Abstract
Atherosclerosis is a chronic inflammatory disease characterized by the accumulation of lipid-laden macrophages in the vessel wall. One of the major transcription factors in inflammation is nuclear factor κB (NF-κB), and we have studied its role in the development of atherosclerosis. Bone marrow from mice targeted in the NF-κB1 gene encoding for the p50 subunit was used to reconstitute irradiated LDLR-/- mice as a model for atherosclerosis. After feeding the mice a high-fat diet, those deficient in NF-κB1 had a 41% lower rate of atherosclerosis than control mice, as judged by the sizes of the lesions. Furthermore, in the absence of NF-κB1, the lesions were characterized by an inflammatory phenotype, contained increased numbers of small cells, and were almost devoid of normal foam cells. In vitro studies using bone marrow (BM)-derived macrophages showed that macrophages lacking p50 had a prolonged production of tumor necrosis factor (TNF) in response to lipopolysaccharide (LPS), and other cytokines were also affected. Interestingly, the uptake of oxidized low-density lipoprotein (LDL) was greatly reduced in activated p50-deficient macrophages, probably because of a reduction in the expression of scavenger receptor class A. The effects on atherosclerosis might have resulted from the changes in cytokine production and the uptake of modified lipoproteins, making p50 a pivotal regulator of atherogenesis. (Blood. 2004;103:934-940)
Introduction
Atherosclerosis is an inflammatory disease of the large arteries. An initiating event is the retention and subsequent modification of lipoproteins in the vessel wall.1,2 This leads to the attraction of monocytes that migrate through the vessel wall and eventually differentiate into macrophages engorged with modified lipoproteins, turning them into foam cells. These processes mark the start of atherosclerosis, in which the immune system plays a key role.3 Macrophages play a dual role in atherogenesis. First, they become foam cells upon uptake of modified lipoproteins in the vessel wall. Second, they are the major immune cells in the lesion, and they mediate the inflammatory response accompanying atherosclerotic plaque formation by producing cytokines, chemokines, and growth factors. These inflammatory mediators contribute to the attraction of more monocytes and other cells of the immune system and can influence other cells in the vicinity, such as smooth muscle cells and endothelial cells. Furthermore, they can produce factors that affect plaque development, morphology, and stability. Hence, macrophages have essential functions in all phases of plaque development, from fatty streaks to advanced plaques and eventually to plaque rupture.
The transcription factor nuclear factor κB (NF-κB) plays a major role in inflammation and immune regulation. NF-κB complexes consist of heterodimers or homodimers that are kept in the cytoplasm in an inactive form. On activation, NF-κB translocates to the nucleus, mediating the transcription of a wide range of genes, including cytokines, growth factors, and antiapoptotic proteins.4 Activated NF-κB has been found in human atherosclerotic lesions.5,6 NF-κB dimers are formed by combinations of 5 different subunits: p65, cRel, RelB, p50, and p52. The p65, RelB, and cRel subunits are transcriptionally active members. The p50 and p52 family members do not exert transcriptional activity but are involved in transcriptional regulation.7 P50 and p52 are generated by the proteolytic cleavage of precursor proteins p105 and p100, which are coded by the NF-κB1 and the NF-κB2 gene, respectively. In general, NF-κB has been regarded as a transcription factor involved in the cellular activation and production of proinflammatory factors. However, the p50 subunit can also have a different effect and can form homodimers, which block the transcription of proinflammatory genes and play a repressive role in cellular activation, especially in macrophages.8,9 In this paper we describe the effect of NF-κB1 deficiency in hematopoietic cells on atherogenesis in low-density lipoprotein receptor-deficient mice, an excellent model for atherosclerosis.10 By creating bone marrow chimeras, we were able to investigate the process of atherosclerosis in the absence of NF-κB1 in hematopoietic cells, including macrophages. Our results indicate that the deletion of NF-κB1 results in a significant reduction in lesion size and a remarkable alteration toward a more inflamed phenotype. In vitro p50-deficient bone marrow macrophages showed altered cytokine secretion and reduced lipoprotein uptake on activation.
Materials and methods
Mice and diet
The generation of mice deficient in NF-κB1 has been described.11 Mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Low-density lipoprotein (LDL) receptor-deficient mice (LDLR-/-) have been described elsewhere.10 The background of each strain was 129, and the mice were backcrossed 4 times onto C57Bl/6 mice. Some in vitro assays were performed using C57Bl/6;129 NF-κB1-deficient animals and C57Bl/6;129 F2 controls, provided by the Jackson Laboratory. High-fat diet feed (Hope Farms, Woerden, The Netherlands) contained 16% fat, 0.15% cholesterol, and no cholate. All experiments were approved by the Committee for Animal Welfare of Maastricht University.
Bone marrow transplantation
One week before transplantation, 32 LDLR-/- female mice were housed in filter-top cages and put on acidified water containing neomycin (100 mg/L; Gibco, Breda, The Netherlands) and polymyxin B sulfate (6 × 104 U/L; Gibco). The mice were treated with 10 Gy total body irradiation. The next day bone marrow was isolated from donor mice (4 NF-κB1-deficient mice and 4 wild-type littermates). Bone marrow cells (107 cells) were injected intravenously into the irradiated mice to rescue their hematopoietic systems. Mice were housed in filter-top cages for 4 weeks to reconstitute the hematopoietic system, after which they were housed conventionally under specific pathogen-free (SPF) conditions for the remainder of the experiment.
Atherosclerosis assessment
Four weeks after transplantation mice were put on the high-fat diet for 10 weeks. Two weeks before humane killing, the animals were fasted overnight, and blood was collected from the tail veins. Plasma cholesterol and triglyceride levels were determined by enzymatic methods (nos. 401 and 337; Sigma Aldrich, Zwijndrecht, The Netherlands). Analysis of the percentages of white blood cells was performed by flow cytometry. Fifty microliters blood was collected and washed with erythrocyte lysis buffer (156 mM NH4Cl, 10 mM NaHCO3). For total leukocyte counts, cells were counted in a standard hemocytometer. Antibody staining for monocytes (Mac1-phycoerythrin [PE]), granulocytes (Gr1-fluorescein isothiocyanate [FITC]), B cells (6B2-PE), and T cells (KT3-FITC) (all from PharMingen, Roosendaal, The Netherlands) was performed in phosphate-buffered saline (PBS) containing 5% normal mouse serum and 2% fetal calf serum. Fourteen weeks after transplantation, the animals were killed. Hearts and aortic arches were removed from the body and bisected perpendicularly to the heart axis, just below the atrial tips. Tissue was frozen in tissue-tec (Shandon, Veldhoven, The Netherlands) with the base facing downward, and sectioning was performed toward the aortic valve area. Sections of 7 μm were collected, starting from where the atrioventricular valves were visible. Aortic lesion areas were quantified using serial cross-sections obtained every 42 μm, beginning at the start of the atrioventricular valves and spanning 250 μm. Six sections were stained with toluidine blue, and lesion areas were quantified using computer-aided morphometry (Scion Image; Scion Corp, Frederick, MD).
Immunohistochemical staining
Lesions from the aortic root were fixed in acetone and incubated with antibodies against macrophages, granulocytes, B cells, and T cells. Antibodies used were FA11, NIMP, 6B2, and KT3, respectively, and all were cultured and purified in house. Detection was performed using a peroxidase-conjugated antirat antibody according to standard procedures.
BM-derived macrophages
To obtain bone marrow (BM)-derived macrophages, hind legs from 2 mice were cleaned thoroughly, and bone marrow from the femurs and tibiae was flushed using ice-cold PBS. The collected bone marrow cells were pooled, washed once in ice-cold PBS, and resuspended in R10-LCM (RPMI supplemented with 10% heat-inactivated fetal calf serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM glutamine [BioWhittaker Europe, Verviers, Belgium], and 15% L929-conditioned medium [LCM]).12 Cells were transferred to 145-mm bacterial plastic culture plates (Greiner Bio-One, Alphen a/d Rijn, The Netherlands) and cultured for 8 days to differentiate them in macrophages. They were then harvested with PBS supplemented with 10 mM EDTA (ethylenediaminetetraacetic acid) and 4 mg/mL lidocaine. All experiments were performed in R10-LCM.
Cytokine secretion
Macrophages were seeded onto bacteriologic plastic 24-well plates (Greiner Bio-One) at 5 × 105 cells/well. Tumor necrosis factor (TNF), interleukin-12 (IL-12), IL-6, IL-10, interferon-γ (IFN-γ), and monocyte chemoattractant protein-1 (MCP1) secretion and expression were quantified in response to treatment with 10 ng/mL lipopolysaccharide (LPS) (O111:B4; Sigma). TNF and IL-12 secretions were assayed in the supernatants by enzyme-linked immunosorbent assay (ELISA; Biosource, Etten-Leur, The Netherlands) according to the manufacturer's instructions. IL-10, IL-6, IFN-γ, and MCP1 secretions were measured by mouse inflammation cytometric bead array (CBA; BD Biosciences, Alphen a/d Rijn, The Netherlands) according to the manufacturer's instructions.
Quantitative gene expression
For gene expression, total RNA was isolated from bone marrow cells using Tri-Reagent (Sigma) according to the manufacturer's instructions. Reverse transcription-polymerase chain reaction (RT-PCR) reactions using 750 ng total RNA were performed using the RevertAid First-Strand cDNA Synthesis Kit (MBI Fermentas, St Leon-Rot, Germany). A standard curve for each amplicon was obtained using serial dilutions of plasmid DNA containing the amplicon. To standardize for the amount of cDNA, the housekeeping gene cyclophilin A was used. Samples were assayed in triplicate in the 7700 Sequence Detector (Applied Biosystems, Nieuwerkerk a/d ijssel, The Netherlands) using quantitative PCR (qPCR) Mastermix (Eurogentec, Seraing, Belgium), 25 ng cDNA, 300 nM each primer, and 200 nM probe. Specific primer/probe sets for CD36, SR-A, IL-6, TNF, IL-12, MCP1, and cyclophilin A were developed with Primer Express version 1.5 (Applied Biosystems) using the default settings. Data were analyzed with the Sequence Detection Software version 1.9 (Applied Biosystems) according to the relative standard curve method (Applied Biosystems).
Uptake of modified LDL and macrophage staining
Uptake of modified LDL was performed with BM-derived macrophages seeded onto bacteriologic plastic 24-well plates at 2.5 × 105 cells/well. Oxidized LDL (oxLDL) was generated and labeled with the fluorescent lipid 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) (Molecular Probes, Leiden, The Netherlands), as described.13 Macrophages were stained with macrophage markers Mac1, Mac3, FA-11, SER-4 and ERTR9 as primary antibodies and as secondary donkey antirat immunoglobulin G (IgG)-PE. Surface marker staining and uptake of modified LDL were quantified by flow cytometry.
Statistical analysis
All statistical analyses were performed using Graphpad Prism (Graphpad Software). Gaussian distributions of the data were established by using the Kolmogorov-Smirnov test. Differences between 2 groups were evaluated using a Welch corrected t test, unless stated otherwise. Values are represented as mean ± SD. A P value of less than .05 was considered statistically significant.
Results
Mice with NF-κB1-deficient hematopoietic cells develop smaller atherosclerotic lesions
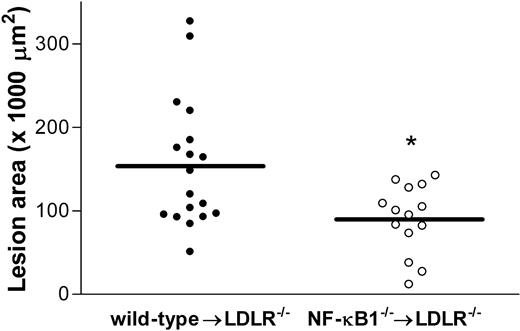
To examine the role of p50 in atherogenesis, bone marrow transplantation was performed to generate atherosclerosis-susceptible LDL knockout mice (LDLR-/-) with NF-κB1-deficient hematopoietic cells. After a 4-week period to allow engraftment of the hematopoietic system, mice were fed a high-fat diet for 10 weeks. Plasma cholesterol and triglyceride levels were analyzed after 8 weeks, and no differences were found between NF-κB1-/- and wild-type mice that underwent transplantation (Table 1). In addition, blood leukocytes were analyzed using flow cytometry, and relative percentages of T cells, B cells, monocytes, and granulocytes were similar between the 2 groups (Table 1), as were total number of leukocytes (data not shown). After 10 weeks on the diet, the mice were killed for atherosclerosis analysis. The extent of atherosclerosis was determined by measuring the lesion size in the aortic root. Lesion area quantification revealed that lesions of the NF-κB1-deficient mice were 41% smaller than in the control group (Figure 1). NF-κB1-deficient mice had a lesion area of 95.4 ± 10.3 × 103 μm (n = 14) compared with 160.1 ± 21.08 × 103 μm (n = 18) for the wild type (P < .01). Thus, NF-κB1 deficiency in hematopoietic cells decreases atherosclerotic lesion formation.
Plasma cholesterol and triglyceride levels and blood leukocyte percentages
. | Wild type . | NF-κB1−/− . |
|---|---|---|
| Cholesterol, mM | 20.7 ± 1.6 | 19.8 ± 1.5 |
| Triglycerides, mM | 1.0 ± 0.4 | 1.0 ± 0.3 |
| B cells, % | 35.6 ± 54.4 | 33.4 ± 7.2 |
| T cells, % | 34.6 ± 6.2 | 30.8 ± 5.8 |
| Monocytes, % | 13.4 ± 6.3 | 10.7 ± 3.0 |
| Granulocytes, % | 7.6 ± 4.0 | 8.2 ± 4.1 |
. | Wild type . | NF-κB1−/− . |
|---|---|---|
| Cholesterol, mM | 20.7 ± 1.6 | 19.8 ± 1.5 |
| Triglycerides, mM | 1.0 ± 0.4 | 1.0 ± 0.3 |
| B cells, % | 35.6 ± 54.4 | 33.4 ± 7.2 |
| T cells, % | 34.6 ± 6.2 | 30.8 ± 5.8 |
| Monocytes, % | 13.4 ± 6.3 | 10.7 ± 3.0 |
| Granulocytes, % | 7.6 ± 4.0 | 8.2 ± 4.1 |
Fasting cholesterol and triglyceride levels and relative levels of B cells, T cells, monocytes, and granulocytes were determined in wild-type and NF-κB1−/− transplanted LDLR−/− mice after 8 weeks on a high-fat diet. No significant differences were observed.
Atherosclerosis is reduced in NF-κB1 transplanted LDLR-/- mice compared with controls. Atherosclerotic lesion area in wild-type (•) and NF-κB1-/- (○) transplanted LDLR-/- mice. Individual circles represent individual mice; n = 18 and n = 14 for wild-type and knockout mice, respectively. Mean of the 2 groups is indicated. *P < .01.
Atherosclerosis is reduced in NF-κB1 transplanted LDLR-/- mice compared with controls. Atherosclerotic lesion area in wild-type (•) and NF-κB1-/- (○) transplanted LDLR-/- mice. Individual circles represent individual mice; n = 18 and n = 14 for wild-type and knockout mice, respectively. Mean of the 2 groups is indicated. *P < .01.
NF-κB1-deficient lesions show increased inflammation
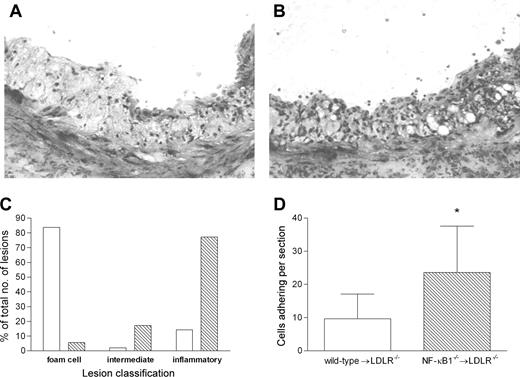
Pathologic examination of the lesions revealed a striking difference between the NF-κB1-deficient mice and the wild-type mice that underwent transplantation. Toluidine staining showed that lesions in NF-κB1 animals that underwent transplantation contained a greater number of small inflammatory cells (Figure 2A-B). To quantify this difference, 3 types of lesions were distinguished: (1) normal atherosclerotic lesions primarily containing large lipid-laden foam cells; (2) intermediate lesions containing small inflammatory cells and large foam cells; and (3) lesions containing large numbers of small cells but no foam cells and resembling an inflammatory infiltrate. The amount of each type is depicted as the percentage of the total lesions (Figure 2C). It is clear that there is a dramatic shift toward the inflammatory plaque phenotype in the NF-κB1-deficient group. Lesions in the NF-κB1-/- mice that underwent transplantation are further characterized by a 3-fold increase in the adherence of cells to the cap of the lesion (Figure 2D).
Lesions in LDLR-/- mice transplanted with NF-κB1-/- bone marrow have a more inflammatory phenotype. Representative lesions from wild-type (A) or NF-κB1-/- (B) transplanted mice showing clear cellular differences. Toluidin staining; original magnification, × 200. (C) Lesion classification was categorized as 3 groups: foam cell lesions containing mainly large foam cells; inflammatory lesions containing large numbers of small cells resembling an inflammatory cellular infiltrate; and an intermediate group. Lesion type for wild-type (□) and NF-κB1-/- (▧) transplanted mice is shown as a percentage of the total lesions. All 3 groups were analyzed by Fisher exact test, and a significant shift toward inflammatory lesions was observed in NF-κB1-/- transplanted mice (P < .0001). (D) Cell adherence at the cap of the lesion was counted and indicated as the average number of cells per lesion. *P < .01. Error bars indicate SD.
Lesions in LDLR-/- mice transplanted with NF-κB1-/- bone marrow have a more inflammatory phenotype. Representative lesions from wild-type (A) or NF-κB1-/- (B) transplanted mice showing clear cellular differences. Toluidin staining; original magnification, × 200. (C) Lesion classification was categorized as 3 groups: foam cell lesions containing mainly large foam cells; inflammatory lesions containing large numbers of small cells resembling an inflammatory cellular infiltrate; and an intermediate group. Lesion type for wild-type (□) and NF-κB1-/- (▧) transplanted mice is shown as a percentage of the total lesions. All 3 groups were analyzed by Fisher exact test, and a significant shift toward inflammatory lesions was observed in NF-κB1-/- transplanted mice (P < .0001). (D) Cell adherence at the cap of the lesion was counted and indicated as the average number of cells per lesion. *P < .01. Error bars indicate SD.
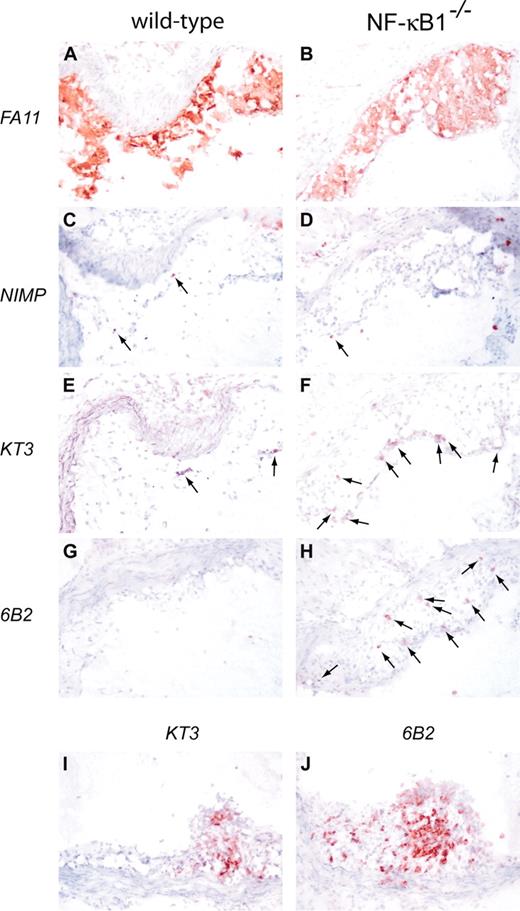
Qualitative differences between the NF-κB1-/- and wild-type plaques were analyzed by staining lesions for macrophages, granulocytes, T cells, and B cells (Figure 3). Knockout and wild-type lesions consist mainly of macrophages, with virtually no granulocytes (Figure 3A-D). Interestingly, lesions from the NF-κB1-/- transplantation group showed relatively large numbers of T cells and B cells (Figure 3E-H). Lymphocyte content of the lesions after transplantation was 2.9% ± 1.0% in wild-type animals and 16.5% ± 2.6% in NF-κB1-/- mice (P < .05). In 4 animals of the NF-κB1-/- group, even an occasional tertiary lymphoid structure could be detected (Figure 3I-J), characterized by the organization of B cells and T cells reminiscent of lymphoid organs.14
Qualitative analysis of atherosclerotic lesions from wild-type and NF-κB1-/- transplanted LDLR-/- mice. Atherosclerotic lesions from wild-type (A, C, E, G) and NF-κB1-/- (B, D, F, H) transplanted mice were stained for macrophages (A-B), granulocytes (C-D), T cells (E-F), and B cells (G-H). Arrows indicate positive cells. Occasionally, a tertiary lymphoid organization was found in NF-κB1-/- transplanted mice stained for T cells (I) and B cells (J). Hematoxylin counterstained; original magnification × 100.
Qualitative analysis of atherosclerotic lesions from wild-type and NF-κB1-/- transplanted LDLR-/- mice. Atherosclerotic lesions from wild-type (A, C, E, G) and NF-κB1-/- (B, D, F, H) transplanted mice were stained for macrophages (A-B), granulocytes (C-D), T cells (E-F), and B cells (G-H). Arrows indicate positive cells. Occasionally, a tertiary lymphoid organization was found in NF-κB1-/- transplanted mice stained for T cells (I) and B cells (J). Hematoxylin counterstained; original magnification × 100.
NF-κB1 deficiency in macrophages leads to altered cytokine secretion
To study the in vitro phenotype of NF-κB1-deficient macrophages, BM-derived macrophages from NF-κB1-deficient and wild-type mice were cultured. Macrophage yields of both genotypes were identical (not shown), and macrophage differentiation, analyzed by macrophage-specific differentiation markers, showed no significant differences (Figure 4A). BM-derived macrophages from NF-κB1-/- and wild-type mice were stimulated with LPS for different times. Cell death after 24-hour LPS stimulation was quantified by propidium iodide staining and showed low levels of dead cells, with some increases in NF-κB1-/- macrophages (7.7% ± 1.4% vs 12.5% ± 3.3% in wild-type and NF-κB1-/- cells, respectively). TNF secretion clearly showed that there were no severe differences in the initial TNF response (Figure 4B, 2 hours and 8 hours). However, TNF secretion at later time points remained higher in the NF-κB1-/- macrophages than in wild-type macrophages (Figure 4B, 17 hours and 24 hours). RNA analysis revealed the same pattern of prolonged TNF expression in the NF-κB1-/- macrophages as measured by qPCR (Figure 4C). Intracellular cytokine staining after 3 hours of LPS stimulation also showed identical TNF production (not shown). Measuring other cytokines revealed a decrease in MCP1, IL-6, and IL-12 and an increase in IL-10 and IFN-γ secretion by the NF-κB1-/- macrophages (Figure 4D). These differences were found at several time points (3, 6, and 24 hours) except for IFN-γ, which only showed induction after 24 hours in NF-κB1-/- cells. Expression analysis by qPCR showed similar results (not shown).
In vitro characterization of NF-κB1-/- and wild-type macrophages. (A) Expression of macrophage differentiation markers was quantified by flow cytometry after staining for the indicated markers. (B) Secretion of TNF by wild-type and NF-κB1-/- macrophages after stimulation with LPS (10 ng/mL) for the indicated times. (C) TNF expression of LPS (10 ng/mL)-stimulated wild-type and NF-κB1-/- macrophages, assessed by qPCR. (D) Secretion of cytokines by wild-type and NF-κB1-/- macrophages after LPS (10 ng/mL) stimulation for 6 hours (MCP1, IL-6, IL-12, IL10) or 24 hours (IFN-γ). Bars represent wild-type (□) and NF-κB1-/- (▧) BM-derived macrophages. (A-B) Representative for 3 experiments. (C-D) Representative for 2 experiments. Error bars indicate SD.
In vitro characterization of NF-κB1-/- and wild-type macrophages. (A) Expression of macrophage differentiation markers was quantified by flow cytometry after staining for the indicated markers. (B) Secretion of TNF by wild-type and NF-κB1-/- macrophages after stimulation with LPS (10 ng/mL) for the indicated times. (C) TNF expression of LPS (10 ng/mL)-stimulated wild-type and NF-κB1-/- macrophages, assessed by qPCR. (D) Secretion of cytokines by wild-type and NF-κB1-/- macrophages after LPS (10 ng/mL) stimulation for 6 hours (MCP1, IL-6, IL-12, IL10) or 24 hours (IFN-γ). Bars represent wild-type (□) and NF-κB1-/- (▧) BM-derived macrophages. (A-B) Representative for 3 experiments. (C-D) Representative for 2 experiments. Error bars indicate SD.
NF-κB1 deficiency in activated macrophages leads to reduced endocytosis of modified lipoproteins and decreased expression of scavenger receptor class A
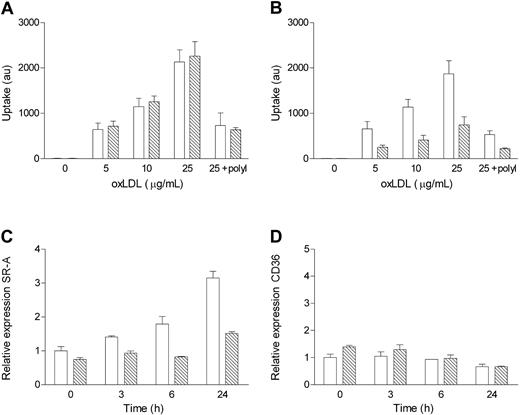
Because of the absence of large foam cells in the lesions of NF-κB1-deficient mice that underwent transplantation, we hypothesized that the uptake of modified lipoproteins by these macrophages was affected. Therefore, we quantified the uptake of oxLDL by BM-derived macrophages from NF-κB1 and wild-type mice. The uptake of oxidized lipoproteins was similar between NF-κB1-deficient and wild-type macrophages if the cells were not stimulated (Figure 5A). However, overnight treatment with LPS (10 ng/mL) greatly reduced the uptake of oxLDL in NF-κB1-/- macrophages (Figure 5B). As a control, polyI was added for scavenger-receptor-dependent uptake15 and for blocking endocytosis of oxLDL. Moreover, overnight activation of macrophages with IFN-γ and TNF also reduced the uptake of oxLDL by NF-κB1-/- macrophages (1329 ± 83 vs 794 ± 57 in wild-type and NF-κB1-/- cells, respectively; P < .01). Thus, on activation, NF-κB1-/- macrophages are greatly hampered in their uptake of modified LDL. To elucidate which receptors were responsible for the difference in uptake of modified LDL, the expression of scavenger receptor class A (SR-A) and CD36 was assessed by quantitative PCR. Wild-type macrophages showed a strong increase in SR-A expression in response to LPS treatment (Figure 5C). Interestingly, the increase was severely impaired in NF-κB1-/- macrophages, resulting in a 2-fold difference after 24 hours. CD36 expression decreased to some extent on LPS activation, but no major differences between wild-type and NF-κB1-/- cells were observed (Figure 5D).
In vitro characterization of oxLDL uptake by NF-κB1-/- and wild-type macrophages. (A) Uptake of DiI-labeled oxLDL by wild-type and NF-κB1-/- macrophages. (B) Uptake of DiI-labeled oxLDL by wild-type and NF-κB1-deficient macrophages stimulated overnight with LPS before uptake. Expression of (C) scavenger receptor class A (SR-A) and (D) CD36 after indicated times of activation with LPS (10 ng/mL) was quantified using qPCR. Bars represent wild-type (□) and NF-κB1-/- (▧)) BM-derived macrophages. (A-B) Representative for 3 experiments. (C-D) Representative for 2 experiments. Error bars indicate SD.
In vitro characterization of oxLDL uptake by NF-κB1-/- and wild-type macrophages. (A) Uptake of DiI-labeled oxLDL by wild-type and NF-κB1-/- macrophages. (B) Uptake of DiI-labeled oxLDL by wild-type and NF-κB1-deficient macrophages stimulated overnight with LPS before uptake. Expression of (C) scavenger receptor class A (SR-A) and (D) CD36 after indicated times of activation with LPS (10 ng/mL) was quantified using qPCR. Bars represent wild-type (□) and NF-κB1-/- (▧)) BM-derived macrophages. (A-B) Representative for 3 experiments. (C-D) Representative for 2 experiments. Error bars indicate SD.
Discussion
In this paper we studied the role of NF-κB1 in hematopoietic cells in the development of atherosclerosis. We observed a striking decrease in lesion size in LDLR-/- mice that received NF-κB1-/- bone marrow compared with control bone marrow (Figure 1). Moreover, when the lesions of the 2 groups were compared qualitatively, we found remarkable differences. NF-κB1-/- lesions do not show the predominant presence of characteristic foam cells but instead contain large numbers of small cells with a highly inflammatory appearance (Figure 2A-C). In addition, increased numbers of T cells and B cells were found in the lesions (Figure 3).
The phenotype of the lesions could be the result of an increase in systemic inflammation.11 However, flow cytometry of leukocytes showed that relative leukocyte percentages in the blood were identical between the 2 groups (Table 1). In addition, immunohistochemical analysis of spleens did not reveal any major changes in cellular composition or in other signs of inflammation (not shown). This was also true for the adventitia of the vessels in which atherosclerosis was analyzed. In contrast, levels of soluble TNF receptor 55 (TNFR55) and TNFR75 in the sera of mice that underwent transplantation were even decreased in the NF-κB1-/- mice (data not shown). Interestingly, minor differences were observed in the liver macrophages—their numbers were slightly increased, and they were smaller—a mild reflection of what we observed in the atherosclerotic lesions. No differences in plasma cholesterol and triglyceride levels were found that could account for the reduction in foam cells (Table 1). An additional observation in the NF-κB1-/- lesions was the increased adhesion of cells to the cap of the lesion (Figure 2D). This adhesion could indicate an increased attraction of blood leukocytes to the inflamed site because of the differential expression of chemokines and cytokines. Thus, our results show increased inflammation in the NF-κB1-deficient lesions and reduced lesion size. The increased inflammation does not reflect the general health status of the mice because leukocyte percentages and immunohistochemical analysis of organs from the 2 groups show similar results.
The absence of the characteristic foam cells in NF-κB1-/- lesions led us to question whether NF-κB1-/- macrophages were altered in their ability to take up modified lipoproteins. Endocytosis of labeled modified lipoproteins was identical between NF-κB1-/- and wild-type BM-derived macrophages, and there were no differences in cell death in oxLDL-treated macrophages, nor did oxLDL induce the secretion of any of the cytokines studied (data not shown). Interestingly, on stimulation of the NF-κB pathway with LPS, uptake was severely reduced in NF-κB1-/- macrophages compared with activated wild-type controls (Figure 5A-B). Modified lipoproteins are taken up by scavenger receptors. The increase in the expression of SR-A normally seen in LPS-treated wild-type cells16 was hampered in the NF-κB1-/- macrophages. In contrast, there were no strong differences in CD36 expression, which was decreased in response to LPS, as was described previously.17,18 These data indicate that the reduced uptake is likely the result of a difference in SR-A expression. Whether this effect is direct (the SR-A promoter is dependent on p50) or indirect (through differential activation of the macrophages) remains to be elucidated. Because atherosclerotic lesions are regarded as sites of chronic inflammation, we speculate that macrophages are activated19 that may lead to a reduced uptake of modified lipoproteins in NF-κB1-/- lesions. In the vessel wall, the reduced removal of pathogenic lipoproteins may lead to an increased inflammatory response because of the modified lipids,20,21 resulting in the observed inflammatory phenotype of the lesions; however, this remains a matter of speculation.
P50 has a complex role in the NF-κB pathway. It dimerizes with RelA to form the most abundant dimer, well studied for its role in cellular activation. However, p50 can also form homodimers that lack transactivation activity and that thereby play a repressive role in cellular activation.9 It has been shown that p50 can shut down the expression of TNF after the cell is activated.8,22 This also implies a role for p50 in LPS tolerance.23 In agreement with these studies, we observed increased TNF expression in our NF-κB1-/- macrophages after stimulation with LPS. In contrast to the prolonged elevation of TNF, cytokines IL-6 and IL-12 and the chemokine MCP1 all showed a reduction in the NF-κB1-/- macrophages. IFN-γ and the anti-inflammatory cytokine IL-10 were increased in the NF-κB1-/- macrophages. These data show that NF-κB1-/- macrophages have a disturbed cytokine balance. Whether this results directly in the smaller size and the more inflammatory phenotype of the lesions remains to be elucidated.
Results obtained from the NF-κB1-/- mice with regard to the role of NF-κB1 in inflammatory disease models show diverse results. In a model for inflammatory arthritis, the NF-κB1-/- mice did not develop the disease, but in a model for colitis it was shown that NF-κB1-/- mice developed more severe colitis.24,25 These findings imply that p50 plays a specific role in different inflammatory diseases, of which the exact mechanisms are still poorly understood. The deletion of genes in the NF-κB pathway led to a diversity of phenotypes and opposing results in disease models. We found that disrupting NF-κB activation by deleting IκB kinase 2 (IKK2) in macrophages aggravated atherosclerosis.26 IKK2 is mainly involved in cellular activation by proinflammatory stimuli,27 whereas p50 might be a player at different levels. It mediates NF-κB activation as a partner in the most abundant NF-κB dimer, p65-p50. However, as also discussed earlier in this section, p50 can have an opposite role and may, especially in macrophages, be involved in repressing cellular activation.8,9 Furthermore, deleting p50 may lead to the differential use of other NF-κB subunits, also changing NF-κB activation. The exact mechanisms regulating inflammatory responses in the absence of p50 are poorly understood. Deleting other players in the NF-κB pathway may well result in a better understanding of the role of NF-κB in atherosclerosis.
Although macrophages are the major cell type in early lesions and are key players in the atherosclerotic process, p50 is also absent in B cells and T cells after bone marrow transplantation. The effects of p50 deficiency on lymphocytes have been documented. Under normal conditions, p50 knockout mice are not affected in the phenotypes and numbers of B and T cells, and analysis of lymphoid organs shows no differences compared with wild-type cells.11 These results indicate that p50 does not play a role in the general development of T or B cells. However, Cariappa et al28 later showed that the marginal zone B cells were absent in mice lacking p50. Proliferative responses have also been studied. The proliferation of the NF-κB1-/- B cells is defective in response to LPS and CD40 activation but normal in response to antigen receptor cross-linking.29 Besides the defect in proliferation, NF-κB1-/- B cells also undergo defective immunoglobulin secretion and class switching and increased cell death, which could lead to an observed increased turnover of knockout B cells.11,29,30 T cells of NF-κB1-/- animals also undergo defective proliferation with or without stimulation24,31 and may experience a slight increase in spontaneous cell death.31 In a model for experimental autoimmune encephalomyelitis, splenocytes from immunized mice also showed a reduced proliferation.32 Because lymphocytes have been implicated in atherosclerosis,33 it cannot be ruled out that these cells may act in concert with macrophages, leading to our results. Future experiments will focus on the role of NF-κB1 in B- and T-cell proliferation and cytokine production in atherosclerotic plaque development.
In conclusion, using bone marrow transplantation we have shown that NF-κB1 plays a crucial role in atherosclerosis. Our findings indicate that NF-κB1 affects lipid uptake and inflammation during atherogenesis. The observed reduction in lesion size could be the result of reduced lipoprotein uptake by macrophages in the absence of NF-κB1. In addition, modified lipoproteins remaining in the vessel wall might act in a proinflammatory manner and cause the inflammatory phenotype of the lesions. Moreover, p50 deficiency could also lead to altered cytokine production in the atherosclerotic lesion. The reduced removal of modified lipids and a changed secretion of inflammatory factors may result in a different cytokine milieu, leading to the inflammatory lesions we observed. Determining whether a more proinflammatory phenotype of the plaque is good or bad for eventual clinical outcome and plaque stability will be the focus of future experiments.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-05-1450.
Supported by the Dutch Organization for Scientific Research (NWO 902-26-194) and the European Union (MAFAPS-QLG1-99-001007). M.H.H. is an established investigator of the Dutch Heart Association (NHS D95022). M.P.J. de W. is an NWO fellow (906-02-075).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr B. de Vries for help with soluble TNFR assays.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal