Abstract
HIV-1- and cytomegalovirus (CMV)-specific CD4 T-cell-mediated antiviral immunity was evaluated by assessing the frequency of interleukin 2 (IL-2)- and interferon γ (IFN-γ)-secreting cells following antigen-specific stimulation in blood and lymph node. HIV-1-infected subjects with progressive disease at early stage of infection with no previous history of antiretroviral therapy (ART), subjects with nonprogressive disease, and HIV-negative subjects were studied. On the basis of the ability to secrete IL-2 and IFN-γ, 3 functionally distinct populations of CD4 T cells were identified: (1) IL-2-secreting cells; (2) IL-2/IFN-γ-secreting cells; and (3) IFN-γ-secreting cells. CMV-specific CD4 T cells were almost equally distributed within the 3 functionally distinct cell populations in the 3 study groups as well as HIV-1-specific CD4 T cells in subjects with nonprogressive disease. However, a skewing toward IFN-γ-secreting cells (70% of HIV-1-specific CD4 T cells) was observed in subjects with progressive disease, and IL-2- and IL-2/IFN-γ-secreting cells were almost absent. The frequencies of IL-2- and of IL-2/IFN-γ-secreting HIV-1-specific CD4 T cells were negatively correlated with the levels of viremia. Interestingly, prolonged ART was able to correct the skewed representation of different populations of HIV-1-specific CD4 T cells but was associated with only a partial recovery of IL-2-secreting cells. These results indicate that the composition of the pool of functionally distinct virus-specific CD4 T cells is important for virus control. (Blood. 2004;103:966-972)
Introduction
A large number of studies have clearly demonstrated that both CD4 and CD8 T-cell-mediated functions are major components of the antiviral immune response.1 In addition to the critical antiviral role played by CD8 T cells,1 the importance of virus-specific CD4 T cells and its association with a protective antiviral immune response has been demonstrated in mice and humans.2-10 In humans, the protective role of virus-specific CD4 T cells has been extensively investigated in HIV-1, hepatitis C virus (HCV), and cytomegalovirus (CMV) infections.2,3,6-10 The presence of virus-specific CD4 T cells able to proliferate in response to viral antigens (eg, helper CD4 T cells) has been shown to be associated with the control of HIV-1 replication,9,10 whereas the presence of interferon γ (IFN-γ)-secreting CD4 T cells is not associated necessarily with protective antiviral immunity.11-13 However, studies have shown the presence of helper virus-specific CD4 T-cell responses in patients with chronic infection in the absence of the control of virus replication, thus challenging the importance of helper CD4 T-cell responses in antiviral control.14-16
A precise quantification of the number of virus-specific CD4 T cells cannot be obtained by the evaluation of cell proliferation, and there is only limited information regarding the distribution of HIV-1- and CMV-specific interleukin 2 (IL-2)- and IFN-γ-secreting cells within the CD4 T-cell populations and in different anatomic compartments. To address these issues, we determined the frequency of IL-2 and IFN-γ virus-specific CD4 T cells in different conditions of virus infection: HIV-1 and CMV coinfected subjects with (1) uncontrolled HIV-1 replication, such as progressors; (2) with controlled HIV-1 replication, such as subjects with nonprogressive disease (defined as long-term nonprogressors [LTNPs]); and (3) HIV-negative subjects with controlled chronic CMV infection. The quantification of IL-2- and IFN-γ-secreting CD4 T cells was performed in blood and lymph node of the same subjects. The relationship between functionally distinct populations of CD4 T cells and certain parameters of activity of HIV-1 disease such as levels of viremia and the effects of prolonged antiretroviral therapy on the different functionally distinct populations of HIV-1-specific CD4 T cells were investigated.
Patients, materials, and methods
Study groups
The 15 subjects with progressive chronic HIV-1 infection enrolled in this study were infected for at least 4 years, were naive to antiviral therapy, and each had a CD4 T-cell count of 250 cells/μL or more (mean ± SE CD4 T-cell count, 790 ± 241 cells/μL) and plasma viremia of 5000 HIV-1 RNA copies/mL or more (mean ± SE viremia, 4.39 ± 0.43 log10 HIV-1 RNA copies). In addition, 7 LTNPs as defined by documented HIV-1 infection for more than 14 years, stable CD4 T-cell counts more than 500 cells/μL (mean ± SE, 1070 ± 524 per μL), and plasma viremia less than 1000 HIV-1 RNA copies/mL (mean ± SE, 1.95 ± 0.74 log10) were also included in this study. Ten randomly selected HIV-1-infected subjects treated with antiretroviral therapy (ART) regimens, containing nucleoside inhibitors of reverse transcriptase and protease inhibitors, were also studied 12 to 15 months after initiation of therapy. At the time of investigation the levels of viremia were below 50 HIV-1 RNA copies/mL plasma. Eight HIV-negative subjects undergoing vascular surgery who agreed to donate blood and lymph node following written informed consent were also studied. Therefore, the lymph nodes obtained from the latter were neither neoplastic nor inflammatory. All HIV-1-infected and HIV-negative subjects were coinfected with CMV and had no clinical signs of CMV-associated organ disease. HIV-1-infected patients with progressive disease were enrolled in therapeutic clinical trials with antiretroviral regimens comprising 2 nucleoside reverse transcriptase inhibitors and 1 or 2 protease inhibitors.17,18 According to the study protocol, excisional lymph node biopsies were performed in all patients prior to the initiation of antiviral therapy. Inguinal lymph nodes were obtained in all patients. These trials were open-label, observational, nonrandomized prospective studies carried out at a single site (Lausanne, Switzerland). These studies were approved by the Institutional Review Board of the Centre Hospitalier Universitaire Vaudois (Lausanne, Switzerland), and all subjects gave written informed consent.
Isolation of blood and lymph node mononuclear cells
Isolation of blood and lymph node mononuclear cells was performed as previously described.19
FACS analysis
Cryopreserved cells stored in liquid nitrogen were thawed and used for flow cytometry. Mononuclear cell preparations were stained for a panel of cell surface markers, including CD4 and CD69. Data were acquired on a fluorescence activated cell sorting (FACS)Calibur system and analyzed by using CellQuest software (Becton Dickinson, Franklin, NJ). Flow cytometric analysis was performed as previously described.20,21 The number of lymphocyte-gated events ranged between 150 000 and 600 000 in the flow cytometry experiments shown, including intracellular cytokine staining (ICS). With regard to the criteria for the positivity of ICS, the background in the unstimulated controls never exceeded 0.01% to 0.02% as shown in Figures 1, 2, and 5. An ICS to be considered positive had to have a background less than 20% of the total percentage of cytokine-positive cells in the stimulated samples.
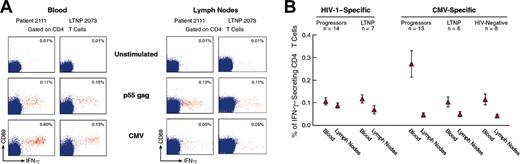
Analysis of HIV-1- and CMV-specific IFN-γ-secreting CD4 T cells in HIV-1-infected progressors, LTNPs, and HIV-negative subjects. (A) Flow cytometry profiles of HIV-1- and CMV-specific IFN-γ-secreting CD4 T cells in the blood and lymph nodes of representative progressors and LTNPs. Blood and lymph node mononuclear cells were stimulated either with p55 gag HIV-1 protein or with CMV lysate, stained with anti-CD4 PerCp Cy5.5, anti-CD69 FITC, and anti-IFN-γ APC and analyzed by flow cytometry. The cluster of events shown in red corresponds to the responder CD4 T cells, ie, coexpressing CD69 and IFN-γ, whereas the cluster of events in blue corresponds to the nonresponder CD4 T cells. Data are expressed as the percentage of cells coexpressing IFN-γ and CD69 within CD4 T cells. (B) Mean ± SE of cumulative data on the percentage of HIV-1- and CMV-specific IFN-γ-secreting CD4 T cells in blood and lymph node of progressors, LTNPs, and HIV-negative subjects. In HIV-1-infected subjects, IFN-γ-secreting cells were assessed in blood and lymph node mononuclear cells obtained at the same time point. In the case of progressors, the cell samples analyzed were collected at study entry prior to the initiation of antiviral therapy. At least 1 × 106 events were analyzed.
Analysis of HIV-1- and CMV-specific IFN-γ-secreting CD4 T cells in HIV-1-infected progressors, LTNPs, and HIV-negative subjects. (A) Flow cytometry profiles of HIV-1- and CMV-specific IFN-γ-secreting CD4 T cells in the blood and lymph nodes of representative progressors and LTNPs. Blood and lymph node mononuclear cells were stimulated either with p55 gag HIV-1 protein or with CMV lysate, stained with anti-CD4 PerCp Cy5.5, anti-CD69 FITC, and anti-IFN-γ APC and analyzed by flow cytometry. The cluster of events shown in red corresponds to the responder CD4 T cells, ie, coexpressing CD69 and IFN-γ, whereas the cluster of events in blue corresponds to the nonresponder CD4 T cells. Data are expressed as the percentage of cells coexpressing IFN-γ and CD69 within CD4 T cells. (B) Mean ± SE of cumulative data on the percentage of HIV-1- and CMV-specific IFN-γ-secreting CD4 T cells in blood and lymph node of progressors, LTNPs, and HIV-negative subjects. In HIV-1-infected subjects, IFN-γ-secreting cells were assessed in blood and lymph node mononuclear cells obtained at the same time point. In the case of progressors, the cell samples analyzed were collected at study entry prior to the initiation of antiviral therapy. At least 1 × 106 events were analyzed.
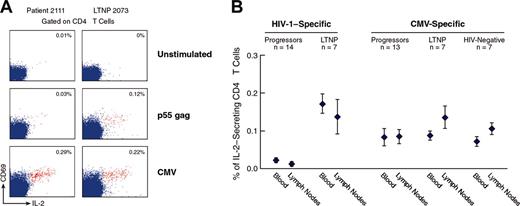
Analysis of HIV-1- and CMV-specific IL-2-secreting CD4 T cells in the blood and lymph nodes of progressors, LTNPs, and HIV-negative subjects. (A) Flow cytometry profiles of HIV-1- and CMV-specific IL-2-secreting CD4 T cells in blood of representative progressors and LTNPs. Blood mononuclear cells were stimulated with p55 gag and CMV lysates and stained with anti-CD4 PerCp Cy5.5, anti-CD69 FITC, and anti-IL-2 PE. The data show the expression of CD69 and IL-2 within CD4 T-cell populations. The cluster of events shown in red corresponds to the responder CD4 T cells, ie, coexpressing CD69 and IL-2, whereas the cluster of events in blue corresponds to the nonresponder CD4 T cells. The data are expressed as the percentage of cells coexpressing CD69 and IL-2 within CD4 T cells. (B) Mean ± SE of cumulative data on the percentage of HIV-1- and CMV-specific IL-2-secreting CD4 T cells in blood and lymph node of progressors, LTNPs, and HIV-negative subjects. At least 1.5 × 106 events were analyzed.
Analysis of HIV-1- and CMV-specific IL-2-secreting CD4 T cells in the blood and lymph nodes of progressors, LTNPs, and HIV-negative subjects. (A) Flow cytometry profiles of HIV-1- and CMV-specific IL-2-secreting CD4 T cells in blood of representative progressors and LTNPs. Blood mononuclear cells were stimulated with p55 gag and CMV lysates and stained with anti-CD4 PerCp Cy5.5, anti-CD69 FITC, and anti-IL-2 PE. The data show the expression of CD69 and IL-2 within CD4 T-cell populations. The cluster of events shown in red corresponds to the responder CD4 T cells, ie, coexpressing CD69 and IL-2, whereas the cluster of events in blue corresponds to the nonresponder CD4 T cells. The data are expressed as the percentage of cells coexpressing CD69 and IL-2 within CD4 T cells. (B) Mean ± SE of cumulative data on the percentage of HIV-1- and CMV-specific IL-2-secreting CD4 T cells in blood and lymph node of progressors, LTNPs, and HIV-negative subjects. At least 1.5 × 106 events were analyzed.
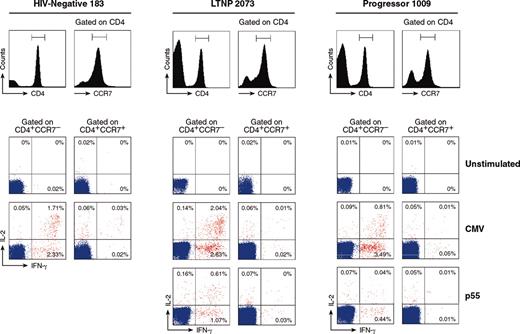
Analysis of the distribution of functionally distinct CMV-specific CD4 T cells within CCR7- and CCR7+ memory cell populations. Flow cytometry profiles of the distribution of blood CMV- and HIV-1-specific IL-2-, IFN-γ-, and IL-2/IFN-γ-secreting cells within gated CD4+CCR7- and CD4+CCR7+ cell populations are shown in representative HIV-negative subjects, LTNPs, and progressors.
Analysis of the distribution of functionally distinct CMV-specific CD4 T cells within CCR7- and CCR7+ memory cell populations. Flow cytometry profiles of the distribution of blood CMV- and HIV-1-specific IL-2-, IFN-γ-, and IL-2/IFN-γ-secreting cells within gated CD4+CCR7- and CD4+CCR7+ cell populations are shown in representative HIV-negative subjects, LTNPs, and progressors.
HIV-1-specific proliferation assays
Blood mononuclear cells were thawed and resuspended in RPMI 1640 Gutamax-1 medium containing 2% inactivated AB human serum. Mononuclear cells were plated at 2 × 105 cells/well in U-bottom 96-well cell culture plates (Costar, Bucks, United Kingdom) and incubated with HIV-1 p24 gag protein (1 μg/well) (Protein Sciences, Meriden, CT) for 5 days. Then, cell cultures were pulsed with [3H]thymidine (1.0 μCi [0.037 MBq] per well) for 18 hours. Cell cultures with a stimulation index (SI) of 5 or greater compared with the unstimulated control cell cultures were considered to be positive for HIV-1-specific proliferation.21 In addition, background levels had to be less than 1000 cpm, and phytohemagglutinin (PHA) response levels had to have an SI more than 100.
IFN-γ detection
Intracellular IFN-γ production was assessed as previously described.11,20 Blood mononuclear cells (2-4 × 106 cells in 2 mL RPMI 1640 Gutamax-1 medium containing 10% inactivated fetal calf serum) were stimulated with 5 μg/mL p55 gag (Protein Sciences) or 1:200 final dilution of CMV lysates (BioWhittaker, Walkersville, MD) or 200 ng/mL staphylococcal enterotoxin B (SEB; positive control; Calbiochem, La Jolla, CA) or phosphate-buffered saline (PBS) for unstimulated negative controls for 12 hours at 37°C, in the presence 0.5 μg/mL purified anti-CD28 antibody (Becton Dickinson, Franklin, NJ) and, as of the second hour, with 10 μg/mL Brefeldin A (Sigma, St Louis, MO). Cell surface staining (anti-CD4 CyChrome; RPA-T4; Pharmingen, San Diego, CA) was completed as described following the 12 hours of in vitro activation. Cells were then permeabilized with Intrastain (Dako, Glostrup, Denmark) and labeled with antihuman IFN-γ allo-phycocyanin (APC; IgG1, B27; Pharmingen). Simultaneously, cell activation was assessed by staining with anti-CD69 fluorescein isothiocyanate (FITC; L78; Becton Dickinson). The background was never more than 0.01% in p55-or CMV-stimulated mononuclear cells of HIV- and CMV-negative subjects (data not shown).
Interleukin-2 detection
Blood mononuclear cells (2-4 × 106 cells in 2 mL RPMI 1640 Gutamax-1 medium containing 10% inactivated fetal calf serum) were stimulated with 5 μg/mL p55 gag, 1:200 final dilution of CMV lysates, 200 ng/mL SEB or PBS for unstimulated negative controls for 12 hours at 37°C, in the presence of 0.5 μg/mL purified anti-CD28 antibody. Cell surface staining was completed as described in “FACS analysis” following the 12 hours of in vitro activation. IL-2 secretion was assessed by using either the IL-2 secretion assay (Miltenyi Biotech, Cologne, Germany) or the intracellular IL-2 detection by using an antihuman IL-2 phycoerythrin (PE; 5344.111; Becton Dickinson). Simultaneously, cell activation was assessed by staining with anti-CD69 FITC. The background was never more than 0.02% in p55- or CMV-stimulated mononuclear cells of HIV- and CMV-negative subjects (data not shown).
Statistical analysis
Statistical significance (P values) of the results was calculated by 2-tailed t test. A 2-tailed P of less than .05 was considered significant. The correlations among variables were tested by simple regression analysis.
Results
Study groups
The frequency of virus-specific (eg, HIV-1 and CMV) CD4 T cells was investigated in the blood and in the lymph nodes obtained from a cohort of 15 HIV-1-infected progressors, from 7 LTNPs, and from 8 HIV-negative subjects. Both HIV-1-infected and HIV-negative subjects were selected on the basis of preexisting chronic CMV infection. Furthermore, the frequency of virus-specific CD4 T cells was investigated in 10 randomly selected progressors before and 12 to 15 months after successful ART.
Frequency of HIV-1- and CMV-specific IFN-γ-secreting CD4 T cells in the blood and in the lymph node
Blood and lymph node mononuclear cells were stimulated with either p55 gag HIV-1 protein or with CMV lysate, and the frequency of IFN-γ-secreting CD4 T cells was determined by flow cytometry. Flow cytometry profiles of representative progressors and LTNPs are shown in Figure 1A. The mean percentage of p55-specific IFN-γ-secreting CD4 T cells was 0.11 ± 0.02 and 0.12 ± 0.02 in blood, and 0.09 ± 0.02 and 0.07 ± 0.02 in lymph nodes of progressors (n = 14) and of LTNPs (n = 7), respectively (Figure 1B). The mean percentage of CMV-specific IFN-γ-secreting CD4 T cells was very similar (about 0.04 ± 0.01) in the lymph nodes of progressors, LTNPs, and HIV-negative subjects (n = 8) (Figure 1B), whereas it was significantly higher (P = .036) in the blood of progressors (0.27% ± 0.09%) as compared with LTNPs (0.10% ± 0.02%) and with HIV-negative subjects (0.10% ± 0.03%) (Figure 1B). Furthermore, CMV-specific IFN-γ-secreting CD4 T cells accumulated preferentially in the blood compared with the lymph nodes (P = .0007) (Figure 1B). The percentage of HIV-1-specific IFN-γ-secreting CD4 T cells found in progressors (0.11%) was almost identical (0.12% or 0.09%) to that observed in previous studies using flow cytometry.11-13 Taken together, these results indicate that there is little evidence for a quantitative defect of IFN-γ-secreting CD4 T cells in HIV-1-infected subjects with progressive disease at early stage of chronic infection.
Frequency of virus-specific IL-2-secreting CD4 T cells
The quantification of antigen-specific IL-2-secreting CD4 T cells was performed in the same number of progressors, LTNPs, and HIV-negative subjects who were investigated for the quantification of antigen-specific IFN-γ CD4 T cells. The flow cytometry profiles of IL-2 secretion in CD4 T cells following stimulation with p55 gag HIV-1 protein and CMV lysate are shown for representative progressors (patient 2111) and LTNPs (patient 2073) (Figure 2A). Following stimulation with p55, a well-defined population of IL-2-secreting cells was detected in the blood of the LTNPs, whereas the percentage of IL-2-secreting CD4 T cells was very low in the progressors (Figure 2A). In contrast, CMV-specific IL-2-secreting CD4 T cells were found in both progressors and LTNPs (Figure 2A-B). Similarly, no differences in the percentage of SEB-specific IL-2-secreting cells were found between progressors and LTNPs (data not shown). The mean frequency of HIV-1-specific IL-2-secreting cells was very low in blood (0.03%) and lymph node (0.02%) of progressors (n = 14) compared with 0.17% ± 0.03% (blood) and 0.14% ± 0.05% (lymph node) in the LTNPs (n = 7) (P < .0002) (Figure 2B). Interestingly, the mean frequencies of CMV-specific IL-2-secreting cells were very similar in progressors, LTNPs, and HIV-negative subjects in both blood and lymph node (Figure 2B).
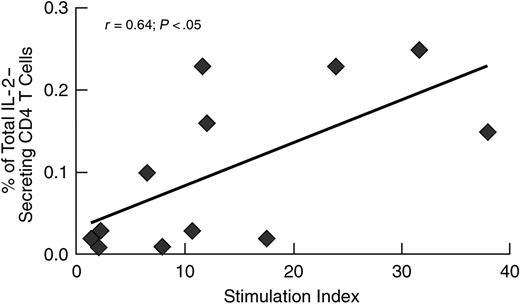
In addition, we had the opportunity to determine the relationship between the frequency of HIV-1-specific IL-2-secreting cells and the proliferation capacity in 12 (6 progressors and 6 LTNPs) subjects. Of interest, there was a good correlation (r = 0.64, P < .05) between these 2 parameters of helper CD4 T-cell function (Figure 3).
Correlation between the frequency of HIV-1-specific IL-2-secreting cells and the capacity to proliferate. HIV-1-specific stimulation with p55 antigen, ICS, and proliferation assay were carried out as described in “Patients, materials, and methods.”
Correlation between the frequency of HIV-1-specific IL-2-secreting cells and the capacity to proliferate. HIV-1-specific stimulation with p55 antigen, ICS, and proliferation assay were carried out as described in “Patients, materials, and methods.”
Functionally distinct population of virus-specific IL-2- and IFN-γ-secreting CD4 T cells
To characterize further the virus-specific IL-2- and IFN-γ-secreting CD4 T cells, blood mononuclear cells were stimulated with CMV or HIV-1 p55 gag and simultaneously assessed for the secretion of IL-2 and IFN-γ. Three populations of CD4 T cells (eg, single IL-2-secreting cells, single IFN-γ-secreting cells, and IL-2/IFN-γ-secreting cells) were identified. Representative flow cytometry profiles of these functionally distinct populations of CMV- and HIV-1-specific CD4 T cells are shown in Figure 4A. The populations of HIV-1-specific single IL-2- and IL-2/IFN-γ-secreting CD4 T cells were severely defective in progressors compared with LTNPs and with CMV-specific CD4 T cells (Figure 4A). Of interest, a skewing toward HIV-1-specific single IFN-γ-secreting CD4 T cells was observed in progressors. This latter population represented about 70% of total HIV-1-specific CD4 T cells in progressors (n = 10) (Figure 4B). In contrast, HIV-1-specific CD4 T cells were almost equally distributed within the functionally distinct cell populations in LTNPs (Figure 4B). Furthermore, similar results were also obtained for CMV-specific CD4 T cells (Figure 4B).
Analysis of functionally distinct populations of virus-specific CD4 T cells. (A) Flow cytometry profiles of the distribution of blood HIV-1- and CMV-specific CD4 T cells within IL-2-, IFN-γ-, and IFN-γ/IL-2-secreting cell populations in representative progressors, LTNPs, and HIV-negative subjects. The cluster of events shown in red correspond to the responder CD4 T cells, which express IL-2 and/or IFN-γ. (B) Mean ± SE of cumulative data on the proportion of HIV-1- and CMV-specific CD4 T cells within the different cytokine-secreting cell populations in progressors, LTNPs, and HIV-negative subjects.
Analysis of functionally distinct populations of virus-specific CD4 T cells. (A) Flow cytometry profiles of the distribution of blood HIV-1- and CMV-specific CD4 T cells within IL-2-, IFN-γ-, and IFN-γ/IL-2-secreting cell populations in representative progressors, LTNPs, and HIV-negative subjects. The cluster of events shown in red correspond to the responder CD4 T cells, which express IL-2 and/or IFN-γ. (B) Mean ± SE of cumulative data on the proportion of HIV-1- and CMV-specific CD4 T cells within the different cytokine-secreting cell populations in progressors, LTNPs, and HIV-negative subjects.
We then investigated the distribution of these functionally distinct CMV-specific CD4 T cells within memory T-cell populations defined by the expression of CCR7. In a representative (1 of 4) HIV-negative subject, the majority (> 90%) of CD4+CCR7- CMV-specific cells were either single IFN-γ or IL-2/IFN-γ (Figure 5), whereas single IL-2-secreting cells were almost absent (Figure 5). CD4+CCR7+ CMV-specific cells contained predominantly (about 70%) single IL-2-secreting cells (Figure 5), 20% to 30% IL-2/IFN-γ-secreting cells, whereas the single IFN-γ cells were poorly represented (Figure 5). Similar distribution of CMV-specific CD4 T cells within the 2 memory cell populations were also observed in LTNPs and progressors (Figure 5). Most (about 80%) of HIV-1-specific CD4 T cells were single IFN-γ-secreting cells and exclusively contained within the CCR7- cell population in progressors (Figure 5). Phenotypic and functional distributions of HIV-1-specific T cells in the LTNPs were similar to those observed for CMV (Figure 5).
Biologic importance of the different components of the virus-specific CD4 T-cell immune response
To evaluate the biologic importance of the different functionally distinct populations of virus-specific CD4 T cells, we analyzed the relationship between these immunologic parameters and markers of disease activity such as the levels of HIV-1 RNA in the plasma (eg, viremia). The percentage of total (single IFN-γ- plus IL-2/IFN-γ-secreting cells) and of single blood IFN-γ-secreting CD4 T cells did not correlate with the levels of viremia (Figure 6A-B). This finding is consistent with previous studies and with the lack of evidence of an association between the magnitude of the IFN-γ CD4 T-cell response and a protective immune response.11-13 In contrast, the percentages of total and single IL-2-secreting cells and of IL-2/IFN-γ-secreting CD4 T cells were negatively correlated with the levels of viremia (Figure 6C-E). Similar to the blood, also in the lymph node the percentage of IL-2- but not of IFN-γ-secreting CD4 T cells were negatively correlated with the levels of viremia (data not shown).
Correlation between the percentage of cytokine-secreting CD4 T cells in blood and viremia. (A) Total IFN-γ-secreting CD4 T cells. (B) Single IFN-γ-secreting CD4 T cells. (C) Correlation between the percentage of total (single IL-2- plus IL-2/IFN-γ-secreting cells) IL-2-secreting cells and viremia. (D) Correlation between the percentage of single IL-2-secreting cells and viremia. (E) Correlation between the percentage of IL-2/IFN-γ-secreting cells and viremia. The regression lines are shown. A 2-tailed P < .05 was considered significant.
Correlation between the percentage of cytokine-secreting CD4 T cells in blood and viremia. (A) Total IFN-γ-secreting CD4 T cells. (B) Single IFN-γ-secreting CD4 T cells. (C) Correlation between the percentage of total (single IL-2- plus IL-2/IFN-γ-secreting cells) IL-2-secreting cells and viremia. (D) Correlation between the percentage of single IL-2-secreting cells and viremia. (E) Correlation between the percentage of IL-2/IFN-γ-secreting cells and viremia. The regression lines are shown. A 2-tailed P < .05 was considered significant.
Effects of ART on HIV-1-specific CD4 T-cell functional populations
Ten randomly selected HIV-1-infected subjects were also studied 12 months after successful ART. The percentage of total IFN-γ-secreting CD4 T cells substantially decreased following ART; for example, mean 0.13% at baseline (prior to therapy) and mean 0.06% after ART (Figure 7A), whereas there was a tendency to an increase of total IL-2-secreting CD4 T cells between baseline (mean, 0.03%) and after ART (mean, 0.06%) (Figure 7A). Of interest, this resulted in a change of the distribution of HIV-1-specific CD4 T cells within the 3 functionally distinct cells populations compared with prior to initiation of ART (Figure 7B). Similar to CMV-specific CD4 T cells, HIV-1-specific CD4 T cells were almost equally distributed within IFN-γ-, IL-2-, and IL-2/IFN-γ-secreting CD4 T-cell populations after ART (Figure 7B). No changes were observed in the distribution of functionally distinct CMV-specific CD4 T-cell populations before and after ART (Figure 7B). The changes in the distribution of HIV-1-specific CD4 T cells within the different cytokine-secreting cell populations were mostly due to the decrease in the percentage of single IFN-γ-secreting cells and to the increase of IL-2-secreting cells, whereas IL-2/IFN-γ-secreting CD4 T cells remained unchanged (data not shown).
Effects of ART on the different populations of HIV-1-specific cytokine-secreting CD4 T cells. (A) Changes in the percentage of HIV-1-specific total IFN-γ- and IL-2-secreting CD4 T cells at baseline (prior to ART) and after 12 months of treatment. (B) Mean ± SE of cumulative data on the proportion of HIV-1- and CMV-specific CD4 T cells within the different cytokine-secreting cell populations before and after ART.
Effects of ART on the different populations of HIV-1-specific cytokine-secreting CD4 T cells. (A) Changes in the percentage of HIV-1-specific total IFN-γ- and IL-2-secreting CD4 T cells at baseline (prior to ART) and after 12 months of treatment. (B) Mean ± SE of cumulative data on the proportion of HIV-1- and CMV-specific CD4 T cells within the different cytokine-secreting cell populations before and after ART.
Analysis of the absolute number of virus-specific functionally distinct CD4 T-cell populations
The results shown earlier were confirmed further by the analysis of the absolute number of HIV-1- and CMV-specific functionally distinct CD4 T cells in HIV-1-infected subjects with progressive disease before and after ART and in LTNPs. With regard to HIV-1-specific CD4 T cells, the number of IFN-γ+ cells was not significantly different (P > .05) between LTNPs and progressors, whereas the number of both IL-2+-IFN-γ+ and IL-2+ was significantly higher (P < .01) in LTNPs (Table 1). After ART, there was a significant increase in the number of IL-2+ cells (P = .009), a decrease toward significance for IFN-γ+ cells (P = .07), and no major change in IL-2+-IFN-γ+ cells (P = .1) (Table 1). The absolute numbers of the 3 HIV-1-specific CD4 T-cell populations remained significantly higher (P < .03) in LTNPs compared with those observed after ART.
Absolute number of HIV-1- and CMV-specific single IFN-γ, IL-2/IFN-γ, and single IL-2-secreting CD4 T cells
. | CD4 T cells/μL . | IFN-γ . | IL-2/IFN-γ . | IL-2 . |
|---|---|---|---|---|
| HIV-1-p55 stimulation | ||||
| Progressors BSL, n = 8 | 739 ± 111 | 0.8 ± 0.24 (0.12) | 0.13 ± 0.03 (0.02) | 0.18 ± 0.043 (0.03) |
| After ART, n = 8 | 1004 ± 108 | 0.42 ± 0.1 (0.04) | 0.21 ± 0.04 (0.02) | 0.4 ± 0.07 (0.04) |
| LTNPs, n = 5 | 960 ± 186 | 1.41 ± 0.6 (0.12) | 0.63 ± 0.15 (0.07) | 1.08 ± 0.4 (0.1) |
| CMV stimulation | ||||
| Progressors BSL, n = 5 | 739 ± 111 | 2.25 ± 0.96 (0.24) | 1.60 ± 0.84 (0.17) | 0.7 ± 0.24 (0.08) |
| After ART, n = 5 | 1004 ± 108 | 1.92 ± 0.85 (0.16) | 1.58 ± 1.06 (0.14) | 0.87 ± 0.29 (0.08) |
| LTNPs, n = 5 | 960 ± 186 | 4.70 ± 3.47 (0.36) | 2.70 ± 1.48 (0.24) | 1.51 ± 0.61 (0.13) |
. | CD4 T cells/μL . | IFN-γ . | IL-2/IFN-γ . | IL-2 . |
|---|---|---|---|---|
| HIV-1-p55 stimulation | ||||
| Progressors BSL, n = 8 | 739 ± 111 | 0.8 ± 0.24 (0.12) | 0.13 ± 0.03 (0.02) | 0.18 ± 0.043 (0.03) |
| After ART, n = 8 | 1004 ± 108 | 0.42 ± 0.1 (0.04) | 0.21 ± 0.04 (0.02) | 0.4 ± 0.07 (0.04) |
| LTNPs, n = 5 | 960 ± 186 | 1.41 ± 0.6 (0.12) | 0.63 ± 0.15 (0.07) | 1.08 ± 0.4 (0.1) |
| CMV stimulation | ||||
| Progressors BSL, n = 5 | 739 ± 111 | 2.25 ± 0.96 (0.24) | 1.60 ± 0.84 (0.17) | 0.7 ± 0.24 (0.08) |
| After ART, n = 5 | 1004 ± 108 | 1.92 ± 0.85 (0.16) | 1.58 ± 1.06 (0.14) | 0.87 ± 0.29 (0.08) |
| LTNPs, n = 5 | 960 ± 186 | 4.70 ± 3.47 (0.36) | 2.70 ± 1.48 (0.24) | 1.51 ± 0.61 (0.13) |
The absolute number of cytokine-secreting cells was calculated from the CD4 T-cell counts. Data are presented as mean ± SE, and values in parentheses represent the mean percentage of cytokine-secreting cells in CD4 T cells. BSL indicates baseline; ART, antiretroviral therapy; and LTNPs, long-term nonprogressors.
With regard to CMV-specific CD4 T cells, no significant differences (P > .05) were observed for any of the comparisons analyzed earlier (Table 1).
Discussion
The present study has investigated virus-specific CD4 T-cell responses in healthy and HIV-1-infected subjects. In particular, (1) it provides for the first time a quantification of the antigen-specific IL-2- and IFN-γ-secreting CD4 T cells in the blood and in the lymph nodes, (2) it analyses the distribution of 3 functionally distinct virus-specific cell populations within CD4 T cells, and (3) it evaluates the effects of prolonged ART on the different HIV-1-specific CD4 T-cell populations.
According to recent studies,12,13,22 we have not found any correlation between the frequency of IFN-γ-secreting HIV-1-specific CD4 T cells and protective immunity as previously proposed.11 Some differences in the percentage of HIV-1- (in LTNPs) and CMV-specific IFN-γ-secreting CD4 T cells found in the present as compared with previous studies11 can be also explained by (1) the different way to express the results (percentage of IFN-γ-secreting cells within the total CD4 T-cell population versus the CD45RO+ CD4 T-cell population) and (2) in the present study, subjects were randomly selected on the basis of CMV serology and not on a positive flow cytometry analysis. This latter result likely represents a bias for the selection of subjects with higher frequencies of CMV-specific CD4 T cells. In the present study, we show that, in addition to the blood, the frequencies of HIV-1-specific IFN-γ-secreting CD4 T cells found in lymph nodes of progressors and LTNPs were also similar.
HIV-1-specific IFN-γ CD4 T cells were equally distributed in the blood and lymph node compartments, whereas CMV-specific IFN-γ CD4 T cells predominantly accumulated in the blood. These findings were consistent, as clearly demonstrated in mice,23,24 with the preferential accumulation of CD4 and CD8 T cells with potential effector function in the target organ of the pathogen away from the lymphoid organs. Therefore, the results obtained are compatible with the different target organs of HIV-1 (eg, the lymphoid tissue)25,26 and of CMV, (eg, lung, cervix, salivary glands, and the retina).27 In this regard, it is worthy to mention that similar results were obtained for CD8 T cells.28
The virus-specific helper CD4 T-cell function has been generally assessed by using antigen-specific proliferation assays.9,10 This is certainly a valid experimental strategy and, in fact, the detection of virus-specific proliferation generally correlates with protective immunity. However, this strategy does not allow precise quantification of the actual frequencies of helper CD4 T cells and presents a series of technical constraints such as the source of antigen, the number of mononuclear cells used in the assay, the use of fresh versus frozen cell preparations, the efficiency of antigen-presenting cells, and the length of the assay (eg, 6 days in vitro culture) that have made difficult its standardization. For these reasons we performed a flow cytometry analysis which allowed us to quantify precisely the frequency of virus-specific CD4 T cells on the basis of their ability to secrete IL-2 following stimulation with viral antigens. In the present study, we assessed the frequencies of HIV-1- and CMV-specific helper CD4 T cells by their ability to secrete IL-2 after specific antigen stimulation using flow cytometry. We demonstrated a selective defect in the percentage of blood and lymph node HIV-1-specific IL-2-secreting CD4 T cells in progressors. In fact, the percentage of CMV-specific IL-2-secreting CD4 T cells in progressors was not different from that found in the LTNPs and in the HIV-negative subjects.
Interestingly, the CD4 T-cell populations secreting IFN-γ and IL-2 were partially overlapping. The large majority (70%) of IL-2-secreting cells was also able to secrete IFN-γ, and 50% of IFN-γ-secreting cells were also IL-2 producers. On the basis of this analysis the following populations were identified: (1) single IFN-γ-secreting cells, (2) single IL-2-secreting cells, and (3) IL-2/IFN-γ-secreting cells. In HIV-negative subjects, LTNPs, and progressors, most of the CMV-specific single IFN-γ- and IL-2/IFN-γ-secreting CD4 T cells were CCR7- memory cells (eg, memory T cells with potential effector function),29 whereas single IL-2-secreting cells were the predominant cells within the CCR7+ cell population. Most of the HIV-1-specific CD4 T cells were also CCR7- cells in LTNPs and progressors, but IL-2/IFN-γ-secreting cells were absent only in progressors.
Taken together, these results indicate that (1) HIV-1-specific CD4 T cells in LTNPs and CMV-specific CD4 T cells in the 3 study groups investigated were almost equally distributed within the 3 functionally distinct CD4 T-cell populations, (2) a skewing toward HIV-1-specific IFN-γ-secreting CD4 T cells was observed in HIV-1-infected progressors and the single IL-2- and IL-2/IFN-γ-secreting CD4 T cells were severely depleted, and (3) there was an accumulation of CD4 T cells with effector function associated with progressive HIV infection.
Overall, HIV-1-specific IL-2-secreting cells seem to represent a potential marker of protective immunity because the percentage of total and single HIV-1-specific IL-2-secreting CD4 T cells and of IL-2/IFN-γ-secreting cells were negatively correlated with the levels of viremia.
Prolonged and successful ART was able to correct the skewed distribution of HIV-1-specific CD4 T cells within the 3 functionally distinct cell populations. This correction was the result of the substantial decrease of single IFN-γ-secreting cells and of the partial recovery of single IL-2-secreting cells, whereas the population of IL-2/IFN-γ-secreting cells showed no major changes after 1 year of ART. However, the absolute number of IL-2-secreting cells remained significantly lower compared with LTNPs. These observations support the conclusions that the recovery is largely incomplete after 12 to 15 months of successful ART.
The association between elevated frequencies of IFN-γ-secreting CD4 T cells and active virus replication suggests that these cells likely represent the reactive component of the immune response to the encountering pathogen rather than an indicator of the effectiveness of the ongoing immune response. The selective severe reduction of HIV-1-specific IL-2-secreting CD4 T cells, which likely belong to the same subset of CD4 T cells with proliferation capacity, is consistent with the impairment of CD4 T-cell helper function at the time of primary infection.9
We have shown a strong negative correlation between the frequency of different populations of HIV-1-specific IL-2-secreting cells and viral load. These observations support previous findings using the proliferation assay that quantitative parameters of the virus-specific helper function are reliable measures of antigen-specific immune responses that are generally associated with protective immunity.10 However, whether the lack of HIV-1-specific IL-2-secreting and -proliferating CD4 T cells is a consequence and not the cause of progressive HIV infection remains to be determined.
The results obtained after ART are of interest because they indicate that suppression of virus replication is associated with the correction of the skewed representation of different populations of HIV-1-specific CD4 T cells and with the partial recovery of IL-2-secreting cells. At the present, it is unclear whether IL-2-secreting cells will be recovered further after several years of virus suppression.
These results represent a further step in the understanding of memory T-cell immune responses and provide new tools to evaluate the effectiveness of the immune response during natural virus infections and/or induced by new vaccine candidates.
Prepublished online as Blood First Edition Paper, September 4, 2003; DOI 10.1182/blood-2003-04-1203.
Supported by research grants from the Swiss National Foundation (FN 3100-058913/2) and the European Commission (QLK2-CT-1999-01321).
A.H. and S.P. are listed in alphabetic order and contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal