Abstract
The only proven cure for Fanconi anemia (FA)-associated bone marrow failure is successful allogeneic hematopoietic stem cell transplantation (HSCT). However, HSCT with donors other than HLA-identical siblings is associated with high morbidity and poor survival. Therefore, we used preimplantation genetic diagnosis (PGD) to select an embryo produced by in vitro fertilization (IVF) that was unaffected by FA and was HLA-identical to the proband. The patient was a 6-year-old girl with FA and myelodysplasia previously treated with oxymetholone and prednisone. After her parents underwent 5 cycles of IVF with intrauterine transfer of 7 embryos over a span of 4 years, successful pregnancy ensued. Twenty-eight days after delivery, the patient underwent transplantation with her newborn sibling donor's HLA-identical umbilical cord blood hematopoietic stem cells (HSCs). Neutrophil recovery occurred on day 17 without subsequent acute or chronic graft-versus-host disease. Currently, 2.5 years after transplantation, the patient is well and hematopoiesis is normal. In summary, we have described the first successful transplantation, using IVF and PGD, of HSCs from a donor selected on the basis of specific, desirable disease and HLA characteristics. The medical, legal, and ethical issues involved with this approach are discussed. (Blood. 2004;103:1147-1151)
Introduction
Fanconi anemia (FA), an autosomal recessive disease, is characterized by varied congenital physical anomalies, progressive bone marrow failure, and increased predisposition for acute leukemia and other cancers.1-4 The only proven long-term cure of the bone marrow manifestations is successful allogeneic hematopoietic stem cell transplantation (HSCT). HSCT in FA is associated with a particularly high risk for transplantation-related events, including graft failure, graft-versus-host disease (GVHD) and opportunistic infections.1,5-8 Best results have been achieved with HLA-genotype-identical sibling donors.1,7,8 However, most FA patients do not have unaffected HLA-identical sibling donors. HSCT using non-genotype-identical donors is associated with increased cost, transplantation-related events, and poor survival.1,5-7,9,10
Preimplantation genetic diagnosis (PGD) was developed to help couples at high risk for transmitting genetic disease to accurately identify unaffected embryos before implantation and thereby eliminate the potential need for termination.11,12 However, the technology also allows for positive selection of other genetic traits, such as specific HLA haplotypes.
We present the case of a 6-year-old girl with FA-associated bone marrow failure and myelodysplasia who did not have an HLA-identical related donor. Using PGD and in vitro fertilization (IVF) techniques, embryos HLA-identical to the patient and unaffected by FA were selected for intrauterine transfer. In the fifth clinical cycle, a single preselected embryo was transferred, and pregnancy established. The umbilical cord blood (UCB) of the healthy infant was harvested at birth and used as the source of HLA-identical hematopoietic stem cells (HSCs) to reconstitute normal hematopoiesis in his affected sister.
Patient, materials, and methods
History
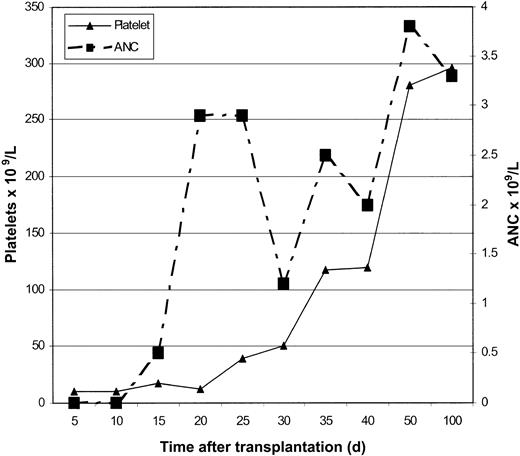
A 3-year-old girl was brought to the University of Minnesota for evaluation of bone marrow failure associated with FA. At birth it was noted that she had multiple congenital malformations, including bilateral radial ray anomalies with absent thumbs, bilateral congenital hip dislocation, and deafness in the left ear. The diagnosis of FA was established based on excessive chromosomal breakage in lymphocytes cultured with diepoxybutane (DEB).13 When she was 2, pancytopenia was first observed (Figure 1); her absolute neutrophil count (ANC) was 0.81 × 109/L, and her platelet count was 104 × 109/L. Therapies included trials of oxymetholone, oral steroids, and a short course of erythropoietin. Subsequently, though the hemoglobin values remained stable (10-12 g/dL), the platelet count persisted typically below 50 × 109/L, and the ANC persisted below 0.5 × 109/L. Bone marrow examination when she was 4 showed features of myelodysplasia (MDS) without an excess of blasts.
Progression of hematologic disease in the patient (bottom) and time course of 5 PGD/IVF attempts by her parents (top).
Progression of hematologic disease in the patient (bottom) and time course of 5 PGD/IVF attempts by her parents (top).
Selection of the donor
Both parents are heterozygotes, and the patient is homozygous for the IVS 4 + 4 A>T mutation. Standard IVF techniques (Colorado Center for Reproductive Medicine, Denver, CO) were combined with PGD (Reproductive Genetics Institute, Chicago, IL).14 Embryo genotyping was performed by obtaining single blastomere biopsy samples of day 3 cleaving embryos using micromanipulation techniques as detailed elsewhere.14 In brief, single-cell polymerase chain reaction (PCR)-amplified DNA was analyzed for IVS 4 + 4 A>T using polyacrylamide gel analysis of ScaI restriction-enzyme-digested product, distinguishing the mutant allele (131-base pair [bp] band) from the wild-type allele (108-bp band) and for HLA type using previously described nested PCR techniques15,16 for allele-level identification of the HLA-A and -B subtype of each embryo.14
Embryos HLA identical to the patient and unaffected by FA (homozygous wild-type allele or heterozygous mutation) were selected for transfer. In the fifth clinical cycle, a single preselected embryo was transferred, and full-term pregnancy resulted (Table 1). Confirmatory genetic testing was performed using chorionic villus sampling (CVS) in the first trimester for FA mutation and HLA type. The UCB was harvested from the placenta after the birth of the child in Denver, Colorado and was delivered to the University of Minnesota, where it was cryopreserved.17 An aliquot was obtained for testing before the UCB unit was frozen (Table 2).
Summary of the 5 PGD/IVF cycles
IVF cycle . | Embryos formed . | HLA-matched, FA-unaffected embryos . | Embryos transferred . | Pregnancies . |
|---|---|---|---|---|
| 1 | 8 | 3 | 2 | 0 |
| 2 | 7 | 2 | 2 | 0 |
| 3 | 4 | 1 | 1 | 0 |
| 4 | 8 | 1 | 1 | 0 |
| 5 | 14 | 1 | 1 | 1 |
IVF cycle . | Embryos formed . | HLA-matched, FA-unaffected embryos . | Embryos transferred . | Pregnancies . |
|---|---|---|---|---|
| 1 | 8 | 3 | 2 | 0 |
| 2 | 7 | 2 | 2 | 0 |
| 3 | 4 | 1 | 1 | 0 |
| 4 | 8 | 1 | 1 | 0 |
| 5 | 14 | 1 | 1 | 1 |
In the 5 clinical cycles, 41 embryos were formed and analyzed. PGD results could be obtained on 38 embryos. All FA-unaffected embryos not transferred were cryopreserved. Results of cycles 2 to 5 were previously reported (JAMA. 2001;285: 3130-3133) and are shown with permission.
Characteristics of the sibling umbilical cord blood
| Collection to infusion time | 28 days |
| FA genotype | IVS 4 + 4 A>T heterozygous (carrier) |
| DEB and mitomycin C testing | In normal range and similar to healthy control sample |
| HLA-type | |
| UCB | HLA-A2, -A26 -B35, -B44, -DRB1*0402, DRB1*1001 |
| Patient | HLA-A2, -A26 -B35, -B44, -DRB1*0402, DRB1*1001 |
| Blood group | |
| UCB | O Rh-positive |
| Patient | O Rh-positive |
| CMV and EBV immunoglobulin M | Negative |
| Nucleated cells | |
| At harvest | 2.5 × 107/kg of recipient |
| After thawing | 1.6 × 107/kg of recipient |
| CD34+ cells (after thawing) | 1.8 × 105/kg of recipient |
| CD3+ cells | 0.4 × 107/kg of recipient |
| Clonogenic assays (after thawing) | 22.5 CFU/100 000 cells plated |
| Collection to infusion time | 28 days |
| FA genotype | IVS 4 + 4 A>T heterozygous (carrier) |
| DEB and mitomycin C testing | In normal range and similar to healthy control sample |
| HLA-type | |
| UCB | HLA-A2, -A26 -B35, -B44, -DRB1*0402, DRB1*1001 |
| Patient | HLA-A2, -A26 -B35, -B44, -DRB1*0402, DRB1*1001 |
| Blood group | |
| UCB | O Rh-positive |
| Patient | O Rh-positive |
| CMV and EBV immunoglobulin M | Negative |
| Nucleated cells | |
| At harvest | 2.5 × 107/kg of recipient |
| After thawing | 1.6 × 107/kg of recipient |
| CD34+ cells (after thawing) | 1.8 × 105/kg of recipient |
| CD3+ cells | 0.4 × 107/kg of recipient |
| Clonogenic assays (after thawing) | 22.5 CFU/100 000 cells plated |
The patient weighed 20.6 kg at admission for HSCT.
Evaluation for HSCT
The diagnosis of FA was reconfirmed at our institution using DEB and mitomycin C testing of peripheral blood lymphocytes.13 Routine laboratory tests revealed serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) values of 228 U/L and 60 U/L, respectively (normal ranges, 0-35 U/L and 0-50 U/L, respectively). Serum bilirubin and alkaline phosphatase values were in the normal ranges. Cardiac function assessment using 2-dimensional echocardiography with M-mode and glomerular filtration rate was normal. Bone marrow examination revealed 60% cellularity with dysgranulopoiesis and dyserythropoiesis. No excess of blasts was seen. Cytogenetic evaluation revealed 46,XX,der(1)t(1;3) (p36.1;q21),der(17)t(1;17)(q23;p11.2), which was detected in all 20 metaphase cells analyzed.
Conditioning regimen and HSCT
The University of Minnesota Institutional Review Board reviewed and approved the protocol and the informed consent documents. The patient was admitted to a high-efficiency particulate air (HEPA)-filtered room in the Pediatric Blood and Marrow Transplantation unit. Because she also had MDS, conditioning therapy consisted of cyclophosphamide and total body irradiation. Cyclophosphamide 10 mg/kg per day was given intravenously over 1 to 2 hours on days -6 to -3 (total dose, 40 mg/kg). Mesna 10 mg/kg was administered intravenously on the days of cyclophosphamide infusion. Intravenous hyperhydration was maintained during the administration and for 24 hours after the administration of the last dose of cyclophosphamide. Total body irradiation of 450 cGy in a single fraction was administered using a linear accelerator on day -1, at a dose of 26 cGy/min. It was prescribed to the midplane of the patient, at the level of the umbilicus, and was delivered with right and left lateral fields.
Cyclosporine A was given as GVHD prophylaxis. Peripheral blood hemoglobin and platelet values were maintained above 8 gm/dL and 10 × 109/L, respectively. Leukocytes were filtered from all blood products, and the blood products were then irradiated with 2500 cGy before infusion. Oral itraconazole (antifungal prophylaxis) 3 mg/kg daily was prescribed for the month preceding transplantation, and intravenous cefazolin was given for streptococcal prophylaxis until the neutrophil count exceeded 0.5 × 109/L. Intravenous and oral acyclovir and oral cotrimoxazole were given to prevent herpes simplex virus and Pneumocystis carinii infection, respectively. Parenteral nutrition was provided for the duration of anorexia.
On the day of transplantation, the sibling donor cord blood was infused through a central venous catheter after standard processing.17 In brief, the cryopreserved UCB unit was identified, retrieved, placed in a sterile zip-locked bag, and sealed. The bag was inserted in a 37°C bath; thawing was accelerated by moving the product in the bath and by gentle kneading. The liquefied product was washed (with 10% dextran 40 and 5% albumin) and resuspended in 10% dextran with 5% albumin before infusion.
Engraftment
Myeloid engraftment was defined as an ANC of 0.5 × 109/L or higher on the first of 3 consecutive days. Platelet engraftment was defined as an untransfused platelet count of 50 × 109/L or higher on the first of 7 consecutive days. Hematopoietic chimerism was estimated on bone-marrow-derived DNA by quantitative PCR analysis of an informative variable number of tandem-repeat regions.18
Results
Engraftment and immune reconstitution
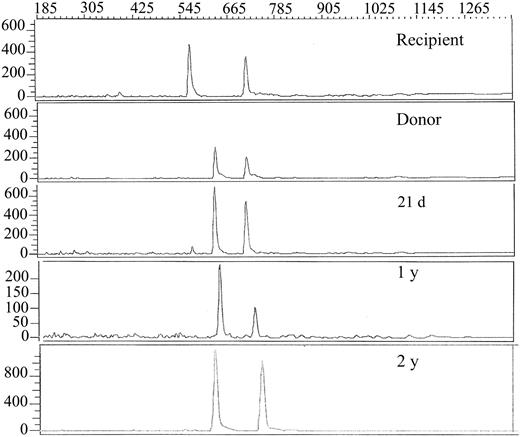
Neutrophil and platelet engraftment occurred at days 17 and 30, respectively (Figure 2), with more than 90% donor chimerism at all time points and 100% donor chimerism at 1- and 2-year studies (Figure 3). The last blood product transfusion occurred 26 days after HSC infusion. Assessment at 24 months after HSCT showed an ANC of 3.1 × 109/L, hemoglobin of 14.8 gm/dL, and platelet count of 304 × 109/L; bone marrow evaluation showed 70% cellularity with normal trilineage hematopoiesis and no evidence of myelodysplasia. Serum immunoglobulin levels were in the normal range 6 months after transplantation.
Assessment of donor chimerism. Electrophoretic profiles of informative variable number of tandem repeat PCR products18 of the recipient and donor (before transplant) and serial posttransplant samples from the recipient bone marrow at indicated time points after HSCT. The donor and recipient are both heterozygous with one shared allele. Thus, one informative donor allele (644 bp) and one informative recipient allele (578 bp) are observed.
Assessment of donor chimerism. Electrophoretic profiles of informative variable number of tandem repeat PCR products18 of the recipient and donor (before transplant) and serial posttransplant samples from the recipient bone marrow at indicated time points after HSCT. The donor and recipient are both heterozygous with one shared allele. Thus, one informative donor allele (644 bp) and one informative recipient allele (578 bp) are observed.
Transplantation-related events
Acute or chronic GVHD did not develop in the patient. Adenovirus-associated gastroenteritis, which developed 1 month after HSC infusion, was clinically mild and resolved by 3 months after HSCT. No opportunistic infections were documented after recovery from adenovirus infection. Asymptomatic hypertransaminasemia, first detected 3 months before transplantation, persisted after HSCT, with serum ALT levels typically between 100 and 200 U/L (range, 20-385 U/L). The etiology has been unclear. No infectious agent was identified. Liver biopsy 1 year after HSCT showed a mild inflammatory pattern consistent with drug-related toxicity. At last follow-up (24 months after HSCT), all medications had been discontinued, the serum ALT level was 141 U/L, the total serum bilirubin level was 0.3 mg/dL, and the serum alkaline phosphatase level was normal.
Cost and time involved
The 5 attempts at IVF with PGD and the period of gestation spanned approximately 4.5 years (Figure 1). The average cost for each cycle was approximately $20 000 ($5300 for medications, $5000 each for HLA identification and IVS4 mutation testing, and intercity travel-related costs).
Discussion
The International Fanconi Anemia Registry data show that 73% of FA patients have overt bone marrow disease by 10 years of age and that the subsequent median survival time is 7 years.3 At present, the only long-term treatment for bone marrow failure and the prevention of acute leukemia in FA patients is successful allogeneic HSCT. Two critical factors associated with favorable outcome are the use of an HLA-genotype-identical donor and HSCT before the development of MDS, leukemia, or infection.1,7-10 In a large International Bone Marrow Transplant Registry series, the 2-year probabilities of survival were 66% and 29% after HLA-matched sibling and alternative donor HSCT, respectively.7 More recent data from the National Marrow Donor Program underscores the poor overall survival associated with unrelated donor HSCT for FA (22% ± 9% at 2 years).10 Unfortunately, as did our patient, most FA patients do not have HLA-matched sibling donors and face the option of higher-risk, alternative-donor HSCT. In addition, patients with FA undergoing alternative-donor HSCT require more intensive conditioning regimens to ensure engraftment. Preparative regimens for unrelated donor HSCT are typically radiation based,10 which increases the risk for late effects such as sterility, endocrinopathies, and potentially cancer, a particularly relevant risk in patients with FA.
PGD was introduced as a viable alternative to prenatal diagnosis for couples at known high risk for conceiving a child with a genetic disease, averting the need to consider the termination of an established pregnancy.11,12 Candidate couples carry structural chromosomal rearrangements or single-gene mutations, and diagnosis is established using fluorescence in situ hybridization or PCR-based techniques, respectively. For monogenic disorders, sufficient sequence information of the particular mutation to design specific primers or probes is essential. For couples with heritable disorders in which the genetic defect is unknown, PGD cannot be offered. Other limitations to the widespread use of PGD include the high expense and the paucity of centers able to analyze a single cell with high reliability in a short enough time period to allow embryo transfer within 24 hours. PGD has now been successfully tested for several single-gene disorders, among them FA, sickle cell anemia, β-thalassemia, cystic fibrosis, and hemophilia.19-21
A newer application is the concept known as IVF with therapeutic intent, which consists of using standard IVF and PGD techniques together to select embryos HLA identical to the affected sibling but free of the genetic disease, thus providing a source of HLA-genotype-identical HSCs. UCB is collected from an otherwise discarded placenta and can be harvested with no risk to the newborn donor. This index case is the first reported use of IVF and PGD with therapeutic intent, with use of the UCB harvested at birth for successful HSCT. Now, more than 2 years after HSCT, the recipient sibling with FA sustains normal donor-derived trilineage hematopoiesis and attends regular school.
Although this case demonstrates the success and feasibility of the technologies used, it also raises a number of practical and ethical issues. For transplantation physicians, there is a question of how to counsel high-risk couples on the use of PGD for the express purpose of creating an HSC donor. It must be made clear that the overall success rate of this approach is as yet unknown. Nonetheless, maternal age is known to have a major impact on the number of retrievable oocytes. Although not an absolute criterion for eligibility, it is known that the success rate diminishes with maternal age. In addition, initiation of the IVF procedure may be delayed if new probes and procedures must be developed to detect the mutant gene. These studies also raised ethical issues. Before the initiation of this project, the investigators met to discuss whether it would be ethically acceptable to extend PGD to include HLA typing. Before and during the application of IVF and PGD to select an HLA-identical embryo, 3 primary ethical concerns were discussed: (1) Should there be restrictions regarding which genetic traits should be tested (eg, HLA alone, sex) using PGD? (2) What will limit equitable access to the combined technologies of IVF and PGD? (3) How do we assess parental motivation?
Selection of traits using PGD
Practically speaking, eligible diseases warranting the use of PGD must be heritable disorders with known genetic mutations and must be potentially benefited by HSCT. Furthermore, the rate of disease progression has to be sufficiently slow to allow the procedure to occur. The time required for IVF and PGD, followed by the period of gestation, has particular relevance for patients in need of HSCT, for whom timing is frequently critical. This practical issue will likely limit the use of the technology to diseases such as FA, sickle cell disease, and β-thalassemia, which are amenable to delayed transplantation and for which HSCT can be performed in a relatively elective manner.1,22 Even when limited to these diseases, the recipient child faces medical risks because of the length and unpredictability of the process with the current technology.
In our patient, because of the time it took to conceive an FA-negative and HLA-matched child, combined with the period of gestation, treatment may have been delayed longer than would have been considered optimal; her bone marrow disease progressed to MDS and could have further progressed to acute leukemia. The development of MDS necessitated the use of a radiation-based conditioning regimen rather than a potentially less toxic, nonradiation-based regimen.23 In certain other genetic diseases, such as the mucopolysaccharidoses and the leukodystrophies, in which disease manifestations may be rapidly progressive, an approach like this may sufficiently delay transplantation to the point that it would defeat the purpose of HSCT. Although improvements in techniques may substantially decrease the number of attempts and the time required for success, the process requires a minimum of approximately 10 months (1 month for IVF plus the period of gestation). This underscores the need to carefully weigh the risk for delayed treatment against the hoped-for benefit of an HLA-matched donor.
Significant ethical issues are also raised by the use of a 2-stage process of PGD—to avoid selecting an embryo with a genetic disease and to select an embryo that is HLA compatible with the eventual HSC recipient. Should there be limits on what characteristics are included in embryo testing? For the therapeutic use discussed in this case report, traits would be limited to the disease and the HLA type. However, it can be foreseen that other traits could be selected through the genetic tests performed, including sex and possibly attributes affecting the physical and behavioral qualities of the future child.
More relevant to what is possible today was a discussion among the investigators, before the project began, about the use of PGD to select a specific HLA genotype if there is no known risk for genetic disease. This would allow couples to “create” an HLA-identical HSC donor for themselves or for other family members. In this instance, HLA compatibility rather than disease prevention is the primary motivation for PGD. Because selection on the basis of HLA has no known impact on the child-to-be, our discussions concluded that this was acceptable. However, it raises the question of whether limits should be imposed regarding which traits should be allowed for embryo selection. There is no federal or other regulatory body oversight or law that would enforce such limits in the United States.
Equitable access
The costs of pursuing PGD for the creation of a stem cell donor are substantial, both in terms of monetary costs and in the investment of time and energy. The cost of each clinical cycle with IVF and dual PGD is estimated at $15 000 to 20 000, with multiple cycles usually required for success. This cost is likely to be prohibitive for all but the most well-to-do patients. Third-party reimbursement has been limited because most payers do not reimburse for IVF use in infertile couples. Therefore, if the creation of stem cell donors using this combination of technologies is appropriate, how can equitable access be ensured for all those patients who could benefit from it? Patient families have begun to make the case to insurers that IVF combined with PGD is a therapeutic use of IVF and should be covered. To date, at least one insurer has agreed to pay all costs; in other cases, there has been partial coverage.
Parental motivation
Finally, the motives of parents who engage in the creation of an HLA-compatible donor raise important ethical issues. Will parents conceive a child with the intention of raising him as a loved and cherished member of the family, or will they be motivated merely by the prospect of creating a life-saving hematopoietic stem cell donor for their sick child? Although it is unethical to use a person as a means to an end, either for parents or for a sick sibling, policy protections are insufficient to prevent parents from placing the donor child up for adoption after birth and after collection of the UCB.
Even when parents love and cherish the donor child, there are concerns regarding the level of risk potentially placed on the donor. For example, if the UCB transplant fails, parents may be faced with a decision about a bone marrow harvest from the infant in the first months of life, exposing the child to procedure-associated risks. At what point would the risk to the donor child be ethically unacceptable, and who should decide? Parents are conflicted in that they must consider the interests of the donor child and the recipient child. A final concern is that some couples may use PGD to select a disease-free or an HLA-compatible embryo with the intent to harvest tissue only and not to bring another child into the world. This scenario would, for example, entail an induced abortion at some point during gestation and the collection of HSCs from the fetal liver. Although such directed donation of tissue from an induced abortion would violate federal law,24 some couples have already inquired about this possibility. Clinics performing PGD and physicians working with patients attempting to create a stem cell donor must be aware of this possibility.
Based on discussions of the issues outlined, the first attempts to use PGD to produce an unaffected HLA-identical donor were limited to 3 highly motivated couples after considerable individualized counseling. Each of these couples had an affected child who could benefit from HSCT, and they had a heritable disorder that could be diagnosed using PGD. In addition, all 3 couples independently desired additional healthy children. After 5 unsuccessful attempts, the first couple (β-thalassemia) continues to pursue IVF with PGD. After 9 unsuccessful attempts, the second couple (FA) withdrew and proceeded to HSCT with an unrelated donor graft. The third couple represents the index case.
With the success and publicity surrounding the index case, there have been a large number of requests regarding this procedure. Over time a number of other issues have surfaced, including the potential use of surrogate (family) gamete donors, collection of fetal liver as a source of hematopoietic stem cells in the event of a spontaneous abortion, and requests for gender selection in addition to HLA. Requests have also been received for selection and storage of HLA-identical embryos for potential future use as a source of embryonic stem cells. Some parents of children with newly diagnosed leukemia have asked to use this technology to have an HLA-identical sibling donor available in the possible event of disease relapse, when the time may be too short to initiate this process.
Clearly, these are not the only issues to consider. For example, what is the fate of the unused embryos? As demonstrated in this case (Table 1), the use of IVF plus PGD to create a stem cell donor leads to the creation of many excess embryos. When IVF is used for infertility, small numbers of embryos are often left and frozen for later disposition. However, to have sufficient probability of success in finding an embryo that is disease free and HLA matched, many more embryos must be made and tested, adding to the estimated 400 000+ embryos frozen in laboratories in the United States alone.25
These are among the ethical issues posing challenges for the wider application of the methodology described. Although the true range of applications of this technology is yet to be fully realized, it is clear that (1) there is significant interest in using this technology by couples who might consider HSCT for a child in the family, (2) physicians must be aware that such technology exists and that families who might have benefited from the technology have been involved in lawsuits against their physicians and medical institutions for not counseling them about it,26,27 (3) HSCT physicians must be intimately involved from the outset because the patient's condition may require HSCT before the birth of an HLA-identical sibling, and (4) significant ethical, legal, and policy issues must be addressed, from the clinic and institution levels to the government and society levels.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-02-0587.
Supported by the Fanconi Anemia Research Fund and the Children's Cancer Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal