Abstract
Despite the popularity of adeno-associated virus 2 (AAV2) as a vehicle for gene transfer, its efficacy for liver-directed gene therapy in hemophilia A or B has been suboptimal. Here we evaluated AAV serotypes 2, 5, 7, and 8 in gene therapy of factor VIII (FVIII) deficiency in a hemophilia A mouse model and found that AAV8 was superior to the other 3 serotypes. We expressed canine B domain-deleted FVIII cDNA either in a single vector or in 2 separate AAV vectors containing the heavy- and light-chain cDNAs. We also evaluated AAV8 against AAV2 in intraportal and tail vein injections. AAV8 gave 100% correction of plasma FVIII activity irrespective of the vector type or route of administration.
Introduction
Hemophilia A is an X-linked bleeding disorder caused by mutations in the factor VIII (FVIII) gene (F8) that lead to deficiency of FVIII, an essential cofactor in the coagulation cascade.1,2 The clinical manifestations of severe FVIII deficiency are frequent spontaneous bleeds into joints and soft tissue, leading to serious complications and death. Current treatment is intravenous infusion of FVIII protein, either prophylactically or during a bleeding episode. However, the risk of transmission of viral blood-borne diseases from plasma-derived products and the high cost of recombinant protein have propelled researchers to look for alternative gene-based approaches mediated either through viral or nonviral delivery. Because a very low concentration of FVIII in plasma greatly ameliorates the clinical phenotype, the goal of gene therapy for hemophilia A is to safely and stably achieve levels of plasma FVIII activity greater than 5%.
Adeno-associated virus of serotype 2 (AAV2) is considered safe and is characterized by prolonged transgene expression. However, its application to liver-directed hemophilia A gene therapy has been limited by poor transgene expression and its 5-kb packaging constraint (FVIII cDNA is 6.9 kb).
Recently, we reported partial correction of hemophilia A mice using a single AAV2 vector expressing B domain-deleted murine FVIII.3 Despite long-term phenotypic correction, plasma FVIII activity peaked at only 8% with intraportal administration and declined to 2% to 3% at 9 months. We attributed these modest levels to the use of a short promoter lacking regulatory elements necessary for greater FVIII expression and poor transduction efficiency of the AAV2 serotype.
Despite the ability of AAV to direct stable gene transfer and expression in hepatocytes, the search for alternative AAV serotypes has become imperative to obtain enhanced expression levels. Indeed, one study demonstrated supranormal levels of canine FIX with AAV1 serotype in immunodeficient mice following intramuscular injection.4 Another study reported improved hepatic gene transfer of human FIX in mice using AAV5 serotype.5 At the same time Gao et al reported the isolation of 2 immunologically distinct AAV capsid proteins, AAV7 and AAV8, from rhesus monkeys.6 Furthermore, they found that human sera with pre-existing immunity to human-derived AAVs had very little neutralizing activity against AAV7 and AAV8 vectors. In that report, the efficacy of AAV7 in gene delivery to skeletal muscle was equivalent to that of AAV1, and AAV8 was 10- to 100-fold more effective in liver delivery than any other AAV serotype.6
Therefore, we have evaluated AAV serotypes 2, 5, 7, and 8 in FVIII delivery to hemophilia A mice. In this study, we had 3 objectives: (1) to compare AAV serotypes expressing B domain-deleted canine FVIII cDNA administered intraportally to hemophilia A mice; doses used were 1 × 1010, 3 × 1010, and 1 × 1011 genome copies of each vector per mouse (gc/vector/mouse); (2) to assess the efficacy of FVIII cDNA delivered in either 2 vectors (heavy and light chain) expressed via large liver-specific promoters, or a single-chain vector expressed via a short liver-specific promoter; and (3) to compare the efficacy of these constructs in 2 different routes of administration, namely, intraportal and tail vein injection. We found that AAV8 was superior to the other serotypes. Using AAV8, we obtained 100% correction of FVIII deficiency regardless of the vector type or route of administration.
Materials and methods
Cloning of canine FVIII cDNA
Three different canine FVIII B domain-deleted expression cassettes were made: (1) a B domain-deleted single-chain construct (FVIIIBDD), driven by a synthetically derived short liver-specific promoter/enhancer of 368 bp, the insulin-like growth factor-binding protein, followed by a 175-bp chimeric intron (IGBP/enh/intron; Figure 1A); (2) a canine FVIII heavy-chain vector (Figure 1B); and (3) a canine FVIII light-chain vector (Figure 1C). Unlike the single-chain vector, these latter 2 vectors were expressed by 695 bp of the liver-specific human thyroxine-binding globulin gene promoter/α1-microglobulin/bikunin enhancer (TBG/enh).
FVIII constructs. (A) Single-chain FVIII construct. A 4.5-kb B domain-deleted canine FVIII cDNA was engineered such that residue S743 is in-frame with Q1630 while deleting most of the B domain. The cDNA is driven by synthetically derived 368-bp promoter/enhancer from insulin-like growth factor-binding protein, fused to 175 bp of a chimeric intron (IGBP/enh/intron, total size 543 bp). The FVIII sequence is followed by 263 bp of SV40 poly A signal and the entire cassette is flanked at both ends by the 145-bp ITRs. The total size of the expression cassette is 5.596 kb. (B) FVIII heavy-chain construct. The 2.5-kb cDNA contains the canine FVIII Kozak, initiator codon (bent arrow), signal peptide (57 bp), A1 and A2 domains followed by a stop codon after residue S743. The cDNA is driven by a 695-bp thyroxine-binding globulin gene promoter/enhancer fused to a 175-bp intron (TBG/enh/intron, total size 856 bp), has an SV40 poly A (263 bp) and 145-bp flanking ITRs. The size of the entire expression cassette is 4 kb. (C) FVIII light-chain construct. The 2.4-kb cDNA contains the canine FVIII Kozak sequence, initiator codon (bent arrow), and the signal peptide (57 bp) in-frame with the C-terminus of the B domain at residue G1555 through residue I1690, followed by A3, C1, and C2 domains. The light-chain construct is also driven by the 856 bp of TBG/enh/intron promoter, has the 263-bp SV40 poly A signal, and the 145-bp ITRs. The size of this expression cassette is 3.9 kb.
FVIII constructs. (A) Single-chain FVIII construct. A 4.5-kb B domain-deleted canine FVIII cDNA was engineered such that residue S743 is in-frame with Q1630 while deleting most of the B domain. The cDNA is driven by synthetically derived 368-bp promoter/enhancer from insulin-like growth factor-binding protein, fused to 175 bp of a chimeric intron (IGBP/enh/intron, total size 543 bp). The FVIII sequence is followed by 263 bp of SV40 poly A signal and the entire cassette is flanked at both ends by the 145-bp ITRs. The total size of the expression cassette is 5.596 kb. (B) FVIII heavy-chain construct. The 2.5-kb cDNA contains the canine FVIII Kozak, initiator codon (bent arrow), signal peptide (57 bp), A1 and A2 domains followed by a stop codon after residue S743. The cDNA is driven by a 695-bp thyroxine-binding globulin gene promoter/enhancer fused to a 175-bp intron (TBG/enh/intron, total size 856 bp), has an SV40 poly A (263 bp) and 145-bp flanking ITRs. The size of the entire expression cassette is 4 kb. (C) FVIII light-chain construct. The 2.4-kb cDNA contains the canine FVIII Kozak sequence, initiator codon (bent arrow), and the signal peptide (57 bp) in-frame with the C-terminus of the B domain at residue G1555 through residue I1690, followed by A3, C1, and C2 domains. The light-chain construct is also driven by the 856 bp of TBG/enh/intron promoter, has the 263-bp SV40 poly A signal, and the 145-bp ITRs. The size of this expression cassette is 3.9 kb.
Single-chain construct
The FVIII cDNA was cloned by a reverse transcriptase-polymerase chain reaction (RT-PCR) method7 from total RNA of normal dog liver (Veterinary School, University of Pennsylvania, Philadelphia). The primers were designed such that the heavy and light chains could be amplified separately from the same first-strand cDNA. The 2 PCR products, 5′ ClaI-3′ SmaI (canine heavy chain with the N-terminus of B domain) and 5′ SmaI-3′ XbaI (canine light chain with the C-terminus of B domain), were brought in-frame by a 3-way ligation to yield a 4.5-kb canine B domain-deleted version (canine BDD). The fusion points of the heavy and light chains were residues S743 and Q1630, respectively, so as to retain thrombin cleavage sites at residues R740 and R1648. The cDNA was initially cloned in pBSII KS at 5′ ClaI and 3′ XbaI, and sequenced to confirm its fidelity. The cDNA was transferred to the shuttle vector containing the synthetic IGBP promoter, liver-specific α1-microglobulin/bikunin enhancer, and intron, a total of 543 bp.8-10 The cDNA was followed by 263 bp of SV40 poly A signal sequence. The entire expression cassette was flanked by 145 bp of AAV inverted terminal repeats (ITRs; Figure 1A). The total size of the vector is 5.6 kb.
FVIII heavy- and light-chain constructs
The canine heavy chain encoding the A1 and A2 domains was obtained as a 2.5-kb PCR fragment. A stop codon was introduced in the 3′ primer after residue S743. The forward and reverse primers were 5′-ccatcgattgaattcccaccatgcaagtagagctctacacctgc-3′ and 5′-ggaagatcttcagcccttgtttctttctattgctccacg-3′, respectively. The fragment was cloned in the shuttle vector containing 695 bp of TBG promoter/enhancer9 followed by 175 bp of chimeric intron. The SV40 poly A signal and AAV ITRs were those used for the single-chain construct (Figure 1B).
The canine light-chain construct encoding the A3, C1, and C2 domains was obtained after a 2-step PCR resulting in a 2.4-kb fragment. First, the Kozak and the signal peptide sequences necessary to express the light-chain protein independent of the heavy chain were obtained by PCR from the existing heavy chain. The 5′ primer used was the same as that used for the heavy-chain construct. However, the 3′ primer was different, with an AluI site introduced after residue L18 to recreate residue S19 of the signal peptide (5′-tggcagctaaggctgaagggcaaaag-3′). The PCR for the second step was carried out with another set of primers to bring residue S19 in-frame with the C-terminus of the B domain beginning at residue G1555. SmaI, BglII. and XbaI were added in that order to the 3′ primer to enable the final cloning step, a 3-way ligation to bring the fragment in-frame with the light chain present in the backbone (used to construct the canine single-chain FVIII BDD). The 5′ and 3′ primers were 5′-tggtgtccttgcttgggataaccactatgatacc-3′ and 5′-gctctagatctcccgggcaggggcaaaatg-3′, respectively. The final 2.4-kb light-chain fragment was transferred into the shuttle vector containing the 695-bp TBG promoter/enhancer, 175-bp intron, 263-bp SV40 poly A signal and the flanking ITRs (Figure 1C).
Production of AAV vector
The canine AAV-FVIII vectors were packaged with AAV7 and AAV8 capsid proteins by pseudotyping. The recombinant AAV-FVIII genomes equipped with AAV2 ITRs were packaged by triple transfection of 293 cells with cis-plasmid, adenovirus helper plasmid, and a chimeric packaging construct in which the AAV2 rep gene was fused to the cap genes derived from either AAV serotype 5, 7, or 8.6 Recombinant AAV2/2 vector was purified by single-step heparin column chromatography. All other serotype vectors were purified by the standard cesium chloride (CsCl2) sedimentation method.11
TaqMan PCR (Applied Biosystems, Foster City, CA) analysis was adopted to determine genome copy (gc) titers of AAV vectors. The primers and probes were targeted to the SV40 poly A region.12
Study design and animal procedures
Mice were housed in a specific pathogen-free environment. All surgical procedures were carried out in accordance with institutional guidelines under approved protocols at the University of Pennsylvania. The exon 16 factor VIII knockout (E16 FVIII KO) mice used were generated in our laboratory.13 Males and females were equally represented in the study. To avoid any potential immune responses to the canine FVIII transgene, all FVIII KO mice were immune suppressed with cyclophosphamide injected intraperitoneally in 3 doses of 100 μg each on days –3, +1, and +3. The hemophilia A mice were also covered with 1.2 U recombinant human FVIII (Refacto; Wyeth BioPharma, Madison, NJ) administered via tail vein injection about 15 minutes prior to surgery.
A total of 131 FVIII KO mice between 6 and 8 weeks of age were given recombinant AAV-canine FVIII vector (Table 1); 120 mice were given intraportal injections (90 mice dosed with heavy- and light-chain vectors and 30 mice dosed with the single-chain vector) and 11 mice were given tail vein injections. Of the 90 mice receiving FVIII in 2 vectors, 32 were injected with AAV8, 21 with AAV7, 16 with AAV5, and 21 with AAV2 vectors. The efficacy of AAV8 and AAV2 in delivering canine FVIII as a single chain was evaluated in 30 mice (15 for each serotype). The vector doses tested for each serotype were 1 × 1010, 3 × 1010, and 1 × 1011 gc/mouse. Additionally, mice were also tested with AAV8 vectors at 8 × 1011 gc/vector/mouse. Mice tolerated surgery for intraportal injection very well with no mortality. At each vector dose a minimum of 5 mice were treated.
Experimental design
Serotype . | Route of administration/vector form . | No. of mice tested . |
|---|---|---|
| Intraportal | ||
| 8 | 2 chains | 32 |
| 8 | Single chain | 15 |
| 7 | 2 chains | 21 |
| 5 | 2 chains | 16 |
| 2 | 2 chains | 21 |
| 2 | Single chain | 15 |
| Tail vein | ||
| 8 | 2 chains | 3 |
| 8 | Single chain | 4 |
| 2 | Single chain | 4 |
Serotype . | Route of administration/vector form . | No. of mice tested . |
|---|---|---|
| Intraportal | ||
| 8 | 2 chains | 32 |
| 8 | Single chain | 15 |
| 7 | 2 chains | 21 |
| 5 | 2 chains | 16 |
| 2 | 2 chains | 21 |
| 2 | Single chain | 15 |
| Tail vein | ||
| 8 | 2 chains | 3 |
| 8 | Single chain | 4 |
| 2 | Single chain | 4 |
AAV vector administration into FVII KO mice. Serotypes 2, 5, 7, and 8 expressing B domain-deleted canine FVIII cDNA were evaluated in hemophilia A mice. Details of the total number of mice tested, routes of vector administration, and numbers of mice receiving FVIII either in 2 vectors (heavy and light chain) or as a single-chain vector are listed.
In addition to these 120 mice, another small group of 11 mice were assessed by tail vein injections of AAV8 and AAV2 vectors. Three mice received AAV8 expressing FVIII in 2 vectors. Eight mice received the single-chain vector, 4 with AAV8 serotype and 4 with AAV2 serotype. The tail vein route was tested at a single vector dose of 1 × 1011 gc/mouse.
Assays for FVIII activity and phenotypic correction
Plasma used for assay of FVIII activity was collected at regular intervals from tail clip bleeds using sodium citrate (0.38% wt/vol) as an anticoagulant. FVIII activity was assayed using the Coatest C/4 kit (Chromogenix, Milan, Italy). In measuring murine FVIII activity in FVIII KO mice, the standard curve was generated by mixing varying amounts of normal pooled mouse plasma in knockout mouse plasma to yield a range of FVIII activity between 20% and 150% for the high-range curve and between 1% and 20% for the low-range curve. However, in the present study KO mice were injected with a recombinant AAV expressing the canine FVIII transgene. Hence, in addition to the mouse standard curve, a second standard curve was developed using normal pooled canine plasma diluted in mouse FVIII KO plasma to generate the same high- and low-range curves. We reasoned that the canine standard curve thus generated would be more pertinent because it should reflect a more realistic measure of canine FVIII activity in the KO mice. In our assay, FVIII activity in canine plasma is about 3-fold greater than FVIII activity in murine plasma. Phenotypic correction was assayed after anesthetizing mice with methoxyflurane and clipping about 2 cm of their tails. Clot formation and survival beyond 24 hours confirmed correction.
Biodistribution of vector genomes
At 4 months after vector administration, a single mouse was killed for each AAV vector and dose group. gDNA was extracted from liver, spleen, stomach, ileum, heart, lung, kidney, muscle, and gonads. Biodistribution of the vectors as measured by copy number of the vector genomes was analyzed by real-time PCR with the primers and probe targeting the SV40 poly A region.11
Southern blot analysis
Liver tissue from treated mice was harvested for Southern blot analysis14 at 4 months after vector administration. DNA from uninjected KO mouse liver was used as negative control. A total of 12 μg gDNA was restricted with a single enzyme (XhoI for 2-vector delivery and AscI for single-vector delivery) to determine the molecular status of the AAV vector genome and with 2 enzymes (XhoI and NotI for 2-vector delivery and AscI and KpnI for single-vector delivery) to determine the transgene copy numbers. XhoI cuts within the vector resulting in a 4-kb or 3.9-kb fragment (unit length of the vector for the canine heavy-chain and light-chain vectors, respectively). A single cut within the vector allows distinction between head-to-tail (4 kb and 3.9 kb for heavy chain and light chain, respectively) and tail-to-tail (5.9 kb and 5.6 kb for heavy and light chain, respectively) concatemeric arrangements (Figure 5A-D). Similarly, a single cut with AscI results in a 5.6-kb head-to-tail fragment (unit length of the vector) and a 9.5-kb tail-to-tail concatemer (Figure 5E-F). For controls, untreated FVIII KO mouse liver DNA, spiked with canine FVIII-AAV plasmid DNA (20, 10, and 1 copy/haploid genome) was restricted as described. After DNA digestion, samples were run on a 0.8% agarose gel, Southern blotted, and hybridized with 32P-labeled probes. The heavy-chain probe was a 700-bp PCR fragment encoding the A1 domain of the canine FVIII protein. The 1-kb light chain probe encodes the A3 domain of the canine FVIII protein, and the 1.2-kb single-chain probe spans the junction between the IGBP promoter and the A1 domain of the canine FVIII protein.
Southern blots of liver DNA from FVIII KO mice treated with various AAV serotypes. One mouse per serotype treated at a dose of 1 × 1011 gc/vector/mouse, injected either intraportally or via tail vein, was killed at 4 months after injection. A total of 12 μg gDNA was used for Southern blotting. Untreated FVIII KO mouse liver DNA either unspiked or spiked with 20, 10, or 1 copy of canine FVIII-AAV was used as control. Liver DNA from mice treated with the 2 vectors (heavy and light chains of FVIII) was digested with XhoI to determine the molecular status of the vector genome and XhoI plus NotI to determine the copy numbers. Similarly liver DNA from the single-vector treatment was digested with AscI for molecular status and AscI plus KpnI for copy numbers. Panels A and C (Southern blot: canine FVIII-heavy chain and Southern blot: canine FVIII-light chain, respectively) represent mice treated intraportally with either AAVs 8, 7, 5, or 2, administered in 2 vectors. The blot in panel A was probed with a 700-bp heavy chain probe (B, canine FVIII heavy chain in AAV vector) encoding the A1 domain of the canine FVIII cDNA. A single digest with XhoI in the blot in panel A revealed 2 different molecular configurations, present in equal ratio, 4-kb head-to-tail molecules and 5.9-kb tail-to-tail molecules for all serotypes. The copy numbers were 9, 7, 1, and 5 copies/cell for AAVs 8, 7, 5, and 2. The blot in panel C was probed with 1-kb light-chain probe (D, canine FVIII light chain in AAV vector), encoding the A3 domain. The copy numbers for the blot in panel C were 9, 8, 0.2, and 5 for AAVs 8, 7, 5, and 2. Also, an equal numbers of head-to-tail (3.9 kb) and tail-to-tail (5.6 kb) concatamers were present. The blot in panel E (Southern blot: canine FVIII-single chain) represents mice treated both intraportally and via tail vein with AAV8 and AAV2 single-chain vectors. The blot in panel E was probed with 1.2-kb single-chain probe (F, canine FVIII single chain in AAV vector), which spans the IGBP promoter and the 5′ end of the FVIII transgene. The copy numbers were 8, 5, 6, and 0.5 for AAV8 intraportal, AAV8 tail vein, AAV2 intraportal, and AAV2 tail vein injections, respectively. DNA digested with AscI revealed 5.6-kb head-to-tail concatemers.
Southern blots of liver DNA from FVIII KO mice treated with various AAV serotypes. One mouse per serotype treated at a dose of 1 × 1011 gc/vector/mouse, injected either intraportally or via tail vein, was killed at 4 months after injection. A total of 12 μg gDNA was used for Southern blotting. Untreated FVIII KO mouse liver DNA either unspiked or spiked with 20, 10, or 1 copy of canine FVIII-AAV was used as control. Liver DNA from mice treated with the 2 vectors (heavy and light chains of FVIII) was digested with XhoI to determine the molecular status of the vector genome and XhoI plus NotI to determine the copy numbers. Similarly liver DNA from the single-vector treatment was digested with AscI for molecular status and AscI plus KpnI for copy numbers. Panels A and C (Southern blot: canine FVIII-heavy chain and Southern blot: canine FVIII-light chain, respectively) represent mice treated intraportally with either AAVs 8, 7, 5, or 2, administered in 2 vectors. The blot in panel A was probed with a 700-bp heavy chain probe (B, canine FVIII heavy chain in AAV vector) encoding the A1 domain of the canine FVIII cDNA. A single digest with XhoI in the blot in panel A revealed 2 different molecular configurations, present in equal ratio, 4-kb head-to-tail molecules and 5.9-kb tail-to-tail molecules for all serotypes. The copy numbers were 9, 7, 1, and 5 copies/cell for AAVs 8, 7, 5, and 2. The blot in panel C was probed with 1-kb light-chain probe (D, canine FVIII light chain in AAV vector), encoding the A3 domain. The copy numbers for the blot in panel C were 9, 8, 0.2, and 5 for AAVs 8, 7, 5, and 2. Also, an equal numbers of head-to-tail (3.9 kb) and tail-to-tail (5.6 kb) concatamers were present. The blot in panel E (Southern blot: canine FVIII-single chain) represents mice treated both intraportally and via tail vein with AAV8 and AAV2 single-chain vectors. The blot in panel E was probed with 1.2-kb single-chain probe (F, canine FVIII single chain in AAV vector), which spans the IGBP promoter and the 5′ end of the FVIII transgene. The copy numbers were 8, 5, 6, and 0.5 for AAV8 intraportal, AAV8 tail vein, AAV2 intraportal, and AAV2 tail vein injections, respectively. DNA digested with AscI revealed 5.6-kb head-to-tail concatemers.
Results
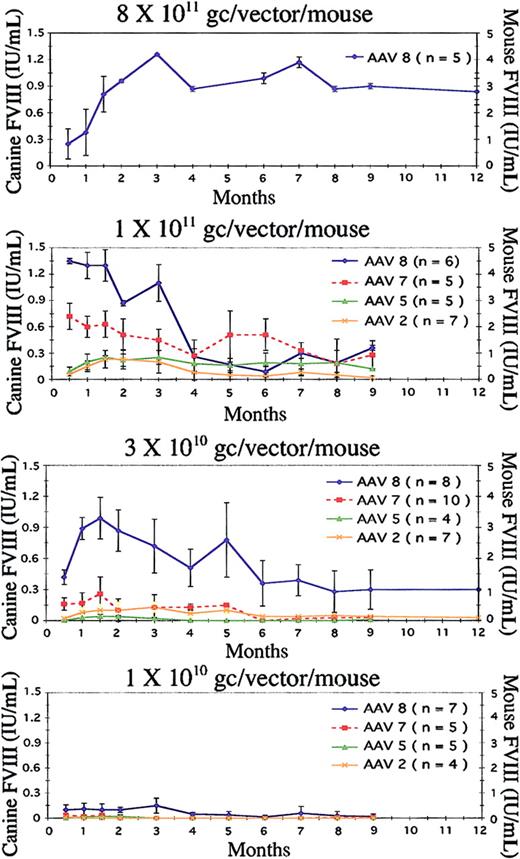
We compared efficacies of 4 different AAV serotypes, 2, 5, 7, and 8, expressing the heavy and light chains of canine FVIII in separate constructs (Figure 1B-C). These were administered intraportally at 3 different doses (1 × 1011, 3 × 1010, and 1 × 1010 of each vector gc/mouse; Figure 2). Furthermore, the AAV8-mediated vectors were also tested by tail vein injection at the high dose (1 × 1011 gc/mouse; Figure 4). Second, we evaluated the performance of AAV8 in comparison to AAV2, delivering the single-chain version of canine FVIII (Figure 1A). The single-chain vector was administered intraportally at the same doses as in the 2-chain vector experiment (Figure 3). Third, we compared AAV 8 and 2 in the single-chain version delivered via tail vein injection at 1 × 1011 gc/mouse (Figure 4).
Serotype and dose comparison in canine 2-chain intraportal delivery. AAV serotypes 2, 5, 7, and 8 expressing canine FVIII in 2 separate AAV vectors (heavy and light chains) were evaluated in FVIII KO mice at different vector doses. Because the vector injections were staggered for experimental convenience, data on some mice are available beyond 12 months, whereas the data on others end at 9 months. (A) Mice injected with 8 × 1011 gc/vector/mouse have long-term normal levels of FVIII expression when measured on the canine standard curve and supraphysiologic levels based on the murine standard curve. (B) Evaluation of the 4 serotypes at 1 × 1011 gc/vector/mouse. At this dose, both AAV8 and AAV7 express normal levels of FVIII when determined on the canine standard curve and supraphysiologic levels for AAV8 based on the murine standard curve. FVIII expression with AAV5 and AAV2 were at much lower levels. (C) The FVIII levels at a dose of 3 × 1010 gc/vector/mouse. AAV8 is clearly superior, expressing normal levels of FVIII over the long-term, whereas AAV7, 5, and 2 express at a much lower level that diminishes over time. This panel shows absence of activity at the lowest dose (1 × 1010 gc/vector/mouse) from AAV7, 5, and 2. On the other hand, AAV8 maintains a low, but respectable, level of FVIII activity. Error bars represent the mean ± SD.
Serotype and dose comparison in canine 2-chain intraportal delivery. AAV serotypes 2, 5, 7, and 8 expressing canine FVIII in 2 separate AAV vectors (heavy and light chains) were evaluated in FVIII KO mice at different vector doses. Because the vector injections were staggered for experimental convenience, data on some mice are available beyond 12 months, whereas the data on others end at 9 months. (A) Mice injected with 8 × 1011 gc/vector/mouse have long-term normal levels of FVIII expression when measured on the canine standard curve and supraphysiologic levels based on the murine standard curve. (B) Evaluation of the 4 serotypes at 1 × 1011 gc/vector/mouse. At this dose, both AAV8 and AAV7 express normal levels of FVIII when determined on the canine standard curve and supraphysiologic levels for AAV8 based on the murine standard curve. FVIII expression with AAV5 and AAV2 were at much lower levels. (C) The FVIII levels at a dose of 3 × 1010 gc/vector/mouse. AAV8 is clearly superior, expressing normal levels of FVIII over the long-term, whereas AAV7, 5, and 2 express at a much lower level that diminishes over time. This panel shows absence of activity at the lowest dose (1 × 1010 gc/vector/mouse) from AAV7, 5, and 2. On the other hand, AAV8 maintains a low, but respectable, level of FVIII activity. Error bars represent the mean ± SD.
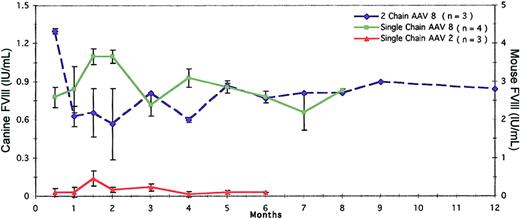
Tail vein delivery of single-chain and 2-chain AAV vectors. The efficacy of AAV8 as a single vector and 2 vectors delivered via tail vein was evaluated at 1 × 1011 gc/mouse. However, AAV2 was tested only as a single-chain vector by this route. Tail vein administration of AAV8 was as efficacious as intraportal delivery irrespective of the single- or 2-vector approach. In contrast, this route was not efficacious for the AAV2 single-chain vector. Error bars represent the mean ± SD.
Tail vein delivery of single-chain and 2-chain AAV vectors. The efficacy of AAV8 as a single vector and 2 vectors delivered via tail vein was evaluated at 1 × 1011 gc/mouse. However, AAV2 was tested only as a single-chain vector by this route. Tail vein administration of AAV8 was as efficacious as intraportal delivery irrespective of the single- or 2-vector approach. In contrast, this route was not efficacious for the AAV2 single-chain vector. Error bars represent the mean ± SD.
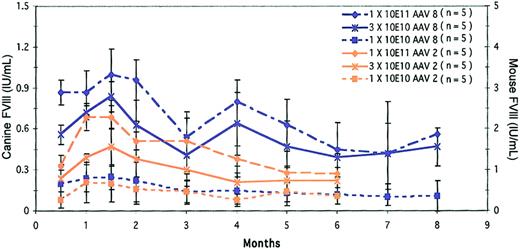
Dose comparison of AAV8 and AAV2 in canine single-chain intraportal delivery. AAV serotypes 8 and 2 were evaluated at 3 different doses, 1 × 1011, 3 × 1010, and 1 × 1010 gc/mouse. At the high and mid doses, AAV8 expresses normal levels of FVIII activity as measured against the canine standard curve and supraphysiologic levels based on the murine standard curve. Interestingly, AAV2 shows improved levels of expression when delivered as a single vector compared to the 2-vector approach at the same doses and time points.
Dose comparison of AAV8 and AAV2 in canine single-chain intraportal delivery. AAV serotypes 8 and 2 were evaluated at 3 different doses, 1 × 1011, 3 × 1010, and 1 × 1010 gc/mouse. At the high and mid doses, AAV8 expresses normal levels of FVIII activity as measured against the canine standard curve and supraphysiologic levels based on the murine standard curve. Interestingly, AAV2 shows improved levels of expression when delivered as a single vector compared to the 2-vector approach at the same doses and time points.
Because canine FVIII activity is several-fold greater than that of murine FVIII, it was important to measure FVIII activity using 2 standard curves, (1) normal canine plasma reconstituted in mouse KO plasma and (2) normal mouse plasma reconstituted in mouse KO plasma (y-axes in Figures 2, 3, 4). When FVIII activity is determined on the canine standard curve, the activity (as a percentage of normal) is about one third that determined on the mouse standard curve (Figures 2, 3, 4). This means that there is 3 times as much FVIII activity per milliliter in canine plasma as in mouse plasma.
Our results clearly demonstrated that at all 3 doses, AAV8 surpassed the other 3 serotypes for long-term, liver-directed, FVIII gene delivery. Second, the routes of administration did not significantly affect the level of FVIII activity for AAV8-mediated delivery, and lastly, the single-chain BDD canine construct with a short synthetic promoter/enhancer (368 bp) was equally as effective in KO mice as the heavy- and light-chain constructs delivered separately. At 6 months after vector administration, single-chain AAV8 vector at a dose of 1 × 1011 gc/mouse resulted in FVIII expression, based on the dog standard curve, of normal physiologic levels (0.58 ± 0.2 IU/mL; Figure 3). However, when that activity is plotted on the mouse standard curve, it is supraphysiologic (1.93 ± 0.67 IU/mL; Figure 3). Phenotypic correction was also determined at 4 months after vector administration. All mice treated with AAV8 (all doses) survived tail clipping, whereas all untreated (n = 5) KO mice died within 12 hours.
Delivery of FVIII in 2 separate vectors via portal vein
Initially AAV8 was tested at a relatively high vector dose of 8 × 1011gc/mouse of each vector (n = 5). Mice in this cohort did exceptionally well, expressing 1.2 ± 0.01 IU/mL at 3 months and 0.9 ± 0.15 IU/mL at 9 months (Figure 2A). The same data plotted on the mouse standard curve give supraphysiologic levels, 4.2 ± 0.01 IU/mL and 3 ± 0.05 IU/mL, respectively (Figure 2A).
Serotype efficacies were compared at the lower doses of 1 × 1011, 3 × 1010, and 1 × 1010 gc of each vector per mouse. Although AAV8 was the highest expressing vector at all 3 doses (Figure 2B-D), AAV7 was also promising at the high dose (1 × 1011), but FVIII levels declined at the mid and low doses (Figure 2B-D). Similarly, with AAV serotypes 5 and 2 measurable levels of FVIII activity were observed at the high dose, but levels were barely detectable at the lower doses (Figure 2C-D). For instance, with a vector dose of 1 × 1011 gc/mouse, AAV8 expressed at 1.1 ± 0.21 IU/mL at 3 months and 0.4 ± 0.03 IU/mL at 9 months (Figure 2B). At these time points, AAV7 expressed at 0.45 ± 0.12 IU/mL and 0.33 ± 0.09 IU/mL, respectively. The values for AAV5 were 0.30 ± 0.08 IU/mL and 0.19 ± 0.01 IU/mL, and for AAV2 they were 0.2 ± 0.13 IU/mL and 0.02 ± 0.01 IU/mL (Figure 2B). All values discussed are based on the canine standard curve.
At the mid dose (3 × 1010 gc/mouse), there was a clear distinction between AAV8 and the other serotypes (Figure 2C). The level peaked at 2 months (0.9 ± 0.2 IU/mL), at 5 months it was 0.78 ± 0.36 IU/mL, and at 9 months it had reached a plateau at 0.36 ± 0.19 IU/mL. FVIII could not be measured with AAV5 and was just above the baseline with AAV7 and AAV2 (0.13 ± 0.01 and 0.07 ± 0.05 IU/mL, respectively; Figure 2C).
At the low dose (1 × 1010 gc/mouse), FVIII expression from AAV8 measured 0.15 ± 0.09 IU/mL at 3 months and declined to 0.03 ± 0.01 IU/mL at 9 months after vector dosing. There was no activity detected when serotypes AAV 7, 5, and 2 were used (Figure 2D).
Delivery of FVIII in a single-chain vector via portal vein
We evaluated AAV8 and AAV2 expressing canine FVIII in a single vector at the same 3 doses, namely, 1 × 1011, 3 × 1010, and 1 × 1010 gc/mouse (Figure 3). Clearly, at all 3 doses the levels of expression were much higher with AAV8 than with AAV2 and exhibited a dose response. The levels at 1.5 months were 1.1 ± 0.10 IU/mL, 0.84 ± 0.11 IU/mL, and 0.25 ± 0.09 IU/mL for the respective doses. At 4 months, the levels persisted at 0.8 ± 0.16 IU/mL, 0.64 ± 0.13 IU/mL, and 0.15 ± 0.01 IU/mL, respectively. At 6 months these mice had maintained steady levels of expression with 0.58 ± 0.02 IU/mL, 0.39 ± 0.07 IU/mL, and 0.12 ± 0.01 IU/mL, respectively (Figure 3). All values mentioned here are based on the canine standard curve. Interestingly, AAV2-mediated delivery at 1.5 months also showed a dose response. The values were 0.69 ± 0.08 IU/mL, 0.47 ± 0.13 IU/mL, and 0.2 ± 0.13 IU/mL for the high, mid, and low doses, respectively, and these values differed little from those obtained with 2-vector delivery. At 6 months, single-chain AAV8 maintained levels of 0.45 ± 0.24 IU/mL, 0.39 ± 0.07 IU/mL, and 0.12 ± 0.01 IU/mL for the high, mid, and low doses, respectively. These values would read about 3 times higher when plotted on the mouse standard curve (Figure 3). However, unlike AAV8, the levels with AAV2 were somewhat lower. The levels at 6 months were 0.27 ± 0.15 IU/mL, 0.22 ± 0.1 IU/mL, and 0.11 ± 0.06 IU/mL for the high, mid and low doses, respectively.
Single-vector and 2-vector delivery by tail vein administration for AAV8 and AAV2
To demonstrate further that AAV8 is a high-expressing vector with tropism to liver, we evaluated its efficacy by tail vein injection compared to the efficacy of AAV2. Both single-vector and 2-vector injections were tested, but only at the high dose (1 × 1011 gc/mouse). Single-chain AAV8 vector did remarkably well in that FVIII levels were higher than with 2-vector delivery (Figure 4). With single-vector tail vein administration via AAV8, the activity peaked at 2 months (1.1 ± 0.05 IU/mL) and was maintained at 0.93 ± 0.7 IU/mL and 0.78 ± 0.04 IU/mL at 4 and 6 months, respectively (Figure 4). In contrast, when FVIII was delivered in 2 vectors via AAV8 by the same route and dose, the levels were 0.57 ± 0.28 IU/mL, 0.6 ± 0.02 IU/mL, and 0.77 ± 0.0 IU/mL for 2, 4, and 6 months, respectively (Figure 4). It was also noteworthy that comparable levels of expression were seen when single-chain AAV8 vector was delivered by either tail vein or portal vein route. For example, at 4 months, mice given single-chain AAV8 vector via tail vein had 0.93 ± 0.07 IU/mL, whereas mice given the same dose via portal vein had 0.8 ± 0.16 IU/mL (Figures 3 and 4). Interestingly, extremely low FVIII levels were seen with single-chain AAV2 vector delivered via tail vein in contrast to the same vector delivered via portal vein. For example, 2 months after portal vein administration of the single-chain AAV2 vector, FVIII activity was 0.51 ± 0.15 IU/mL, whereas after tail vein injection it was barely detectable (Figures 3 and 4).
Tissue biodistribution: liver tropism of AAV8
Tissue biodistribution of AAV8-injected mice revealed tropism to liver following a dose response. In the mice injected intraportally with 2 vectors, the copy numbers/cell based on TaqMan PCR at 4 months were 9.6, 3.9, and 0.5 versus barely detectable copy numbers in other tissues for the high, mid, and low doses, respectively. The same vectors injected via tail vein had 42.2 copies/cell for 1 × 1011 gc/mouse. AAV7 also showed tropism to liver, but did not follow a dose-response curve. In the liver the copy numbers were 4.2, 0.5, and 4.7 copies/cell for the high, mid, and low doses. No signals were obtained from other tissues. The biodistribution of the AAV5 serotype was interesting in that it demonstrated a wide tissue distribution without a dose correlation. Using TaqMan PCR at the mid dose, the copy numbers were highest (1.4) in muscle followed by 0.7, 0.6, and 0.2 copies/cell for liver, lung, and heart, respectively (Table 2). The 2-vector intraportal delivery of AAV2 serotype resulted in 7, 3.8, and 0.006 copies/cell in the liver for the high, mid, and low doses, respectively. On the other hand, AAV2 single vector when injected intraportally had only 0.428, 0.028, and 0.004 for the high, mid, and low doses, respectively. In contrast, the same vector when administered via tail vein (1 × 1011 gc/mouse) had only 0.089 copies/cell in the liver. Biodistribution was not assessed for the mid and low doses administered via the tail vein.
Tissue biodistribution of canine FVIII transgene 4 months after injection
Dose and route of administration . | . | Tissue biodistribution, copies/cell . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | AAV serotype . | Liver . | Spleen . | Stomach . | Ileum . | Heart . | Lung . | Kidney . | Muscle . | Gonads . | ||||||||
| 1 × 1011 gc/vector/mouse | ||||||||||||||||||
| Portal vein | 8 (2 chains) | 9.60 | 0.01 | 0.02 | 0.00 | 0.26 | 0.01 | 0.07 | NA | 0.64 | ||||||||
| 8 (single chain) | 5.70 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | |||||||||
| 7 (2 chains) | 4.20 | 0.01 | 0.00 | 0.02 | 0.03 | 0.06 | 0.04 | 0.01 | 0.00 | |||||||||
| 5 (2 chains) | 0.03 | 0.05 | 0.01 | 0.08 | 0.70 | 0.77 | 0.05 | 0.08 | 0.01 | |||||||||
| 2 (2 chains) | 7.00 | 0.00 | 0.10 | 0.01 | 0.01 | 0.01 | 0.01 | 0.05 | 0.00 | |||||||||
| 2 (single chain) | 0.43 | 0.05 | 0.04 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | |||||||||
| Tail vein | 8 (2 chains) | 42.20 | 0.01 | 0.01 | 0.01 | 0.06 | 0.03 | 0.03 | 0.01 | 0.00 | ||||||||
| 8 (single chain) | 2.70 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.17 | |||||||||
| 2 (single chain) | 0.09 | 0.01 | 0.05 | 0.01 | 0.01 | 0.01 | 0.04 | 0.01 | NA | |||||||||
| 3 × 1010 gc/vector/mouse | ||||||||||||||||||
| Portal vein | 8 (2 chains) | 3.97 | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | ||||||||
| 8 (single chain) | 0.60 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 | 0.00 | 0.00 | 0.02 | |||||||||
| 7 (2 chains) | 0.51 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.02 | |||||||||
| 5 (2 chains) | 0.77 | 0.02 | 0.04 | 0.20 | 0.20 | 0.60 | 0.04 | 1.40 | 0.07 | |||||||||
| 2 (2 chains) | 3.80 | 0.01 | 0.01 | 0.17 | 0.01 | 0.41 | 0.02 | 0.01 | 0.15 | |||||||||
| 2 (single chain) | 0.03 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.04 | 0.02 | |||||||||
| 1 × 1010 gc/vector/mouse | ||||||||||||||||||
| Portal vein | 8 (2 chains) | 0.47 | 0.00 | 0.01 | 0.03 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | ||||||||
| 8 (single chain) | 0.06 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||
| 7 (2 chains) | 4.74 | 0.00 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||
| 5 (2 chains) | 0.57 | 0.02 | 0.02 | 0.06 | 0.10 | 0.22 | 0.01 | NA | 0.02 | |||||||||
| 2 (2 chains) | 0.01 | 0.05 | 0.00 | 0.05 | 0.03 | 0.04 | 0.01 | 0.19 | 0.01 | |||||||||
| 2 (single chain) | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.02 | 0.04 | |||||||||
Dose and route of administration . | . | Tissue biodistribution, copies/cell . | . | . | . | . | . | . | . | . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | AAV serotype . | Liver . | Spleen . | Stomach . | Ileum . | Heart . | Lung . | Kidney . | Muscle . | Gonads . | ||||||||
| 1 × 1011 gc/vector/mouse | ||||||||||||||||||
| Portal vein | 8 (2 chains) | 9.60 | 0.01 | 0.02 | 0.00 | 0.26 | 0.01 | 0.07 | NA | 0.64 | ||||||||
| 8 (single chain) | 5.70 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | |||||||||
| 7 (2 chains) | 4.20 | 0.01 | 0.00 | 0.02 | 0.03 | 0.06 | 0.04 | 0.01 | 0.00 | |||||||||
| 5 (2 chains) | 0.03 | 0.05 | 0.01 | 0.08 | 0.70 | 0.77 | 0.05 | 0.08 | 0.01 | |||||||||
| 2 (2 chains) | 7.00 | 0.00 | 0.10 | 0.01 | 0.01 | 0.01 | 0.01 | 0.05 | 0.00 | |||||||||
| 2 (single chain) | 0.43 | 0.05 | 0.04 | 0.01 | 0.01 | 0.04 | 0.01 | 0.01 | 0.01 | |||||||||
| Tail vein | 8 (2 chains) | 42.20 | 0.01 | 0.01 | 0.01 | 0.06 | 0.03 | 0.03 | 0.01 | 0.00 | ||||||||
| 8 (single chain) | 2.70 | 0.00 | 0.00 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.17 | |||||||||
| 2 (single chain) | 0.09 | 0.01 | 0.05 | 0.01 | 0.01 | 0.01 | 0.04 | 0.01 | NA | |||||||||
| 3 × 1010 gc/vector/mouse | ||||||||||||||||||
| Portal vein | 8 (2 chains) | 3.97 | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | ||||||||
| 8 (single chain) | 0.60 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 | 0.00 | 0.00 | 0.02 | |||||||||
| 7 (2 chains) | 0.51 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.02 | |||||||||
| 5 (2 chains) | 0.77 | 0.02 | 0.04 | 0.20 | 0.20 | 0.60 | 0.04 | 1.40 | 0.07 | |||||||||
| 2 (2 chains) | 3.80 | 0.01 | 0.01 | 0.17 | 0.01 | 0.41 | 0.02 | 0.01 | 0.15 | |||||||||
| 2 (single chain) | 0.03 | 0.02 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.04 | 0.02 | |||||||||
| 1 × 1010 gc/vector/mouse | ||||||||||||||||||
| Portal vein | 8 (2 chains) | 0.47 | 0.00 | 0.01 | 0.03 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | ||||||||
| 8 (single chain) | 0.06 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||
| 7 (2 chains) | 4.74 | 0.00 | 0.02 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | |||||||||
| 5 (2 chains) | 0.57 | 0.02 | 0.02 | 0.06 | 0.10 | 0.22 | 0.01 | NA | 0.02 | |||||||||
| 2 (2 chains) | 0.01 | 0.05 | 0.00 | 0.05 | 0.03 | 0.04 | 0.01 | 0.19 | 0.01 | |||||||||
| 2 (single chain) | 0.00 | 0.00 | 0.00 | 0.17 | 0.00 | 0.00 | 0.00 | 0.02 | 0.04 | |||||||||
A single mouse for each serotype per dose and injection route was humanely killed at 4 months after vector administration. gDNA was extracted from 9 different tissues for TaqMan DNA PCR. The primers and probes were targeted to the SV40 poly A region.
NA indicates not assessed.
Southern blot analysis
Southern blot analysis of liver DNA was performed to determine the copy number and molecular status of AAV vectors at 4 months after vector administration. Liver DNA was subjected to Southern blotting with 3 different probes, each specific for the single-chain, heavy-chain, or light-chain vectors. Control lanes contain untreated FVIII KO mouse liver DNA either unspiked or spiked with canine FVIII-AAV plasmid DNA in 20, 10, or 1 copy/haploid genome. A double digest with XhoI and NotI, revealed 9, 7, 1, and 5 copies/cell for AAVs 8, 7, 5, and 2, respectively. A single digest with XhoI revealed 2 different molecular configurations, present in equal ratio, 4-kb head-to-tail molecules and 5.9-kb tail-to-tail molecules for all serotypes (Figure 5A). The blot was probed with a 700-bp heavy-chain probe encoding the A1 domain of canine FVIII cDNA (Figure 5B). When the blots were probed with a 1-kb light-chain probe, encoding the A3 domain (Figure 5D), the copy numbers were 9, 8, 0.2, and 5 for AAVs 8, 7, 5, and 2, respectively. Also, equal numbers of head-to-tail (3.9 kb) and tail-to-tail (5.6 kb) concatamers were present (Figure 5C). Blots analyzed with a 1.2-kb single-chain probe (Figure 5F) revealed copy numbers of 8, 5, 6, and 0.5 for AAV8 intraportal, AAV8 tail vein, AAV2 intraportal, and AAV2 tail vein injections, respectively. DNA digested with AscI revealed 5.6-kb head-to-tail concatemers (Figure 5F).
Discussion
Some unique features of AAV vectors, namely, their nonpathogenicity, their ability to transduce both dividing and nondividing cells, and their ability to direct long-term expression while persisting as an episome, have made AAV2 the vector of choice for several gene therapy trials, including hemophilia B. Nonetheless, poor levels of gene expression with AAV2 serotype3,15 reduce its attractiveness for liver-directed gene delivery. Because FVIII is normally synthesized in the liver, our focus was to evaluate efficacies of AAV serotypes for liver-directed FVIII gene delivery. Using the hemophilia A mouse model, we have shown that AAV8 delivered intraportally or via tail vein as either 2 separate vectors or a single vector surpasses all other known serotypes in terms of efficiency of gene transfer to the liver and achieves high levels of plasma FVIII activity. In addition, at relatively low vector doses (3 × 1010 gc/mouse), which were clearly inefficient with AAV7, 5, and 2 vectors, AAV8 demonstrated sustained long-term FVIII expression with normal levels of activity and complete phenotypic correction of the mice.
In a recent study,3 we reported partial correction in hemophilia A mice with AAV2 expressing single-chain BDD murine FVIII. In this study, FVIII levels were 2% to 3% from 2 to 9 months after intraportal dosing. Factor VIII activity with vector administered intrasplenically or via tail vein was even more disappointing with poor levels of expression. We believe that these modest levels were due to the lack of liver-specific enhancer elements necessary to augment FVIII expression in our constructs. Here we have obtained levels of FVIII activity that are 30 times greater than those we achieved previously. In another study using AAV2 to deliver canine BDD FVIII intraportally, FVIII activity of 2.8% was obtained at 4 months.16
In another collaborative study,17 we demonstrated that coinjection of AAV2 vectors encoding the heavy and light chains of murine FVIII resulted in the secretion of biologically active FVIII protein, peaking at about 30% of normal murine FVIII activity at 8 weeks. The levels later dropped to about 7%, albeit well above the therapeutic levels (2%), and were sustained throughout the 22-week study period. This study was unique in that 1-day-old FVIII KO mice were injected via the superficial temporal vein with 2.4 × 108 infectious particles (IPs) of 2 vectors, one expressing the murine FVIII heavy chain and the other expressing the murine FVIII light chain. Despite this success, it is important to bear in mind that a total dose of 4.8 × 108 IP/pup (4.8 × 1010 gc/pup; IP/gc ratio being 1:100) for a 1-day-old pup, weighing less than a gram, is a relatively high dose, about 1012 gc/mouse for an adult mouse weighing 20 g.17 Hence, to avoid vector toxicity (an issue not addressed in that paper), high efficacy with the lowest vector dose is most desirable. Although the above 2 studies were done with AAV2 serotype expressing murine FVIII cDNA, the levels of FVIII activity were not comparable due to key differences in the design of the cDNA constructs as well as in the time and route of vector administration.
In yet another study,18 the use of AAV2 serotype expressing canine FVIII in the treatment of hemophilia A mice was reported. FVIII was delivered as separate heavy- and light-chain constructs driven by the human α1-antitrypsin promoter and apolipoprotein E hepatic control region. Each vector also contained a cytomegalovirus [CMV]/β-globin chimeric intron and a human growth hormone polyadenylation sequence. These regulatory sequences were used because of a report that showed stabilization of hepatic FIX gene expression when the construct included a hepatic locus control region.19 At 12 weeks apparently normal levels of FVIII activity were obtained with a very high dose of the 2 vectors. However, at this time point a dose of 3 × 1010 gc/mouse gave FVIII activity that was 6.5% based on their standard curve or about 1% of normal canine FVIII activity.18 In contrast, in the present study using AAV8 serotype with the 2-vector delivery approach at the same dose (3 × 1010 gc/vector/mouse), the results were astounding. The plasma FVIII level was 0.78 IU/mL after 5 months after dosing. This level of FVIII activity is well within the normal range (0.5-1.5 IU/mL) and corresponds to 78% of normal canine FVIII (Figure 2C). Clearly, the efficacy of AAV8-mediated FVIII gene delivery to liver is much greater than with all other known serotypes in terms of high levels of expression and efficiency of gene transfer.
Analysis of tissue biodistribution by TaqMan PCR (Table 2) as well as data from Southern blots demonstrated that the transgene copy numbers were highest in the liver with AAV8 vectors (8-9 copies/cell) and lowest with AAV 5 (0.2-1 copies/cell; Figure 5A,C,E). Although one would expect a direct correlation between copy numbers and FVIII expression, our study did not demonstrate such a relationship. For example AAV8 and AAV2 administered intraportally (1 × 1011 gc/vector/mouse) had copy numbers of 9.6 and 7.0 copies/cell, respectively (Table 2). However, their FVIII levels at 4 months were 0.26 and 0.08 IU/mL, respectively (Figure 2B), clearly demonstrating vector internalization but absence of transduction that may be related to postentry blocks.
Furthermore, we were surprised that AAV8 vector delivered via tail vein was as effective as portal vein administration and resulted in normal levels of FVIII (Figures 2A-C and 4). In essence, using AAV8 in a 2-vector approach achieved 100% correction in mice. The mechanisms for improved efficiency of AAV8 vectors remain unclear although we speculate that AAV8 uptake is receptor mediated and is more active on the basolateral surface of hepatocytes.6 Plausibly, the molecular status of the AAV vector genome as well as its localization within the different cellular fractions of liver tissue may play a significant role in affecting transgene expression.
On obtaining success with AAV8 in 2-vector delivery, we evaluated AAV8 for delivery of canine FVIII as a single-chain vector. We reasoned that indeed if AAV8 is superior to other existing AAV serotypes in terms of more stable transduction in the liver, we should then obtain higher levels of FVIII expression even with minimal regulatory elements. Above all, we might observe sustained high levels of FVIII over the long-term. Because the 2-vector approach greatly increases the vector load and the potential for toxicity due to contaminants in the viral preparations, this factor provided another reason for attempting FVIII correction with a single chain vector. Consequently, we compared AAVs 8 and 2 at a single dose (1 × 1011 gc/mouse) by 2 routes, intraportal and tail vein injections, and again found that AAV8 was superior.
The 5-kb packaging limit of AAV vectors has remained a major disadvantage and a challenge for delivering large therapeutic transgenes, such as FVIII. However, several groups16-27 as well as we3 have shown that it is possible to package more than 5 kb of DNA with fairly high titers. In this study, we demonstrate packaging of 5.6 kb of DNA in an AAV vector. Although fairly high titers (average 1 × 1013 gc/mL) were obtained, the ratio between genome copies and infectious center assay (GC/ICA) was very high, ranging between 32 000 and 50 000, suggesting inefficient packaging due to the large size may have led to many empty capsids. Nonetheless, we achieved 100% correction of hemophilia status in mice with these viral preparations. It was also encouraging that the single-chain vector was as effective as the 2 vectors, administered via tail vein with plasma levels of 0.83 ± 0.01 IU/mL at 8 months (Figure 4). Interestingly, the single-chain AAV8 vector, administered intraportally at the same dose (1 × 1011 gc/mouse) resulted in 0.56 ± 0.38 IU/mL at 8 months. These levels are within the normal range of FVIII activity (0.5-1.5 IU/mL).
This is the first study demonstrating complete correction in mice with AAV8-mediated gene delivery. The next challenge will be to treat hemophilia A dogs either with the 2 vectors or the single-chain vector and to achieve long-term expression of plasma FVIII levels comparable to those obtained in mice.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-08-2954.
Supported by grants from the National Institutes of Health and the Programs of Excellence in Gene Therapy (PEGT) grant of the Cell Morphology Core at the University of Pennsylvania.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Julio Sanmiguel, Deirdre McMenamin, Marcia Huston-Leslie, Philip Zoltick, and Di Wu for their help.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal