM-ALK and several other proteins.
Besides NPM-ALK, one of the major phosphotyrosyl proteins was observed at approximately 60 kDa (Figure 1A). This led us to identify Src-kinase family members as new elements involved in the signaling pathway initiated by NPM-ALK in various cell types, including cell lines established from patients with ALCL. Coimmunoprecipitation experiments demonstrated an interaction between NPM-ALK and an Src-kinase. This association was observed both after NPM-ALK or Src-kinase immunoprecipitation, suggesting a stable association. Using various NPM-ALK mutants, we demonstrated that the kinase activity of NPM-ALK is required for this association and we mapped the tyrosine 418 of NPM-ALK as the site of Src-kinases' direct or indirect interactions. The Y418, within the sequence pYRIMTQ, is not a consensus binding sequence for the Src homology 2 (SH2) domain of pp60c-src. It is rather a putative binding site for the p85 subunit of PI 3-kinase, although the Y418F mutation does not prevent the binding of p85 to NPM-ALK12 and the activation of Akt.15 These results suggest that an intermediate protein recruited by the active form of NPM-ALK may be involved in the complex formation of NPM-ALK and Src-kinases. Interestingly, NPM-ALK expression in Ba/F3 cells led to a robust phosphorylation of the tyrosine 418, a conserved signature of maximal catalytic activity of all Src-family kinases. The phosphorylation of tyrosine 215 of pp60c-src was also clearly detected in these cells, indicating the activation of this particular member of the Src-family. The tyrosines 418 and 215 (Figure 4B) were also phosphorylated in 3 patient-derived ALCL cell lines expressing NPM-ALK, COST, SU-DHL1, and KARPAS but not in an NPM-ALK–negative ALCL cell line. The mechanism of NPM-ALK–mediated Src-kinase activation remains to be established, but several possibilities may be envisaged. NPM-ALK may activate a protein tyrosine phosphatase (PTPase) able to dephosphorylate the pY527, leading to the disruption of an intramolecular interaction and the subsequent activation of Src-kinases. However, the role of PTPase and NPM-ALK–mediated oncogenesis remains unknown. Another possibility is that the Src-kinase SH2 or SH3 domains could associate with a protein recruited by NPM-ALK and contain an adequate phosphotyrosyl residue or a proline-rich sequence, respectively. Physiologically, the activation of the Src-family kinases generally occurs transiently during cell activation and is required for growth factor–induced mitogenesis.30,31 Constitutive activation of the tyrosine kinase activity of pp60c-src is a potent transforming signal, and a rise in its expression level or specific activity has been observed in a variety of human cancers.32-35 In T lymphocytes, pp60c-src expression is induced by activation of the T-cell receptor,26 suggestive of a role for pp60c-src in the normal T-cell activation pathway. Moreover, activated pp60c-src can induce hematopoietic growth factor independence (IL3 and granulocytemacrophage colony-stimulating factor) in a bone marrow–derived cell line, FDC-P1.36
Expression of the NPM-ALK Y418F mutant, which impairs the association of NPM-ALK with Src-kinases in Ba/F3 cells, severely impaired the cells' potential to grow in IL3-free medium. In agreement, the Src-kinase inhibitors strongly affected the growth of NPM-ALK–positive cells, including cell lines established from patients with ALCL. Wild-type Ba/F3 cells or an NPM-ALK–negative ALCL cell line were not or were weakly sensitive to these inhibitors. Using an siRNA approach, we observed that reduction of pp60c-src expression in KARPAS cells affects the proliferation of this NPM-ALK–positive ALCL. Moreover, although we succeeded in stably expressing a dominant-negative form of pp60c-src in NPM-ALK–negative ALCL cells, this was not possible in NPM-ALK–positive ALCL cells. These findings strongly suggest that Src-kinases are important elements in NPM-ALK lymphomagenesis. However, although we identified pp60c-src as a downstream effector of NPM-ALK, our results also indicate that, due to the high level of redundancy and selectivity of expression, different members of the Src-family may be involved in NPM-ALK–mediated transformation depending on the cell type (Ba/F3 or ALCL cells).
pp60c-src plays a critical role in the relay of signals by several other tyrosine kinases, including the PDGF receptor31,37 or the non–receptor tyrosine kinase Pyk2.38 A recent report indicates that the cytoplasmic tyrosine kinase c-Abl is an effector of pp60c-src for growth factor–induced c-myc expression and DNA synthesis,39 highlighting the complex relationships that exist between various tyrosine kinases in controlling cell proliferation. Recently, Hck, another member of the Src-family, was shown as a primary protein kinase responsible for BCR/ABL-mediated activation of STAT5 in myeloid leukemia cells.40 In fibroblasts, v-Src has been shown to activate STAT3.41 The expression and phosphorylation of STAT3 is increased in patient-derived ALCL cell lines, and evidence is accumulating that this transcription factor plays an important role in the mitogenic signaling initiated by NPM-ALK.17,18 Based on these observations, it is tempting to propose a potential role for Src-kinases in STAT3 activation in ALCL. Moreover, our results suggest that Src-kinases may phosphorylate NPM-ALK. Addition of phosphotyrosyl residues on NPM-ALK would increase the efficiency of this oncogene to recruit effector molecules involved in the transformation process.
In conclusion, we have identified Src-family kinases, particularly pp60c-src, as novel important effectors of the NPM-ALK oncoprotein associated withALCL. Further studies are now necessary to identify the exact role and the target of Src-kinases in NPM-ALK–mediated cell transformation. Analysis of the status of pp60c-src activation in ALCL patient tumor biopsies should be of great interest to determine whether this kinase might become a pharmacologic target for the treatment of NPM-ALK–positive ALCL.
Prepublished online as Blood First Edition Paper, October 16, 2003; DOI 10.1182/blood-2003-04-1038.
Supported by Association pour la Recherche Contre le Cancer (ARECA-Toulouse and contract 4794), by National Institutes of Health (NIH) R01 CA-69129 (S.W.M.), and by the American Lebanese Syrian Associated Charities, St Jude Children's Research Hospital.
D.C. and C.G. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr K. Pulford for providing KARPAS 299 cells, F. Capilla for technical assistance in immunohistochemistry, X. Cui for technical assistance in preparing Ba/F3 cells transfected with pcDNA3 NPM-ALK, and Drs C. Racaud-Sultan and S. Manenti (INSERM U563) for valuable discussions and thoughtful review of the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal