Abstract
We have recently identified a novel mechanism of hematopoietic cell survival that involves site-specific serine phosphorylation of the common beta subunit (βc) of the granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5 receptors. However, the downstream components of this pathway are not known, nor is its relationship to survival signals triggered by tyrosine phosphorylation of the receptor clear. We have now found that phosphorylation of Ser585 of βc in response to GM-CSF recruited 14-3-3 and phosphatidyl inositol 3-OH kinase (PI 3-kinase) to the receptor, while phosphorylation of the neighboring Tyr577 within this “viability domain” promoted the activation of both Src homology and collagen (Shc) and Ras. These are independent processes as demonstrated by the intact reactivity of phosphospecific anti-Ser585 and anti-Tyr577 antibodies on the cytotoxic T-lymphocyte–ecotrophic retroviral receptor neomycin (CTL-EN) mutants βcTyr577Phe and βcSer585Gly, respectively. Importantly, while mutants in which either Ser585 (βcSer585Gly) or all tyrosines (βcF8) were substituted showed a defect in Akt phosphorylation, nuclear factor κB (NF-κB) activation, bcl-2 induction, and cell survival, the mutant βcTyr577Phe was defective in Shc, Ras, and extracellular signal-related kinase (ERK) activation, but supported CTL-EN cell survival in response to GM-CSF. These results demonstrate that both serine and tyrosine phosphorylation pathways play a role in hematopoietic cell survival, are initially independent of each other, and converge on NF-κB to promote bcl-2 expression.
Introduction
Hematopoiesis is a dynamic process undergoing constant flux where the enormous proliferative capacity of hematopoietic cells is precisely balanced against cell death programs. At the heart of this process lie the hematopoietic cytokines, which are central regulators of both cell proliferation and survival. Many hematopoietic cell types remain poised to activate intrinsic cell death programs and require constant survival signals provided by cytokines. There are 3 such cytokines, granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-3 (IL-3), and IL-5, that are potent regulators of not only myeloid cell proliferation but also cell survival through their ability to suppress apoptotic programs and as such can play a role also in certain inflammatory conditions and leukemia.1,2
The receptors for GM-CSF, IL-3, and IL-5 share a subunit, βc, which is required for most, if not all, of the signaling including cell survival.1,2 However, the molecular basis and signaling cascades that emanate from βc and underpin this prosurvival effect are not fully understood. While some experiments have implicated tyrosine phosphorylation of βc (below), we noted that Ser585 was phosphorylated in response to ligand, resulting in the recruitment of the adaptor molecule 14-3-3.3 Importantly, we demonstrated using mutations at this position that Ser585 was required for phosphatidyl inositol 3-OH kinase (PI 3-kinase) activation and stimulation of hematopoietic cell survival, however, the downstream components of this pathway were not characterized.4
PI 3-kinase phosphorylates phosphatidyl inositol phosphates that act as docking sites in the cell membrane for the recruitment of proteins containing pleckstrin-homology (PH) domains.5 One such PH-domain target protein is the serine-threonine kinase Akt (or protein kinase B), which is an important transducer of survival signals.6 For example, the activation of Akt in response to IL-3 has been shown to result in the phosphorylation of the proapoptotic regulator BAD, resulting in 14-3-3 binding and its sequestration in the cytoplasm.7 In the absence of IL-3, PI 3-kinase and Akt are not activated and BAD remains unphosphorylated, allowing it to translocate to the mitochondria where it can bind bcl-2 or bcl-xL and exert its proapoptotic activities. More recently, Akt has also been shown to regulate the transcriptional activity of nuclear factor κB (NF-κB). Normally, NF-κB is held in an inactive, latent state in the cytoplasm by the inhibitor protein IκB. Akt has been shown to phosphorylate IκB kinase (IKK), which in turn phosphorylates IκB.8,9 Phosphorylated IκB is then targeted for degradation by the proteosome allowing NF-κB to translocate to the nucleus and regulate gene expression. NF-κB has been proposed to regulate the expression of a broad range of genes, some of which are involved in cell survival such as bcl-2, bcl-xL, and A1/bfl1.10-12 The expression of bcl-2, bcl-xL, and A1/bfl1 has been clearly linked to the regulation of survival of a range of hematopoietic cell lineages,13-15 and the expression of these genes is induced by a range of cytokines including GM-CSF and IL-3, although the signaling events involved are not known.16-19
Although the Ser585:14-3-3 pathway is a major component of the prosurvival activity of GM-CSF and IL-3, early studies suggested a role for tyrosine phosphorylation of βc. Using a series of βc mutants with cytoplasmic tail truncations, previous works have delineated domains important in regulating viability in response to GM-CSF and IL-3.16,20-22 We noted that the minimal “viability domain” defined by these studies encompasses amino acids Asp574 to Leu610 and is distinct from the region important for regulating cell proliferation. One expectation arising from these earlier truncation studies was that tyrosine residues within distinct βc domains would be responsible for regulating specific signaling pathways and biologic responses. The cytoplasmic domain of βc contains 8 tyrosines (Tyr450, Tyr452, Tyr577, Tyr612, Tyr695, Tyr750, Tyr806, and Tyr866). In some cases, individual βc tyrosines have been shown to mediate specific receptor proximal signaling events, however, linking these individual tyrosines with specific biologic responses has been difficult to establish. Tyr577 of βc lies within the viability domain and has been shown to be important for the binding and tyrosine phosphorylation of Src homology and collagen (Shc).23 The binding of Shc to Tyr577 has been suggested to be important for the regulation of at least 2 signaling pathways. First, Shc is a known regulator of the ras–mitogen activated protein (MAP) kinase pathway through its ability to recruit the adaptor protein, growth factor receptor binding protein-2 (Grb-2), and the nucleotide exchange factor, SOS.24 In fact, the binding of Shc to Tyr577 of βc has been shown to be important for the recruitment of Grb-2.25 In addition, using a βc mutant in which only a single tyrosine residue is left intact, it was shown that either Tyr577, Tyr612, or Tyr695 was sufficient to promote Src homology 2–containing tyrosine phosphatase 2 (SHP2) tyrosine phosphorylation, Grb-2 association, and MAP kinase activation.25,26 Secondly, Tyr577 of βc has also been proposed to recruit a PI 3-kinase signaling complex via the adaptors Shc, Grb-2, and Grb2-associated binder 2 (Gab2).27 Thus, Tyr577 of βc has the potential to integrate signaling through the ras–MAP kinase and PI 3-kinase pathways and may also play an important role in regulating GM-CSF prosurvival activities.
In the current studies, we have evaluated the receptor-proximal signaling events regulated by Ser585 and Tyr577 of βc and the downstream signaling pathways that lead to hematopoietic cell survival. We show that while Tyr577 of βc is essential for the activation of ras, it is not critical for promoting cell proliferation and survival in response to GM-CSF. On the other hand, our findings establish a direct link between the phosphorylation of Ser585 of βc, the activation of a PI 3-kinase pathway that leads to NF-κB activation and the expression of the prosurvival gene, bcl-2.
Materials and methods
Antibodies and reagents
Anti–14-3-3 (EB1) antibodies were generated in New Zealand white rabbits using glutathione S-transferase-14-3-3ζ (GST-14-3-3ζ) as the immunogen. Polyclonal antibodies (pAbs) were purified from rabbit serum using a GST-14-3-3ζ affinity column. Antibodies that specifically recognize phospho-Tyr577 of βc were generated by immunizing New Zealand white rabbits with a phospho-peptide (Mimotopes, Clayton, Victoria, Australia) (CDFNGPYLGPPH, where Y is phosphorylated) conjugated to keyhole limpet hemocyanin. Phospho-specific pAbs were affinity-purified by sequential passage over a nonphosphopeptide column (CDFNGPYLGPPH coupled to Sepharose) and a phosphopeptide column (CDFNGPYLGPPH coupled to Sepharose) as previously described.3 Anti-p85 and 4G10 antiphosphotyrosine monoclonal antibodies (mAbs) from Upstate Biotech (Lake Placid, NY), anti-MAP2 mAb (MK12) from Pharmingen (San Diego, CA), anti-Shc pAb from Transduction Laboratories (San Jose, CA), anti–H-ras and anti–K-ras from Santa Cruz (Santa Cruz, CA), anti–phospho-Akt-Thr308, anti–phospho-Akt-Ser473, and phospho-IκB-α (Ser32/36) antibody from Cell Signaling (Beverly, MA), anti–active–extracellular signal-related kinase (ERK) pAb from Promega (Madison, WI), antiphosphorylated signal transducer and activator of transcription 5 (STAT5) mAb from Zymed (South San Francisco, CA), and the anti–phospho–Janus kinase 2 (JAK2) pAb from Affinity Bioreagents (Golden, CO) were used according to the manufacturer's conditions unless otherwise stated.
Immunoprecipitations and immunoblot analysis
Cytotoxic T-lymphocyte–ecotrophic retroviral receptor neomycin (CTL-EN) cells expressing wild-type (wt) or mutant GM-CSF receptors were factor deprived for 12 hours in RPMI containing 0.5% fetal calf serum (FCS) and then stimulated with 50 ng/mL human GM-CSF. Cells were lysed in either radioimmunoprecipitation assay buffer (150 mM NaCl, 1.0% nonidet P-40 [NP-40], 0.5% deoxycholate, 0.1% sodium dodecylsulphate [SDS], 50 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.4) or NP-40 lysis buffer (137 mM NaCl, 1.0% NP-40, 10% glycerol, 50 mM Tris-HCl, pH 7.4) as previously described.4 Cell lysates were subjected to immunoprecipitation (1 × 107 cells/immunoprecipitation) using specific antibodies coupled to protein A Sepharose (Amersham Pharmacia, Piscataway, NJ). Immunoprecipitates were subjected to SDS polyacrylamide gel electrophoresis (PAGE) and immunoblot analysis using standard conditions, and signals were developed using enhanced chemiluminescence (ECL; Amersham Pharmacia or West Dura from Pierce [Rockford, IL]). PI 3-kinase assays were performed as previously described.4
Cell survival and proliferation assays
CTL-EN cells were transduced with retroviral constructs for the coexpression of the α subunit of the GM-CSF receptor (GMRα) together with wt and mutant βc subunits (pRuf-IRES-αβc, pRuf-IRES-αβcSer585Gly, pRuf-IRES-αβcTyr577Phe, pRuf-IRES-αβcF8) as previously described.28 Pools of transduced cells were selected in 500 μg/mL G418 for 2 weeks, after which GMRα-expressing cells were purified by fluorescence-activated cell sorting (FACS) using the 4H1 anti-GMRα mAb antibody. At least 2 separate pools of transfected cells were generated for each receptor mutant and the results shown are typical for both lines. Cell viability was assessed by annexin V–fluos (Roche, Indianapolis, IN) and propidium iodide staining as previously described.4 Briefly, CTL-EN cells expressing either wt or mutant GM-CSF receptors were washed in phosphate-buffered saline (PBS) and plated out at 5 × 105 cells/mL in RPMI containing 0.1% FCS and either no factor, 50 ng/mL GM-CSF, or 20 ng/mL IL-2. Apoptotic cells were examined over 48 hours by annexin V/propidium iodide staining and flow cytometry. The ability of GM-CSF to maintain cell viability in the presence of a number of pharmacologic inhibitors was also examined using the MTS CellTiter 96 AQueous one solution assay (Promega). CTL-EN cells expressing the wt GM-CSF receptor were plated out at 5 × 105 cell/mL in RPMI containing 0.1% FCS and either no GM-CSF, 50 ng/mL GM-CSF, or 50 ng/mL GM-CSF plus increasing doses of the PI 3-kinase inhibitor, LY294002 (Cayman Chemical, Ann Arbor, MI), the mitogen-activated protein kinase kinase (MEK) inhibitor, PD98059 (Biomol, Plymouth Meeting, PA), the JAK2 inhibitor, AG-490 (Biomol), the p38 MAP kinase inhibitor, SB203580 (Calbiochem, San Diego, CA), and the NF-κB inhibitors, SN50 and SN50M (Calbiochem), or BAY 11-7082 and BAY 1107085 (Biomol). The ability of GM-CSF to promote cell cycle progression was determined by 5-Bromo-2′-deoxyuridine (BrdU) incorporation using the in situ cell proliferation kit (Roche) according to the manufacturer's recommended conditions.
NF-κB reporter assays
CTL-EN cells (107 cells/electroporation) expressing either wt or mutant GM-CSF receptors were electroporated (270 mV, 975 μF) with 20 μg immunoglobulin (Ig)–κB-firefly luciferase reporter plasmid (pTK81-IgK) and 1 μg Renilla luciferase control vector (pRL). After 24 hours of culture, the cells were washed in PBS and plated at 105 cells/mL in RPMI/10% FCS containing either no factor, 20 ng/mL GM-CSF, or 20 ng/mL IL-2. The cells were then cultured for 12 hours, after which cell extracts were made and reporter gene activity was determined by the dual-luciferase assay system (Promega) according to the manufacturer's instructions. The luciferase activity for each cell line was normalized for transfection efficiency and expressed as a percentage of the IL-2 control cytokine response.
Northern blots
The ability of GM-CSF to regulate gene expression was examined by Northern blot analysis as previously described.29 Briefly, CTL-EN cells expressing wt or mutant GM-CSF receptors were washed in RPMI and factor-deprived in RPMI/0.5% FCS for 18 hours and then stimulated with 100 ng/mL GM-CSF for up to 24 hours. Total RNA was isolated from cells using the Trizol method (Gibco-BRL, Grand Island, NY), electrophoresed on a formaldehyde-agarose gel (20 μg/lane), and transferred to Hybond-N nylon membrane (Amersham Pharmacia). 32P-labeled cDNA probes were used to probe filters, and signals were detected by X-ray film and autoradiography.
Ras binding domain pull-down assay
The activation of ras in response to GM-CSF was examined using a pull-down assay using a fusion protein composed of GST and the ras-binding domain (RBD) of c-Raf (GST-RBD).30 CTL-EN cells expressing wt or mutant GM-CSF receptors were factor deprived for 18 hours in RPMI containing 0.5% FCS and then stimulated with 50 ng/mL GM-CSF for up to 10 minutes. Cells were lysed in NP-40 lysis buffer as described above and lysates were incubated with glutathione resin bound to either 10 μg GST or GST-RBD for 3 hours, after which the pulldowns were washed 3 times in NP-40 lysis buffer. Pulldowns were subjected to SDS-PAGE and immunoblot analysis using anti–H-ras and anti–K-ras antibodies.
Results
Ser585 and Tyr577 of βc selectively couple to distinct signaling pathways
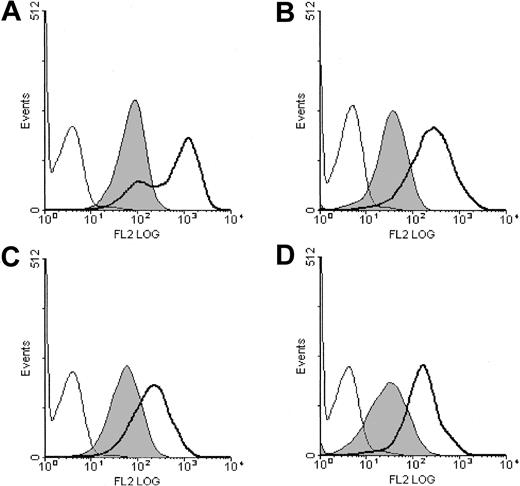
We constructed CTL-EN cell lines coexpressing the GM-CSF receptor α subunit (GMRα) with either wild-type βc (wtβc) or mutant βc in which Ser585 or Tyr577 was substituted (βcSer585Gly, βcTyr577Phe, respectively). While we have not examined the role of other individual tyrosine residues in βc besides Tyr577, we have included a βc mutant in which all 8 tyrosine residues were substituted for phenylalanine (βcF8). These cells are able to grow in the presence of mouse IL-2, or following expression of human GM-CSF receptors are able to grow in the presence of human GM-CSF. At least 2 different pools of cells were analyzed. The surface expression of both the GMRα and the βc subunits of the GM-CSF receptor was examined by flow cytometry and was not significantly different between the cell lines (Figure 1).
Surface expression of the human GM-CSF receptor subunits in transduced CTL-EN cells. CTL-EN cell lines expressing GMRαβc (A), GMRαβcSer585Gly (B), GMRαβcTyr577Phe (C), or GMRαβcF8 (D) were stained for the α subunit (GMRα) with the 4H1 monoclonal antibody (thick line) and the βc subunit using the 1C1 monoclonal antibody (filled) as described in “Materials and methods.” As a control, cells were also stained with an isotype-matched control antibody (thin line). Cells were then stained with antimouse PE-conjugated secondary antibodies and analyzed by flow cytometry.
Surface expression of the human GM-CSF receptor subunits in transduced CTL-EN cells. CTL-EN cell lines expressing GMRαβc (A), GMRαβcSer585Gly (B), GMRαβcTyr577Phe (C), or GMRαβcF8 (D) were stained for the α subunit (GMRα) with the 4H1 monoclonal antibody (thick line) and the βc subunit using the 1C1 monoclonal antibody (filled) as described in “Materials and methods.” As a control, cells were also stained with an isotype-matched control antibody (thin line). Cells were then stained with antimouse PE-conjugated secondary antibodies and analyzed by flow cytometry.
We first examined the role of Ser585 and βc tyrosine phosphorylation for the activation of receptor-proximal signaling events by GM-CSF stimulation. CTL-EN cells expressing wtβc, βcSer585Gly, βcTyr577Phe, and βcF8 were factor deprived overnight and then stimulated with GM-CSF. Following stimulation, the cells were lysed and βc immunoprecipitated and subjected to immunoblot analysis using anti–phospho-βcSer585, anti-p85, and anti–14-3-3 antibodies. GM-CSF stimulation of CTL-EN cells expressing the wtβc induced Ser585 phosphorylation and the recruitment of both 14-3-3 and p85 (Figure 2A). No specific signal was detected with the anti–phospho-βcSer585 antibody in cells expressing the βcSer585Gly mutant and no recruitment of either 14-3-3 or p85 to the βc was observed. On the other hand, cells expressing the βcTyr577Phe mutant demonstrated Ser585 phosphorylation and the recruitment of 14-3-3 and p85 in response to GM-CSF in a manner essentially identical to that observed in cells expressing the wtβc. Cells expressing βcF8 exhibited slightly delayed Ser585 phosphorylation and no detectable recruitment of 14-3-3 or p85. To confirm the independent regulation of Ser585 and Tyr577 phosphorylation we also developed antibodies to phosphorylated Tyr577 using βc peptides as described in “Materials and methods.” This anti–phospho-βcTyr577 antibody does not recognize other phosphorylated peptides and is specific for Tyr577 of βc. As can be seen in Figure 2B, we found that Tyr577 of βc is phosphorylated in response to GM-CSF and that this phosphorylation occurs independently of the phosphorylation of Ser585. These βc mutants were also examined for their ability to regulate Shc tyrosine phosphorylation in response to GM-CSF. While GM-CSF stimulation of cells expressing either the wtβc or the βcSer585Gly mutant resulted in tyrosine phosphorylation of Shc, no such phosphorylation was observed in cells expressing either the βcTyr577Phe or the βcF8 mutant (Figure 2C).
Ser585 of βc selectively couples to 14-3-3 and p85. CTL-EN cells expressing either the wtβc or the indicated βc mutants were factor deprived for 12 hours in RPMI containing 0.5% FCS, after which the cells (2 × 107 cells/stimulation) were stimulated with 50 ng/mL GM-CSF for 0, 5, and 15 minutes. Following stimulation, cells were lysed and cleared lysates were subjected to immunoprecipitation with either anti-βc antibodies (A-B) or anti-Shc antibodies (C). Immunoprecipitates were then subjected to SDS-PAGE and immunoblotted with anti–phosphoserine-585, anti-p85, anti–14-3-3 (EB1), anti-βc (1C1), anti–phospho-Tyr577, anti–phosphotyrosine (4G10), or anti-Shc.
Ser585 of βc selectively couples to 14-3-3 and p85. CTL-EN cells expressing either the wtβc or the indicated βc mutants were factor deprived for 12 hours in RPMI containing 0.5% FCS, after which the cells (2 × 107 cells/stimulation) were stimulated with 50 ng/mL GM-CSF for 0, 5, and 15 minutes. Following stimulation, cells were lysed and cleared lysates were subjected to immunoprecipitation with either anti-βc antibodies (A-B) or anti-Shc antibodies (C). Immunoprecipitates were then subjected to SDS-PAGE and immunoblotted with anti–phosphoserine-585, anti-p85, anti–14-3-3 (EB1), anti-βc (1C1), anti–phospho-Tyr577, anti–phosphotyrosine (4G10), or anti-Shc.
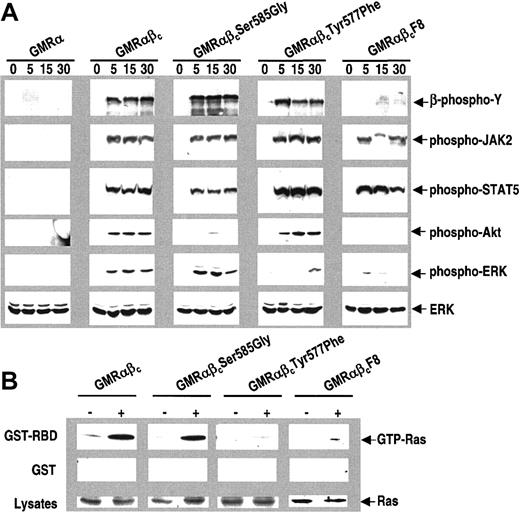
These results show that proximal phosphorylation events within the viability domain of βc couple to different signaling pathways and that Ser585 but not Tyr577 recruits the p85 subunit of PI 3-kinase. To biochemically characterize these separate pathways further, we performed Western blots using phospho-specific antibodies against some key signaling molecules. Increased phosphorylation of JAK2, STAT5, Akt, and ERK in response to GM-CSF stimulation was observed in CTL-EN cells expressing the wtβc (Figure 3A). In CTL-EN cells expressing the βcSer585Gly mutant, stimulation with GM-CSF also resulted in increased phosphorylation of JAK2, STAT5, and ERK, however, phosphorylation of Akt was markedly reduced. In cells expressing the βcTyr577Phe mutant, the phosphorylation of JAK2, STAT5, and Akt was similar to wtβc, however, in this case phosphorylation of ERK was greatly decreased. The βcF8 mutant behaved as a combination of the βcSer585Gly and the βcTyr577Phe mutants being able to induce phosphorylation of JAK2 and STAT5 but defective in phosphorylation of both Akt and ERK (Figure 3A).
Ser585-mediated but not Tyr577-mediated signaling selectively affects Akt activation. (A) CTL-EN cells expressing either GMRα alone, the wt GM-CSF receptor (GMRαβc), or the indicated βc mutants were factor deprived for 12 hours in RPMI containing 0.5% FCS, after which the cells were stimulated with 50 ng/mL GM-CSF for 0, 5, 15, and 30 minutes. Following stimulation, cells were lysed and cleared lysates were subjected to SDS-PAGE and immunoblotted sequentially using anti–phospho-tyrosine antibodies (4G10), anti–phospho-JAK2, anti–phospho-STAT5, anti–phospho-Akt, anti–phospho-ERK, and anti-ERK antibodies as described in “Materials and methods.” To examine the activation of ras in response to GM-CSF (B), cells were factor deprived, stimulated with GM-CSF, and lysed as described above. Cell lysates were then subjected to a pull-down experiment using either 10 μg GST (middle panel) or GST-RBD (top panel). The relative amounts of ras in the lysates were determined by Western blot analysis (bottom panel). The pulldowns were washed and the association of ras was examined by Western blot analysis.
Ser585-mediated but not Tyr577-mediated signaling selectively affects Akt activation. (A) CTL-EN cells expressing either GMRα alone, the wt GM-CSF receptor (GMRαβc), or the indicated βc mutants were factor deprived for 12 hours in RPMI containing 0.5% FCS, after which the cells were stimulated with 50 ng/mL GM-CSF for 0, 5, 15, and 30 minutes. Following stimulation, cells were lysed and cleared lysates were subjected to SDS-PAGE and immunoblotted sequentially using anti–phospho-tyrosine antibodies (4G10), anti–phospho-JAK2, anti–phospho-STAT5, anti–phospho-Akt, anti–phospho-ERK, and anti-ERK antibodies as described in “Materials and methods.” To examine the activation of ras in response to GM-CSF (B), cells were factor deprived, stimulated with GM-CSF, and lysed as described above. Cell lysates were then subjected to a pull-down experiment using either 10 μg GST (middle panel) or GST-RBD (top panel). The relative amounts of ras in the lysates were determined by Western blot analysis (bottom panel). The pulldowns were washed and the association of ras was examined by Western blot analysis.
We also examined the ability of these βc mutants to regulate ras activation using the ras-binding domain of c-Raf (GST-RBD) to pull down GTP-ras following GM-CSF stimulation. While GST-RBD was able to pull down activated ras following GM-CSF stimulation of cells expressing wtβc and βcSer585Gly, no association of GST-RBD with activated ras was observed for the βcTyr577Phe or the βcF8 mutants (Figure 3B).
Ser585 of βc and PI 3-kinase activity are required for promoting survival but not proliferation in response to GM-CSF
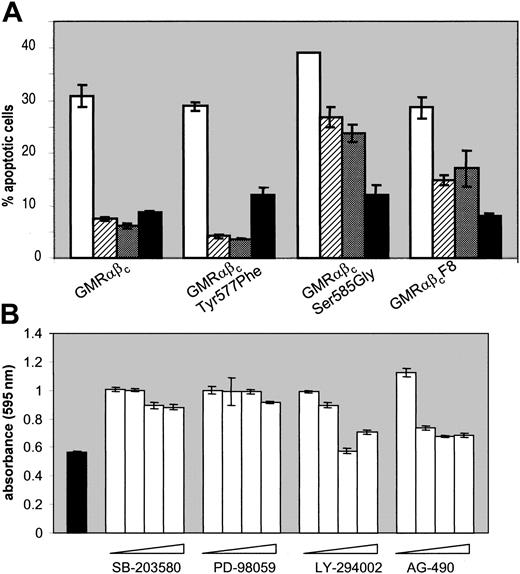
To determine the functional significance of the signaling pathways regulated by βc serine and tyrosine phosphorylation we then examined the ability of the mutant βc receptors to promote cell survival. For these experiments, CTL-EN cells expressing wtβc, βcSer585Gly, βcTyr577Phe, or βcF8 were washed and plated out in medium containing either no factor, GM-CSF, or IL-2 control cytokine. Cell survival was examined after 48 hours by combined annexin V and propidium iodide staining followed by flow cytometry. While wtβc and βcTyr577Phe receptors were able to transduce survival signals in response to GM-CSF, the βcSer585Gly mutant was clearly defective in mediating these signals (Figure 4A).
Ser585 of βc and PI 3-kinase activity are required for GM-CSF–mediated cell survival. (A) Cells were washed and plated out at 5 × 105 cells/mL in medium containing either no factor (white bars), 2 ng/mL GM-CSF (hatched bars), 20 ng/mL GM-CSF (dark gray bars), or 20 ng/mL control cytokine, IL-2 (black bars). After 48 hours, cells were costained with annexin V and propidium iodide and analyzed by flow cytometry as described in “Materials and methods.” The average of duplicate samples ± the standard deviations (SDs) are indicated. (B) CTL-EN cells expressing the wt GM-CSF receptor were washed and plated out as above in medium containing either no factor (solid) or 50 ng/mL GM-CSF (open) with increasing amounts of pharmacologic inhibitor. The SB203580 p38 MAP kinase inhibitor was used at 0, 0.2, 0.6, and 1.2 μM. The PD98059 MEK inhibitor was used at 0, 6, 12, and 20 μM. The LY294002 PI 3-kinase inhibitor was used at 0, 10, 30, and 50 μM. The AG490 JAK2 inhibitor was used at 0, 5, 20, and 30 μM. After 48 hours, cell viability was examined by the MTS reduction assay and measured at 490 nm. The average of triplicate samples ± SD are plotted.
Ser585 of βc and PI 3-kinase activity are required for GM-CSF–mediated cell survival. (A) Cells were washed and plated out at 5 × 105 cells/mL in medium containing either no factor (white bars), 2 ng/mL GM-CSF (hatched bars), 20 ng/mL GM-CSF (dark gray bars), or 20 ng/mL control cytokine, IL-2 (black bars). After 48 hours, cells were costained with annexin V and propidium iodide and analyzed by flow cytometry as described in “Materials and methods.” The average of duplicate samples ± the standard deviations (SDs) are indicated. (B) CTL-EN cells expressing the wt GM-CSF receptor were washed and plated out as above in medium containing either no factor (solid) or 50 ng/mL GM-CSF (open) with increasing amounts of pharmacologic inhibitor. The SB203580 p38 MAP kinase inhibitor was used at 0, 0.2, 0.6, and 1.2 μM. The PD98059 MEK inhibitor was used at 0, 6, 12, and 20 μM. The LY294002 PI 3-kinase inhibitor was used at 0, 10, 30, and 50 μM. The AG490 JAK2 inhibitor was used at 0, 5, 20, and 30 μM. After 48 hours, cell viability was examined by the MTS reduction assay and measured at 490 nm. The average of triplicate samples ± SD are plotted.
In addition, the ability of the βcF8 mutant to promote cell survival was reduced, but not abolished. Although CTL-EN cells expressing the βcSer585Gly mutant failed to activate PI 3-kinase signaling, as evidenced by the lack of Akt phosphorylation (Figure 3A), and underwent apoptosis (Figure 4A), the role of PI 3-kinase in regulating the survival of these cells was not known. We therefore examined the ability of GM-CSF to promote cell viability in the presence of pharmacologic inhibitors that block PI 3-kinase and others that block p38 MAPK, MEK, or JAK2 activity. CTL-EN cells expressing the wtβc were plated out as above in the presence or absence of GM-CSF, with increasing doses of drug and cell viability measured using the MTS reduction assay. Blockade of either p38 MAPK with SB203580 (1.2 μM) or MEK with PD98059 (12 μM) had no detectable effect on cell viability (Figure 4B). However, both the PI 3-kinase inhibitor, LY294002 (30 μM), and the JAK2 inhibitor, AG490 (5 μM), were able to inhibit cell survival. These results indicate that the ability of GM-CSF to regulate PI 3-kinase and JAK2 is critical for regulating cell survival, while the p38 and MEK pathways are not, and directly link Ser585 to a distinct survival pathway.
We also examined the role of Ser585 and tyrosine residues of the βc in regulating cell proliferation in response to GM-CSF. The percentage of cells positive for BrdU and with more than 2N DNA was determined by flow cytometry and the results are shown in Figure 5. GM-CSF promoted DNA synthesis in cells expressing wtβc, βcSer585Gly, and βcTyr577Phe but not in the βcF8 mutant lacking all cytoplasmic tyrosines, indicating that Ser585 and Tyr577 alone are not essential for regulating cell proliferation.
Ser585 or Tyr577 of βc are not required for GM-CSF–mediated cell cycle progression. CTL-EN cells expressing the wt GM-CSF receptor or the indicated mutants were washed and plated out at 1 × 105 cells/mL in RPMI containing 10% FCS and either no factor (nil), 20 ng/mL positive control cytokine IL-2 (IL-2), or 50 ng/mL GM-CSF (GM-CSF) for 20 hours, after which the cells were pulsed for 4 hours with 10 μM BrdU. The cells were then fixed, stained with anti–BrdU-Fluos, and counterstained with propidium iodide (PI). Cells positive for both BrdU and PI were analyzed by flow cytometry, and duplicate samples ± SD are plotted.
Ser585 or Tyr577 of βc are not required for GM-CSF–mediated cell cycle progression. CTL-EN cells expressing the wt GM-CSF receptor or the indicated mutants were washed and plated out at 1 × 105 cells/mL in RPMI containing 10% FCS and either no factor (nil), 20 ng/mL positive control cytokine IL-2 (IL-2), or 50 ng/mL GM-CSF (GM-CSF) for 20 hours, after which the cells were pulsed for 4 hours with 10 μM BrdU. The cells were then fixed, stained with anti–BrdU-Fluos, and counterstained with propidium iodide (PI). Cells positive for both BrdU and PI were analyzed by flow cytometry, and duplicate samples ± SD are plotted.
Ser585 of βc is important for NF-κB activation and the regulation of bcl-2 expression
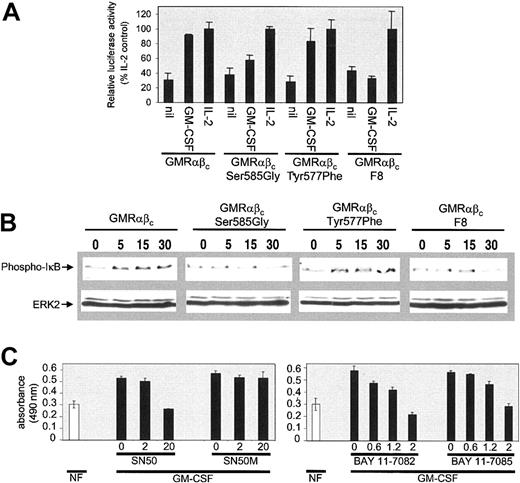
During the course of our investigations we noted that the ability of GM-CSF to promote cell viability was, at least in part, regulated through de novo gene expression, as inhibitors of gene transcription (actinomycin D) or mRNA translation (cycloheximide) blocked GM-CSF–mediated cell survival (data not shown). In addition, the onset of apoptosis for the CTL-EN cells expressing the βcSer585Gly mutant was relatively slow (Figure 4 and data not shown), which suggested that the ability of GM-CSF to regulate cell survival was regulated through the induction of a viability gene program rather than the direct regulation of proapoptotic proteins such as BAD or caspase-9. We therefore examined the regulation of NF-κB transcriptional activity in response to GM-CSF and its role in mediating CTL-EN survival. CTL-EN cells expressing wtβc, βcSer585Gly, βcTyr577Phe, or βcF8 were cotransfected with plasmid constructs containing a promoter containing 6 NF-κB–binding DNA consensus sites linked to a luciferase reporter gene together and a Renilla luciferase control vector. For each cell line, luciferase activity was normalized to an IL-2 control stimulation. While CTL-EN cells expressing the wtβc and the βcTyr577Phe mutant demonstrated NF-κB induction of luciferase activity in response to GM-CSF, this regulation was markedly reduced in cells expressing the βcSer585Gly and the βcF8 mutant (Figure 6A).
Ser585 of βc is important for NF-κB activation in response to GM-CSF. (A) Cells were electroporated (107 cells/electroporation) with 20 μg Ig-κB-firefly luciferase reporter plasmid (pTK81-IgK) and 1 μg Renilla luciferase control vector (pRL). After 24 hours of culture, the cells were plated in medium containing either no factor (nil), 20 ng/mL GM-CSF, or 20 ng/mL IL-2 for a further 12 hours. Cell extracts were then made and luciferase activity was measured using the dual-luciferase assay system. Triplicate samples ± SD were assayed. To examine the ability of GM-CSF to regulate IκB phosphorylation (B), cells were factor deprived for 18 hours in medium containing 0.5% FCS and then stimulated with 50 ng/mL GM-CSF for 0, 5, 15, and 30 minutes. Following stimulation, cells were lysed and lysates were subjected to SDS-PAGE and immunoblotted sequentially with anti–phospho-IκB (B, top panel) and anti-MAP2 (B, bottom panel) antibodies to demonstrate equal loading. The ability of 2 independent inhibitors of NF-κB to block GM-CSF–mediated survival was also examined (C). CTL-EN cells expressing the wt GM-CSF receptor were washed and plated out at 4 × 105 cells/mL in medium containing either no factor (open bar) or 50 ng/mL GM-CSF (solid bar). The peptide inhibitor of NF-κB translocation, SN50, and a control peptide, SN50M, were used at 0, 2, and 20 μM. The inhibitors of IκB phosphorylation, BAY 11-7082 and BAY 11-7085, were used at 0, 0.6, 1.2, and 2 μM. After 48 hours, cell viability was examined by the MTS reduction assay at 490 nm. Triplicate samples ± SD were plotted.
Ser585 of βc is important for NF-κB activation in response to GM-CSF. (A) Cells were electroporated (107 cells/electroporation) with 20 μg Ig-κB-firefly luciferase reporter plasmid (pTK81-IgK) and 1 μg Renilla luciferase control vector (pRL). After 24 hours of culture, the cells were plated in medium containing either no factor (nil), 20 ng/mL GM-CSF, or 20 ng/mL IL-2 for a further 12 hours. Cell extracts were then made and luciferase activity was measured using the dual-luciferase assay system. Triplicate samples ± SD were assayed. To examine the ability of GM-CSF to regulate IκB phosphorylation (B), cells were factor deprived for 18 hours in medium containing 0.5% FCS and then stimulated with 50 ng/mL GM-CSF for 0, 5, 15, and 30 minutes. Following stimulation, cells were lysed and lysates were subjected to SDS-PAGE and immunoblotted sequentially with anti–phospho-IκB (B, top panel) and anti-MAP2 (B, bottom panel) antibodies to demonstrate equal loading. The ability of 2 independent inhibitors of NF-κB to block GM-CSF–mediated survival was also examined (C). CTL-EN cells expressing the wt GM-CSF receptor were washed and plated out at 4 × 105 cells/mL in medium containing either no factor (open bar) or 50 ng/mL GM-CSF (solid bar). The peptide inhibitor of NF-κB translocation, SN50, and a control peptide, SN50M, were used at 0, 2, and 20 μM. The inhibitors of IκB phosphorylation, BAY 11-7082 and BAY 11-7085, were used at 0, 0.6, 1.2, and 2 μM. After 48 hours, cell viability was examined by the MTS reduction assay at 490 nm. Triplicate samples ± SD were plotted.
To further examine the regulation of NF-κB signaling we also examined the phosphorylation of IκB using an IκB phospho-specific antibody. While IκB phosphorylation was regulated by GM-CSF in cells expressing the wtβc and the βcTyr577Phe mutants, no such regulation was observed in cells expressing the βcSer585Gly or the βcF8 mutants (Figure 6B). To determine whether NF-κB activation in response to GM-CSF was required for promoting cell viability, we examined the effect of 2 independent families of pharmacologic inhibitors of NF-κB: SN50, a cell-permeable peptide that binds to the nuclear localization sequence of NF-κB and blocks its nuclear translocation and its transcriptional activity,31 and BAY 11-7082 or BAY 11-7085,32,33 which have been shown to specifically inhibit IκB phosphorylation and therefore block NF-κB activation.34-36 Inhibition of NF-κB activity by SN50, BAY 11-7082, and BAY 11-7085 blocked the ability of GM-CSF to promote cell survival in CTL-EN cells (Figure 6C). No effect on cell survival was observed for the SN50M control peptide.
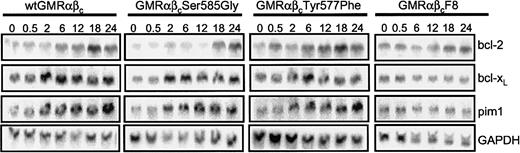
The ability of GM-CSF to regulate the mRNA expression of members of the bcl-2 family of prosurvival genes that exhibit NF-κB binding sites was then investigated.10-12 In addition, we also examined the regulation of pim1 mRNA, which is a known GM-CSF–regulated gene. GM-CSF induced the expression of bcl-2, bcl-xL, and pim1 in cells expressing the wt and βcTyr577Phe with the onset of expression occurring at 2 to 6 hours after stimulation (Figure 7). No expression of A1/bfl1 mRNA was detectable in these cells (data not shown). The βcSer585Gly mutant showed regulation of bcl-xL and pim1 mRNA, but the induction of bcl-2 was clearly reduced with the onset of expression occurring at 18 hours (Figure 7). In 3 independent experiments, the levels of bcl-2 expression (normalized to GAPDH [glyceraldehyde-3-phosphate dehydrogenase]) in the βcSer585Gly mutant were 37%, 21%, and 54% of the wtβc at 12 hours. No clear regulation of any of these genes was observed in response to GM-CSF in cells expressing the βcF8 mutant. Thus, Ser585 is important in regulating cell survival through a pathway that promotes NF-κB activation and bcl-2 expression.
Ser585 of βc is important for the regulation of bcl-2 expression. CTL-EN cells expressing wt or mutant GM-CSF receptors were factor deprived for 18 hours, stimulated with 100 ng/mL GM-CSF for up to 24 hours, and then analyzed for mRNA expression by Northern blot analysis. Total RNA was purified and probed with 32P-labeled cDNA for bcl-2, bcl-xL, pim1, and GAPDH. Results were visualized by exposing the filters to X-ray film and autoradiography.
Ser585 of βc is important for the regulation of bcl-2 expression. CTL-EN cells expressing wt or mutant GM-CSF receptors were factor deprived for 18 hours, stimulated with 100 ng/mL GM-CSF for up to 24 hours, and then analyzed for mRNA expression by Northern blot analysis. Total RNA was purified and probed with 32P-labeled cDNA for bcl-2, bcl-xL, pim1, and GAPDH. Results were visualized by exposing the filters to X-ray film and autoradiography.
Discussion
The findings reported in these studies now trace a novel βc survival pathway from its initiator point at the cell membrane where Ser585 is phosphorylated in response to GM-CSF to its downstream effector points in the nucleus, which include NF-κB activation and the induction of expression of bcl-2. This pathway is shown to selectively control cell survival and to be regulated independently of tyrosine phosphorylation, in particular from Tyr577 within the previously described viability domain.
In the present studies we have shown that Ser585 of βc is phosphorylated in response to GM-CSF stimulation and that this leads to the recruitment of 14-3-3 and p85 (Figure 2A). These events are important for the activation of a PI 3-kinase pathway that leads to the phoshorylation of Akt (Figure 3A) and cell survival (Figure 4A). While PI 3-kinase has been proposed to serve a wide variety of functions in cells, its ability to regulate cell survival is now well established.5 PI 3-kinase phosphorylates phosphatidyl inositols, which then act as membrane docking sites for the recruitment of proteins containing pleckstrin homology (PH) domains. The serine-threonine kinase Akt is one such PH domain signaling partner of PI 3-kinase and is pivotal in the regulation of cell survival in response to a variety of cytokines and growth factors.6 Akt has been reported to phosphorylate and regulate the activity of a growing list of proteins involved in modulating cell viability.6 One such target is the critical regulator of NF-κB activity, IκB kinase (IKK).8,9 NF-κB is normally retained in the cytoplasm by the IκB inhibitory proteins. Following stimulation, IκB becomes phosphorylated by IKK and degraded by the proteosome releasing NF-κB to translocate to the nucleus and regulate gene transcription. Our results show that Ser585 of βc is not only important for PI 3-kinase signaling, but also for regulating NF-κB activation (Figure 6). Furthermore, the induction of bcl-2 mRNA was reduced in CTL-EN cells expressing the βcSer585Gly mutant (Figure 7). We have also demonstrated the importance of NF-κB in regulating CTL-EN survival, as GM-CSF was unable to promote cell survival in the presence of 2 independent families of pharmacologic inhibitors of NF-κB: the inhibitor peptide SN-50 or the drugs BAY 11-7082 and BAY 11-7085 (Figure 6C). Although NF-κB has been shown to regulate bcl-2 transcription,12 our results do not exclude the possibility that NF-κB may regulate other prosurvival genes not examined in these studies. For example, the inhibitor-of-apoptosis (IAP) proteins c-IAP1 and c-IAP2 have been shown to be gene targets of NF-κB.37 Furthermore, additional NF-κB–independent mechanisms for the regulation of prosurvival genes may also contribute to CTL-EN cell viability. For example, NF-κB has been shown to regulate not only bcl-2 but also bcl-xL and A1/bfl1 gene transcription.10,11 However, the induction of bcl-xL was unaffected in the βcSer585Gly mutant, whereas we were unable to detect the induction of A1/bfl1 in response to GM-CSF in CTL-EN cells. It is therefore likely that the induction of bcl-xL by GM-CSF occurs through a mechanism independent of PI 3-kinase and NF-κB, and may involve the JAK/STAT pathway.19
Our results now provide a molecular explanation for the observations made in earlier studies that defined a minimal viability domain in βc encompassing amino acids Asp574-Leu610.16,20-22 We noted that both Ser585 and Tyr577 lay within this domain, and in the present studies we have defined Ser585 as being critical for regulating survival in response to GM-CSF. It is important to note that in addition to the survival pathway regulated by Ser585, an alternative pathway exists that uses βc tyrosine phosphorylation as CTL-EN cells expressing the βcF8 mutant failed to regulate Akt phosphorylation (Figure 3A) and demonstrated diminished viability (Figure 4A). This alternate tyrosine-dependent pathway does not appear to use Tyr577, as Akt phosphorylation and cell survival in cells expressing the βcTyr577Phe mutant were not affected. In addition, signaling through ras and ERK were essentially abolished in cells expressing the βcF8 mutant (Figure 3). Somewhat surprisingly, the phosphorylation of both JAK2 and STAT5 was normal in CTL-EN cells expressing the βcF8 mutant (Figure 3A). The binding of STAT SH2 domains to phospho-tyrosine residues in cytokine receptors has been proposed to be important for STAT tyrosine phosphorylation and activation, however, STAT activation has been shown to occur in the absence of cytokine receptor tyrosine phosphorylation.38 The βcF8 mutant also failed to mediate cell proliferation in response to GM-CSF, while no detectable defect in cell proliferation was observed for the βcSer585Gly or the βcTyr577Phe mutants. Although we cannot rule out an abnormal βc conformation due to the substitution of 8 tyrosines, our results nevertheless suggest that βc tyrosine phosphorylation can serve a number of general roles in the regulation of both cell survival and proliferation.
The results presented in these studies highlight the ability of the βc to use specific residues to independently regulate different signaling pathways. This is best exemplified by the GM-CSF–mediated phosphorylation of Ser585 in the βcTyr577Phe and βcF8 mutants and the phosphorylation of Tyr577 in the βcSer585Gly mutant. Thus, Ser585 is important for 14-3-3/PI 3-kinase signals and cell survival and Tyr577 for Shc/ras/ERK signals. Shc is known to bind Tyr577 of βc via its phosphotyrosine binding domain (PTB) and has been suggested to be important for the regulation of both ERK and PI 3-kinase signaling in response to GM-CSF through its ability to recruit and/or activate a number of signaling molecules such as Grb-2, SOS1, SHP2, Src homology 2–containing inositol phosphatase (SHIP), Gab2, and p85.23,25-27 However, the biologic significance of the Shc/ras/ERK signals generated by Tyr577 remains unclear as cell survival and proliferation were unperturbed in CTL-EN cells expressing the βcTyr577Phe mutant. In fact, the role of ras signaling in response to GM-CSF or IL-3 (both of which signal via the same βc subunit) remains controversial. From experiments using dominant-negative and dominant-active forms of ras, Okuda et al39 have proposed that IL-3 promotes cell proliferation via a ras-dependent pathway, while Terada et al40 have suggested that ras is not important for the regulation of cell proliferation in response to IL-3.
It is interesting to note that until now studies on the mechanism of cytokine receptor activation have focused almost exclusively on the role of receptor tyrosine phosphorylation. In fact, understanding the functional significance of receptor serine phosphorylation has lagged behind considerably. Despite this shortfall, it is worth noting that tyrosine phosphorylation of a number of cytokine receptors is not essential for mediating at least some of the biologic activities of their respective ligands. Such receptors include the thrombopoietin (TPO) receptor (c-mpl), the erythropoietin receptor, the granulocyte colony-stimulating factor receptor, and the growth hormone receptor.41-44 In the case of c-mpl, careful analysis of the biologic role of receptor serine phosphorylation has been undertaken with the phosphorylation of 4 specific serine residues identified as important in regulating proliferation in response to TPO.45 In addition, serine phosphorylation of the insulinlike growth factor I (IGF-I) receptor and 14-3-3 binding have been proposed to be important in the ability of IGF-I to regulate cell survival.46,47 Thus, these receptors as well as others shown to bind 14-3-348-53 may use this novel mode of signaling involving receptor serine phosphorylation and 14-3-3 binding to perform critical tasks in initiating intracellular pathways that lead to the regulation of specific cellular responses.
One expectation that arises from the degree of specificity observed for the binding of individual SH2 or PTB domains to specific receptor phospho-tyrosine motifs is that specific receptor tyrosine residues would be responsible for regulating specific signaling pathways and biologic responses.54 While there are clear examples in which this is the case, the high degree of functional redundancy often observed for receptor tyrosine residues in many cases leaves the question of how receptors achieve signaling specificity to mediate multiple biologic responses unanswered.55 A striking example of the functional redundancy in receptor tyrosine signaling is in the βc subunit itself. Apart from the ability of Tyr577 to regulate Shc/ras/ERK signaling, no individual tyrosine residue has been shown to be involved in any specific signaling pathway or biologic response. Use of a mechanism that involves receptor serine phosphorylation may provide at least part of the explanation by which some receptors achieve both diversity and specificity in signaling.
Prepublished online as Blood First Edition Paper, August 14, 2003; DOI 10.1182/blood-2003-06-1999.
Supported by grants from the Anti-Cancer Foundation of South Australia, the National Health and Medical Research Council of Australia, and a National Institute of Allergy and Infectious Diseases (NIAID) grant from the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Constructs for the NF-κB reporter assays were provided by Pu Xia, Institute of Medical and Veterinary Science, Adelaide. The construct for βcF8 was provided by James D. Griffin, Dana-Farber Cancer Institute, Boston. The construct for GST-RBD was provided by J. L. Bos, Universiteitsweg, Utrecht, the Netherlands.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal