Abstract
The survival of patients with aggressive non-Hodgkin lymphoma (NHL) is increasing, but the incidence of secondary cancer and late toxicity is poorly defined for those treated with cyclophosphamide-hydroxydaunomycin/doxorubicin-Oncovin-prednisone (CHOP)–like chemotherapy. From February 1984 to January 1998, 2837 patients with aggressive NHL received the control-arm chemotherapy adriamycin-cyclophosphamide-vindesine-bleomycin-prednisone (ACVBP) in 3 consecutive Groupe d'Etude des Lymphomes de l'Adulte (GELA) studies. With a median follow-up time of 74 months, the 5-year overall and event-free survival rates were 60% and 52%. Two hundred two occurrences of nonneoplastic late toxicity were reported, resulting in a 5.35% cumulative probability of incidence at 7 years. Eighty-one second tumors developed, for which the 7-year cumulative incidence rate was 2.75%; 64 were solid tumors, and 17 were hematologic malignancies. In multivariate analysis, age was the only risk factor for the second development of cancer. Epidemiologic analysis allowed a comparison of this NHL group with the general population. Considering all tumors, no excess of second cancer was observed. In the male population, however, there was an excess of lung cancer (standardized incidence ratio [SIR], 2.45; P < .001) and myelodysplastic syndrome/acute myelocytic leukemia (MDS/AML) (SIR, 5.65; P = .006), and in the female population there was an excess of MDS/AML (SIR, 19.9; P < .001). With a long follow-up, the ACVBP regimen was highly effective for the treatment of aggressive NHL. Increases occurred in secondary MDS/AML and in lung cancer among men.
Introduction
Because of the successful treatment of Hodgkin disease for many years, late sequelae, including secondary cancers and major organ dysfunction, have been extensively studied. Second cancers, including acute nonlymphoblastic leukemia, caused by chemotherapy, radiotherapy, or both, have been reported by different groups.1-3 Fewer studies have been published about non-Hodgkin lymphoma (NHL) because this disease generally occurs at a more advanced age, and therapies have not been as effective. Nevertheless, recent advances in the definition of risk groups and in therapies have improved the prognosis of the disease, and an increasing number of patients can now expect relatively long-term survival. According to the International Prognostic Index (IPI), 5-year survival rates for patients with NHL are between 26% and 73%,4 and recent therapeutic improvements, including the use of monoclonal antibodies, will probably ameliorate these results.5 Even in patients who experience relapse, high-dose chemotherapy with stem cell support will allow patients to be cured and, therefore, will expose them to the risk for late toxicities.6
The adriamycin–cyclophosphamide–vindesine–bleomycin–prednisone (ACVBP) regimen has been used as the control arm by the Groupe d'Etude des Lymphomes de l'Adulte (GELA) since 1984 in multicenter trials to treat aggressive NHL, and it has recently shown better results than conventional cyclophosphamide-hydroxydaunomycin/doxorubicin-Oncovin-prednisone (CHOP) chemotherapy.7,8 This prompted us to perform a retrospective cohort study of a homogenous population of 2837 NHL patients included in 3 different GELA protocols to evaluate the occurrence of second cancers and late toxicities. Special attention focused on secondary myelodysplasia and acute leukemia.
Patients, materials, and methods
This retrospective analysis was performed on 2837 patients included in 3 consecutive GELA trials, namely LNH84, LNH87, and LNH93, between February 1984 and January 1998. The ACVBP regimen consisted of 4 cycles of adriamycin 75 mg/m2 day (D) 1, cyclophosphamide 1200 mg/m2 D1, vindesine 2 mg/m2 D1 and 5, and bleomycin 10 mg D1 and 5, followed by 2 cycles of methotrexate 3000 mg/m2, 4 cycles of ifosfamide 1500 mg/m2 and VP-16 300 mg/m2, and 2 cycles of cytarabine 100 mg/m2 D1-4. Prophylactic intrathecal administration of 15 mg methotrexate was performed during the first 4 cycles. The first trial, LNH84, was a multicenter, nonrandomized trial. All patients on this protocol were included in the present study.9
The second trial, LNH87, was a multicenter, randomized trial based on prognostic factors different from those of IPI because this trial was initiated before the publication of IPI. Risk factors were age, Eastern Cooperative Oncology Group (ECOG) score greater than 1, large tumor mass (10 cm or larger), extranodal localization 2 or greater, and bone marrow and neurologic involvement. Group 1 included patients younger than 70 years who had no risk factors. The reference chemotherapy was ACVBP, and those patients were included in this study.10 Group 2 included patients younger than 55 years who had at least one adverse prognostic factor.11 Only patients who received the Adriamycin-containing chemotherapy and conventional consolidation were included in the present study. Group 3 included patients from 55 to 69 years who had at least one adverse prognostic factor. The reference chemotherapy was ACVBP, and those patients were included in the present study.12
The third trial, LNH93 was a multicenter, randomized trial based on IPI. Groups 1, 2, and 3 included patients 60 years and younger. In group 1 patients without any adverse prognostic factors were randomly assigned between CHOP and radiotherapy compared with ACVBP.9 The patients who received ACVBP were included in this study. In group 2, patients with one adverse prognostic factor were randomly assigned between ACVBP and an epirubicin-containing regimen. Patients from the ACVBP arm were included in the present study. In group 3, patients with 2 or more adverse prognostic factors were randomly assigned between ACVBP and an intensive high-dose chemotherapy with stem cell support. The patients from the ACVBP arm were included in the present study.13 Group 5 included patients ranging in age from 61 to 69 years who had at least one adverse prognostic factor. They were randomly assigned between CHOP and ACVBP. The patients from the ACVBP arm were included in the present study.8
The initial staging procedure included bone marrow biopsy, cerebrospinal fluid examination, and computed tomography of the chest and abdomen. Patients were classified according to the Ann Arbor system. Serum lactate dehydrogenase (LDH) was expressed as a percentage of the highest normal value. Central histologic examination was performed, and B and T phenotypes were determined on deparaffinized tissue sections for patients in LNH87 and LNH93. The institutional ethics committee approved these protocols, and all patients gave informed consent.
Using the database of the GELA, patients assigned to the ACVBP regimen in the 3 different protocols were identified. Patients identified during histologic examination to have HIV-positive lymphoma or to be without lymphoma were excluded from the study. Files were reviewed, and data about radiotherapy, occurrence of second cancer, and late toxicity (National Cancer Institute [NCI] grades II-IV) were collected by means of a standardized form. Data were reviewed for each patient at the GELA data center. If necessary, additional information was requested from the responsible investigator. Systematic review of all original files was not performed. For women younger than 40, pregnancy was also recorded.
Second cancer included any tumor occurring after the inclusion date. Previous cancer was an exclusion criterion for the protocol, except for basal skin carcinoma or noninvasive cervical cancer. Late toxicities included any new toxicity occurring after the completion of chemotherapy and any toxicity developing while the patient was on therapy and continuing after the end of therapy.14
Statistical methods
Follow-up started at the end of the first NHL treatment and ended at the date of death, date of second cancer, or date of the last examination, whichever came first. Observed cancers were classified by site and histologic type in accordance with the oncology section of the International Classification of Diseases.15 Diagnoses used were those provided by centers in which the patient was followed up.
The primary end point of the study was the occurrence of a solid tumor or a hematologic cancer. It was calculated as a cumulative probability or as a cumulative incidence of second cancer. Cumulative probability was calculated using the Kaplan-Meier estimate in which the data of patients who died were censored16 and in which curves were compared using the log-rank test.17 Effects of potential risk factors on second cancer rates were examined in a Cox proportional-hazards model.18 The cumulative incidence curve, however, explicitly accounted for other causes of death and was computed by the method of Gooley et al.19 Demographic and baseline laboratory data were compared for consistency among subpopulations with 2 tests for nominal variables and t tests for ordinal variables. All statistical tests were two-sided. Analyses were performed with SAS software (version 8.0; SAS Institute, Cary, NC) and S-Plus (version 2000; Insightful, Seattle, WA).
The incidence of second cancer in the NHL population was compared with the incidence of cancer in the general population using the 1995 French registry of cancer after matching for age and sex. In this part of the analysis, synchronous cancer, defined as cancer occurring during the first year after the diagnosis of lymphoma, was excluded. Therefore, the time at risk started 12 months after randomization and ended at the date of occurrence of second cancer, the date of death, or the date of last known vital status. Basal skin carcinoma has not been reported to the French registry of cancer since 1990 and, therefore, was excluded from this part of the analysis. Standardized incidence ratio (SIR) was calculated as the ratio of observed numbers to expected numbers based on cancer incidence data in France in 1995. The 95% confidence interval (95% CI) was calculated assuming a binomial distribution of the observed numbers. Finally, the excess number of cases was reported as the excess number of malignancies per 104 person-years at risk.
Results
Patient characteristics and survival
Eight patients who had HIV-positive lymphoma and 42 patients who did not have non-Hodgkin lymphoma at histologic examination were excluded from the analysis. LNH84, LNH87, and LNH93 contributed, respectively, for 26%, 34%, and 40% of the total cohort. The main patient characteristics (Table 1) were median age, 54 years (range, 8-79 years); male sex, 59%; stage III to IV disease, 60%; ECOG greater than 1, 21%; elevated LDH level, 49%; more than 1 extranodal involvement, 36%; and bone marrow involvement, 21%. The 5 factors of the IPI were distributed as 0 (19%), 1 (24%), 2 (23%), 3 17%), 4 (13%), and 5 (4%). Histology was diffuse large cell lymphoma in 55% of the patients. Phenotype was not available for the LNH84 group. In the LNH87 and LNH93 groups, 83% of patients had B-cell lymphoma and 17% had T-cell lymphoma. Additional radiotherapy was allowed by protocols and was administered as part of the initial treatment to 4.3% of the patients. Patients with refractory or relapsing disease received supportive care (31%) or additional treatments: chemotherapy alone, 40%; radiotherapy alone, 2.5%; chemotherapy and radiotherapy, 10.2%; chemotherapy and high-dose chemotherapy with stem cell support, 10.3%; chemotherapy, radiotherapy, and high-dose chemotherapy with stem cell support, 6%. Among the 207 patients who underwent transplantation, 195 received autologous transplants and 12 received allogeneic transplants.
Patient characteristics
Characteristics . | Total cohort N = 2837 . | LNH84 N = 779 . | LNH87 N = 954 . | LNH93 N = 1104 . |
|---|---|---|---|---|
| Median age, y (range) | 54 (8-79) | 48 (8-79) | 58 (15-70) | 52 (15-69) |
| Male sex, % | 59 | 62 | 56 | 60 |
| ECOG score greater than 1, % | 21 | 26 | 19 | 18 |
| Stages III-IV, % | 60 | 62 | 59 | 59 |
| LDH above normal, % | 49 | 48 | 48 | 50 |
| Marrow involvement, % | 21 | 29 | 19 | 18 |
| Extranodal greater than 1, % | 36 | 39 | 37 | 34 |
| IPI score, % | ||||
| 0-2 | 66 | 66 | 65 | 67 |
| 3-5 | 34 | 34 | 35 | 33 |
| Diffuse large cell, % | 43 | 53 | 64 | 55 |
| Phenotype cell B, % | NA | NA | 84 | 83 |
| Initial radiotherapy, % | 4 | 6 | 5 | 3 |
| Additional treatments for refractory or relapsing disease | ||||
| No treatment, % | 31 | 34.2 | 36.9 | 21.3 |
| CT, % | 40 | 44 | 37.9 | 37.3 |
| RDTH, % | 2.5 | 3 | 2 | 2.5 |
| RDTH, CT, % | 10.2 | 8.3 | 11.6 | 11.3 |
| CT, ASCT, % | 10.3 | 8.7 | 6.5 | 15.7 |
| CT, ASCT, RDTH, % | 6 | 1.8 | 5.1 | 11.9 |
Characteristics . | Total cohort N = 2837 . | LNH84 N = 779 . | LNH87 N = 954 . | LNH93 N = 1104 . |
|---|---|---|---|---|
| Median age, y (range) | 54 (8-79) | 48 (8-79) | 58 (15-70) | 52 (15-69) |
| Male sex, % | 59 | 62 | 56 | 60 |
| ECOG score greater than 1, % | 21 | 26 | 19 | 18 |
| Stages III-IV, % | 60 | 62 | 59 | 59 |
| LDH above normal, % | 49 | 48 | 48 | 50 |
| Marrow involvement, % | 21 | 29 | 19 | 18 |
| Extranodal greater than 1, % | 36 | 39 | 37 | 34 |
| IPI score, % | ||||
| 0-2 | 66 | 66 | 65 | 67 |
| 3-5 | 34 | 34 | 35 | 33 |
| Diffuse large cell, % | 43 | 53 | 64 | 55 |
| Phenotype cell B, % | NA | NA | 84 | 83 |
| Initial radiotherapy, % | 4 | 6 | 5 | 3 |
| Additional treatments for refractory or relapsing disease | ||||
| No treatment, % | 31 | 34.2 | 36.9 | 21.3 |
| CT, % | 40 | 44 | 37.9 | 37.3 |
| RDTH, % | 2.5 | 3 | 2 | 2.5 |
| RDTH, CT, % | 10.2 | 8.3 | 11.6 | 11.3 |
| CT, ASCT, % | 10.3 | 8.7 | 6.5 | 15.7 |
| CT, ASCT, RDTH, % | 6 | 1.8 | 5.1 | 11.9 |
CT indicates chemotherapy; RDTH, radiotherapy; ASCT, autologous stem cell transplantation; and NA, not available.
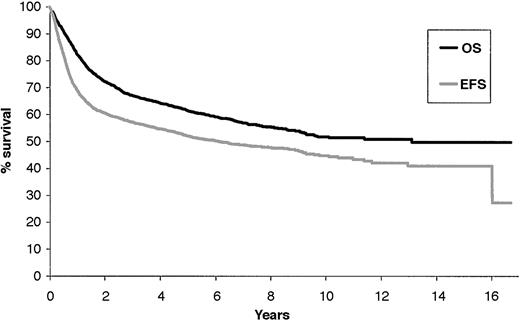
With a median follow-up of 74 months (range, 1-203 months), the 5-year overall survival rate was 60% (± 2%), and the 5-year event-free survival rate was 52% (± 2%) (Figure 1).
Overall survival and event-free survival of the 2837 NHL patients treated with the ACVBP regimen, with a median follow-up of 74 months.
Overall survival and event-free survival of the 2837 NHL patients treated with the ACVBP regimen, with a median follow-up of 74 months.
Second cancers
Eighty-one second tumors were observed, and the 7-year cumulative incidence rate was 2.75% (Figure 2). Eight (5 solid tumors and 3 MDS/AML) of these 81 second cancers occurred after additional treatment for relapse or progression. There were 64 solid tumors (lung 22, skin 8, head and neck 5, uterus 5, colorectal 4, breast 4, kidney 2, bladder 1, prostate 1, gastric 1, biliary tract 1 esophagus 1, ovary 2, brain 2, and unknown origin 5). Among the 22 lung cancers, 9 were squamous cell carcinoma, 3 were adenocarcinoma, 1 was large-cell carcinoma, and 7 were small cell lung cancer; for 2 patients the histology was unavailable. Among the 8 skin tumors, 2 were melanoma and 6 were not. Only 1 tumor, a bladder cancer, occurred in the previously irradiated field. A third solid tumor developed in each of 2 patients, but these tumors were not included in the present analysis.
Cumulative probability of incidence of second cancer after the ACVBP regimen in 2837 NHL patients.
Cumulative probability of incidence of second cancer after the ACVBP regimen in 2837 NHL patients.
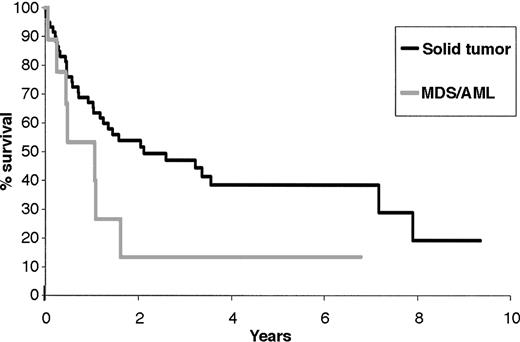
The median time from the diagnosis of NHL to solid tumor was 47 months (range, 2-183 months). Thirty-one patients died of second solid tumor; the median time from the diagnosis of solid tumor was 2.1 years (95% CI, 1.18-7.2) (Figure 3).
Survival from the time of diagnosis of second solid tumor and secondary MDS/AML.
Survival from the time of diagnosis of second solid tumor and secondary MDS/AML.
Seventeen hematologic malignancies (AML 7, MDS 4, chronic myeloid leukemia [CML] 2, chronic myelomonocytic leukemia [CMML] 1, polycythemia vera 1, essential thrombocythemia 1, myeloma 1) were observed. For the 12 patients with AML and MDS, karyotype was available only in 2 patients with AML, showing either monosomy 7 or t(8;21), and in 1 patient with MDS, showing monosomy 7 associated with other abnormalities. All patients had pancytopenia at diagnosis. Types 2 and 4 in the French-American-British (FAB) classification were predominant. Of the 12 patients, 5 (4 AML, 1 MDS) were in first complete remission (CR) from NHL, and 3 were in second complete remission. Eight of 12 patients died of leukemia, 1 died of relapsed NHL while he was in complete remission from secondary AML, and 1 died of MDS without evidence of leukemia.
The median time from NHL diagnosis to leukemia was 40 months (range, 18-100 months), and the median survival time from MDS/AML diagnosis was 1 year (95% CI, 0.45-1.62). Only 1 patient experienced prolonged survival after the diagnosis of leukemia (Figure 3).
During the course of the disease, patients experienced several competing events such as death, second cancer, or late toxicity. Risk factors associated with these different events were evaluated. For death, prognostic factors were those of IPI (age, stage, LDH level, ECOG, and more than 1 extranodal localization; P < .001). In multivariate analysis, age was the only significant risk factor for second cancer (P < .0001). Sex, LDH, stage, ECOG, extranodal involvement, relapse, stem cell transplantation at relapse, radiotherapy at relapse, and chemotherapy at relapse were not shown to have significant influence.
Comparison with healthy population
Among the 81 cases reported, 9 synchronous tumors that developed less than 12 months after the diagnosis of NHL were excluded from analysis, as were 6 cases of basal skin cancer. Forty-nine solid tumors (21 lung, 3 head and neck, 6 digestive, 4 breast, 7 genitourinary, 2 melanoma, and 6 miscellaneous) and all cases of hematologic cancer were analyzed. This allowed us to analyze 2267 patients, accounting for 9847 person-years at risk. Observed second neoplasms, SIR, and excess numbers are reported for male and female patients in Tables 2 and 3 with various groupings of cancer. For all second cancers, no excess risk was detected for the whole population (males and females respectively: SIR, 0.92; 95% CI, 0.67-1.24; SIR, 0.94; 95% CI, 0.59-1.42; P = NS for both). A significant excess of risk for secondary MDS/AML was detected in the male population (SIR, 5.65; 95% CI, 1.54-14.46; P = .006) and was even stronger in the female population (SIR, 19.89; 95% CI, 7.98-40.97; P < .001). For all solid tumors, no excess of risk was identified (males and females respectively: SIR 0.77; 95% CI, 0.54-1.07; SIR 0.63; 95% CI, 0.35-1.06; P = NS for both). In the male population, we observed a highly significant excess of risk for lung cancer (SIR, 2.45; 95% CI, 1.48-3.83; P < .001). This resulted in an excess of 19.9 cases of lung cancer per 104 person-years at risk (Table 3).
SIR and excess number of second cancer in the female population (947 patients)
Second cancer . | No. observed . | SIR . | 95% CI . | P . | Excess numbers . |
|---|---|---|---|---|---|
| All types | 22 | 0.94 | 0.59-1.42 | .64 | — |
| Solid tumors | 14 | 0.63 | 0.35-1.06 | .97 | — |
| Breast | 4 | 0.47 | 0.13-1.20 | .97 | — |
| AML/MDS | 7 | 19.89 | 7.98-40.97 | < .001 | 15.9 |
Second cancer . | No. observed . | SIR . | 95% CI . | P . | Excess numbers . |
|---|---|---|---|---|---|
| All types | 22 | 0.94 | 0.59-1.42 | .64 | — |
| Solid tumors | 14 | 0.63 | 0.35-1.06 | .97 | — |
| Breast | 4 | 0.47 | 0.13-1.20 | .97 | — |
| AML/MDS | 7 | 19.89 | 7.98-40.97 | < .001 | 15.9 |
— indicates not applicable.
SIR and excess number of second cancer in the male population (1320 patients)
Second cancer . | No. observed . | SIR . | 95% CI . | P . | Excess numbers . |
|---|---|---|---|---|---|
| All types | 44 | 0.92 | 0.67-1.24 | .73 | — |
| Solid tumors | 35 | 0.77 | 0.54-1.07 | .95 | — |
| Lung | 19 | 2.45 | 1.48-3.83 | < .001 | 19.9 |
| Head and neck | 2 | 0.38 | 0.005-1.37 | .97 | — |
| Digestive | 5 | 0.52 | 0.17-1.12 | .96 | — |
| AML/MDS | 4 | 5.65 | 1.54-14.46 | .006 | 5.8 |
Second cancer . | No. observed . | SIR . | 95% CI . | P . | Excess numbers . |
|---|---|---|---|---|---|
| All types | 44 | 0.92 | 0.67-1.24 | .73 | — |
| Solid tumors | 35 | 0.77 | 0.54-1.07 | .95 | — |
| Lung | 19 | 2.45 | 1.48-3.83 | < .001 | 19.9 |
| Head and neck | 2 | 0.38 | 0.005-1.37 | .97 | — |
| Digestive | 5 | 0.52 | 0.17-1.12 | .96 | — |
| AML/MDS | 4 | 5.65 | 1.54-14.46 | .006 | 5.8 |
— indicates not applicable.
Late nonneoplastic toxicities
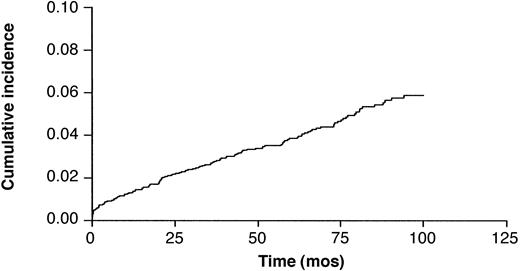
There were 222 reported late nonneoplastic toxicities, resulting in a 5.35 ± 0.76 cumulative probability of incidence at 7 years (Figure 4). Of these late toxicities, 8.2% occurred after additional treatment for refractory or relapsed lymphoma. In multivariate analysis, age (P < .0001) and relapse (P = .02) were significant risk factors for late nonneoplastic toxicities. Sex, LDH level, stage, ECOG score, extranodal involvement, stem cell transplantation at relapse, radiotherapy at relapse, and chemotherapy at relapse were not significant risk factors in this multivariate analysis. The different causes of toxicities and death are reported in Table 4. Late nonneoplastic toxicities resulted in 61 deaths and were the second cause of death after NHL recurrence or progression. Late second cancers (31 deaths) were the third cause of death. Of these deaths from late nonneoplastic toxicity, 17% occurred in patients who received additional treatments after ACVBP for resistance or relapse after first-line treatment. The 2 major causes of late toxicity and death from late toxicity were infections and cardiovascular events, resulting in 29 and 14 deaths, respectively.
Cumulative probability of incidence of late nonneoplastic toxicity after the ACVBP regimen in 2837 patients.
Cumulative probability of incidence of late nonneoplastic toxicity after the ACVBP regimen in 2837 patients.
Late nonneoplastic toxicities and related death
Causes . | Toxicities, no. . | Deaths, no. . |
|---|---|---|
| Infectious | 61 | 29 |
| Cardiovascular | 54 | 14 |
| Neurologic | 20 | 2 |
| Psychologic | 19 | 3 |
| Pulmonary | 12 | 6 |
| Miscellaneous | 36 | 7 |
| Total | 222 | 61 |
Causes . | Toxicities, no. . | Deaths, no. . |
|---|---|---|
| Infectious | 61 | 29 |
| Cardiovascular | 54 | 14 |
| Neurologic | 20 | 2 |
| Psychologic | 19 | 3 |
| Pulmonary | 12 | 6 |
| Miscellaneous | 36 | 7 |
| Total | 222 | 61 |
Hepatitis was the most frequent infectious complication. Because the different studies recruited patients through the period of discovery of hepatitis C virus and HIV, several cases of viral transmission through transfusions probably occurred. There were 12 occurrences of hepatitis C transmission with 1 death, 2 of hepatitis B transmission, and only 1 of HIV transmission. Two patients had hepatitis of unknown etiology. Seven late deaths were attributed to aspergillosis, and 4 to Pneumocystis carinii. Angina pectoris was the most frequently reported cardiovascular complication (20 patients), but only 3 patients died of myocardial infarction. Three other patients died of cardiac failure and 3 of arrhythmia. Ten patients presented ischemic stroke, resulting in 4 deaths. Eighteen patients reported disabling peripheral neuropathy attributed to vindesine, and 2 patients died of encephalopathy (1 after having undergone brain radiotherapy. Nineteen patients experienced severe psychological problems after treatment, and 3 committed suicide while they were in CR. Three patients acquired severe pulmonary fibrosis, and 2 of them died.
The other most frequently reported late complications were thyroid dysfunction (6 patients), renal failure (5 patients), alcohol abuse (5 patients), benign tumor (3 patients), and cataract (3 patients). Five patients died of toxicity from autologous stem cell transplantation and 6 from allogenic transplantation.
Pregnancies
Among the 2837 patients, 609 women were younger than 40 at the time of diagnosis of NHL, and 344 were in CR at the time of analysis. Thirty-one pregnancies were reported in 26 patients. There were 2 therapeutic abortions and 1 death in utero. No malformations were reported among the 28 children. The mean age at the time of pregnancy was 26 years, and no pregnancy occurred after the age of 33.
Discussion
In this study, the risk for second malignancies and other late toxicities was analyzed in 2837 patients treated with the same ACVBP regimen for NHL. These trials, covering almost 20 years, were retrospectively analyzed. With 9847 person-years at risk, this represents one of the largest studies ever reported on the subject. However, results must be interpreted cautiously because of the retrospective design of this study. Eighty-one second tumors were observed, and the 7-year cumulative incidence rate was 3.5%. Overall, there was no increased risk for second cancer compared with the general population, but in this study there was a 5.6-fold risk for MDS/AML in males and a 19.9-fold risk in females compared with the general population. In the male population, there was a strong 2.45-fold excess of risk for lung cancer. However, with a median follow-up of 74 months, nonneoplastic toxicities were accountable for more deaths than second malignancies.
Therapy-related MDS/AML has been extensively described after the treatment of Hodgkin20 disease or after the use of high-dose therapy with stem cell support.21-23 However, the impact of high-dose chemotherapy by itself is unlikely to increase the risk generated by conventional therapy.3,19 Several investigations focused on secondary MDS/AML after conventional chemotherapy for NHL.24-30 In Travis et al,25 despite the excess of MDS/AML associated with therapy, secondary leukemia remained a rare event after NHL. Of 10 000 NHL patients treated for 6 months with various chemotherapy agents and followed up for 10 years, an excess of 4 leukemia could be expected.25 Various alkylating agents are used in the treatment of NHL. The increased incidence of MDS/AML after prednimustine, chlorambucil, mechlorethamine, and procarbazine for the treatment of NHL is well known. The risk increases with cumulative dose and duration of therapy. Cyclophosphamide-based regimens at a median cumulative dose of 12.5 g were associated with a non-significant 1.8-fold risk of AML compared with treatments that did not include alkylating agents. Increasing cumulative dose or duration of treatment with cyclophosphamide was not associated with increasing risk for AML (P = .57 and .33, respectively).25 This weak association between small cumulative doses of cyclophosphamide and AML is consistent with the results of other studies31 and is reassuring given the frequent use of this alkylating agent in the current therapy for NHL. Epi-podophyllotoxins have clearly demonstrated a leukemogenic potential after the treatment of acute lymphoblastic leukemia. In NHL and in our studies, they were not administered without alkylating agents; therefore, the individual leukemogenic risk for cyclophosphamide, ifosfamide, and VP-16 could not be evaluated in this study. However, as suggested by one of our patient with t(8;21), a possible contribution of epipodophyllotoxins to the occurrence of secondary MDS/AML cannot be ruled out.
The increased risk for lung cancer associated with radiotherapy is well known.32,33 A recent large study on lung cancer after Hodgkin disease clearly linked this cancer to alkylating agents and distinguished this toxicity from that of radiotherapy and tobacco use.34 Travis et al25 reported that treatment with alkylating agents without radiotherapy was associated with increased lung cancer risk (RR = 4.2). Statistically significant elevated risk was apparent within 1 to 4 years of treatment with chemotherapy. In another case-control study, Kaldor et al35 showed that patients with Hodgkin disease who were treated with chemotherapy alone had approximately twice the risk for lung cancer as those treated with radiotherapy and with both modalities. The finding that the risk for lung cancer after chemotherapy was higher than after radiotherapy was unexplained and unexpected, as it was in our study. After therapy with alkylating agents, the risk for squamous cell lung cancer and small-cell lung cancer was statistically significantly elevated.31 In our series, these 2 histologic types represented most of the tumors. A major limitation of our study, and of previous studies of lung cancer in Hodgkin disease, is the uncertainty of risk associated with treatment, in view of the great risk conferred by tobacco use. Although information on radiotherapy and chemotherapy was consistently available in medical records, information on smoking habits was not systematically reported in formats used for epidemiologic purposes. During the time of this study (1984-2000), the incidence of smoking ranged between 25% and 36% (male, 30%-47%; female, 19%-27%).36 The excess of lung cancers among male patients may partially be explained by the consistent, 10% higher incidence of cigarette smoking among men. However, the difference in the rates of lung cancer among male and female patients was major in this study. Therefore, the possibility of a difference between males and females for this tumor, despite different smoking habits, may not be ruled out. A recent review of the literature on the association between smoking and NHL found no evidence that tobacco use could increase the risk for NHL.37 The probability that our large NHL population would have different smoking habits than the general population is low and might have reflected a true increase of lung cancer secondary to chemotherapy in the male population.
Only one bladder cancer was reported in this series, although cyclophosphamide is known to induce this late complication. This low incidence is related to the low cumulative dose (4.8 g/m2) and short duration of treatment. In the largest study reported on bladder cancer after cyclophosphamide, the risk was strongly associated with cumulative dose, and patients receiving less than 20 g had only a 2.4-fold increased risk.38
Among agents used in the treatment of non-Hodgkin lymphoma, anthracyclines are the most important in causing direct injury to the heart. However, because the heart has considerable compensatory reserves, a long time elapses before heart failure becomes manifest. In the study by Miller et al,39 left ventricular dysfunction was found in 7 of 201 patients treated with 8 cycles of CHOP (cumulative dose of doxorubicin, 400 mg/m2), whereas in the group treated with 3 cycles combined with radiotherapy, none of 200 patients had left ventricular dysfunction. Seven patients treated with the 8 cycles of CHOP but only 2 treated with 3 CHOP and radiotherapy subsequently died of heart disease. In our study, left ventricular function was not systematically assessed, but only 3 patients died of cardiac failure. They received a cumulative dose of doxorubicin of 300 mg/m2.
Ovarian injury caused by radiation or chemotherapy can cause sterilization and suppressed sexual hormone production. In women, assessing gonadal function after lymphoma treatment is difficult because the ova and ovary are not easily accessible for study. Therefore, the surrogate markers of a woman's fertility are regular menses, estrogen levels higher than 20 pmol/L, and the ability to become pregnant. Bokemeyer et al40 reported 10 women with NHL treated with CHOP and radiotherapy. One of them showed elevated levels of serum gonadotrophins and decreased levels of estradiol as indicators of gonadal toxicity, with abnormalities of the menstrual cycle. In the Müller and Stahel series,41 1 of 7 women was found to have gonadal dysfunction after MACOP-B or VACOP-B.41 In our series, we showed that fertility may be preserved after 4.8 g/m2 cyclophosphamide, but no pregnancy was observed in women older than 33 at the time of NHL diagnosis. Several reports have shown the preservation of fertility even after high-dose chemotherapy with stem cell support.42
After treatment, patients with NHL experienced elevated risks for late toxicities, therapy-related leukemia, and several solid tumors. These risks were substantially lower than they were for patients with Hodgkin disease, especially regarding the occurrence of second cancer. Genetic predisposition to drug-induced second cancers may, in the future, be a valuable tool to tailor therapy to individual patients.43 In the meantime, careful, continuous follow-up of lymphoma patients is recommended to correctly evaluate late toxicities and second cancers and to allow the design of new, less toxic regimens.
Appendix
The following investigators of the GELA participated in the study: N. Albin, C. Allard, M. Aoudjane, D. Assouline, M. Attal, B. Audhuy, M. Azagury, J.C. Barats, C. Beaumont, E. Baumelou, P. Biron, M. Blanc, D. Bordessoulle, R. Bouabdallah, C. Bouleuc, P. Bourquard, P. Bourquelot, F. Boué, D. Boulat, J. Brière, G. Brun, D. Caillot, O. Casasnovas, P. Carde, S. Castaigne, S. Chèze, B. Christian, P. Colin, C. Collet, T. Conroy, T. Cosnard, B. Corront, H. Curé, A. Delmer, V. Delwail, L. Detourmignies, A. Devidas, H. Dombret, J.F. Dor, C. Doyen, F. Dreyfus, S. Dront, G. Dupont, B. Dupriez, JC. Eisenmann, M. Fabbro, G. Fillet, M. Flesch, C. Fruchart, J. Gabarre, C. Guettier, C. Haioun, R. Herbrecht, O. Hermine, F. Huguet, M. Janvier, E. Jourdan, J.M. Karsenti, Y. Kerneis, F. Kohser, V. Leblond, P. Lederlin, S. Lefort, P. Lenain, E. Lepage, G. Lepeu, S. Lepretre, X. Levaltier, A. Le Rol, G. Marit, C. Martin, F. Mayer, C. Nouvel, T. Molina, M. Moriceau, J.N. Munck, G. Nedelec, F. Offner, J.M. Pavlovitch, I. Plantier, P.Y. Péaud, A.M. Peny, J. Pico, C. Platini, J.P. Pollet, B. Quesnel, O. Reman, B. Richard, R. Riou, P. Rodon, JF. Rossi, B. Salles, G. Salles, C. Sarazin, D. Schlaifer, C. Sebban, M. Simon, P. Solal-Celigny, J.J. Sotto, A. Stamatoullas, G. Tertian, A. Thyss, J.D. Tigaud, G. Tobelem, C. Traullé, P. Travade, A. Van Hoof, A. Zaniboni, and J.M. Zini.
The following members of the Groupe d'Etude des Lymphomes de l'Adulte performed the pathological review: T. Molina, C. Guettier, J. Brière, J. Diebold, B. Fabiani, and T. Petrella.
Prepublished online as Blood First Edition Paper, October 23, 2003; DOI 10.1182/blood-2003-04-1124.
Supported by grants from the Programme Hospitalier de Recherche Clinique (AOM95061) from the Ministère de la Santé and from Amgen, Roche, Schering-Plough, and Asta Medica.
A complete list of the members of the Groupe des Lymphomes de l'Adulte appears in the “Appendix.”
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal