Abstract
A plausible mechanism to explain thrombotic risk differences associated with the use of second- and third-generation oral contraceptives (OCs), particularly in carriers of factor VLeiden, is still lacking. In a double-blind trial, 51 women without and 35 women with factor VLeiden were randomized to either a second- (30 μg ethinylestradiol/150 μg levonorgestrel) or third- (30 μg ethinylestradiol/150 μg desogestrel) generation OC. After 2 cycles of use and a wash-out of 2 cycles, the participants continued with the corresponding progestagen-only preparation. Hemostatic variables that probe the activity of the anticoagulant protein C system were determined. Compared with levonorgestrel, desogestrel-containing OCs significantly decreased protein S and increased activated protein C (APC) resistance in both groups. OCs with desogestrel had the most pronounced effects in carriers of factor VLeiden. Progestagen-only preparations caused changes of anticoagulant parameters opposite to those of combined OCs, which in a number of cases were more pronounced with levonorgestrel. Our data show that progestagens in combined OCs counteract the thrombotic effect of the estrogen component. The higher thrombotic risk associated with third-generation OCs compared with second-generation OCs may be explained by the fact that desogestrel appeared less antithrombotic than levonorgestrel, especially in women with factor VLeiden. (Blood. 2004;103:927-933)
Introduction
Epidemiologic studies clearly demonstrated an association between the use of oral contraceptives (OCs) and an increased risk of venous1-3 and arterial thrombosis.4-6 Over the years, there have been many important changes in the composition and use of these preparations to reduce the vascular and metabolic side effects. In the early 1980s, third-generation oral contraceptives, containing the progestagens desogestrel or gestodene, were developed in an attempt to reduce the risk of cardiovascular diseases and to decrease androgenic side effects such as weight gain, acne, and adverse changes in metabolism of lipoprotein. However, in 1995 and 1996, 4 studies reported that women who used so-called third-generation oral contraceptives containing desogestrel or gestodene were at higher risk of venous thromboembolism than users of oral contraceptives with the second-generation progestagen, levonorgestrel.7-10 The absolute risk of venous thrombosis associated with the use of particularly third-generation oral contraceptives is further elevated among carriers of the factor VLeiden mutation,9-11 a hereditary disorder in which activated factor V is inactivated by activated protein C (APC) at a lower rate than normal factor Va.12-14 This hereditary defect, also called APC resistance, is a common risk factor for venous thrombosis, which is associated with a 3- to 7-fold increased risk in heterozygous individuals.12-14
A plausible biologic mechanism for the thrombotic effects of second- and third-generation oral contraceptives is still lacking. Rosing et al15,16 reported that oral contraceptive use leads to acquired APC resistance. Women using third-generation oral contraceptives were more resistant to the anticoagulant action of APC than users of second-generation oral contraceptives. Besides that, they reported that the effects of oral contraceptives and factor VLeiden on the APC sensitivity ratio (a hemostatic variable that quantifies the efficacy with which APC down-regulates thrombin generation in plasma; APC-sr) were additive.15 The APC resistance test used in these studies has since then been clinically validated,17 lending support to the proposal that the more pronounced acquired APC resistance observed in users of third-generation OCs may explain why they are exposed to a higher thrombotic risk than second-generation OC users.
A recent cross-over study indicated that second- and third-generation oral contraceptives not only cause differences in acquired APC resistance, but also have different effects on a large number of other procoagulant, anticoagulant, and fibrinolytic parameters.18-20 Particularly, the differential changes of the proteins involved in the protein C system may contribute to the thrombotic effects of oral contraceptives.18 The protein C system, which comprises the plasma proteins protein C and protein S, down-regulates in vivo blood coagulation14 via proteolytic inactivation of coagulation factor Va and VIIIa. Protein S, which acts as cofactor of APC, also exhibits anticoagulant activity independent of APC by directly inhibiting thrombin formation.15 The anticoagulant activity of protein S is modulated by C4b-binding protein, which binds some 60% of plasma protein S.16,18 The physiologic importance of the protein C system is illustrated by the fact that defects in this pathway are associated with an increased risk of venous thromboembolism.21-23 The thrombotic risk of women with hereditary defects in the protein C pathway is enhanced by oral contraceptive use.11
For a long time, the increased thrombotic risk associated with oral contraceptive use was attributed to the estrogen component. Because low-dose second- and third-generation oral contraceptives contain the same estrogen dose, the differences may reflect a progestagen-specific effect. Although it has been proposed that progestagens have estrogen-like effects,24 among others on hemostasis,25 there are as yet no data on the influence of levonorgestrel or desogestrel in the absence of the estrogen component on the protein C pathway.
To gain more insight in the biologic basis of an enhanced risk of venous thrombosis in oral contraceptive users, particularly in carriers of factor VLeiden, we performed a double-blind randomized trial in which we determined the effects of second- and third-generation progestagens, alone or in combination with estrogens, on the protein C pathway in the absence or presence of the factor VLeiden mutation.
Patients, materials, and methods
Study design and participants
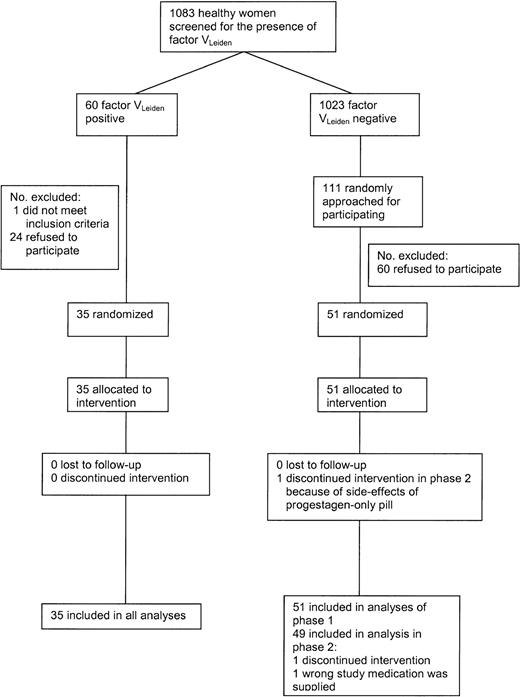
Figure 1 shows the flow of the participants during the study. In 1998, 1083 female students and employees of Utrecht University aged 18 to 40 years were screened for the presence of the factor VLeiden mutation. Of the women, 60 (5.5%) were heterozygous carriers of the factor VLeiden mutation. These women and 111 women without factor VLeiden were approached for the trial; 36 women with and 51 women without VLeiden agreed to participate and were tested whether or not they met the exclusion criteria. Exclusion criteria included the following: a history of any malignant disorder; cardiovascular, cerebrovascular, hepatic or renal diseases; venous thrombosis; epilepsy or classical migraine; vaginal bleeding of unknown etiology; pregnancy; rheumatoid arthritis; diabetes mellitus; psychiatric disorders; alcohol abuse or drug abuse within the last 12 months; heavy smoking; excessive obesity; chronic infectious diseases; and any other serious disease. Moreover, volunteers with a contraindication to estrogens and/or progestagens were excluded. One woman with VLeiden was excluded because of a psychiatric disorder. From the women without factor VLeiden no one met the exclusion criteria.
One participant was excluded from phase 2 because the wrong medication was supplied. Another woman withdrew consent in phase 2 because of side effects of the progestagen-only pill (symptoms of depression). Both were factor VLeiden-negative. The trial started in January 1999 and ended in October 1999.
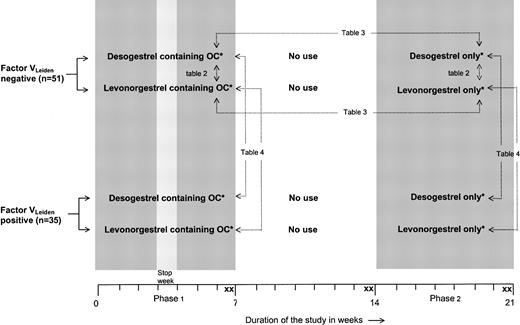
Figure 2 illustrates the design of the trial. Women with and without factor VLeiden were randomly assigned to 1 of 2 different combination pills containing either 30 μg ethinyloestradiol and 150 μg levonorgestrel or 30 μg ethinyloestradiol and 150 μg desogestrel. These formulations are identical to those commercially marketed. The oral contraceptives were used for a period of 2 menstruation cycles (phase 1). After a wash-out of 2 menstrual periods, the participants were treated with a corresponding progestagen-only preparation containing either 150 μg levonorgestrel or 150 μg desogestrel. These progestagens were used for a period of 2 menstruation cycles without a stop week to provide full contraceptive safety (phase 2). The study medication was labeled in blocks of 10 according to a randomization list generated by the medication manufacturer. Participants were enrolled and assigned to their groups by the investigators. Participants, those administering the intervention, and those assessing the outcomes were not aware of group assignment. There were 2 blood samples taken at the end of phase 1 (days 47 ± 2 and 49 ± 2), at the end of the wash-out period (days 101 ± 2 and 105 ± 2), and at the end of phase 2 (days 152 ± 2 and 154 ± 2). We used duplicate sampling to reduce random measurement error. For reasons of safety, ultrasound examination of the veins of both legs was performed before the start of the study, after the wash-out period, and at the end of phase 2, to exclude venous thrombosis.
Trial profile.*Desogestrel-containing OCs, 30 μg ethinylestradiol + 150 μg desogestrel; levonorgestrel-containing OCs, 30 μg ethinylestradiol + 150 μg levonor gestrel; desogestrel only, 150 μg desogestrel; and levonorgestrel only, 150 μg levonorgestrel. X indicates blood sampling. → indicates comparisons described in the tables. Comparisons referring to Tables 2-3 are also made for women with the factor VLeiden mutation.
Trial profile.*Desogestrel-containing OCs, 30 μg ethinylestradiol + 150 μg desogestrel; levonorgestrel-containing OCs, 30 μg ethinylestradiol + 150 μg levonor gestrel; desogestrel only, 150 μg desogestrel; and levonorgestrel only, 150 μg levonorgestrel. X indicates blood sampling. → indicates comparisons described in the tables. Comparisons referring to Tables 2-3 are also made for women with the factor VLeiden mutation.
A power calculation showed that if 25 women were allocated in each treatment group, assuming an APC-sr of 380 nM/minute ± 50 in healthy individuals, a differences in APC-sr of 40 nM/minute or more could be detected (α = 0.05; β = 0.20). This difference is enough to achieve statistical significance in the APC-sr calculation. With this sample size, we also expected to be able to demonstrate differences in the other hemostatic variables.
Before start of the study, all selected women were informed about the aims and potential risks of the study. All participants gave written informed consent. The study was approved by the Ethics Committee of the University Medical Center Utrecht.
Laboratory methods
All blood samples were drawn in the morning after an overnight abstinence from intake of food, caffeine, alcohol, or nicotine. Cell-free, citrated plasma was prepared and centrally stored at -80°C. Anticoagulant parameters were determined after all participants had completed the treatment regimen and were done in duplicate.
All commercially available assays were carried out according to the manufacturer's instructions. Protein C was determined using the Coamatic protein C activity kit from Chromogenix (Mölndal, Sweden). Total protein S antigen was assayed by an enzyme-linked immunosorbent assay (ELISA) using antibodies from DAKO (Glostrup, Denmark). Free protein S was measured by precipitating the C4b-binding protein-bound fraction with polyethylene glycol 8000 and measuring the concentration of free protein S in the supernatant. C4b-binding protein antigen levels were determined by enzyme-linked immunosorbent assay using a combination of monoclonal antibodies against C4b-binding protein (8C11 and horseradish peroxidase-labeled 9H10). The APC-independent anticoagulant activity of protein S (PSAPCind) was determined as described by van Wijnen et al26 with 2 modifications. To avoid contamination with endogenous phospholipids, all plasma samples were centrifuged for 10 minutes at 13 000g at 20°C. Plasma aliquots taken from the bottom of the tube were used for the assay. Recombinant human tissue (Innovin; Dade Behring, Marburg, Germany) diluted 100-fold was used to initiate coagulation. Antigen levels of protein C inhibitor (PCI) were determined by ELISA using a monoclonal antibody against PCI (API-93) as capturing antibody and rabbit polyclonal anti-PCI serum as secondary antibody.27 APC sensitivity ratios (APC-sr) were determined by quantifying the effect of APC on thrombin generation as described before.18 The plasma levels of the proteins determined (antigen or activity) were expressed as percentage of that present in normal pooled plasma determined in the same experiment. The presence of the factor VLeiden mutation was assessed by DNA analysis.28
All tests were performed without knowledge of the carrier status of the individual and the oral contraceptive preparation used.
Statistical analysis
To reduce random measurement error, the results of the 2 visits at the end of phase 1, the wash-out period, and phase 2 were averaged. If only one sample was available, the values obtained for that sample were used in the analysis.
According to the design of the trial (Figure 1), 3 comparisons were made in the data analysis and represented in the corresponding tables. To assess the effect of progestagens in combined oral contraceptives, mean differences in anticoagulant parameters between the 2 treatment groups relative to values when no oral contraceptives were used were tested with unpaired t tests for noncarriers and carriers of factor VLeiden separately.
The effect of combined oral contraceptives (progestagens plus estrogen) versus progestagen-only preparations was calculated by testing differences in means between phase 1 and phase 2 using paired t tests. Again, these analyses were performed separately for noncarriers and carriers of factor VLeiden.
Differential effects of a hormone preparation between women with and without the factor VLeiden mutation were assessed from the mean change in anticoagulant parameters in the period of oral contraceptive use relative to no use using unpaired t tests.
For all estimates 95% confidence intervals were calculated.
In addition to the parametric t tests we also performed nonparametric tests, which yielded similar results to the parametric ones.
Results
Characteristics of the volunteers participating in the study
No clinically relevant differences in general characteristics were present between the study groups (Table 1). The values of the anticoagulant parameters determined at the various stages in the trial are summarized in Table 2. During the study none of the volunteers showed clinical signs or symptoms of venous thromboembolism or abnormalities on ultrasound examination of the leg veins.
Baseline characteristics according to factor VLeidenand type of oral contraceptive (OC)
. | Factor VLeiden-negative . | . | Factor VLeiden-positive . | . | ||
|---|---|---|---|---|---|---|
| Characteristic . | Levonorgestrel-containing OC, n = 24 . | Desogestrel-containing OC, n = 27 . | Levonorgestrel-containing OC, n = 19 . | Desogestrel-containing OC, n = 16 . | ||
| Age, y | 22.9 ± 3.4 | 24.1 ± 5.9 | 23.3 ± 2.7 | 22.6 ± 2.8 | ||
| Body weight, kg | 67.6 ± 10.6 | 67.6 ± 9.5 | 64.0 ± 7.9 | 65.9 ± 10.6 | ||
| Height, cm | 168.6 ± 5.5 | 170.9 ± 6.5 | 169.2 ± 6.4 | 171.5 ± 6.7 | ||
| BMI, kg/m2 | 23.4 ± 3.4 | 23.2 ± 3.4 | 22.3 ± 2.5 | 22.4 ± 3.5 | ||
| Previous OC use | 19 (79) | 20 (74) | 15 (79) | 15 (94) | ||
| Second generation | 5 (26) | 9 (45) | 6 (40) | 3 (20) | ||
| Third generation | 13 (68) | 5 (25) | 9 (60) | 5 (33) | ||
| Others | 1 (5) | 6 (30) | 0 | 7 (47) | ||
. | Factor VLeiden-negative . | . | Factor VLeiden-positive . | . | ||
|---|---|---|---|---|---|---|
| Characteristic . | Levonorgestrel-containing OC, n = 24 . | Desogestrel-containing OC, n = 27 . | Levonorgestrel-containing OC, n = 19 . | Desogestrel-containing OC, n = 16 . | ||
| Age, y | 22.9 ± 3.4 | 24.1 ± 5.9 | 23.3 ± 2.7 | 22.6 ± 2.8 | ||
| Body weight, kg | 67.6 ± 10.6 | 67.6 ± 9.5 | 64.0 ± 7.9 | 65.9 ± 10.6 | ||
| Height, cm | 168.6 ± 5.5 | 170.9 ± 6.5 | 169.2 ± 6.4 | 171.5 ± 6.7 | ||
| BMI, kg/m2 | 23.4 ± 3.4 | 23.2 ± 3.4 | 22.3 ± 2.5 | 22.4 ± 3.5 | ||
| Previous OC use | 19 (79) | 20 (74) | 15 (79) | 15 (94) | ||
| Second generation | 5 (26) | 9 (45) | 6 (40) | 3 (20) | ||
| Third generation | 13 (68) | 5 (25) | 9 (60) | 5 (33) | ||
| Others | 1 (5) | 6 (30) | 0 | 7 (47) | ||
Values are means ± SD or numbers with percentage in parentheses. BMI indicates body mass index.
Effects of combined oral contraceptives (OCs) and progestagen-only preparations on anticoagulant parameters in the absence or presence of the factor VLeidenmutation
. | Levonorgestrel-containing OC . | . | Desogestrel-containing OC . | . | Mean difference in change (95% CI) . | Levonorgestrel only . | . | Desogestrel only . | . | Mean difference in change (95% CI)‡ . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No use, mean ± SD . | OC use, mean ± SD . | No use, mean ± SD . | OC use, mean ± SD . | . | No use,†mean ± SD . | OC use, mean ± SD . | No use, mean ± SD . | OC use, mean ± SD . | . | ||||
| Factor VLeiden-negative | ||||||||||||||
| Protein C | 96.0 ± 18.3 | 108.8 ± 21.7 | 92.0 ± 14.0 | 103.7 ± 17.8 | −1.1 (−7.2 to 5.0) | 96.7 ± 18.3 | 92.7 ± 18.6 | 92.0 ± 14.0 | 84.9 ± 11.5 | −3.1 (−6.8 to 0.6) | ||||
| Protein S total | 88.5 ± 13.2 | 83.4 ± 13.6 | 85.9 ± 9.9 | 71.7 ± 10.2 | − 9.1 (−13.4 to −4.7) | 88.6 ± 13.4 | 94.9 ± 13.4 | 85.9 ± 9.9 | 92.5 ± 13.2 | 0.3 (−4.2 to 4.7) | ||||
| Protein S free | 39.9 ± 7.0 | 43.9 ± 8.0 | 38.9 ± 5.8 | 33.8 ± 7.5 | −9.2 (−12.0 to −6.4) | 40.0 ± 7.1 | 47.9 ± 8.4 | 38.9 ± 5.8 | 42.7 ± 7.1 | −4.0 (−6.4 to −1.7) | ||||
| C4BP | 89.9 ± 20.1 | 77.1 ± 17.3 | 88.8 ± 15.8 | 79.1 ± 15.1 | 2.6 (−1.5 to 6.6) | 89.2 ± 20.3 | 82.0 ± 15.8 | 88.8 ± 15.8 | 87.5 ± 17.8 | 6.0 (0.03 to 12.0) | ||||
| PSAPCind | 1.32 ± 0.12 | 1.19 ± 0.08 | 1.34 ± 0.13 | 1.14 ± 0.07 | −0.08 (−0.13 to −0.02) | 1.32 ± 0.12 | 1.46 ± 0.23 | 1.34 ± 0.13 | 1.35 ± 0.13 | −0.13 (−0.20 to −0.06) | ||||
| PCI | 90.6 ± 16.6 | 101.0 ± 15.0 | 90.5 ± 13.7 | 99.5 ± 18.1 | −1.4 (−8.4 to 5.6) | 88.9 ± 14.6 | 90.4 ± 17.7 | 90.5 ± 13.7 | 90.0 ± 14.4 | −2.0 (−7.8 to 3.9) | ||||
| APC-sr | 1.92 ± 0.61 | 3.26 ± 0.72 | 2.04 ± 0.63 | 3.76 ± 0.77 | 0.38 (0.08 to 0.67) | 1.93 ± 0.63 | 1.58 ± 0.61 | 2.04 ± 0.63 | 1.80 ± 0.52 | 0.11 (−0.09 to 0.30) | ||||
| Factor VLeiden-positive | ||||||||||||||
| Protein C | 92.1 ± 12.3 | 102.5 ± 16.3 | 106.6 ± 15.9 | 122.7 ± 22.2 | 5.7 (−1.8 to 13.3) | 92.1 ± 12.3 | 86.2 ± 11.1 | 106.6 ± 15.9 | 96.5 ± 14.9 | −4.2 (−9.0 to 0.7) | ||||
| Protein S total | 88.2 ± 11.4 | 85.9 ± 12.9 | 96.9 ± 10.9 | 77.6 ± 11.1 | −16.9 (−23.8 to −10.1) | 88.2 ± 11.4 | 95.2 ± 10.5 | 96.9 ± 10.9 | 100.6 ± 11.2 | −3.2 (−8.1 to 1.6) | ||||
| Protein S free | 42.2 ± 9.4 | 45.9 ± 9.9 | 42.1 ± 8.1 | 35.3 ± 8.4 | −10.5 (−15.0 to − 5.9) | 42.2 ± 9.4 | 48.9 ± 7.8 | 42.1 ± 8.1 | 45.9 ± 6.8 | −2.9 (−5.9 to 0.1) | ||||
| C4BP | 80.5 ± 11.2 | 72.3 ± 9.8 | 101.5 ± 12.7 | 85.4 ± 16.6 | −8.0 (−13.9 to −2.1) | 80.5 ± 11.2 | 75.1 ± 9.7 | 101.5 ± 12.7 | 95.3 ± 12.1 | −0.8 (−6.4 to 4.9) | ||||
| PSAPCind | 1.24 ± 0.10 | 1.16 ± 0.10 | 1.33 ± 0.12 | 1.14 ± 0.05 | −0.10 (−0.15 to −0.05) | 1.24 ± 0.10 | 1.33 ± 0.16 | 1.33 ± 0.12 | 1.33 ± 0.10 | −0.09 (−0.14 to − 0.03) | ||||
| PCI | 86.0 ± 14.9 | 92.2 ± 16.3 | 92.5 ± 17.0 | 106.9 ± 17.6 | 8.2 (2.2 to 14.1) | 86.0 ± 14.9 | 85.7 ± 18.3 | 92.5 ± 17.0 | 91.0 ± 20.8 | −0.6 (−8.0 to 6.8) | ||||
| APC-sr | 4.60 ± 1.02 | 5.95 ± 1.38 | 4.29 ± 1.00 | 7.06 ± 1.40 | 1.41 (0.76 to 2.07) | 4.60 ± 1.02 | 4.09 ± 0.97 | 4.29 ± 1.00 | 4.10 ± 1.04 | 0.32 (−0.05 to 0.68) | ||||
. | Levonorgestrel-containing OC . | . | Desogestrel-containing OC . | . | Mean difference in change (95% CI) . | Levonorgestrel only . | . | Desogestrel only . | . | Mean difference in change (95% CI)‡ . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | No use, mean ± SD . | OC use, mean ± SD . | No use, mean ± SD . | OC use, mean ± SD . | . | No use,†mean ± SD . | OC use, mean ± SD . | No use, mean ± SD . | OC use, mean ± SD . | . | ||||
| Factor VLeiden-negative | ||||||||||||||
| Protein C | 96.0 ± 18.3 | 108.8 ± 21.7 | 92.0 ± 14.0 | 103.7 ± 17.8 | −1.1 (−7.2 to 5.0) | 96.7 ± 18.3 | 92.7 ± 18.6 | 92.0 ± 14.0 | 84.9 ± 11.5 | −3.1 (−6.8 to 0.6) | ||||
| Protein S total | 88.5 ± 13.2 | 83.4 ± 13.6 | 85.9 ± 9.9 | 71.7 ± 10.2 | − 9.1 (−13.4 to −4.7) | 88.6 ± 13.4 | 94.9 ± 13.4 | 85.9 ± 9.9 | 92.5 ± 13.2 | 0.3 (−4.2 to 4.7) | ||||
| Protein S free | 39.9 ± 7.0 | 43.9 ± 8.0 | 38.9 ± 5.8 | 33.8 ± 7.5 | −9.2 (−12.0 to −6.4) | 40.0 ± 7.1 | 47.9 ± 8.4 | 38.9 ± 5.8 | 42.7 ± 7.1 | −4.0 (−6.4 to −1.7) | ||||
| C4BP | 89.9 ± 20.1 | 77.1 ± 17.3 | 88.8 ± 15.8 | 79.1 ± 15.1 | 2.6 (−1.5 to 6.6) | 89.2 ± 20.3 | 82.0 ± 15.8 | 88.8 ± 15.8 | 87.5 ± 17.8 | 6.0 (0.03 to 12.0) | ||||
| PSAPCind | 1.32 ± 0.12 | 1.19 ± 0.08 | 1.34 ± 0.13 | 1.14 ± 0.07 | −0.08 (−0.13 to −0.02) | 1.32 ± 0.12 | 1.46 ± 0.23 | 1.34 ± 0.13 | 1.35 ± 0.13 | −0.13 (−0.20 to −0.06) | ||||
| PCI | 90.6 ± 16.6 | 101.0 ± 15.0 | 90.5 ± 13.7 | 99.5 ± 18.1 | −1.4 (−8.4 to 5.6) | 88.9 ± 14.6 | 90.4 ± 17.7 | 90.5 ± 13.7 | 90.0 ± 14.4 | −2.0 (−7.8 to 3.9) | ||||
| APC-sr | 1.92 ± 0.61 | 3.26 ± 0.72 | 2.04 ± 0.63 | 3.76 ± 0.77 | 0.38 (0.08 to 0.67) | 1.93 ± 0.63 | 1.58 ± 0.61 | 2.04 ± 0.63 | 1.80 ± 0.52 | 0.11 (−0.09 to 0.30) | ||||
| Factor VLeiden-positive | ||||||||||||||
| Protein C | 92.1 ± 12.3 | 102.5 ± 16.3 | 106.6 ± 15.9 | 122.7 ± 22.2 | 5.7 (−1.8 to 13.3) | 92.1 ± 12.3 | 86.2 ± 11.1 | 106.6 ± 15.9 | 96.5 ± 14.9 | −4.2 (−9.0 to 0.7) | ||||
| Protein S total | 88.2 ± 11.4 | 85.9 ± 12.9 | 96.9 ± 10.9 | 77.6 ± 11.1 | −16.9 (−23.8 to −10.1) | 88.2 ± 11.4 | 95.2 ± 10.5 | 96.9 ± 10.9 | 100.6 ± 11.2 | −3.2 (−8.1 to 1.6) | ||||
| Protein S free | 42.2 ± 9.4 | 45.9 ± 9.9 | 42.1 ± 8.1 | 35.3 ± 8.4 | −10.5 (−15.0 to − 5.9) | 42.2 ± 9.4 | 48.9 ± 7.8 | 42.1 ± 8.1 | 45.9 ± 6.8 | −2.9 (−5.9 to 0.1) | ||||
| C4BP | 80.5 ± 11.2 | 72.3 ± 9.8 | 101.5 ± 12.7 | 85.4 ± 16.6 | −8.0 (−13.9 to −2.1) | 80.5 ± 11.2 | 75.1 ± 9.7 | 101.5 ± 12.7 | 95.3 ± 12.1 | −0.8 (−6.4 to 4.9) | ||||
| PSAPCind | 1.24 ± 0.10 | 1.16 ± 0.10 | 1.33 ± 0.12 | 1.14 ± 0.05 | −0.10 (−0.15 to −0.05) | 1.24 ± 0.10 | 1.33 ± 0.16 | 1.33 ± 0.12 | 1.33 ± 0.10 | −0.09 (−0.14 to − 0.03) | ||||
| PCI | 86.0 ± 14.9 | 92.2 ± 16.3 | 92.5 ± 17.0 | 106.9 ± 17.6 | 8.2 (2.2 to 14.1) | 86.0 ± 14.9 | 85.7 ± 18.3 | 92.5 ± 17.0 | 91.0 ± 20.8 | −0.6 (−8.0 to 6.8) | ||||
| APC-sr | 4.60 ± 1.02 | 5.95 ± 1.38 | 4.29 ± 1.00 | 7.06 ± 1.40 | 1.41 (0.76 to 2.07) | 4.60 ± 1.02 | 4.09 ± 0.97 | 4.29 ± 1.00 | 4.10 ± 1.04 | 0.32 (−0.05 to 0.68) | ||||
The individual plasma components are expressed as percent of normal pooled plasma. PCI indicates protein C inhibitor; APC-sr, activated protein C sensitivity ratio; C4BP, C4b-binding protein; and PSAPCind, APC-independent anticoagulant activity of protein S.
Formula: (no use of levonorgestrel containing OC - use of levonorgestrel containing OC) - (no use of desogestrel containing OC - use of desogestrel containing OC).
For this analysis, one woman was excluded due to protocol violation in phase 2.
Formula: (no use of levonorgestrel - use of levonorgestrel) - (no use of desogestrel - use of desogestrel).
Effect of combined oral contraceptives
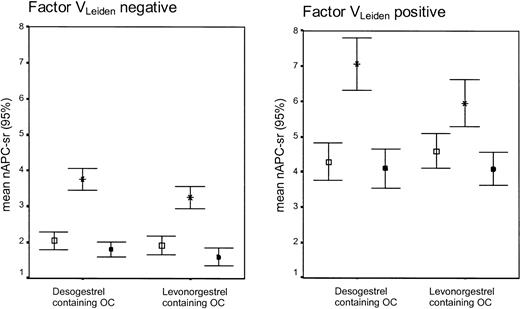
Women without factor VLeiden who used desogestrel-containing oral contraceptives became significantly more resistant to APC (as indicated by the increase of the APC-sr) than users of levonorgestrel-containing oral contraceptives (mean difference in change: 0.38; 95% CI, 0.08 to 0.67) (Table 2; Figure 3). In noncarriers of factor VLeiden, the use of levonorgestrel- and desogestrel-containing oral contraceptives also caused significant differential changes of total protein S (-9.1; 95% CI, -13.4 to -4.7), free protein S (-9.2; 95% CI, -12.0 to -6.4), and protein SAPCind (-0.08; 95% CI, -0.13 to -0.02). No differential changes were observed for protein C, C4b-binding protein, and protein C inhibitor.
APC-sr levels (95% CI) on combined oral contraceptives (OCs), progestagen-only (POP), or during no oral contraceptive use according to the absence or presence of the factor VLeiden mutation. Error bar with open square indicates no oral contraceptive use; error bar with star, oral contraceptive use (end phase 1); and error bar with black square, POP use (end phase 2).
APC-sr levels (95% CI) on combined oral contraceptives (OCs), progestagen-only (POP), or during no oral contraceptive use according to the absence or presence of the factor VLeiden mutation. Error bar with open square indicates no oral contraceptive use; error bar with star, oral contraceptive use (end phase 1); and error bar with black square, POP use (end phase 2).
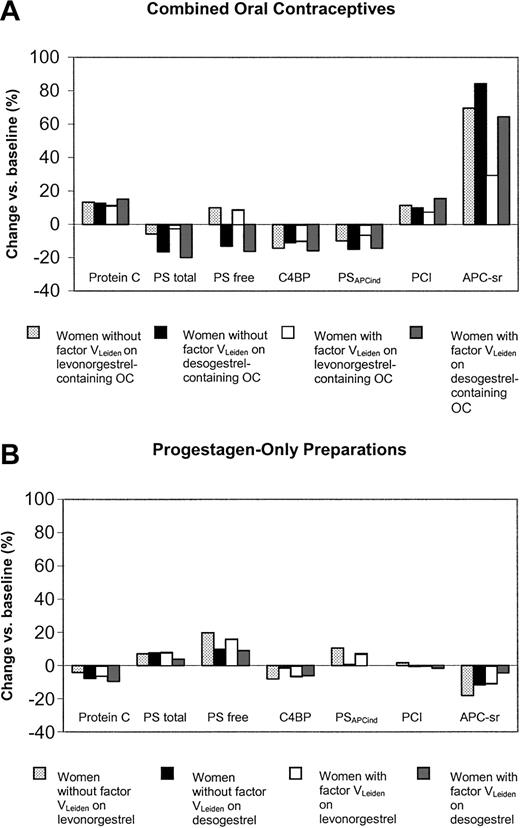
In carriers of factor VLeiden, the use of desogestrel- and levonorgestrel-containing oral contraceptives had significant differential effects on all anticoagulant parameters, except for protein C (Table 2; Figure 4A).
Changes of anticoagulant parameters during the study according to pill type and presence of the factor VLeiden mutation. PS indicates protein S; C4BP, C4b-binding protein; PSAPCind, APC-independent anticoagulant activity of protein S; PCI, protein C inhibitor; and APC-sr, activated protein C sensitivity ratio.
Changes of anticoagulant parameters during the study according to pill type and presence of the factor VLeiden mutation. PS indicates protein S; C4BP, C4b-binding protein; PSAPCind, APC-independent anticoagulant activity of protein S; PCI, protein C inhibitor; and APC-sr, activated protein C sensitivity ratio.
Effect of progestagen-only preparations
The changes observed in noncarriers of factor VLeiden were significantly less pronounced on desogestrel than on levonorgestrel for the increase of free protein S (-4.0; 95% CI, -6.4 to -1.7) and PSAPCind (-0.13; 95% CI, -0.20 to -0.06) and the decrease in C4b-binding protein (6.0; 95% CI, 0.03-12.0) (Table 2; Figure 4B). In carriers of factor VLeiden the only significant differential effect between levonorgestrel and desogestrel was observed for PSAPCind (-0.09; 95% CI, -0.14 to -0.03).
The effects of the combination of estrogens and progestagens versus progestagen-only were estimated from the difference between the anticoagulant parameters in phase 1 and phase 2 (see Supplemental Table S1 on the Blood website; click on the “Data Set” link at the top of the online article). Except for free protein S and C4b-binding protein in carriers of factor VLeiden, all anticoagulant parameters differed significantly between phases 1 and 2.
Comparison of women with and without the factor VLeiden mutation
During the use of levonorgestrel-containing oral contraceptives a significantly more pronounced decrease was found in noncarriers than in carriers for C4b-binding protein and PSAPCind (Supplemental Table S2). In contrast, the changes observed during the use of desogestrel-containing oral contraceptives were more pronounced in carriers than in noncarriers of factor VLeiden, which was statistically significant for the increase in the APC-sr (Figure 3) and the decrease in the plasma level of C4b-binding protein.
Discussion
Oral contraceptive use has repeatedly been associated with an increased risk of venous thrombosis.1-3 Particularly, the differential changes of the proteins involved in the protein C system may contribute to the thrombotic effects of oral contraceptives. In the present study, we found that third-generation oral contraceptives have a stronger effect on anticoagulant parameters than second-generation preparations. These effects were not found with the progestagen components of these pills only. On the contrary, the effects of progestagen-only preparations were in general more pronounced with levonorgestrel compared with desogestrel. Our findings suggest that, compared with levonorgestrel, desogestrel is less effective in counteracting the thrombotic effects induced by the estrogen component in combined oral contraceptives.
Despite the small number of women with factor VLeiden, which may have led to less precise estimates, we showed that earlier reported changes in anticoagulant parameters induced by oral contraceptives in healthy women18,29,30 also occurred in women with factor VLeiden.18,29 In carriers as well as in noncarriers of the factor VLeiden mutation both oral contraceptives induced APC resistance, increased the plasma levels of protein C and protein C inhibitor, and decreased the APC-independent anticoagulant activity of protein S and the plasma levels of total protein S and C4b-binding protein. In both study populations, free protein S increased on levonorgestrel- and decreased on desogestrel-containing oral contraceptives. This increase of free protein S on combined pills with levonorgestrel is explained by the fact that on this contraceptive total protein S hardly changes, which, together with the decrease of C4b-binding protein (which complexes protein S), results in an elevation of the plasma level of free protein S. In both carriers and noncarriers of factor VLeiden the changes in anticoagulant parameters were most pronounced in the women using desogestrel-containing oral contraceptives.
Since hereditary defects of the protein C pathway are associated with an increased risk of venous thromboembolism,21-23 the differential effects of levonorgestrel- and desogestrel-containing oral contraceptives particularly on the plasma levels of protein S and on the degree of acquired APC resistance may well explain the differences in thrombotic risk associated with these preparations.7,8,10,31 Although the effects of third-generation OCs on the plasma levels of total and free protein S is relatively small, it should be emphasized that these concern mean changes and that in some individuals the changes are larger than the mean. Thus, it is possible that in a woman with a low protein S at baseline, a change in protein S larger than the mean may further disturb the hemostatic balance and increase the thrombotic risk.32
With respect to the clinical relevance of acquired APC resistance, it was recently demonstrated that APC-sr determined with the APC resistance test used in this study predicts for venous thrombosis, in men and in women both in the absence and presence of the factor VLeiden mutation.17 This means that acquired APC resistance may well explain the occurrence of venous thrombosis and different thrombotic risks in OC users. Moreover, the pronounced increase in APC resistance in women with factor VLeiden who use desogestrel-containing oral contraceptives may account for the reported elevated risk of venous thrombosis in carriers of this mutation taking third-generation oral contraceptives.9
To determine to what extent the progestagens levonorgestrel and desogestrel are responsible for the changes of anticoagulant parameters induced by combined oral contraceptives, we also investigated the effect of progestagen-only pills on the protein C pathway. Some years ago, Egberg et al33 studied 2 long-term contraceptive implants (containing the biologically active metabolite of desogestrel or levonorgestrel) with respect to hemostatic variables. They found that both implants had similar small effects on hemostasis. Winkler et al34 compared 2 progestagen-only pills containing either 30 μg levonorgestrel or 75 μg desogestrel. They reported increased protein S levels for desogestrel compared with levonorgestrel. However, the doses of progestagen used in these pills differed from those normally used in oral contraceptives. In our study we compared progestagen-only preparations that contained the same dose of progestagen as present in oral contraceptives (150 μg levonorgestrel or desogestrel). Almost all effects of combined oral contraceptives on anticoagulant parameters disappeared or even went in an opposite direction when the participants used progestagen-only preparations. Our observations are supported by reports indicating that the changes of several other hemostatic parameters on progestagen-only pills and implants are the inverse of those induced by combined oral contraceptives,33,34 which may indicate a net antithrombotic effect. Progestagen-only pills may thus be a safer method of contraception. However, during the study many women complained about irregular bleedings when these preparations were used (65% compared with 15% during the use of combined oral contraceptives).
The present study may provide an explanation for the observation that combined oral contraceptives with desogestrel cause more marked changes of the anticoagulant protein C system than levonorgestrel-containing oral contraceptives. We assume that estrogens such as ethinyloestradiol cause changes of anticoagulant parameters that are in the same direction, but more profound than observed with combined oral contraceptives. Due to their androgenic properties, progestagens induce changes in the anticoagulant system that are opposite to those of estrogen and that, due to the higher androgenicity,35,36 are more pronounced with levonorgestrel than with desogestrel. Hence, we propose that combined oral contraceptives with desogestrel induce more profound changes of the anticoagulant system than levonorgestrel-containing oral contraceptives because the effects of ethinyloestradiol on anticoagulant parameters are less well compensated by desogestrel than by levonorgestrel. This view is supported by the observation that APC resistance correlates inversely with the dose of levonorgestrel present in 5 different combined oral contraceptives,37 suggesting that high concentrations of levonorgestrel counteract the increase in APC resistance.
In conclusion, our findings indicate that desogestrel-containing oral contraceptives have a more pronounced effect on the anticoagulant protein C system than levonorgestrel-containing oral contraceptives, especially in women with factor VLeiden. Particularly the decrease in protein S and the profoundly increased resistance to APC might contribute to the elevated risk of venous thrombosis in carriers of factor VLeiden who use third-generation oral contraceptives. The differential effects of second- and third-generation oral contraceptives on the anticoagulant pathway can at least be partially explained by the observation that levonorgestrel is more effective than desogestrel in counteracting the thrombotic effect of ethinyloestradiol.
Prepublished online as Blood First Edition Paper, October 9, 2003; DOI 10.1182/blood-2003-04-1285.
Supported by grant 98.001 from the Dutch Thrombosis Foundation. J.C.M.M. is an established investigator of the Netherlands Heart Foundation (grant D96.021)
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all participants who took part in this study. We are indebted to Esther van Lunteren for her help in the execution of this study and to Winnie Tekelenburg and Stella Thomassen for laboratory analysis.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal