Abstract
Vascular endothelial growth factor (VEGF) is the pivotal angiogenic growth factor activating endothelial cells to migrate, proliferate, and form capillary tubes. For an ordered endothelial cell migration, tissue invasion, and degradation of the extracellular matrix, proteolytic machinery is indispensable. Such machinery, suitable for localized proteolysis, is provided by the prourokinase-urokinase-plasmin system. Prourokinase (pro-uPA), the initial component of this system, is, however, synthesized in its inactive precursor form and as such bound to its cellular receptor uPAR. Here we identify a mechanism via which VEGF165 interacting with its receptor VEGFR-2 rapidly induces prourokinase activation that is dependent on a change in integrin affinity, activation of matrix metalloproteinase 2 (MMP-2), and pro-uPA being bound to its surface receptor uPAR. This VEGF-induced pro-uPA activation on endothelial cells is responsible for VEGF-dependent local fibrinolytic activity and might be one of the initial steps in the angiogenic process. (Blood. 2004;103:955-962)
Introduction
During adult life, angiogenesis is a physiologic process that occurs during the menstrual cycle and wound healing but also has a pathophysiologic role in cancer. VEGF165, a member of the vascular endothelial growth factor (VEGF) family, plays a critical role in angiogenesis by inducing endothelial cell activation, migration and invasion, proliferation, and finally capillary strand and tube formation.1 For invasion, cells have to elaborate a repertoire of proteolytic enzymes allowing cleavage of matrix proteins, whereby both serine proteases of the plasminogen/plasmin system and metalloproteinases are thought to be important.1,2 The key enzyme of the initial step of cell-bound plasmin generation is urokinase (uPA) produced by cells in its inactive precursor form prourokinase (pro-uPA).3 On the cell surface, pro-uPA is bound to its receptor, the glycosylphosphatidylinositol (GPI)-linked urokinase receptor (uPAR; CD-87), via its growth factor domain, allowing receptor-bound conversion of pro-uPA to active uPA. Active uPA in turn cleaves the proenzyme plasminogen yielding active plasmin that, in a positive feedback loop, activates pro-uPA to uPA and prometalloproteinases to active metalloproteinases.2 Therefore activation of pro-uPA to uPA is an initial step in both plasmin- and metalloproteinase-dependent proteolysis.4,5 It is, however, unclear where the first active uPA originates from: low-rate constant generation of active urokinase by plasmin is one working model; alternatively pro-uPA can become activated by additional enzymes including trypsin, kallikrein, factor XIIa, different cathepsins, and matrix metalloproteinases (MMPs).6-8
VEGF transcriptionally up-regulates urokinase in its proenzyme form and VEGF induces invasion of endothelial cells that are dependent on active uPA,1 indicating an initial requirement for VEGF-induced pro-uPA activation. How this initial activation of pro-uPA is achieved is unknown. It was therefore the aim of this study to analyze the mechanism(s) underlying rapid VEGF-induced activation of the proteolytic machinery in endothelial cells.
We found that VEGF165 induces within minutes modulation of integrin activity in a phosphatidylinositol 3-kinase (PI3-kinase)-dependent way that leads to activation of pro-MMP-2, which in turn activates pro-uPA bound to its receptor uPAR. Activated uPA in turn generates fibrinolytic activity of cells seeded on fibrin gels. Inhibition of this sequence of events at different points including the use of a specific MMP-2 inhibitor leads to inhibition of the immediate increase in fibrinolytic activity upon VEGF165 stimulation.
Materials and methods
Cell culture and reagents
Human endothelial cells (skin microvascular or umbilical vein origin) were cultured in M199 (Sigma, St Louis, MO) supplemented with 20% serum and bovine endothelial growth supplement (Technoclone, Vienna, Austria). Experiments were performed using subconfluent cultures up to passage 5, silenced under serum-reduced conditions for 24 hours, followed by 4-hour serum deprivation (1% bovine serum albumin [BSA]). Standard procedures were used for Western blots and enzyme-linked immunosorbent assay (ELISA), whereby ELISA kits for pro-uPA, uPA, and plasminogen activator inhibitor 1 (PAI-1) were from Technoclone. Gelatin zymography was performed as described previously9 (37°C, 18-hour incubation, staining with Coomassie-Blue).
VEGF165 (Promocell, Heidelberg, Germany); pro-uPA and fibronectin (Technoclone); and active uPA (Ebewe-Pharma, Unterach, Austria) were obtained. The inhibitors PD098059 and LY294002, and the MMP inhibitors (MMP-2/MMP-9 Inhibitor I, MMP-8 Inhibitor I, MMP-3 Inhibitor II; Calbiochem, La Jolla, CA); RGD (Arg-Gly-Asp) peptides, wortmannin, and GDP-β-S (Sigma); aprotinin (Bayer, Leverkusen, Germany); and EZ-Link Sulfo-NHS-SS-Biotin (Pierce, Rockford, IL) were obtained as indicated.
Antibodies
Monoclonal antibody against domain 2 of uPAR (American Diagnostica, Greenwich, CT); monoclonal antibodies against inactive pro-uPA (PUK), both forms of uPA (35scuPA), PAI-1 (5PAI), as well as functionally inhibiting monoclonal antibodies against uPA (14scuPA) or tissue plasminogen activator (tPA) (7VPA) (Technoclone); monoclonal antibody, specific for active conformation of human β1-integrins (HUTS-4) (Chemicon International, Temecula, CA); anti-CD29 (Sigma); secondary antibodies: Alexa Fluor 488-conjugated anti-mouse immunoglobulin G (IgG) antibody and biotinylated goat antimouse antibody (Molecular Probes, Leiden, Netherlands); and DAPI (4,6 diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA) were obtained as indicated. To account for unspecific binding in all immunoassays, purified nonimmune mouse IgG or rabbit IgG (Sigma) were used.
The effectiveness of the growth factors and of the respective signal transduction pathway inhibitors used in endothelial cells was analyzed in replicate experiments determining the induced phosphorylation of specific target molecules by Western blots. The used growth factors used induced activation of the respective pathways that was inhibited by the respective pathway inhibitors.10,11
Cell ELISA
Subconfluent, serum-deprived human endothelial cells were incubated in 96-well plates with growth factors in the presence or absence of inhibitors for the indicated time periods at 37°C. Cellular antigens were determined basically as described previously.12 Cells were washed with phosphate-buffered saline (PBS), fixed with 2% paraformaldehyde, and blocked with 5% BSA in PBS. Pro-uPA and uPA were determined using the respective specific antibodies. Horseradish peroxidase (HRP)-labeled secondary antibodies and 3,3′, 5,5′-tetramethylbenzimide (TMB) substrate (Technoclone) were used for detection. The reaction was stopped after 15 minutes with 3 M H2SO4, and optical density was measured at 450 nm in Anthos Reader 2001 (Wals, Austria). Alternatively, fluorimetric detection of specific primary antibodies to different surface molecules was used using fluorescence-conjugated secondary antibodies. Integrin affinities were determined either by measuring reactivity of HUTS-4 antibody and fluorescence-conjugated secondary antibodies13 or by binding of biotinylated fibronectin monomers to endothelial cells using streptavidin Alexa Fluor 488 (Molecular Probes) as detecting reagent.14,15 Briefly, biotinylated fibronectin (20 μg/mL) was added to treated endothelial cells for 30 minutes; reaction was stopped by washing cells with PBS at 4°C and fixation as described above. Unspecific fibronectin binding (5 mM EDTA [ethylenediaminetetraacetic acid]) was subtracted as nonspecific binding.14 Fluorescence intensity was measured with multilabel counter Victor2 1420 (Wallac, Finland).
Saturation of free uPAR with prourokinase (25 ng/mL) was performed after acid treatment of the cell monolayer as described3 at 4°C for 10 minutes. Afterward, cells were transferred to 37°C and experiments were performed immediately as indicated above.
Use of GDP-β-S
Guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S, 20 μM), a GDP analog resistant to phosphorylation and hydrolysis and antagonizing guanosine 5c-triphosphate (GTP), was added for 10 minutes to permeabilized cells with or without VEGF165 stimulation. Permeabilization of endothelial cells was performed using a “minimal cell permeabilization” protocol as described before.16 After washing the cells with PBS they were incubated for 10 minutes with permeabilization buffer containing 15 μg/mL saponin, which was diluted and prewarmed to 37°C before each experiment. The effectiveness of incorporation and functional activity of GDP-β-S was proved by observing complete inhibition of VEGF165-induced actin filament reorganization and stress fiber formation. Actin was stained in endothelial cells, which had been fixed by 4% formaldehyde and permeabilized with 0.1% Triton X-100, by 10 μM fluorescein isothiocyanate (FITC)-phalloidin, and visualized by an Olympus AX70 microscope (Olympus, Vienna, Austria).
Endothelium cell fibrinolysis assay (ECFA)
Plasminogen containing human 0.5% fibrinogen (Sigma) was dissolved in 0.2 mL NaCl (0.9%) before the addition of 7 μL thrombin (30 U/mL) for clot formation. After 2 hours of incubation at 37°C, 105 human endothelial cells were seeded on the fibrin clot under serum-deprived conditions in M199 + 1% BSA. VEGF165 in the presence or absence of inhibitors was incubated for indicated time periods before supernatants were collected and immediately used for analyzing d-dimer concentration by an ELISA (Technoclone).
Immunocytochemistry
Human microvascular endothelial cells seeded on gelatin-coated coverslips, stimulated as described, were fixed with 4% paraformaldehyde, then permeabilized with 0.1% Triton X-100 for 5 minutes and blocked with 5% normal goat serum in Primary Antibody Dilution Buffer (DAKO, Glostrup, Denmark) for one hour. For single antigen detection, primary antibody against d-dimer was applied for one hour at 37°C, washed, and then incubated with Alexa-Fluor 488 antimouse antibodies for one hour at 37°C. Samples were mounted in Vectashield (Vector Laboratories) and visualized on an Olympus AX70 microscope, and digital images were recorded using an F-View camera and the software package AnalySiS Pro (Soft Imaging System, Münster, Germany).
Determination of the proteolytic activity of uPA
Aliquots of samples were normalized with respect to protein concentration (A280nm = 0.1) and analyzed for amidolytic activity on Glu-Gly-Arg-pNA (S-2444; Chromogenix, Mölndal, Sweden) as described previously.17 Activity of uPA (10 ng/mL) in solution in 50 mM Tris (tris(hydroxymethyl)aminomethane)-HCl, 100 mM NaCl, pH 7.4, at 37°C was studied by continuous assay using S-2444. uPA and S-2444 were equilibrated at 37°C in the spectrophotometer sample cuvette. The absorbance at 406 nm was monitored over time. Absorbance measurements were transformed using the first derivative function to yield S-2444 (0.3 mM) hydrolysis velocities (ΔA406/Δt).
Statistics
Statistical significance was analyzed by paired or unpaired t test when one group was compared with the control group. To compare 2 or more groups with the control group, one-way analysis of variance and Dunnett tests as posttests were used. Significance was assessed to P values of less .05.
Results
VEGF increases the fibrinolytic activity of endothelial cells within minutes
Cells react to growth factor stimulation with a rapid migratory response, implying that part of growth factor stimulation occurs independent of protein synthesis.18 Therefore, we were interested in changes of proteolytic activity during short-term stimulation of endothelial cells by VEGF165. When endothelial cells were seeded on a plasminogen-containing fibrin gel and d-dimer generation in cell supernatants as a measure for plasmin-cleaved cross-linked fibrin19 was followed over time in VEGF165-stimulated or control endothelial cells (Figure 1), VEGF165-stimulated endothelial cells elaborated a significantly higher fibrinolytic activity than unstimulated endothelial cells. To examine whether the increase in fibrinolysis depends on protein synthesis or on zymogen activation alone, we used the protein synthesis inhibitor cycloheximide (10 μg/mL). Cycloheximide had no inhibitory effect on VEGF-induced increase in fibrinolysis (Figure 1A). The VEGF165 effect on fibrinolysis was time-dependent, and an initial modest but significant increase was already seen after 30 minutes (d-dimer concentrations: 0.292 ± 0.01 mg/L and 0.432 ± 0.04 mg/L; control and VEGF; P < .05; Figure 1B).
VEGF165 effect on the fibrinolytic potential of endothelial cells is independent of protein synthesis but depends on uPA activity. (A) When cells were seeded on a fibrin gel (0.5%), VEGF165 (50 ng/mL) stimulation of human umbilical vein endothelial cells (HUVECs) for 60 minutes induces an increase in d-dimer concentrations of cell supernatants, measured by a specific ELISA. The VEGF165 effect was not diminished in the presence of cycloheximide (CHX, 10 μM). Single bars represent mean values of 3 independent experiments; error bars represent SDs. (B) The increase in d-dimer concentrations in supernatants of endothelial cells by VEGF165 (50 ng/mL) is time dependent. Already after 30 minutes a statistically significant higher amount of d-dimer was found in supernatants of VEGF165-stimulated compared with unstimulated endothelial cells (0.292 ± 0.01 mg/L and 0.432 ± 0.04 mg/L; control and VEGF; P < .05). (C) The increase in d-dimer concentration by VEGF165 was partially blocked by an antibody, inhibiting urokinase activity (scuPA14), while an antibody, described to inhibit tPA activity (7VPA), could not diminish the d-dimer concentrations. When urokinase receptor was cleaved by PI-PLC (5 U/mL) or urokinase activity was blocked by the urokinase inhibitor benzamidine (benz, 10 μM), the VEGF effect on d-dimer generation was also diminished. (D) Immunocytochemistry of HUVECs seeded on fibrin gel under serum-deprived conditions, exposed for one hour to VEGF (50 ng/mL) or left untreated as control. After cells were fixed in 4% paraformaldehyde, immunostaining was performed with horseradish peroxidase (POX)-conjugated mouse anti-d-dimer monoclonal antibody followed by streptavidin Alexa-Fluor 488. Samples were mounted in Vectashield. Immunofluorescence microscopy was performed with an Olympus AX70 microscope, whereby digital images were recorded using an F-View camera. Scale bar: 10μm.
VEGF165 effect on the fibrinolytic potential of endothelial cells is independent of protein synthesis but depends on uPA activity. (A) When cells were seeded on a fibrin gel (0.5%), VEGF165 (50 ng/mL) stimulation of human umbilical vein endothelial cells (HUVECs) for 60 minutes induces an increase in d-dimer concentrations of cell supernatants, measured by a specific ELISA. The VEGF165 effect was not diminished in the presence of cycloheximide (CHX, 10 μM). Single bars represent mean values of 3 independent experiments; error bars represent SDs. (B) The increase in d-dimer concentrations in supernatants of endothelial cells by VEGF165 (50 ng/mL) is time dependent. Already after 30 minutes a statistically significant higher amount of d-dimer was found in supernatants of VEGF165-stimulated compared with unstimulated endothelial cells (0.292 ± 0.01 mg/L and 0.432 ± 0.04 mg/L; control and VEGF; P < .05). (C) The increase in d-dimer concentration by VEGF165 was partially blocked by an antibody, inhibiting urokinase activity (scuPA14), while an antibody, described to inhibit tPA activity (7VPA), could not diminish the d-dimer concentrations. When urokinase receptor was cleaved by PI-PLC (5 U/mL) or urokinase activity was blocked by the urokinase inhibitor benzamidine (benz, 10 μM), the VEGF effect on d-dimer generation was also diminished. (D) Immunocytochemistry of HUVECs seeded on fibrin gel under serum-deprived conditions, exposed for one hour to VEGF (50 ng/mL) or left untreated as control. After cells were fixed in 4% paraformaldehyde, immunostaining was performed with horseradish peroxidase (POX)-conjugated mouse anti-d-dimer monoclonal antibody followed by streptavidin Alexa-Fluor 488. Samples were mounted in Vectashield. Immunofluorescence microscopy was performed with an Olympus AX70 microscope, whereby digital images were recorded using an F-View camera. Scale bar: 10μm.
In a further step, we wanted to analyze which plasminogen activator was responsible for the VEGF165-induced fibrinolytic effect. Therefore we used 2 different specific inhibitory antibodies, one against uPA (scuPA14) and the other against tPA (7VPA). Only the antibody against uPA had a significant inhibitory effect on VEGF-induced fibrinolysis (Figure 1C). As expected from the kinetic constant of this antibody, the inhibition was incomplete. Furthermore the increase in fibrinolysis could be blocked by the urokinase inhibitor benzamidine, indicating that active urokinase is decisive for this VEGF effect (Figure 1C).
To further examine any possible role of the urokinase receptor, we used phosphoinositol-phospholipase C (PI-PLC), which cleaves the GPI-anchored urokinase receptor. After PI-PLC treatment of the cells, the VEGF165 effect was almost abolished (Figure 1C). The efficiency of PI-PLC-induced uPAR cleavage was ensured by fluorescence-activated cell sorter (FACS) analysis (data not shown). In addition we could not measure an increase in uPA, tPA, or PAI-1 concentration in cell supernatants after respective VEGF165 incubation periods (data not shown). Therefore we conclude that the uPA-uPAR complex on the cell surface is the important molecular component for VEGF165-induced fibrinolysis.
To analyze whether the VEGF165-induced increase in fibrinolytic activity is cell derived, VEGF165-stimulated and unstimulated endothelial cells seeded on a fibrin gel were stained for the presence of d-dimer epitopes in the fibrin gel around the cells. As shown in Figure 1D, d-dimer epitope-positive areas were seen only around endothelial cells treated with VEGF165, while all unstimulated endothelial cells were negative.
VEGF165 induces pro-uPA activation on the surface of human endothelial cells
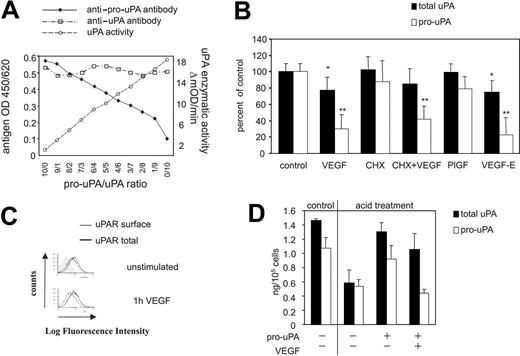
Urokinase is synthesized in its inactive precursor form pro-uPA, which is bound to its receptor uPAR3 on the cell surface. To generate fibrinolytically active plasmin from the zymogen plasminogen, pro-uPA itself has to become activated. Therefore, VEGF165 would have to initially activate pro-uPA in order to finally trigger the events leading to cell-dependent fibrin degradation. To follow that step more directly, we analyzed the effect of VEGF165 on pro-uPA present on endothelial cells using a monoclonal antibody specifically recognizing pro-uPA, but not uPA (Figure 2A). A standard curve obtained with different pro-uPA/uPA ratios at approximately 10 ng/mL urokinase antigen was constructed by blotting pro-uPA or uPA antibody reactivity versus uPA activity on the synthetic paranitroanilide substrate S-2444 (Figure 2A). A decrease in pro-uPA reactivity at a fixed uPA antigen reactivity indicates conversion of pro-uPA to uPA. Upon VEGF165 treatment, pro-uPA levels decreased by 69.2 ± 16.9% (P < .01), while total uPA on the cell surface was reduced by only 23.0 ± 16.3% (P < .05) (Figure 2B). The relative decrease of surface-bound total uPA was comparable with the decrease of surface uPAR (Figure 2C), indicating that the decrease in the amount of uPA on the cell surface upon VEGF165 treatment is caused by uPAR internalization as a regulatory mechanism to clear and degrade uPA-inhibitor complexes from the cell surface.20 The additional loss of pro-uPA immunoreactivity reflects conversion of pro-uPA to uPA. Again cycloheximide could not block the VEGF-induced pro-uPA activation, indicating a protein-synthesis-independent mechanism (Figure 2B). When ligands specific for either VEGFR-1 (PlGF) or VEGFR-2 (VEGF-E) were used, only VEGF-E induced pro-uPA activation. When uPA and pro-uPA were quantified in the respective cell lysates, total uPA decreased upon VEGF165 stimulation from 1.45 ng/105 cells to 1.00 ng/105 cells and pro-uPA from 1.18 ng/105 cells to 0.27 ng/105 cells. Noticeably, pro-uPA, uPA, and PAI-1 levels in endothelial cell supernatants were unaffected (data not shown). These data indicate that VEGF165 induces pro-uPA activation via its receptor VEGFR-2.
VEGF stimulation of endothelial cells leads to pro-uPA activation on the cell surface. (A) Characterization of the anti-pro-uPA antibody PUK. Pro-uPA antibody reactivity versus uPA activity on the synthetic chromogenic substrate S-2444 was blotted in a standard curve obtained with different pro-uPA/uPA ratios in a solution of 10 ng/mL urokinase antigen. These data indicate that only the inactive pro-uPA is recognized by the PUK antibody, while active uPA is not. (B) VEGF165 (50 ng/mL) induced cell surface uPA (dark gray bars) and pro-uPA (light gray bars) changes in HUVECs determined by fluorometric cell ELISA. Single bars represent mean values ± SD of at least 5 independent experiments. A statistically significant loss of the reactivity of the cells with antibodies specific for pro-uPA was seen after one hour of VEGF165 or VEGF-E stimulation (30.2 ± 16.9% or 22.1 ± 20.4% of controls, both P < .01). The decrease to 77 ± 16.3% of initial total uPA reactivity was comparable with the decrease of uPAR reactivity (70.3 ± 5.2%). *P < .05; **P < .01 (n = 5). (C) Representative immunocytofluorimetric histograms of cell surface (gray) and total (black) uPAR in HUVECs after stimulation under serum-free conditions with 50 ng/mL VEGF165 for 60 minutes or untreated as a control. VEGF165 induced a decrease in cell surface uPAR of 29.7 ± 5.2%, whereby total uPAR levels were not affected, indicating an internalization process. (D) Effect of VEGF165 (50 ng/mL) on pro-uPA activation on the surface of HUVECs measured by fluorimetric cell ELISA. Receptor bound urokinase antigen was removed by acid treatment as described by Stoppelli et al,3 following saturation of the free uPAR with exogenously added pro-uPA (25 mg/mL). Washed cells were stimulated with VEGF165 (50 ng/mL) for 2 hours. Growth factor stimulation significantly decreases cell surface pro-uPA levels. P = .002.
VEGF stimulation of endothelial cells leads to pro-uPA activation on the cell surface. (A) Characterization of the anti-pro-uPA antibody PUK. Pro-uPA antibody reactivity versus uPA activity on the synthetic chromogenic substrate S-2444 was blotted in a standard curve obtained with different pro-uPA/uPA ratios in a solution of 10 ng/mL urokinase antigen. These data indicate that only the inactive pro-uPA is recognized by the PUK antibody, while active uPA is not. (B) VEGF165 (50 ng/mL) induced cell surface uPA (dark gray bars) and pro-uPA (light gray bars) changes in HUVECs determined by fluorometric cell ELISA. Single bars represent mean values ± SD of at least 5 independent experiments. A statistically significant loss of the reactivity of the cells with antibodies specific for pro-uPA was seen after one hour of VEGF165 or VEGF-E stimulation (30.2 ± 16.9% or 22.1 ± 20.4% of controls, both P < .01). The decrease to 77 ± 16.3% of initial total uPA reactivity was comparable with the decrease of uPAR reactivity (70.3 ± 5.2%). *P < .05; **P < .01 (n = 5). (C) Representative immunocytofluorimetric histograms of cell surface (gray) and total (black) uPAR in HUVECs after stimulation under serum-free conditions with 50 ng/mL VEGF165 for 60 minutes or untreated as a control. VEGF165 induced a decrease in cell surface uPAR of 29.7 ± 5.2%, whereby total uPAR levels were not affected, indicating an internalization process. (D) Effect of VEGF165 (50 ng/mL) on pro-uPA activation on the surface of HUVECs measured by fluorimetric cell ELISA. Receptor bound urokinase antigen was removed by acid treatment as described by Stoppelli et al,3 following saturation of the free uPAR with exogenously added pro-uPA (25 mg/mL). Washed cells were stimulated with VEGF165 (50 ng/mL) for 2 hours. Growth factor stimulation significantly decreases cell surface pro-uPA levels. P = .002.
In order to analyze whether VEGF165-induced pro-uPA activation occurs on the cell surface or active uPA is already secreted, pro-uPA (25 ng/mL) was added exogenously to acid-washed endothelial cells (Figure 2D). Upon acid treatment, the levels of pro-uPA and uPA decreased to 0.58 ng/105 cells and 0.52 ng/105 cells, respectively. Loading of acid-washed cells with exogenous pro-uPA replenished bound pro-uPA/uPA antigen. Upon VEGF165 stimulation of loaded cells, pro-uPA immunoreactivity decreased to levels of acid-washed cells (P < .002), while total uPA immunoreactivity decreased by only about 20%. These data indicate that VEGF165 induces pro-uPA activation on the cell surface. Pro-uPA activation in response to VEGF165 is here shown for the first time.
VEGF165 induces prourokinase activation in an MMP-2-dependent fashion
Pro-uPA activation mainly occurs by 2 classes of proteases, serine proteinases and matrix-metalloproteinases (MMPs). The serine proteinase plasmin is the major component in a positive feedback loop in which uPA activates plasminogen to plasmin and plasmin in turn activates, among others, pro-uPA to uPA. When we analyzed the mechanism responsible for VEGF165-induced pro-uPA activation, we found that neither the plasmin inhibitor aprotinin nor the uPA inhibitor benzamidine was effective (Figure 3A). This indicates that serine proteases are not participating in VEGF165-induced pro-uPA activation. When, however, the broad metalloproteinase inhibitor 1,10-phenanthroline and the more specific MMP-2/9 (gelatinase A/B) inhibitor ((2R)-2-[(4-biphenylylsulfonyl)amino]-3-phenylpropionic acid) were used, VEGF165-induced pro-uPA activation (Figure 3A) was inhibited.
Metalloproteinase inhibitors inhibit the growth factor effect on pro-uPA activation and fibrinolytic activity. (A) Cell ELISA measurement of pro-uPA changes on the surface of VEGF-stimulated endothelial cells in the presence or absence of different protease inhibitors. 1,10-Phenanthroline (10 μM) as well as a specific gelatinase inhibitor (1 μM) prevented VEGF165 (50 ng/mL)-induced pro-uPA reduction from the cell surface, while benzamidine (10 μM) or aprotinin (50 kIE/mL) had no effect. Results are given in percent of fluorescence units of unstimulated cells, whereby single bars represent average of mean values ± SDs of 3 independent experiments. V indicates VEGF165; benz, benzamidine; apr, aprotinin; phe, 1,10-phenanthroline; M2/9I, ,MMP-2/9 inhibitor; M3I, MMP-3 inhibitor; and M8I, MMP-8 inhibitor. **P < .01 (n = 3). (B) d-dimer concentrations of supernatants from endothelial cells seeded on fibrin gels measured by a specific ELISA. Fresh supernatant was collected after 60 minutes. The VEGF165 effect was diminished in the presence of a specific gelatinase inhibitor (M2/9I, 1 μM). Single bars represent mean values of 3 independent experiments; error bars represent SDs. (C) Western blots for MMP-2 and MMP-9 from VEGF165-stimulated endothelial cell lysates. Specific mAbs against MMP-2 and MMP-9, respectively, were applied and visualized with subsequent chemiluminescence detection. Upper panel represents MMP-9 (zymogen 92 kDa and active form 82 kDa); lower panel, MMP-2 (zymogen 72 kDa and active form 62 kDa). (D) Gelatin zymography of endothelial cell lysates. The 62-kDa MMP-2 band significantly increases in intensity upon VEGF165 stimulation.
Metalloproteinase inhibitors inhibit the growth factor effect on pro-uPA activation and fibrinolytic activity. (A) Cell ELISA measurement of pro-uPA changes on the surface of VEGF-stimulated endothelial cells in the presence or absence of different protease inhibitors. 1,10-Phenanthroline (10 μM) as well as a specific gelatinase inhibitor (1 μM) prevented VEGF165 (50 ng/mL)-induced pro-uPA reduction from the cell surface, while benzamidine (10 μM) or aprotinin (50 kIE/mL) had no effect. Results are given in percent of fluorescence units of unstimulated cells, whereby single bars represent average of mean values ± SDs of 3 independent experiments. V indicates VEGF165; benz, benzamidine; apr, aprotinin; phe, 1,10-phenanthroline; M2/9I, ,MMP-2/9 inhibitor; M3I, MMP-3 inhibitor; and M8I, MMP-8 inhibitor. **P < .01 (n = 3). (B) d-dimer concentrations of supernatants from endothelial cells seeded on fibrin gels measured by a specific ELISA. Fresh supernatant was collected after 60 minutes. The VEGF165 effect was diminished in the presence of a specific gelatinase inhibitor (M2/9I, 1 μM). Single bars represent mean values of 3 independent experiments; error bars represent SDs. (C) Western blots for MMP-2 and MMP-9 from VEGF165-stimulated endothelial cell lysates. Specific mAbs against MMP-2 and MMP-9, respectively, were applied and visualized with subsequent chemiluminescence detection. Upper panel represents MMP-9 (zymogen 92 kDa and active form 82 kDa); lower panel, MMP-2 (zymogen 72 kDa and active form 62 kDa). (D) Gelatin zymography of endothelial cell lysates. The 62-kDa MMP-2 band significantly increases in intensity upon VEGF165 stimulation.
Addition of either MMP-3 inhibitor (N-Isobutyl-N-(4-methoxyphenylsulfonyl)-glycylhydroxamic acid) or MMP-8 inhibitor ((3R)-(+)-[2-(4-methoxybenzenesulfonyl)-1,2,3,4-tetrahydroisoquinoline-3-hydroxamate]) had no effect on VEGF165-induced pro-uPA activation. These data indicate that VEGF165 induces pro-uPA activation in an MMP-2- and/or MMP-9-dependent mechanism.
Measuring d-dimer concentrations in supernatants of endothelial cells stimulated with VEGF165, with or without the specific gelatinase inhibitor, confirmed the gelatinase dependency of VEGF165-induced fibrinolysis (Figure 3B). To determine whether MMP-2 or MMP-9 might be responsible for pro-uPA activation, we analyzed the presence of active forms of MMP-2 and MMP-9 in lysates of VEGF165-stimulated endothelial cells, using Western blots and specific monoclonal antibodies. When endothelial cells were left unstimulated, MMP-2 was present in its 72-kDa precursor form and only trace amounts of active 62-kDa MMP-2 were seen (Figure 3C). Upon VEGF165 stimulation, the 62-kDa MMP-2 band significantly increased in intensity. In contrast, VEGF165 stimulation had no effect on the band seen in Western blots probed with anti-MMP-9 antibody. These results were confirmed by data obtained by gelatin zymography (Figure 3D), which demonstrates that VEGF165 elicits strong pro-MMP-2 activation. As expected, the proenzyme form of MMP-2 is also seen in the gelatin zymography.21 This indicates that MMP-2 and not MMP-9 is the metalloproteinase responsible for VEGF165-induced pro-uPA activation.
VEGF165-induced pro-uPA activation and generation of active MMP-2 are PI3-kinase dependent
Several major signal transduction pathways originate from the VEGF165 receptors.22 VEGF activates the mitogen-activated protein (MAP) kinase p3810 and extracellular signal-related kinase 1/2 (ERK-1/2) pathways. The ERK-1/2 pathway is synergistically activated by phospholipase Cγ and protein kinase C ϵ (PKCϵ) and up-regulates several genes via the transcription factor early growth response 1 (EGR-1). VEGF also induces the PI3-kinase pathway that modulates β1 integrin-dependent cell migration.23
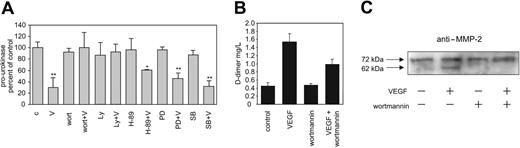
In order to analyze which of the pathways originating from VEGFR-2 might be responsible for VEGF165-induced pro-MMP-2 activation and in turn pro-uPA activation, we used respective inhibitors and analyzed their effects on these VEGF165-induced events. We found that the generation of the active form of uPA, the increase in d-dimer measured fibrinolytic activity, as well as MMP-2 activation in response to VEGF165 are all PI3-kinase dependent (Figure 4A-C) because they could be inhibited by the PI3-kinase inhibitors wortmannin or LY294002, while other pathway inhibitors (H-89, PD098059, and SB203580) were ineffective at the level of pro-uPA activation. From these data, we conclude that VEGF165 induces generation of active MMP-2 and in turn activation of pro-uPA via a PI3-kinase-dependent pathway.
VEGF165 effects on pro-uPA and MMP-2 activation are PI3-kinase dependent. (A) Surface pro-uPA levels of endothelial cells analyzed in fluorocytometric ELISA. VEGF effects on cell surface pro-uPA are abolished by the PI3-kinase inhibitors wortmannin or Ly294002. Serum-deprived human endothelial cells, preincubated for 30 minutes with different inhibitors (100 nM wortmannin, 10 μM Ly294002, 20 μM H-89, 10 μM PD98059, and 10 μM SB203580), were stimulated with 50 ng/mL VEGF165 for 60 minutes, or were left unstimulated. VEGF165 induced a PI3-kinase-dependent (wortmannin- or Ly294002-treated cells) decrease of cell surface pro-uPA level. c indicates control; V, VEGF; wort, wortmannin; Ly, Ly294002; PD, PD98059; and SB, SB203580; mean ± SDs; n = 3; *P < .05; **P < .01. (B) VEGF-induced increase in d-dimer concentration in cell supernatants (measured by an ELISA) was partially blocked by the PI3-kinase inhibitor wortmannin. Serum-deprived human endothelial cells were incubated with or without wortmannin (100 nM) and with or without VEGF (50 ng/mL). Mean ± SDs; n = 2. (C) Western blots for MMP-2 from endothelial cells lysates. The increase in active MMP-2 (62 kDa) in response to VEGF165 is wortmannin (100 nM) dependent.
VEGF165 effects on pro-uPA and MMP-2 activation are PI3-kinase dependent. (A) Surface pro-uPA levels of endothelial cells analyzed in fluorocytometric ELISA. VEGF effects on cell surface pro-uPA are abolished by the PI3-kinase inhibitors wortmannin or Ly294002. Serum-deprived human endothelial cells, preincubated for 30 minutes with different inhibitors (100 nM wortmannin, 10 μM Ly294002, 20 μM H-89, 10 μM PD98059, and 10 μM SB203580), were stimulated with 50 ng/mL VEGF165 for 60 minutes, or were left unstimulated. VEGF165 induced a PI3-kinase-dependent (wortmannin- or Ly294002-treated cells) decrease of cell surface pro-uPA level. c indicates control; V, VEGF; wort, wortmannin; Ly, Ly294002; PD, PD98059; and SB, SB203580; mean ± SDs; n = 3; *P < .05; **P < .01. (B) VEGF-induced increase in d-dimer concentration in cell supernatants (measured by an ELISA) was partially blocked by the PI3-kinase inhibitor wortmannin. Serum-deprived human endothelial cells were incubated with or without wortmannin (100 nM) and with or without VEGF (50 ng/mL). Mean ± SDs; n = 2. (C) Western blots for MMP-2 from endothelial cells lysates. The increase in active MMP-2 (62 kDa) in response to VEGF165 is wortmannin (100 nM) dependent.
Integrins are involved in pro-uPA activation
A major mechanism for pro-MMP-2 activation involves association of the zymogen with MT1-MMP and tissue inhibitor of metalloproteinase 2 (TIMP-2).24 It is assumed that a neighboring MT1-MMP molecule not associated with TIMP-2 cleaves pro-MMP-2 at the Asn37-Leu38 peptide bond within the prodomain.25 This mechanism is described to be regulated by ligand binding of integrins that leads to MT1-MMP and MMP-2 activation.9,21,26,27 Yan et al described a mechanism whereby inducing “on”-conformation of integrins either by clustering integrins by high density of fibronectin or by activation of αvβ3 and β1 integrins by respective antibodies leads to a decrease of pro-MMP-2 activation.28 In addition, a PI3-kinase-dependent effect of VEGF on β1 integrin activity was described.23 These data prompted us to analyze the possible involvement of integrins in VEGF165-induced MMP-2/pro-uPA activation. When the divalent cation manganese, which induces a strong “on”-conformation change of integrins,29 was added, VEGF165-induced pro-MMP-2 (Figure 5A) and pro-uPA activation (Figure 5B) were blocked. In contrast, the soluble integrin ligand mimetic peptide (RGD) by itself induced pro-MMP-2 (Figure 5A) as well as pro-uPA activation (Figure 5B) and was additive with VEGF165 effects on pro-uPA (not shown). Consistently we found that the MMP2/9 inhibitor abolished the effects of RGD on pro-uPA activation, further indicating that pro-uPA activation is brought about in similar ways by RGD and VEGF165, both being dependent on proteolysis by MMP-2. To exclude the possibility that RGD might induce pro-uPA activation via VEGFR signaling, we used the PI3-kinase inhibitor, wortmannin. Wortmannin did not block RGD-induced pro-uPA activation, indicating that the RGD effect occurs downstream of the PI3-kinase effect of VEGF165 and not via a PI3-kinase-dependent intracellular signaling mechanism (Figure 5B). Therefore, we conclude that VEGF165-induced pro-uPA activation involves integrins. In concordance with the integrin involvement of VEGF165-induced pro-uPA activation, d-dimer concentrations also were decreased when manganese was added to VEGF165-stimulated endothelial cells, and soluble RGD peptides were able to increase d-dimer concentrations when they were added to serum-deprived endothelial cells (Figure 5C).
Integrins are involved in pro-uPA activation. (A) Gelatin zymography of endothelial cell lysates. The 62-kDa MMP-2 band significantly increases in intensity upon VEGF165 (50 ng/mL) or S-RGD (10 μM) stimulation, while 2 mM manganese (Mn++) blocks the VEGF165 effect. (B) Pro-uPA levels measured by cell ELISA. Mimicking integrin ligand binding by soluble tetrapeptide S-RGD (10 μM) resulted in pro-uPA activation, while 2 mM manganese (Mn++) inhibited the VEGF165-induced decrease of surface pro-uPA. Wortmannin (100 nM) did not affect the S-RGD-induced pro-uPA activation; also GDP-β-S (20 μM) had no effect on VEGF165-induced pro-uPA activation. Mean ± SDs; n = 4; **P < .01. (C) VEGF165-induced increase in fibrinolytic activity was measured by a d-dimer ELISA. The soluble tetrapeptide S-RGD (10 μM) increased the fibrinolytic activity, while manganese (2 mM) or coincubation of manganese and wortmannin (100 nM) inhibited the VEGF165 effect. Mean ± SDs. *P < .05; **P < .005. (D) Fluorometric detection of β1 integrin in “on”-conformation on endothelial cells by an activity-specific anti-β1 integrin antibody (HUTS-4). Significant change in conformation of β1 integrins by VEGF165 (50 ng/mL, 60 minutes) shows PI3-kinase dependency (100 nM wortmannin). In the presence of 2 mM manganese (Mn++) the effect of VEGF165 was blocked. The soluble tetrapeptide S-RGD (10 μM) decreases HUTS-4 immunoreactivity significantly (n = 3). GDP-β-S (20 μM) could not influence VEGF165-induced decrease of HUTS-4 immunoreactivity. For statistical analysis, one-way analysis of variance and Dunnett tests as posttests were used; significance was assigned to P values less than .05 (*P < .05, **P < .01). Mean ± SDs; n = 7.
Integrins are involved in pro-uPA activation. (A) Gelatin zymography of endothelial cell lysates. The 62-kDa MMP-2 band significantly increases in intensity upon VEGF165 (50 ng/mL) or S-RGD (10 μM) stimulation, while 2 mM manganese (Mn++) blocks the VEGF165 effect. (B) Pro-uPA levels measured by cell ELISA. Mimicking integrin ligand binding by soluble tetrapeptide S-RGD (10 μM) resulted in pro-uPA activation, while 2 mM manganese (Mn++) inhibited the VEGF165-induced decrease of surface pro-uPA. Wortmannin (100 nM) did not affect the S-RGD-induced pro-uPA activation; also GDP-β-S (20 μM) had no effect on VEGF165-induced pro-uPA activation. Mean ± SDs; n = 4; **P < .01. (C) VEGF165-induced increase in fibrinolytic activity was measured by a d-dimer ELISA. The soluble tetrapeptide S-RGD (10 μM) increased the fibrinolytic activity, while manganese (2 mM) or coincubation of manganese and wortmannin (100 nM) inhibited the VEGF165 effect. Mean ± SDs. *P < .05; **P < .005. (D) Fluorometric detection of β1 integrin in “on”-conformation on endothelial cells by an activity-specific anti-β1 integrin antibody (HUTS-4). Significant change in conformation of β1 integrins by VEGF165 (50 ng/mL, 60 minutes) shows PI3-kinase dependency (100 nM wortmannin). In the presence of 2 mM manganese (Mn++) the effect of VEGF165 was blocked. The soluble tetrapeptide S-RGD (10 μM) decreases HUTS-4 immunoreactivity significantly (n = 3). GDP-β-S (20 μM) could not influence VEGF165-induced decrease of HUTS-4 immunoreactivity. For statistical analysis, one-way analysis of variance and Dunnett tests as posttests were used; significance was assigned to P values less than .05 (*P < .05, **P < .01). Mean ± SDs; n = 7.
These results indicate involvement of integrins in VEGF165-induced activation of endothelial fibrinolytic activity. To further analyze the participation of integrins in the VEGF165 response, we used the HUTS-4 antibody, previously described to be specific for the “on”-conformation state of β1 integrins,13 and, alternatively, binding of monomeric fibronectin. In fact, VEGF165 induced a significant change in the immunoreactivity with this antibody that was inhibited by wortmannin (Figure 5D), while immunoreactivity with an antibody recognizing β1 integrins independent of their conformation was not affected by VEGF165 (not shown). The VEGF165 effect on the HUTS-4 epitope was blocked in the presence of manganese that induced a general increase in HUTS-4 reactivity, while RGD peptide by itself reduced HUTS-4 reactivity significantly. RGD peptides do not directly interfere with HUTS-4 binding epitopes.13 We then wanted to demonstrate that the VEGF165 effects on HUTS-4 reactivity are related to integrin conformation and not to internalization or lateral movement of integrins expressing the HUTS-4 epitope. In the presence of guanosine-[beta-thio]diphosphate (GDP-β-S), a nonphosphorylatable form of GDP that inhibits any cellular events dependent on small GTPases and therefore inhibits redistribution of integrins, the total amount of integrin β1 increased in such treated cells by +41.2%, but the immunoreactivity of HUTS-4 still decreased in response to VEGF165 in a similar way as without GDP-β-S (Figure 5D). Similarly while addition of manganese blocked VEGF165-induced pro-uPA activation, GDP-β-S did not (Figure 5B). Therefore, VEGF165-induced decrease in HUTS-4 reactivity as well as pro-uPA activation are related to integrin conformation, but not to GTP/GDP-dependent integrin movement. In addition we wanted to confirm the VEGF-induced change in integrin affinity by fibronectin binding assays.14,15 When endothelial cells were stimulated with VEGF165 for 60 minutes, binding of biotinylated fibronectin to these cells decreased to 59.65 ± 21.17% of binding to unstimulated cells. Again the VEGF effect on fibronectin binding was completely inhibited in the presence of wortmannin (106.24 ± 13.53%). This led us to conclude that VEGF165 induces in a PI3-kinase-dependent fashion a change in β1 integrin conformation. Pro-MMP-2 activation and in turn pro-uPA activation are then related to “on/off”-conformation change of integrins. Whether β1 integrins alone or additionally a conformation change in other integrins is responsible for VEGF165-induced pro-uPA activation cannot be answered from these data.
Discussion
Angiogenesis, a complex process by which new blood vessels arise from preexisting vasculature, is a physiologic process in development, wound healing, and the female reproductive cycle, and it occurs in several pathologic conditions such as tumor-vessel formation. VEGF, a pivotal angiogenic growth factor, increases vascular permeability, leading to extravascular accumulation of plasma proteins such as fibrinogen, but also stimulates endothelial cells to migrate and invade into surrounding tissue by increasing proteolytic activity as well as cytoskeleton reorganization. Therefore, endothelial cells have to elaborate a coordinated localized formation of a proteolytic machinery to degrade fibrin-containing matrices, which were formed by the hemostatic process in wounding or local fibrinogen exudation in inflammatory or malignant processes. It has been shown that focusing the urokinase receptor toward the leading edge of migrating cells provides such armor30 and that inhibition of uPA binding to its receptor inhibited invasion of endothelial cells.31 However, it is not known at which step of the invasive process the inactive zymogen pro-uPA is activated and how this activation is achieved.
We here show that in fact VEGF-induced endothelial cell stimulation leads to increased fibrinolytic activity. To follow that increase in more detail, we introduced an assay quantifying pro-uPA epitopes on the cell surface. VEGF-induced pro-uPA activation was not due to secretion of active uPA, but due to activation of pro-uPA on the cell surface as revealed by activation of exogenously added pro-uPA to acid-washed endothelial cells upon VEGF stimulation. Activation of pro-uPA, while bound to its receptor, was also seen by others previously, who showed that receptor binding indeed favors pro-uPA activation.32 Pro-uPA activation in response to VEGF is shown for the first time here.
Using several protease inhibitors we next analyzed the mechanism leading to activation of pro-uPA and found that pro-uPA activation was inhibited only by a broad spectrum matrix metalloproteinase inhibitor and an MMP-2/9 inhibitor ((2R)-2-[(4-biphenylylsulfonyl)amino]-3-phenylpropionic acid). Neither the plasmin inhibitor aprotinin nor the urokinase inhibitor benzamidine was effective on pro-uPA activation, indicating that VEGF induces pro-uPA activation in an MMP-2/9-dependent fashion. We then went back to our original experiment and analyzed whether MMP-2/9 inhibitors also could inhibit the increase in fibrinolytic activity in response to VEGF. We could show that indeed the MMP-2/9 inhibitor blocked not only VEGF-induced pro-uPA activation but also fibrinolysis.
To determine whether MMP-2 or MMP-9 is responsible for VEGF-induced pro-uPA activation, we analyzed cell lysates of VEGF-stimulated and unstimulated endothelial cells for the appearance of the active forms of MMPs by Western blotting and gelatin zymography. While active MMP-9 was similarly present in Western blots of VEGF-stimulated and unstimulated cells, active MMP-2 appeared in Western blots and on gelatin zymography only. This indicates that MMP-2 is responsible for pro-uPA activation in VEGF-induced endothelial cells.
Based on these findings we wanted to analyze which of the signaling cascades originating from the VEGF receptor might be responsible for MMP-2 and in turn pro-uPA activation. We found that events related to VEGF-induced pro-uPA activation were all inhibited by the PI3-kinase inhibitor wortmannin, while other pathway inhibitors were ineffective.
Dependency of VEGF-induced pro-uPA activation on MMP-2 as well as the involvement of a PI3-kinase pathway prompted us to investigate whether integrins are involved in such a process. It has been shown previously that VEGF causes in a PI3-kinase-dependent manner a change in β1 integrin activity, that pro-MMP-2 is bound to integrins, and that its activation is integrin-ligand dependent.9,27,33 More precisely, it has been shown that inducing an “on”-conformation of integrins, either by clustering integrins or by direct activation with antibodies, diminished pro-MMP-2 activation, while decreased integrin activity leads to a higher amount of active MMP-2.28 Consistently, we can show here that in fact an integrin ligand mimetic peptide (soluble RGD) activates pro-MMP-2 and pro-uPA. The participation of integrins in pro-uPA activation is consistent with our finding that manganese blocks the VEGF effect on pro-MMP-2 and pro-uPA activation. The divalent cation manganese strongly induces the active conformation of integrins29 and prevents the conformation change of integrins induced by VEGF via the PI3-kinase pathway.
To further delineate the involvement of integrins in that process, we used an anti-β1 integrin antibody recognizing only the active conformation of the integrin β1 subunit (HUTS-4) and in addition determined binding of monomeric fibronectin to endothelial cells. Using this approach we could show that VEGF leads in a PI3-kinase-dependent fashion to a decrease in HUTS-4 reactive β1 integrins and in fibronectin binding. This VEGF effect was not due to redistribution of integrins, because it was still seen when cytoskeletal mobility was blocked by the addition of GDP-β-S. An association of MT1-MMP, known to be the major activator of cell-bound pro-MMP-2, and integrins has been reported.21 Preliminary data using antisense oligonucleotides against MMP-2 or MT1-MMP provide evidence that not only MMP-2 but also MT1-MMP is involved in this process (data not shown). Furthermore, the decrease in HUTS-4 reactivity following addition of RGD peptides is consistent with the notion that pro-MMP-2 activation and in turn pro-uPA activation by RGD peptides are due to a decrease in integrin affinity.
In our initial experiments we have demonstrated that VEGF-induced fibrinolytic activity is dependent on urokinase and not on tissue-type plasminogen activator. Therefore it was puzzling that under experimental conditions in which VEGF-induced pro-uPA activation was completely inhibited (wortmannin Figure 4A, or manganese Figure 5B), inhibition of d-dimer formation in response to VEGF was incomplete (wortmannin, Figure 4B, or manganese, Figure 5C, respectively). Similarly, addition of integrin ligand mimetic peptide RGD induced pro-uPA activation in an extent comparable with VEGF (Figure 5B), while induction of fibrinolytic activity as measured by d-dimer formation was significantly lower (Figure 5C). One explanation for this discrepancy could lie in the fact that pro-uPA activation was analyzed in a plasminogen-free system, where the positive feedback loop of plasmin is absent, while in the case of the d-dimer assay, plasmin generated by small amounts of active uPA would lead to further uPA generation and in turn d-dimer formation. In fact, purified pro-uPA (1 ng, 60 minutes) added to the fibrin matrix in a cell-free system generated d-dimer products in an amount of 0.34 ± 0.01 mg/L that was significantly higher than the buffer control (0.07 ± 0.04 mg/L). This feedback loop could explain why neither addition of manganese nor inhibition of the PI3-kinase inhibitor wortmannin nor a combination of both would completely block d-dimer formation. This, however, cannot explain why addition of RGD peptides would not generate a comparable fibrinolytic activity with that of VEGF-activated endothelial cells (Figure 5C). As activation of pro-uPA to uPA would also lead to complex formation with PAI-1 and uPAR internalization20 as shown in Figure 2C, we analyzed the effects of the respective inhibitors and activators of endothelial pro-uPA conversion on the relative amount of uPAR and on total uPA on the cell surface. From these data and from the respective pro-uPA activation we calculated the amounts of active uPA generated. These data are shown as Supplementary-Figure 1 and indicate that in fact RGD peptides—although causing strong pro-uPA activation—generate less active uPA on the cell surface probably due to an increased internalization of uPAR and recycling of unoccupied receptors as reflected by the diminished amount of total uPA on the cell surface. The data shown in Supplementary-Figure 1 also provide an additional explanation for the incomplete inhibition of VEGF-induced fibrinolytic activity by manganese or wortmannin, because in both cases a significant amount of active uPA is still present on the cell surface, probably due to decreased receptor internalization. From these data we can conclude that the total fibrinolytic activity generated on the endothelial cell surface is not only a sole function of pro-uPA activation, but is also influenced by uPAR internalization and recycling.
In conclusion, we here demonstrate that the initial step in VEGF-induced pro-uPA activation is a PI3-kinase-dependent change in integrin conformation, causing activation of integrin-associated pro-MMP-2. Active MMP-2 in turn leads to urokinase-receptor-bound pro-uPA activation, which increases fibrinolysis. The resulting proteolysis may favor matrix component degradation resulting in increased endothelial cell invasion and supporting angiogenesis.30 This mechanism seems not to be restricted to VEGF-receptor-mediated effects, because in preliminary experiments shown in Supplementary-Figure 2 we demonstrate that activation of endothelial cells by fibroblast growth factor 2 (FGF-2) induces pro-uPA activation in a similar manner as VEGF165. This indicates that the mechanism described here in detail for the activation of pro-uPA by VEGF is possibly a more general mechanism for generation of proteolytic activity on the cell surface that might be used by growth factors activating the PI3-kinase pathway.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-07-2214.
Supported in part by grants from the Austrian Science Foundation (SFB5-09) and the Kplus Competence Center for Bio Molecular Therapeutics, BMT.
G.W.P. and J.M.B. contributed equally to this work.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal