Abstract
All-trans-retinoic acid (ATRA) increases the efficacy of chemotherapy when used for induction and maintenance treatment of acute promyelocytic leukemia (APL), but its role in consolidation is unknown. Since November 1996, 426 patients with newly diagnosed APL have received induction therapy with ATRA and idarubicin. Before November 1999 (LPA96 study), consolidation therapy consisted of 3 courses of anthracycline monochemotherapy. After November 1999 (LPA99 study), patients with intermediate and high risks of relapse received consolidation therapy with ATRA and increased doses of anthracyclines. Of the 384 patients who achieved complete remission (90%), 382 proceeded to consolidation therapy. Seven patients died in remission (1.8%). The 3-year cumulative incidence of relapse for patients in the LPA96 and LPA99 studies was 17.2% and 7.5%, respectively (P = .008). Patients treated with ATRA in consolidation therapy showed an overall reduction in the relapse rate from 20.1% to 8.7% (P = .004). In intermediate-risk patients the rate decreased from 14.0% to 2.5% (P = .006). This improved antileukemic efficacy also translated into significantly better disease-free and overall survival. A risk-adapted strategy combining anthracycline monochemotherapy and ATRA for induction and consolidation therapy of newly diagnosed APL results in improved antileukemic efficacy and a high degree of compliance.
Introduction
The current recommendations for treatment of patients with newly diagnosed acute promyelocytic leukemia (APL) include all-trans-retinoic acid (ATRA) and anthracycline-based chemotherapy for remission induction, anthracycline-based chemotherapy for consolidation, and ATRA with low-dose chemotherapy for maintenance.1 Use of ATRA during consolidation has not been explored.
Using ATRA and anthracycline monochemotherapy (LPA96 study), the Spanish cooperative group PETHEMA (Programa de Estudio y Tratamiento de las Hemopatías Malignas) recently reported outcome results2 similar to those obtained in other studies3-8 using ATRA with anthracycline-based chemotherapy combinations. Aiming to improve the antileukemic efficacy of this protocol in patients with increased relapse risk, a new trial based on a risk-adapted strategy was started in November 1999 (LPA99 study). In this study, intermediate- and high-risk patients9 received ATRA during consolidation therapy in combination with moderately reinforced anthracycline monochemotherapy.
We report here the results obtained in 426 consecutive patients with newly diagnosed promyelocytic leukemia/retinoic acid receptor α-positive (PML/RARα+) APL who were enrolled in these 2 consecutive studies.
Patients and methods
Eligibility
The eligibility criteria for both studies were a diagnosis of de novo APL with demonstration of the t(15;17) or PML/RARα rearrangements, normal hepatic and renal function, no cardiac contraindications to anthracycline chemotherapy, and an Eastern Cooperative Oncology Group (ECOG) performance status of less than 4. Informed consent was obtained from all patients. According to the Declaration of Helsinki, the protocol was approved by the Research Ethics Board of each participating hospital.
Study design
Induction therapy. The induction regimen consisted of oral ATRA (45 mg/m2/d), divided into 2 daily doses, which was maintained until complete hematologic remission, or for a maximum of 90 days, and idarubicin (12 mg/m2/d) given as an intravenous bolus dose on days 2, 4, 6, and 8. For patients 20 years of age or younger, the ATRA dose was adjusted to 25 mg/m2/d. From November 1999, the dose of idarubicin on day 8 was omitted for patients older than 70 years of age.
Treatment was started as soon as a diagnosis of APL was made by cytologic criteria.10,11 For patients in whom the diagnosis was not confirmed by genetic studies, ATRA treatment was withdrawn and alternative chemotherapy was given at the physician's discretion.
Consolidation therapy. Between November 1996 and October 1999 (LPA96 study), all patients in complete remission (CR) received 3 monthly consolidation courses. The first course consisted of idarubicin (5 mg/m2/d) on days 1 through 4. The second course consisted of mitoxantrone (10 mg/m2/d) by intravenous bolus daily on days 1 through 5. The third course consisted of idarubicin (12 mg/m2) on day 1. From November 1999 (LPA99 study), intermediate- and high-risk patients (see “Definitions and study end points”) received ATRA combined with reinforced consolidation chemotherapy. ATRA (45 mg/m2/d) was given on days 1 through 15 in combination with the 3 single-agent chemotherapy courses. Reinforcement of chemotherapy consolidation consisted of increasing the idarubicin dose in the first course to 7 mg/m2/d and of administering idarubicin for 2 consecutive days instead of one in the third course.
Maintenance therapy. After completion of consolidation, patients who tested negative for PML/RARα (“Laboratory Studies”) were started on oral mercaptopurine (50 mg/m2/d), intramuscular methotrexate (15 mg/m2/wk), and oral ATRA (45 mg/m2/d) for 15 days every 3 months. Doses of mercaptopurine and methotrexate were decreased by 50% if the white blood cell (WBC) count was less than 3.5 × 109/L and discontinued if it was less than 2.5 × 109/L. Maintenance therapy was continued for 2 years.
Supportive therapy
Coagulopathy was treated with fresh-frozen plasma or fibrinogen. Platelet transfusions were given to maintain a platelet count of more than 30 × 109/L until resolution of significant coagulopathy. Once the coagulopathy was under control, platelet transfusions were only given for patients with infectious or hemorrhagic manifestations, or when the platelet count dropped below 20 × 109/L. Heparin use was not recommended. From November 1999 (LPA99 study), tranexamic acid (100 mg/kg/d) was given by continuous intravenous infusion until the platelet count was higher than 50 × 109/L. As prophylaxis for retinoic acid syndrome, patients in the LPA99 study received prednisone (0.5 mg/kg/d) on days 1 through 15. At the first signs of suspected retinoic acid syndrome, ATRA was discontinued and patients were given 10 mg dexamethasone every 12 hours.
Laboratory studies
Bone marrow samples were obtained at diagnosis, after induction, after consolidation, and periodically during maintenance and beyond, as reported elsewhere.2 Besides morphologic evaluation, samples were processed for RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR) for PML/RARα. RT-PCR tests were carried out by 14 different laboratories involved in an external quality control program, which included interlaboratory exchange of samples, as reported elsewhere.12,13 If a positive PCR result was reported after consolidation or beyond, a new sample was obtained and sent to one of 2 reference laboratories (P.B. and M.G.).
Definitions and study end points
Complete remission and relapse were defined according to the National Cancer Institute criteria.14 Early death was defined as death occurring during induction therapy or during the period of aplasia after chemotherapy. Molecular remission was defined as the disappearance on an ethidium bromide gel of the PML/RARα-specific band visualized at diagnosis, using RT-PCR assays with a sensitivity level of one cell in 10–4. Molecular persistence was defined as PCR positivity in 2 consecutive bone marrow samples collected at the end of consolidation therapy. Molecular relapse was defined as reported elsewhere.9 According to Frankel et al,15 retinoic acid syndrome was defined as “definitely present,” “indeterminate,” or “definitely absent.”
Risk of relapse was established at diagnosis according to a predictive model based on patient leukocyte and platelet counts at diagnosis, as reported elsewhere.9 Low-risk patients had a WBC count less than 10 × 109/L and a platelet count more than 40 × 109/L; intermediate-risk patients had a WBC count less than 10 × 109/L and a platelet count less than 40 × 109/L; and high-risk patients had a WBC count equal to or more than 10 × 109/L.
Statistical analysis
Rates of CR were evaluated using contingency tables. Unadjusted time-to-event analyses were performed using the Kaplan-Meier estimate,16 and, for comparisons, the log-rank tests.17 The probability of relapse was also estimated by the cumulative incidence method (marginal probability).18,19 Overall survival (OS) was calculated from the date of starting induction therapy, while cumulative incidence of relapse (CIR) and disease-free survival (DFS) were calculated from the date of CR. In the analysis of DFS, relapse and death in CR were considered uncensored events, whichever occurred first. For cumulative incidence analysis, death in CR was considered as a competing cause of failure. For all estimates in which the event “relapse” was considered as an end point, hematologic and molecular relapses, as well as molecular persistence, were each considered as uncensored events. For OS, an event was death from any cause, with any patients alive at last follow-up censored. The follow-up of the patients was updated on April 15, 2003. All P values reported are 2-sided. Multivariate analysis was performed using the Cox proportional hazards model.20 Except for the cumulative incidence method, computations were performed using the 4F, 1L, and 2L programs from the BMDP statistical library (BMDP Statistical Software, Los Angeles, CA).
Results
Accrual and patient characteristics
Between November 1, 1996, and August 31, 2002, 481 consecutive patients with morphologic diagnosis of APL were registered from 74 institutions from Spain, Argentina, and the Czech Republic (see “Appendix”). A total of 44 patients (9%) were considered not eligible for the study, 16 (8%) and 28 (10%) in the LPA96 and LPA99 trials, respectively. The distribution of causes for noneligibility was diagnosis not confirmed at the genetic level, 10 cases; poor performance status, 17 cases; and death before starting therapy, 17 cases. Differences in the distribution of noneligibility causes between the 2 studies were not significant. Thus, 437 patients met the previously defined entry criteria and were enrolled in the LPA96 and LPA99 studies. Six of 181 patients in the LPA96 study and 5 of 256 patients in the LPA99 study were not evaluated because of protocol violations during induction therapy. Protocol violations were due to the addition of cytarabine in 8 patients (4 in each trial) and the omission of idarubicin in one 75-year-old patient in the LPA96 trial. Two additional patients (one in each trial) who moved to another hospital during induction therapy were also excluded and considered protocol violations. The main clinical and biologic characteristics of the 426 patients evaluable for induction are shown in Table 1.
Demographic and baseline characteristics of the study population
. | LPA96 study, N = 175 . | . | LPA99 study, N = 251 . | . | . | ||
|---|---|---|---|---|---|---|---|
| Characteristic . | Median (range) . | No. (%) . | Median (range) . | No. (%) . | P . | ||
| Age, y | 40 (2-73) | 39 (2-81) | .4 | ||||
| 15 or younger | 11 (6.3) | 28 (11.1) | |||||
| 16-40 | 77 (44.0) | 106 (42.1) | |||||
| 41-60 | 58 (33.1) | 72 (28.7) | |||||
| 61-70 | 19 (10.9) | 33 (13.1) | |||||
| 71 or older | 10 (5.7) | 12 (4.8) | |||||
| Sex | .01 | ||||||
| Male | 103 (58.9) | 116 (46.2) | |||||
| Female | 72 (41.1) | 135 (53.8) | |||||
| WBC count, × 109/L | 2.1 (0.3-210) | 2.5 (0.3-235) | .8 | ||||
| Less than 3.5 | 108 (61.7) | 145 (57.8) | |||||
| 3.5-10 | 21 (12.0) | 35 (13.9) | |||||
| 10-50 | 33 (18.9) | 50 (19.9) | |||||
| 50 or higher | 13 (7.4) | 21 (8.4) | |||||
| Patelet count, × 109/L | 20 (1-161) | 22 (1-183) | .7 | ||||
| Less than 10 | 38 (21.7) | 46 (18.3) | |||||
| 11-40 | 97 (55.4) | 142 (56.6) | |||||
| 40 or higher | 40 (22.9) | 63 (25.1) | |||||
| Hemoglobin, g/L (range) | 94 (43-152) | 92 (30-169) | |||||
| Morphologic subtype | .5 | ||||||
| Hypergranular | 144 (82.3) | 201 (80.1) | |||||
| Microgranular | 31 (17.7) | 50 (19.9) | |||||
| PML/RARα isoform | .4 | ||||||
| BCR1/BCR2 | 94 (55.9) | 128 (56.4) | |||||
| BCR3 | 74 (44.1) | 99 (43.6) | |||||
. | LPA96 study, N = 175 . | . | LPA99 study, N = 251 . | . | . | ||
|---|---|---|---|---|---|---|---|
| Characteristic . | Median (range) . | No. (%) . | Median (range) . | No. (%) . | P . | ||
| Age, y | 40 (2-73) | 39 (2-81) | .4 | ||||
| 15 or younger | 11 (6.3) | 28 (11.1) | |||||
| 16-40 | 77 (44.0) | 106 (42.1) | |||||
| 41-60 | 58 (33.1) | 72 (28.7) | |||||
| 61-70 | 19 (10.9) | 33 (13.1) | |||||
| 71 or older | 10 (5.7) | 12 (4.8) | |||||
| Sex | .01 | ||||||
| Male | 103 (58.9) | 116 (46.2) | |||||
| Female | 72 (41.1) | 135 (53.8) | |||||
| WBC count, × 109/L | 2.1 (0.3-210) | 2.5 (0.3-235) | .8 | ||||
| Less than 3.5 | 108 (61.7) | 145 (57.8) | |||||
| 3.5-10 | 21 (12.0) | 35 (13.9) | |||||
| 10-50 | 33 (18.9) | 50 (19.9) | |||||
| 50 or higher | 13 (7.4) | 21 (8.4) | |||||
| Patelet count, × 109/L | 20 (1-161) | 22 (1-183) | .7 | ||||
| Less than 10 | 38 (21.7) | 46 (18.3) | |||||
| 11-40 | 97 (55.4) | 142 (56.6) | |||||
| 40 or higher | 40 (22.9) | 63 (25.1) | |||||
| Hemoglobin, g/L (range) | 94 (43-152) | 92 (30-169) | |||||
| Morphologic subtype | .5 | ||||||
| Hypergranular | 144 (82.3) | 201 (80.1) | |||||
| Microgranular | 31 (17.7) | 50 (19.9) | |||||
| PML/RARα isoform | .4 | ||||||
| BCR1/BCR2 | 94 (55.9) | 128 (56.4) | |||||
| BCR3 | 74 (44.1) | 99 (43.6) | |||||
Induction therapy
Hematologic and molecular responses. Of the 426 evaluable patients, 384 achieved hematologic CR (90%; 95% CI, 87%-92%). The remaining 42 were considered as failures due to early death or resistance (3 patients). Eleven early deaths were attributable to infection; 25 to cerebral, intestinal, or pulmonary hemorrhage; 2 to ATRA syndrome; and the remaining one to an unknown cause. The response rates and causes of induction death were similar in both the LPA96 and LPA99 studies (Table 2). For patients older than 70 years, the response rate was 60% (6 of 10) and 75% (9 of 12) for those receiving 4 (LPA96) and 3 doses (LPA99) of idarubicin, respectively. This difference was not statistically significant.
Results of induction therapy for patients with APL
. | . | Study . | . | Age, y . | . | WBC count, × 109/L . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | Overall, no. (%) . | LPA96, no. (%) . | LPA99, no. (%) . | 70 y or younger, no. (%) . | Older than 70 y, no. (%) . | Lower than 10, no. (%) . | 10 or higher, no. (%) . | |||
| Complete remission | 384 (90) | 157 (90) | 227 (90) | 369 (92) | 15 (68) | 287 (93) | 97 (84) | |||
| Failure | 42 | 18 | 24 | 35 | 7 | 22 | 20 | |||
| Early death | 39 | 15 | 24 | 33 | 6 | 21 | 18 | |||
| Hemorrhage | 25 (64) | 9 (60) | 16 (67) | 21 (64) | 4 (66) | 12 (57) | 13 (72) | |||
| Infection | 11 (28) | 5 (33) | 6 (25) | 9 (27) | 2 (33) | 7 (33) | 4 (22) | |||
| Retinoic acid syndrome | 2 (5) | 1 (7) | 1 (4) | 2 (6) | 0 | 1 (5) | 1 (6) | |||
| Other | 1 (3) | 0 | 1 (4) | 1 (3) | 0 | 1 (5) | 0 | |||
| Resistance | 3 | 3 | 0 | 2 | 1 | 1 | 2 | |||
| Retinoic acid syndrome | ||||||||||
| Definitely present | 18 (4.2) | 8 (4.6) | 10 (4.0) | 17 | 1 | 10 | 8 | |||
| Indeterminate | 105 (24.6) | 40 (25.9) | 65 (22.9) | 100 | 5 | 72 | 33 | |||
| Definitely absent | 303 | 127 | 176 | 287 | 16 | 227 | 76 | |||
. | . | Study . | . | Age, y . | . | WBC count, × 109/L . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
. | Overall, no. (%) . | LPA96, no. (%) . | LPA99, no. (%) . | 70 y or younger, no. (%) . | Older than 70 y, no. (%) . | Lower than 10, no. (%) . | 10 or higher, no. (%) . | |||
| Complete remission | 384 (90) | 157 (90) | 227 (90) | 369 (92) | 15 (68) | 287 (93) | 97 (84) | |||
| Failure | 42 | 18 | 24 | 35 | 7 | 22 | 20 | |||
| Early death | 39 | 15 | 24 | 33 | 6 | 21 | 18 | |||
| Hemorrhage | 25 (64) | 9 (60) | 16 (67) | 21 (64) | 4 (66) | 12 (57) | 13 (72) | |||
| Infection | 11 (28) | 5 (33) | 6 (25) | 9 (27) | 2 (33) | 7 (33) | 4 (22) | |||
| Retinoic acid syndrome | 2 (5) | 1 (7) | 1 (4) | 2 (6) | 0 | 1 (5) | 1 (6) | |||
| Other | 1 (3) | 0 | 1 (4) | 1 (3) | 0 | 1 (5) | 0 | |||
| Resistance | 3 | 3 | 0 | 2 | 1 | 1 | 2 | |||
| Retinoic acid syndrome | ||||||||||
| Definitely present | 18 (4.2) | 8 (4.6) | 10 (4.0) | 17 | 1 | 10 | 8 | |||
| Indeterminate | 105 (24.6) | 40 (25.9) | 65 (22.9) | 100 | 5 | 72 | 33 | |||
| Definitely absent | 303 | 127 | 176 | 287 | 16 | 227 | 76 | |||
Populations were as follows: overall, n = 426; LPA96, n = 175; and LPA99, n = 251.
At the end of induction, molecular assays for PML/RARα were carried out for 355 patients (92.4%). Of these, 173 (48.7%) tested positive.
A poorer response rate was observed in elderly patients and in those with higher WBC counts, with the most discriminating cut-off values being 70 years (91% versus 68%; P = .0007) and 10 cells × 109/L (93% versus 83%; P = .006), respectively.
Retinoic acid syndrome. “Definitely present” retinoic acid syndrome was diagnosed in 18 patients (4.2%), 2 of whom died from it, whereas an “indeterminate” retinoic acid syndrome was reported in 105 (24.6%). Despite prednisone prophylaxis, the incidence of “indeterminate” and “definitely present” retinoic acid syndrome in the LPA99 study (22.9% and 4%, respectively) did not differ from those in the LPA96 trial (25.9% and 4.6%, respectively).
The ATRA therapy was temporarily discontinued in 68 patients (16%) due to a presumed retinoic acid syndrome. This therapy was restarted at a median time of 5 days (range, 2-17 days). In 22 additional patients, ATRA was definitively discontinued after a median of 15 days of treatment (range, 7-19 days).
Consolidation therapy
Molecular response. RT-PCR tests for PML/RARα were carried out in 359 cases at the end of consolidation therapy (95%; Table 3). Of the 7 patients with molecular evidence of persistence at this time, 6 were among the 88 high-risk and one among the 199 intermediate-risk patients tested (P = .001). Among the 19 patients who were not tested for RT-PCR at the end of consolidation therapy, 2 early clinical relapses occurred at 1 and 3 months. Both were high-risk patients of the LPA96 trial.
Molecular persistence at the end of consolidation and molecular and clinical relapse according to relapse risk group in LPA96 and LPA99 trials
. | Molecular persistence . | . | Molecular relapse . | . | Clinical relapse . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Relapse risk group and type of consolidation . | No. (%) . | No./tested . | No. . | Time to relapse, mo . | No. (CNS) . | Time to relapse, mo . | |||
| Low | 79 (21) | 0/72 | 1 | 16 | 2 (0) | 32, 39 | |||
| LPA96 study | 33 | 0 | 0 | — | 2 (0) | 32, 39 | |||
| LPA99 study | 46 | 0 | 1 | 16 | 0 | — | |||
| Intermediate | 208 (54) | 1/199 | 5 | 8-21 | 8 (2) | 8-31 | |||
| LPA96 study | 86 | 1 | 4 | 8-21 | 7 (2) | 8-31 | |||
| LPA99 study | 122 | 0 | 1 | 15 | 1 (0) | 19 | |||
| High | 97 (25) | 6/88 | 4 | 5-31 | 12 (4) | 4-32 | |||
| LPA96 study | 38 | 4 | 3 | 5-31 | 6 (3) | 4-14 | |||
| LPA99 study | 59 | 2 | 1 | 9 | 6 (1) | 4-32 | |||
| Overall | 384 | 7/359 | 10 | 5-31 | 22 (6) | 4-39 | |||
| LPA96 study | 157 | 5 | 7 | 5-31 | 15 (5) | 4-39 | |||
| LPA99 study | 227 | 2 | 3 | 9, 15 | 7 (1) | 4-32 | |||
. | Molecular persistence . | . | Molecular relapse . | . | Clinical relapse . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Relapse risk group and type of consolidation . | No. (%) . | No./tested . | No. . | Time to relapse, mo . | No. (CNS) . | Time to relapse, mo . | |||
| Low | 79 (21) | 0/72 | 1 | 16 | 2 (0) | 32, 39 | |||
| LPA96 study | 33 | 0 | 0 | — | 2 (0) | 32, 39 | |||
| LPA99 study | 46 | 0 | 1 | 16 | 0 | — | |||
| Intermediate | 208 (54) | 1/199 | 5 | 8-21 | 8 (2) | 8-31 | |||
| LPA96 study | 86 | 1 | 4 | 8-21 | 7 (2) | 8-31 | |||
| LPA99 study | 122 | 0 | 1 | 15 | 1 (0) | 19 | |||
| High | 97 (25) | 6/88 | 4 | 5-31 | 12 (4) | 4-32 | |||
| LPA96 study | 38 | 4 | 3 | 5-31 | 6 (3) | 4-14 | |||
| LPA99 study | 59 | 2 | 1 | 9 | 6 (1) | 4-32 | |||
| Overall | 384 | 7/359 | 10 | 5-31 | 22 (6) | 4-39 | |||
| LPA96 study | 157 | 5 | 7 | 5-31 | 15 (5) | 4-39 | |||
| LPA99 study | 227 | 2 | 3 | 9, 15 | 7 (1) | 4-32 | |||
CNS indicates central nervous system; and —, not applicable.
Tolerance and treatment feasibility. Except one 81-year-old patient who died before starting consolidation due to volvulum and another 42-year-old patient who developed severe cardiac toxicity during induction (ventricular ejection fraction 40%), all the remaining 382 patients who achieved CR proceeded to receive consolidation therapy. Three deaths from infection occurred during consolidation in the LPA96 study (one in the first and 2 in the second course), and one in the LPA99 study (first course). Three patients did not receive the third course of consolidation (2 because of severe infection during the second consolidation course and one because she became pregnant). The remaining 375 patients (99.2%) completed the 3 consolidation courses as scheduled.
Maintenance therapy
All of the 378 patients alive after completing consolidation therapy proceeded to receive maintenance therapy. Cytopenias, especially neutropenia, and slight liver function test abnormalities were commonly observed in this phase, often requiring dose reduction or temporary discontinuation of chemotherapy. Three deaths in remission occurred during maintenance in patients aged 73, 78, and 33 due to unknown cause, infection, and epilepsy status, respectively. The latter patient had had cerebral hemorrhage at presentation.
Outcome
In addition to the 7 cases of molecular persistence, 32 relapses occurred among the 384 patients who achieved CR, 22 of 157 in the LPA96 study and 10 of 227 in the LPA99 study (Table 3). Five additional patients died after developing other malignancies (3 of acute myeloid leukemia and 2 of colon cancer).
CIR. For patients in the LPA96 study, with a median follow-up of 48 months for surviving patients, the CIR rate was 17.2%, whereas for patients in the LPA99 study, with a median follow-up of 21 months, it was 7.5% (P = .008; Figure 1A). Excluding low-risk patients to better evaluate the impact of risk-adapted consolidation, the 3-year CIR rates were 20.1% in the LPA96 study and 8.7% in the LPA99 study (P = 0.004; Figure 1B).
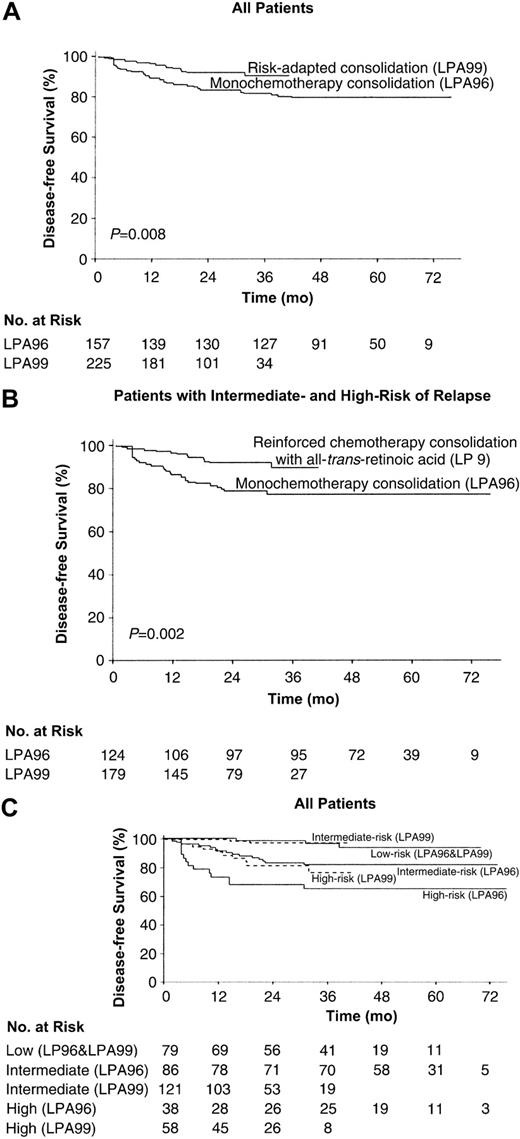
CIR. Cumulative incidence of relapse from the time of CR among all 382 patients (A) and 303 intermediate- and high-risk patients (B), according to whether they received anthracycline monochemotherapy consolidation (LPA96 study) or risk-adapted consolidation (LPA99 study). (C) CIR of all patients according to both the risk group and the trial.
CIR. Cumulative incidence of relapse from the time of CR among all 382 patients (A) and 303 intermediate- and high-risk patients (B), according to whether they received anthracycline monochemotherapy consolidation (LPA96 study) or risk-adapted consolidation (LPA99 study). (C) CIR of all patients according to both the risk group and the trial.
For low-risk patients from both studies, the 6-year CIR was 6.4%. For patients in the LPA99 study, the 3-year CIR for low-, intermediate-, and high-risk patients were 3%, 2.5%, and 21%, respectively, whereas in the LPA96 study, they were 6.1%, 14%, and 34.2% (P = .8, P = .006, and P = .08; Figure 1C). This led to a CIR of 2.6% for both low- and intermediate-risk groups in the LPA99 trial when they were analyzed together.
DFS. The 3-year DFS rates were 81% ± 6% in the LPA96 study and 90% ± 5% in the LPA99 study (P = .008; Figure 2A). Excluding low-risk patients, the 3-year DFS rates were 77% ± 8% in the LPA96 study and 90% ± 6% in the LPA99 study (P = .002; Figure 2B).
DFS. Kaplan-Meier product-limit estimate of DFS among all 382 patients (A) and 303 intermediate- and high-risk patients (B), according to whether they received anthracycline monochemotherapy consolidation (LPA96 study) or risk-adapted consolidation (LPA99 study). (C) DFS of all patients according to both the risk group and the trial.
DFS. Kaplan-Meier product-limit estimate of DFS among all 382 patients (A) and 303 intermediate- and high-risk patients (B), according to whether they received anthracycline monochemotherapy consolidation (LPA96 study) or risk-adapted consolidation (LPA99 study). (C) DFS of all patients according to both the risk group and the trial.
For low-risk patients, the 6-year DFS rate was 86% ± 10%. For patients in the LPA99 study, the 3-year DFS rates for low-intermediate- and high-risk patients were 93% ± 8%, 97% ± 4% and 77% ± 14%, respectively, whereas in the LPA96 study, they were 97% ± 6%, 82% ± 8%, and 66% ± 15% (P = .4, P = .002, and P = .05; Figure 2C).
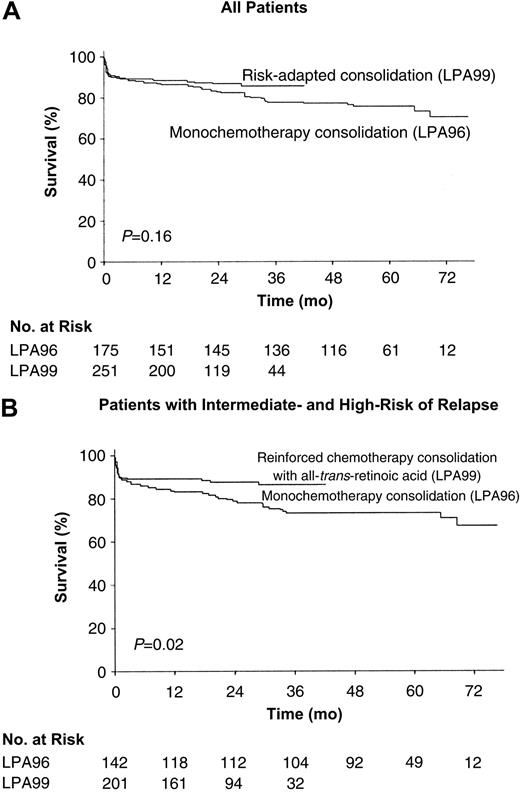
OS. The probability of remaining alive after 3 years was 78% ± 6% in the LPA96 study and 85% ± 5% in the LPA99 study (P = .2; Figure 3A). For patients younger than 70 years, these estimates were 79% ± 6% and 88% ± 5% (P = .1). Excluding low-risk patients, the 3-year OS rate for patients in the LPA99 study (86% ± 5%) was significantly higher than for those in the LPA96 study (73% ± 7%; P = .02; Figure 3B).
OS. Kaplan-Meier product-limit estimate of OS among all 426 patients (A) and 343 intermediate- and high-risk patients (B), according to whether they received anthracycline monochemotherapy consolidation (LPA96 study) or risk-adapted consolidation (LPA99 study). P values were calculated with use of the log-rank test.
OS. Kaplan-Meier product-limit estimate of OS among all 426 patients (A) and 343 intermediate- and high-risk patients (B), according to whether they received anthracycline monochemotherapy consolidation (LPA96 study) or risk-adapted consolidation (LPA99 study). P values were calculated with use of the log-rank test.
Discussion
This study shows that a risk-adapted strategy combining ATRA with anthracycline monochemotherapy for induction and consolidation, followed by ATRA and low-dose methotrexate and mercaptopurine for maintenance therapy, results in extremely high antileukemic efficacy, moderate toxicity, and a high degree of compliance in patients with APL. The novel addition of ATRA to consolidation therapy, combined with a moderate increase in the dose of anthracycline for intermediate- and high-risk patients, resulted in higher antileukemic activity with no additional severe toxicity.
Although these apparent improvements are based on historical comparisons, several features of the present study reduce the chance of bias. All participating institutions registered all patients with newly diagnosed APL, regardless of any criteria that may lead to a selection bias. Moreover, eligibility criteria were unchanged throughout the study, and included obligatory genetic diagnosis, a prerequisite that was accomplished by only 3 of the other major series combining ATRA and chemotherapy.3,6,8 Given the sequential nature of the studies, patients in the LPA99 series had a shorter follow-up than those in the LPA96 study. Notwithstanding, the LPA99 study contains the largest series reported so far for a single therapeutic option, and its follow-up is similar to those reported in the major trials published to date.
Induction results in the LPA96 and LPA99 studies confirmed the virtual absence of leukemia resistance using ATRA and idarubicin treatment alone. Induction failure rates remained unchanged over the study period, despite prophylactic administration of tranexamic acid and corticosteroids in the LPA99 study to reduce hemorrhage- and retinoic acid syndrome-associated mortality, respectively. In line with these findings, Avvisati et al21 demonstrated a reduction of hemorrhagic episodes using tranexamic acid prophylaxis, although they failed to demonstrate any impact on hemorrhage-associated mortality.
That concomitant ATRA and chemotherapy results in higher antileukemic efficacy when used for induction3,5 and maintenance therapy5 provided a rationale for using such a combination in consolidation. In addition, the low toxicity during consolidation therapy observed in the interim analysis of the LPA96 study2 suggested that the idarubicin dose could be raised safely. This reinforcement appeared to improve the rate of molecular remissions after consolidation therapy in intermediate- and high-risk patients while maintaining a very high degree of treatment compliance without increasing toxic deaths. In contrast, most recent trials3-8 reported important toxicity during consolidation, leading to a variable but significant number of patients receiving less treatment than scheduled.
Although the optimal schedule for monitoring minimal residual disease by RT-PCR in APL is not well established,22 the experience of the PETHEMA studies confirms that molecular status after induction has no predictive value on patient outcome. In contrast, molecular persistence of residual disease and molecular relapse after consolidation are almost invariably observed in high-risk patients. These findings suggest that such stringent molecular monitoring may not be cost effective for low- and intermediate-risk patients.
The significant improvement of the antileukemic efficacy in the LPA99 study, compared with the LPA96 study, was certainly caused by the modified consolidation therapy, although it is unclear which part of the reinforced therapy (ATRA or chemotherapy or both) may have led to the impact observed in the outcome. However, there may be a significant role for ATRA, as has been demonstrated for induction3,5 and maintenance therapy.5 Thus, the use of ATRA combined with chemotherapy for consolidation therapy for low-risk patients is warranted. As has been demonstrated for induction and maintenance therapy in other previous studies,3,5 the results of the present study also suggest that the benefit provided by the modified consolidation therapy in terms of relapse rate was less important in patients with initially higher WBC counts (P = .08 versus P = .006).
This 7.5% CIR at 3-years in the overall LPA99 series, with 2.6% in the low and intermediate group (together accounting for 75% of total patients), compares favorably with all other studies published so far. Although the antileukemic benefit provided by the addition of ATRA to consolidation therapy should be definitively settled in randomized studies or with longer follow-up, we believe that the current consensus on the simultaneous administration of ATRA and chemotherapy for induction and maintenance in the front-line therapy for APL could be extended to the consolidation phase. In addition to its higher antileukemic efficacy, this combination also results in a higher degree of compliance and lower toxicity, compared with polychemotherapy combinations. Finally, risk-adapted strategies focusing on patients with hyperleukocytosis at presentation should be the major subject of future studies.
Appendix
The following institutions and clinicians participated in the study: Argentina (Grupo Argentino de Tratamiento de la Leucemia Aguda)—daleu, Buenos Aires: S. Pavlovsky, G. Milone, I. Giere; Hospital Clemente Álvarez, Rosario: S. Ciarlo; Hospital General San Martín, La Plata: M. Gelemur; Hospital Rossi, La Plata: S. Saba; Hospital San Martín de Paraná, Entre Ríos: P. Negri; Instituto de Trasplante de Médula Ósea, La Plata: V. Prates; Czech Republic—Faculty Hospital, Brno: M. Protivankova; Spain (Programa de Estudio y Tratamiento de las Hemopatías Malignas)— Basurtuko Ospitalea, Bilbao: J. M. Beltrán de Heredia; Complejo Hospitalario de Segovia: J. M. Hernández; Complexo Hospitalario Xeral-Calde, Lugo; J. Arias; Complejo Hospitalario, León: F. Ramos; Fundación Jiménez Díaz, Madrid: JF Tomás; Hospital 12 de Octubre, Madrid: J. de la Serna, J. Martínez; Hospital Carlos Haya, Málaga: S. Negri; Hospital Carlos Haya (Materno Infantil), Málaga: E. González; Hospital Central de Asturias, Oviedo: C. Rayón; Hospital Clinic, Barcelona: J. Esteve, D. Colomer; Hospital Clínico de Valladolid: F.J. Fernández-Calvo; Hospital Clínico San Carlos, Madrid: J. Díaz Mediavilla; Hospital Clínico Universitario, Santiago de Compostela: M. Pérez; Hospital Clínico Universitario, Valencia: M. Tormo, I. Marugán; Hospital Clínico Universitario Lozano Blesa, Zaragoza: L. Palomera; Hospital de Cruces, Baracaldo: E. Amutio; Hospital del Mar, Barcelona: C. Pedro; Hospital de Navarra, Pamplona: K. Pérez-Equiza; Hospital Dr Negrín, Las Palmas: T. Molero, M.T. Gómez; Hospital Dr Peset, Valencia: M. J. Sayas; Hospital Dr Trueta, Girona: R. Guardia; Hospital General de Albacete: J. R. Romero; Hospital General de Alicante: C. Rivas; Hospital General de Alicante (Oncología Pediátrica): C. Esquembre; Hospital General de Castellón: G. Cañigral; Hospital General de Especialidades Ciudad de Jaén: A. Alcalá; Hospital General de Jerez de la Frontera: A. León, L. Hermosín; Hospital General de Murcia: J. M. Moraleda; Hospital General de Valencia: M. Linares; Hospital Germans Trias i Pujol, Badalona: J. M. Ribera; Hospital Insular de Las Palmas: J. D. González San Miguel; Hospital Juan Canalejo, A Coruña: G. Debén; Hospital Joan XXIII, Tarragona: L. Escoda; Hospital La Princesa, Madrid: A. Figuera; Hospital Materno-Infantil de Las Palmas: A. Molines; Hospital do Meixoeiro, Vigo: C. Loureiro; Hospital Montecelo, Pontevedra: M. J. Allegue; Hospital Mutua de Terrasa: J. M. Martí; Hospital Niño Jesús, Madrid: L. Madero; Hospital Ntra. Sra. de Sonsoles, Ávila: M. Cabezudo; Hospital Ramón y Cajal, Madrid: J. García-Laraña; Hospital Reina Sofía, Córdoba: J. Román, A. Rodríguez; Hospital Río Carrión, Palencia: F. Ortega; Hospital Río Hortega, Valladolid: M. J. Peñarrubia; Hospital San Jorge, Huesca: F. Puente; Hospital San Rafael, Madrid: B. López-Ibor; Hospital Sant Pau, Barcelona: S. Brunet; Hospital San Pedro de Alcántara, Cáceres: J. M. Bergua; Hospital Santa María del Rosell, Cartagena: J. Ibáñez; Hospital Severo Ochoa, Leganés: P. Sánchez; Hospital Son Dureta, Palma de Mallorca: A. Novo; Hospital de Tortosa: L. L. Font; Hospital Txagorritxu, Vitoria: J. M. Guinea; Hospital Universitario del Aire, Madrid: A. Montero; Hospital Universitario de Salamanca: M. González, M. C. Chillón; Hospital Universitario La Fe, Valencia: M. A. Sanz, G. Martín, P. Bolufer, E. Barragán; Hospital Universitario La Fe (Hospital Infantil), Valencia: V. Castell; Hospital Universitario Marqués de Valdecilla, Santander: E. Conde García; Hospital Universitario Príncipe de Asturias, Alcalá de Henares: J. García; Hospital Universitario Puerta del Mar, Cádiz: F. J. Capote; Hospital Universitario Vall D'Hebron, Barcelona: J. Bueno; Hospital Universitario Materno-Infantil Vall D'Hebron, Barcelona: J. J. Ortega; Hospital Universitario Virgen de la Arrixaca, Murcia: P. Rosique; Hospital Universitario Virgen de la Arrixaca (Pediatría), Murcia: J. L. Fuster; Hospital Universitario Virgen del Rocío, Sevilla: R. Parody; Hospital Universitario Virgen de la Victoria, Málaga: I. Pérez; Universidad de Navarra: M. J. Calasanz.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-07-2462.
Supported in part by grant from the Fondo de Investigación Sanitaria (FIS), Ministerio de Sanidad of Spain (grant 99/0806; M.A.S.).
Presented in part in abstract form at the 44th Annual Meeting of the American Society of Hematology, Philadelphia, PA, December 7, 2002.23
A complete list of the members of the Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA) appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Rodrigo Martino for helpful discussion and critical reading of the manuscript and Luis Benlloch for data collection and management.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal