Abstract
Germ line mutations in the Adenomatous polyposis coli tumor suppressor gene cause a hereditary form of intestinal tumorigenesis in both mice and man. Here we show that in ApcMin/+ mice, which carry a heterozygous germ line mutation at codon 850 of Apc, there is progressive loss of immature and mature thymocytes from approximately 80 days of age with complete regression of the thymus by 120 days. In addition, ApcMin/+ mice show parallel depletion of splenic natural killer (NK) cells, immature B cells, and B progenitor cells in bone marrow due to complete loss of interleukin 7 (IL-7)-dependent B-cell progenitors. Using bone marrow transplantation experiments into wild-type recipients, we have shown that the capacity of transplanted ApcMin/+ bone marrow cells for T- and B-cell development appears normal. In contrast, although the ApcMin/+ bone marrow microenvironment supported short-term reconstitution with wild-type bone marrow, ApcMin/+ animals that received transplants subsequently underwent lymphodepletion. Fibroblast colony-forming unit (CFU-F) colony assays revealed a significant reduction in colony-forming mesenchymal progenitor cells in the bone marrow of ApcMin/+ mice compared with wild-type animals prior to the onset of lymphodepletion. This suggests that an altered bone marrow microenvironment may account for the selective lymphocyte depletion observed in this model of familial adenomatous polyposis. (Blood. 2004;103:1050-1058)
Introduction
The Adenomatous polyposis coli (APC) tumor suppressor gene is mutated in the hereditary form of colorectal cancer, familial adenomatous polyposis (FAP), and in the majority of sporadic colorectal cancers in humans.1-3 FAP individuals inherit one mutant APC allele and tumorigenesis proceeds following loss of the second APC allele. In sporadic colorectal cancers, tumorigenesis involves somatic inactivation of both APC alleles.
Much of our insight into the functions of APC has come from analysis of murine models. The mouse Apc protein exhibits 90% amino acid (aa) homology to human APC.4 Several different mouse models of intestinal polyposis exist, each harboring different truncating mutations of the Apc gene. The ApcMin/+ mouse is one of the most commonly used models to investigate the molecular mechanisms of intestinal tumorigenesis. ApcMin/+ mice carry a heterozygous germ line mutation at codon 850 of the Apc gene,4 and loss of the normal Apc allele in intestinal epithelium precedes adenoma formation.5 These animals develop adenomas along the length of the small intestine and colon. ApcMin/Min homozygotes arrest development at approximately 5 days after coitus and die in utero.6
Apc is a large 310-kDa protein characterized by the presence of several different functional domains that mediate interactions with numerous protein partners.7 Apc is postulated to have many functions including roles in microtubule dynamics, cell cycle control, cell adhesion, and chromosomal stability.8 However, the tumor suppressor function of Apc appears to lie in its regulation of cellular levels of the protooncogene β-catenin.9-11 β-Catenin has dual roles in both cadherin-mediated cell adhesion and in cell signaling as a transcriptional effector of the canonical Wnt signaling pathway. In this pathway, Apc forms part of a high-molecular weight phosphorylation/destruction complex with axin, glycogen synthase kinase 3β (GSK-3β), protein phosphatase 2a, and GBP/Frat1 (GSK-3-binding protein/frequently rearranged in advanced T-cell lymphomas 1) that targets β-catenin for degradation via the ubiquitin/proteasome system.12-15 In response to a Wnt signal, β-catenin escapes phosphorylation by the Apc complex and translocates to the nucleus where it activates expression of target genes through the Tcf/Lef-1 (T-cell factor 1/lymphoid enhancer factor 1) transcription factors.16,17 Apc can also shuttle between nucleus and cytoplasm further regulating the subcellular localization and turnover of β-catenin.18,19 In the intestinal epithelium, loss of function of Apc promotes tumorigenesis via constitutive activation of β-catenin/Tcf-4-mediated transcription of downstream targets including the growth promoting genes c-Myc20 and cyclin D1.21
The Wnt signaling pathway is an evolutionarily conserved mechanism that governs cell fate decisions and patterning during embryogenesis and in adult tissues. Wnt ligands are secreted glycoproteins that act on members of the frizzled and low-density lipoprotein (LDL) receptor families to activate the canonical Wnt pathway.22,23 In the mouse, at least 18 different Wnt genes have been identified, which show overlapping and/or cell-specific patterns of expression. Several Wnt genes are expressed in hematopoietic tissues and recently Wnt signaling has been shown to regulate hematopoiesis by effects on hematopoietic and stromal cells.24-26 Tcf-1 and Lef-1, the nuclear end points of Wnt signaling, are expressed in developing T and B cells. Tcf-1-/- animals show thymic depletion and a block in T-cell maturation.27,28 Lef-1-/- mice die shortly after birth29 and exhibit defects in pro-B-cell proliferation and survival.24
The emerging roles of Wnt signaling and Tcf-1/Lef-1-driven transcription in the immune system suggest that Apc, as a negative regulator of Wnt signaling, may also function in the differentiation of the lymphoid lineage. Apc protein is found in thymi, lymphocytes, and lymphoblastic cell lines,30 although Apc mutations have not been linked to any hematologic malignancies. In transgenic mice, overexpression of Axin, a component of the Apc phosphorylation complex, results in abnormal thymi and a block in normal development of T lymphocytes,31 again suggesting that components of the Wnt pathway, including Apc, may regulate lymphocyte development.
We have been investigating Apc function at the earliest stages of intestinal tumorigenesis using the ApcMin/+ mouse as a model. We noted that in addition to polyposis these animals exhibit thymic and lymph node atrophy that commences at approximately 80 days old. The similarity in phenotype between Tcf-1-/- and Lef-1-/- mice and the ApcMin/+ mouse with respect to lymphoid development suggested that Apc may also play a role in hematopoiesis. However, a number of other factors are known to cause thymic involution. Several lines of evidence suggest that solid tumor growth itself is associated with immune down-regulation in both humans and in animal models. Thymic atrophy in mammary tumor-bearing mice is associated with severe depletion of double-positive (DP) T cells.32,33 Thymic involution has also been reported in mice with large colorectal carcinoma.34 In patients with sporadic colorectal cancer, a dramatic depletion of lymphocytes has been reported with a selective suppression of cytokines that is apparently related to tumor burden.35,36 Severe thymic atrophy can also occur secondary to various causes including stress, malnutrition, graft-versus-host reactions, immunosuppressive or cytotoxic drugs, and chronic viral infection, especially with HIV.37,38
To determine the nature of the lymphocyte defect in the ApcMin/+ mouse and relevant functional effects of Apc mutation, we characterized the lymphodepletion in these animals. Using bone marrow (BM) transplantation experiments, we have shown that there is no intrinsic defect in repopulating hematopoietic stem and progenitor cells of ApcMin/+ mice. However, there is a marked reduction in the incidence of mesenchymal progenitor cells (MPCs) in the BM of ApcMin/+ mice suggesting that the lymphodepletion observed in these animals may be related to an altered BM microenvironment.
Materials and methods
Animals
C57BL/6J-ApcMin/+ mice and wild-type (wt) C57BL/6J littermates were obtained at 6 to 8 weeks of age from The Jackson Laboratory (Bar Harbor, ME). A breeding colony was then established in-house and offspring were genotyped using allele-specific polymerase chain reaction (PCR) of tail DNA, as described previously.39 All animals were housed in isolators and shown to be specific pathogen-free by bacteriologic and serologic testing. Experiments in the United Kingdom were undertaken under Home Office project and personal license approval.
Histology
Specimens of thymi and lymph nodes were fixed in 4% (wt/vol) paraformaldehyde prior to embedding in paraffin wax. Paraformaldehyde-fixed paraffin-embedded sections were mounted on glass slides and stained with hematoxylin and eosin (H and E) or Giemsa by standard protocols.
Flow cytometry
Single cell suspensions were prepared from thymi, spleens, and BM by ammonium chloride lysis. Monoclonal antibodies used were rat antimouse CD4-fluorescein isothiocyanate (CD4-FITC), CD8-phycoerythrin (CD8-PE), B220-FITC, and immunoglobulin M (IgM) heavy-chain biotin (all from Serotec, Kidlington, United Kingdom), SCA-1-FITC, GR-1 biotin, TER-119-PE, natural killer 1.1-PE (NK-1.1-PE; all from Becton Dickinson, Oxford, United Kingdom), and hamster antimouse CD3-FITC (Caltag, Burlingame, CA). Secondary antibodies were PE/Cy5-conjugated Neutravidin (a gift from Dr R. A. Jones, Leeds, United Kingdom) or Streptavidin-red 670 (Invitrogen, Paisley, United Kingdom).
Fluorescent staining was carried out for 15 minutes on ice followed by one wash with phosphate-buffered saline (PBS) supplemented with 2% fetal calf serum (FCS) and 0.1% NaN3 (Sigma, Poole, United Kingdom). In some experiments propidium iodide (PI; Sigma) at 4 μg/mL was used for live-cell gating. Analyses were performed using Epics XL/MCL (Coulter, High Wycombe, United Kingdom) and dual-laser FACScan (Becton Dickinson) flow cytometers.
Colony assays
For colony-forming unit (CFU)-Mix and pre-B-cell colony assays, 5 × 104 BM cells were seeded in duplicate in complete media MethoCult M3434 and MethoCult M3630, respectively (StemCell Technologies, Vancouver, BC, Canada), and colonies were scored on day 7. For fibroblast CFU (CFU-F) colony assays, 5 × 105 BM cells were seeded in duplicate in murine Mesencult basal medium with mesenchymal stem cell stimulatory supplements (StemCell Technologies) and colonies were scored on day 14.
BM transplantation
Wild-type female C57BL/6J animals were lethally irradiated twice with 4.75 Gy from a Caesium-137 source at an interval of 4 hours. BM cells (2 × 106) from male ApcMin/+ donors were then transplanted intravenously into the tail vein of irradiated recipient mice.
Female ApcMin/+ mice were sublethally irradiated twice with 3 Gy and received 2 × 106 BM cells from male wild-type donors in the tail vein. All transplant-recipient mice received neomycin (0.16 g per 100 mL) in drinking water for 4 weeks following irradiation. Animals were killed at different times after transplantation and the thymi, spleens, and BM were analyzed by flow cytometry and Southern blotting.
Results
Thymic and lymph node atrophy in ApcMin/+ mice
ApcMin/+ mice develop adenomas along the length of the small intestine and colon. In our colony, ApcMin/+ mice develop a total of 53 ± 4 (mean ± SEM) adenomas by 130 days of age.39 In addition to intestinal tumorigenesis, we observed that ApcMin/+ mice had a small or absent thymus when compared with wt littermates. One hundred-day-old animals showed a variable reduction in the size of the thymus of between approximately 60% and 90% when compared with wt littermates (Figure 1A). Both lobes of the thymus appeared equally affected. The rate of thymic atrophy was variable in ApcMin/+ siblings and commenced at approximately 80 to 90 days of age with complete regression of the thymus in most animals by 120 days. The size and morphology of the thymus in 27-day-old animals appeared normal (data not shown). The thymus was affected in all ApcMin/+ animals observed, whereas none of the wt animals observed had affected thymi.
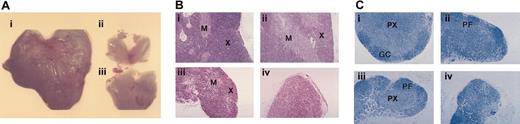
Thymic and lymph node atrophy in ApcMin/+ mice. (A) Thymi from (i) wt and (ii-iii) ApcMin/+ animals at 100 days of age showing the reduction in size and variable rate of atrophy in ApcMin/+ mice. (B) Thymic histology of (i) wt thymus and (ii-iv) ApcMin/+ thymi at 100 days of age. Three different thymi from the same age ApcMin/+ animals are shown demonstrating the initial depletion of cortical thymocytes and the variable rate and stages of atrophy. The most advanced stage of atrophy is shown in panel iv. Sections are stained with H and E. X indicates cortex; and M, medulla. (C) Lymph node histology of (i) wt and (ii-iv) ApcMin/+ lymph nodes at 100 days of age. Inguinal lymph nodes from 3 different ApcMin/+ animals at various stages of atrophy are shown demonstrating the progressive depletion of the paracortex. Sections are stained with Giemsa. PX indicates paracortex; PF, primary follicle; and GC, germinal center. Original magnification, × 10 (A); × 200 (B-C).
Thymic and lymph node atrophy in ApcMin/+ mice. (A) Thymi from (i) wt and (ii-iii) ApcMin/+ animals at 100 days of age showing the reduction in size and variable rate of atrophy in ApcMin/+ mice. (B) Thymic histology of (i) wt thymus and (ii-iv) ApcMin/+ thymi at 100 days of age. Three different thymi from the same age ApcMin/+ animals are shown demonstrating the initial depletion of cortical thymocytes and the variable rate and stages of atrophy. The most advanced stage of atrophy is shown in panel iv. Sections are stained with H and E. X indicates cortex; and M, medulla. (C) Lymph node histology of (i) wt and (ii-iv) ApcMin/+ lymph nodes at 100 days of age. Inguinal lymph nodes from 3 different ApcMin/+ animals at various stages of atrophy are shown demonstrating the progressive depletion of the paracortex. Sections are stained with Giemsa. PX indicates paracortex; PF, primary follicle; and GC, germinal center. Original magnification, × 10 (A); × 200 (B-C).
Histologic analysis of thymi from 100-day-old ApcMin/+ mice revealed cortical thinning of thymocytes in all of the animals observed and complete depletion of cortical and medullary thymocyte populations in the most advanced stages of thymic atrophy (Figure 1B). In thymic remnants, small lymphocytes having small condensed nuclei not typical of thymocytes were present along with stromal epithelial cells (Figure 1B). The epithelial cells in ApcMin/+ thymi at early stages of atrophy appeared normal as judged by immunohistochemistry with an anti-β-catenin antibody, showing a well-established epithelial network that appeared more prominent than in wt animals due to cortical thinning (data not shown). There was no evidence of lipid-laden macrophages in sections of ApcMin/+ thymi, a feature commonly associated with thymic involution induced by glucocorticoids or by stress in normal animals.40
In addition to the thymic atrophy in ApcMin/+ animals, we also observed lymph node atrophy. The inguinal lymph nodes from different 100-day-old ApcMin/+ mice showed a variable reduction in size with associated depletion of the paracortical T-cell population (Figure 1C). The combined thymus and lymph node phenotype observed in ApcMin/+ mice is therefore consistent with a loss of lymphocyte populations in these animals.
Depletion of the double-positive T-cell population in ApcMin/+ mice
The thymic atrophy with initial loss of the immature cortical thymocyte population in ApcMin/+ animals suggested that there may be a disruption in thymocyte development. To determine the nature and stage of any developmental defect, we performed 2-color flow cytometry using anti-CD4 and anti-CD8 antibodies on thymocytes from mice of different ages. Prothymocytes enter the thymus as CD4/CD8- double-negative (DN) cells and then pass through a stage with low CD8+ expression before up-regulating both receptors to become CD4+/CD8+ DP. Following further maturation, DP thymocytes down-regulate one of the coreceptors to become CD4+ or CD8+ single-positive (SP) cells. SP cells migrate out of the thymus to populate peripheral lymphoid organs.41
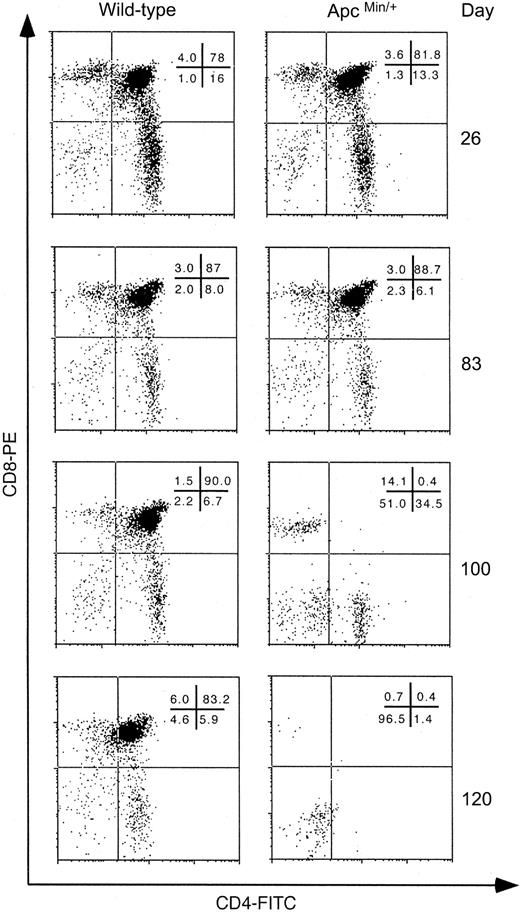
At 26 and 83 days of age, ApcMin/+ mice had similar levels of SP and DP T cells as wt littermates (Figure 2). However, by 100 days of age in ApcMin/+ mice there was complete loss of the DP thymocyte population (Figure 2). This was followed by loss of the more mature CD4+ and CD8+ SP population between 100 and 120 days. This suggests that in the ApcMin/+ mouse there is a defect in T-cell development at the DP stage or earlier.
Depletion of the DP T-cell population in thymi of ApcMin/+ mice. Flow cytometry results for anti-CD4+/CD8+ staining of thymocytes from the different stated ages for wt and ApcMin/+ mice are shown. Expression of surface markers on cells was detected using an Epics XL/MCL flow cytometer and analyzed using the WinMDIv2.7 program (Scripps Research Institute, La Jolla, CA). Forward scatter/side scatter (FCS/SSC) gating was used to exclude debris and doublets and dead cells were gated out on the basis of PI positivity measured on the FL-3 channel. The percentages of cells in each gate are shown in each panel. Each panel is representative of data from at least 3 different animals.
Depletion of the DP T-cell population in thymi of ApcMin/+ mice. Flow cytometry results for anti-CD4+/CD8+ staining of thymocytes from the different stated ages for wt and ApcMin/+ mice are shown. Expression of surface markers on cells was detected using an Epics XL/MCL flow cytometer and analyzed using the WinMDIv2.7 program (Scripps Research Institute, La Jolla, CA). Forward scatter/side scatter (FCS/SSC) gating was used to exclude debris and doublets and dead cells were gated out on the basis of PI positivity measured on the FL-3 channel. The percentages of cells in each gate are shown in each panel. Each panel is representative of data from at least 3 different animals.
Depletion of pro- and pre-B cells in ApcMin/+ mice
The early defect in T-cell development coupled with the lymph node atrophy observed in ApcMin/+ mice raised the possibility that the defect manifested itself prior to T- and B-cell lineage separation and that B-cell development may also be affected.
B-cell development can be divided into distinct phases in mice that can be characterized by the expression of the B220 and IgM surface markers.42 Development proceeds through the pro- and pre-B-cell stages (B220+, IgM-) to immature (B220+, IgM+) and recirculating mature B cells (B220++, IgM+). Analysis of lymphocytes in BM of ApcMin/+ and wt animals using 2-color flow cytometry with anti-B220 and anti-IgM antibodies showed a progressive decrease in the proportions of B220+ B cells in ApcMin/+ animals compared with wt littermates (Figure 3). Up to a 50% reduction in the number of B220+ cells was evident in 100- and 120-day-old animals. This depletion was due to a progressive decrease in the pro- and pre-B cells and immature B cells compared with wt littermates. By 100 days of age, ApcMin/+ animals had a markedly reduced number of pro- and pre-B cells suggesting failure to renew the early B-cell population.
B-cell depletion in the BM of ApcMin/+ mice. To identify B-cell maturation stages, cells were double-stained with monoclonal antibodies anti-B220 and anti-IgM. For analysis, a lymphoid FCS/SSC gate was set up to exclude other cell lineages and the B-cell maturation stages were identified as pro- and pre-B cells with a B220+/IgM- phenotype, immature B cells with a B220++/IgM+ phenotype, and mature B cells with a B220+++/IgM+ phenotype. The proportions of cells present in each subpopulation are given as a percentage of the total B220-positive population. Results are representative of at least 3 different animals per group.
B-cell depletion in the BM of ApcMin/+ mice. To identify B-cell maturation stages, cells were double-stained with monoclonal antibodies anti-B220 and anti-IgM. For analysis, a lymphoid FCS/SSC gate was set up to exclude other cell lineages and the B-cell maturation stages were identified as pro- and pre-B cells with a B220+/IgM- phenotype, immature B cells with a B220++/IgM+ phenotype, and mature B cells with a B220+++/IgM+ phenotype. The proportions of cells present in each subpopulation are given as a percentage of the total B220-positive population. Results are representative of at least 3 different animals per group.
To establish that the block in B-cell development in ApcMin/+ mice occurred prior to the pre-B-cell stage, we measured the number of interleukin 7 (IL-7)-dependent B-cell progenitors in the BM of 100-day-old ApcMin/+ and wt mice. ApcMin/+ mice had 9.6 ± 3.9 (mean ± standard deviation) pre-B-cell progenitors per 105 cells compared with 56.6 ± 10.6 (n = 3) in BM of wt littermates. This suggested that the block in B-cell maturation occurs at the IL-7-dependent stage of B-cell development prior to the pre-B-cell stage.
Myeloid and erythroid differentiation is normal in the BM of ApcMin/+ mice
To determine if there was a parallel impairment in BM myelopoiesis and/or erythropoiesis in ApcMin/+ mice, we measured the total numbers of clonogenic progenitors (granulocyte-erythrocyte-megakaryocyte-macrophage CFUs [CFU-GEMMs], CFU-GMs, and erythroid burst-forming units [BFU-Es]) in a mixed colony assay. No significant difference in total myeloid and erythroid progenitor cell numbers from ApcMin/+ and wt littermates was observed (Figure 4). In order to examine whether myelopoiesis was affected we examined macrophage differentiation in ApcMin/+ and wt littermates by flow cytometric analysis after staining of BM cells with the macrophage progenitor-specific antibodies ER-MP12 and ER-MP20.43 No significant differences were observed (data not shown). Myeloid and erythroid maturation from clonogenic progenitors was also not impaired as proportions of both Gr-1+ (monomyeloid) and TER119+ (erythroid) cells in ApcMin/+ BM remained at similar levels as wt littermates at all stages (data not shown).
Myelopoiesis and erythropoiesis are similar in BM of ApcMin/+ and wt mice. The numbers of clonogenic erythroid and myeloid progenitor cells in a mixed colony assay are shown for mice of different ages. Numbers are shown as mean ± SEM.
Myelopoiesis and erythropoiesis are similar in BM of ApcMin/+ and wt mice. The numbers of clonogenic erythroid and myeloid progenitor cells in a mixed colony assay are shown for mice of different ages. Numbers are shown as mean ± SEM.
ApcMin/+ hematopoietic stem cells have lymphoid differentiation capacity in vivo
The parallel defects in T- and B-cell development observed in ApcMin/+ mice closely resembled those described in Tcf-1-/- and Lef-1-/- mutant mice, respectively, albeit with a later time of onset. This striking similarity suggested that Apc might function directly in T- and B-cell development. The T- and B-cell developmental defects in ApcMin/+ mice could arise from an intrinsic defect in hematopoietic precursor cells or alternatively from changes in the BM microenvironment.
To determine whether the ApcMin/+ genotype had a direct effect on repopulating stem and progenitor cells we assessed the capacity of ApcMin/+ BM cells from different aged mice to reconstitute the hematopoietic system of wt mice. BM from 63-day-old male donor ApcMin/+ mice (ie, prior to the onset of lymphodepletion and from 100-day-old male ApcMin/+ donors, at an advanced stage of lymphodepletion) was transplanted into lethally irradiated wt females. The T-cell population in thymi and spleens and the B-cell populations in BM and spleens were characterized by flow cytometry (Figure 5). Genomic Southern blot analysis on samples isolated at 11 months after transplantation using a Y-chromosome-specific probe showed that the donor contribution was between 80% and 100% (data not shown).
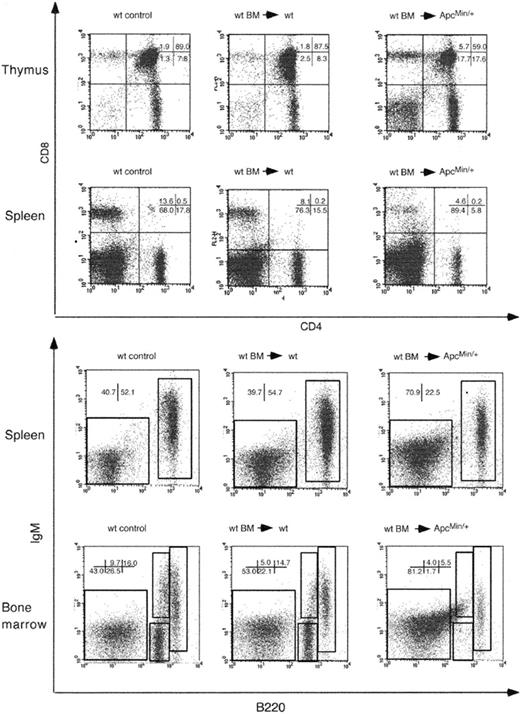
Flow cytometric analysis of lymphocyte populations in wt mice that received ApcMin/+ BM. Analysis of animals that received BM from 63- (63d) or 100-day-old (100d) male donors at 337 days after transplantation. Cells from thymus and spleen were stained with anti-CD4 and anti-CD8 antibodies (top) and from spleen and thymus with anti-B220 and anti-IgM antibodies (bottom). Only cells in the lymphocyte gate were analyzed. The percentages of cells in each gate are shown in each panel. Results are representative of at least 3 different animals per group.
Flow cytometric analysis of lymphocyte populations in wt mice that received ApcMin/+ BM. Analysis of animals that received BM from 63- (63d) or 100-day-old (100d) male donors at 337 days after transplantation. Cells from thymus and spleen were stained with anti-CD4 and anti-CD8 antibodies (top) and from spleen and thymus with anti-B220 and anti-IgM antibodies (bottom). Only cells in the lymphocyte gate were analyzed. The percentages of cells in each gate are shown in each panel. Results are representative of at least 3 different animals per group.
BM grafts of 63- and 100-day-old donors sustained long-term T- and B-lymphoid reconstitution in thymi, spleen, and BM of animals that received transplants when analyzed at 337 days after transplantation. This showed that ApcMin/+ BM could differentiate and maintain T and B cells in a wt environment. Furthermore, as the 100-day-old ApcMin/+ BM graft was capable of lymphoid-lineage reconstitution, this suggested that lymphodepletion in ApcMin/+ mice may be due to an intrinsic stromal cell defect or alternatively to failure of the complete BM microenvironment to support cell survival and/or differentiation.
WT BM can repopulate ApcMin/+ mice in vivo but is still subject to lymphodepletion
To further determine if the lymphodepletion observed in ApcMin/+ animals was due to failure of BM or thymic stroma to support B- and T-cell differentiation, wt BM cells were injected into sublethally irradiated ApcMin/+ animals prior to the onset of lymphodepletion. This would determine first if the animals could be reconstituted and second if the reconstituted immune system would undergo lymphodepletion. Sublethal rather than lethal irradiation was used to ensure survival of the relatively fragile ApcMin/+ animals.
BM from 49-day-old male wt mice was transplanted into sublethally irradiated 51-day-old female ApcMin/+ mice. Flow cytometric analysis of thymi, spleens, and BM of transplant-recipient animals at 93 days of age (42 days after transplantation) showed that ApcMin/+ animals were reconstituted with all T- and B-cell populations to the same extent as wt animals (data not shown). Genomic Southern blotting of samples isolated at 6 weeks after transplantation using a Y-chromosome-specific probe showed that the donor contribution was between 80% and 100% (data not shown). This suggests that the BM and thymic stroma of ApcMin/+ mice were capable of supporting short-term T- and B-cell reconstitution.
Flow cytometric analysis of 143-day-old animals that received transplants (92 days after transplantation) revealed a decrease in the number of DP T cells in thymi with a relative increase in the population of CD4+ and CD8+ SP cells (Figure 6). CD4+ and CD8+ T cells were also reduced in the spleens of recipient ApcMin/+ animals. Similarly, the number of mature B cells in spleen was also reduced. In recipient ApcMin/+ BM, the number of pro- and pre-B cells was reduced by approximately 86% compared with wt recipients. This pattern of depletion mirrored that observed in untreated ApcMin/+ animals. These results show that the reconstituted T- and B-cell populations are subject to lymphodepletion by 142 days of age. The depletion of T- and B-cell populations was not as advanced as that observed in the untreated 120-day-old ApcMin/+ mice (Figures 2, 3). However, as all of the animals tested (n = 3) showed lymphodepletion, albeit at various stages, this suggests that the onset of depletion in these animals was delayed. ApcMin/+ control animals that were sublethally irradiated without transplantation also had a reduced level of lymphodepletion at the same age (data not shown).
Flow cytometric analysis of lymphocyte populations in ApcMin/+ mice that received wt BM. Analysis of animals that received BM from 49-day-old male donors at 142 days after transplantation. Cells from thymi and spleen were stained with anti-CD4 and anti-CD8 antibodies (top) and from spleen and thymus with anti-B220 and anti-IgM antibodies. Only cells in the lymphocyte gate were analyzed. The percentages of cells in each gate are shown in each panel. Results are representative of 3 different animals per group.
Flow cytometric analysis of lymphocyte populations in ApcMin/+ mice that received wt BM. Analysis of animals that received BM from 49-day-old male donors at 142 days after transplantation. Cells from thymi and spleen were stained with anti-CD4 and anti-CD8 antibodies (top) and from spleen and thymus with anti-B220 and anti-IgM antibodies. Only cells in the lymphocyte gate were analyzed. The percentages of cells in each gate are shown in each panel. Results are representative of 3 different animals per group.
Depletion of NK cells in ApcMin/+ mice
The fact that ApcMin/+ mice that received wt BM transplants were fully reconstituted and then underwent lymphodepletion suggested that the depletion might be due to change in the BM microenvironment. One of the major factors regulating the BM microenvironment is the cytokine milieu. IL-7 is crucial for murine T and B lymphopoiesis and the primary source of IL-7 in the BM are stromal cells of nonhematopoietic origin.44 In contrast, IL-7 is not essential for the development of NK cells and IL-7-/- mice have an increased frequency of NK cells in spleen.45,46 We therefore examined NK-cell development in ApcMin/+ mice to determine if defective IL-7 signaling may play a role in the observed lymphodepletion in these animals.
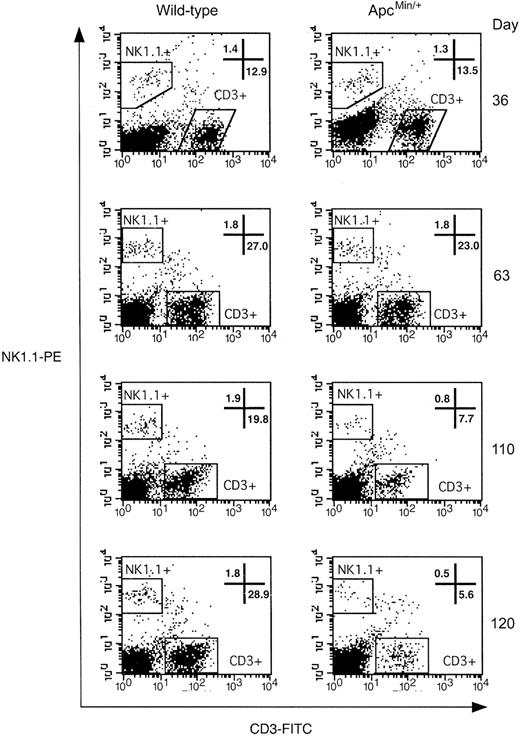
Splenocytes were stained with anti-NK1.1 and anti-CD3 antibodies and defined as NK1.1+ CD3- (Figure 7). At 36 and 63 days of age, ApcMin/+ mice had similar levels of NK cells and CD3+ T cells as wt littermates. However, there was a progressive loss of NK cells in ApcMin/+ mice of approximately 58% and 72% by 110 and 120 days of age, respectively. This was accompanied by depletion of the splenic CD3+ T-cell population. This shows that there is a parallel and progressive depletion of T, B, and NK cells in ApcMin/+ mice, which suggests that defective IL-7 signaling cannot alone account for the observed phenotype.
NK-cell depletion in the spleen of ApcMin/+ mice. Representative dot plots of lymphocyte-gated splenic cells stained with antibodies against mouse CD3 and NK1.1. NK cells are defined as the NK1.1+ CD3- population contained within the top left gate. The bottom right gate contains CD3+ T cells. Numbers indicate the percentage of lymphocytes that fall within the gates shown. Plots shown are representative of at least 3 different animals.
NK-cell depletion in the spleen of ApcMin/+ mice. Representative dot plots of lymphocyte-gated splenic cells stained with antibodies against mouse CD3 and NK1.1. NK cells are defined as the NK1.1+ CD3- population contained within the top left gate. The bottom right gate contains CD3+ T cells. Numbers indicate the percentage of lymphocytes that fall within the gates shown. Plots shown are representative of at least 3 different animals.
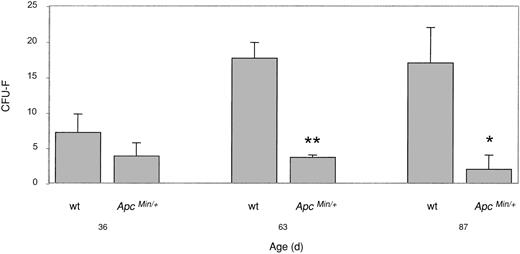
Mesenchymal progenitor cells are reduced in the BM of ApcMin/+ mice
Many of the factors, both secreted and cell associated, that are required for the self-renewal and differentiation of hematopoietic stem cells (HSCs) are produced by the BM stroma.47 The stroma is a complex tissue composed of a heterogeneous population of vascular and connective tissue cell types.48 BM stroma also contains a rare population of MPCs (sometimes referred to as mesenchymal stem cells) that are capable of self-renewal and can differentiate along multiple mesenchymal cell lineages.49 MPCs play a vital role in HSC survival, proliferation, and differentiation. MPCs from whole BM grow as clonal populations derived from a single precursor termed CFU-F.50,51 To determine if the observed lymphodepletion was due to cellular changes in the ApcMin/+ BM stroma, we determined the numbers of CFU-Fs in BM of wt and ApcMin/+ mice.
The number of CFU-Fs derived from BM of ApcMin/+ mice was significantly lower than that of wt animals at 36, 63, and 87 days of age (ie, at stages prior to and at the onset of lymphodepletion) (Figure 8). There was on average a 5-fold decrease in the number of colony-forming cells in ApcMin/+ BM compared with wt animals. There was no difference in the number of colony-forming cells in ApcMin/+ mice of different ages. In wt animals the number of CFU-Fs increased from 36 to 63 days of age and then remained constant (Figure 8).
Colony-forming mesenchymal progenitor cells are reduced in BM of ApcMin/+ mice. The numbers of clonogenic mesenchymal progenitor cells in a CFU-F assay are shown for mice of different ages. Numbers are shown as mean ± SEM. *P ≤ .1; **P ≤ .005; Student t test. n = 3 at 36 days and 63 days, n = 2 at 87 days.
Colony-forming mesenchymal progenitor cells are reduced in BM of ApcMin/+ mice. The numbers of clonogenic mesenchymal progenitor cells in a CFU-F assay are shown for mice of different ages. Numbers are shown as mean ± SEM. *P ≤ .1; **P ≤ .005; Student t test. n = 3 at 36 days and 63 days, n = 2 at 87 days.
Discussion
The Apc tumor suppressor gene codes for a large multidomained protein that is ubiquitously expressed.7 It is mutated in several different tumors of epithelial origin but its function in hematopoietic tissues has not been previously investigated. We show here that ApcMin/+ mice in addition to intestinal tumorigenesis show progressive loss of immature and mature thymocytes from 83 days of age with complete atrophy of the thymus by 120 days. This is accompanied by a parallel and progressive depletion of B-cell progenitors and immature B cells in BM, atrophy of lymph nodes, and depletion of the NK-cell population.
As Apc plays a pivotal role in transduction of the Wnt signaling pathway, we considered that the ApcMin/+ lymphoid phenotype may be related to defective Wnt signaling. Wnt signaling has been shown to be important for T- and B-cell development and for self-renewal of HSCs.26 In Tcf-1 knockout mice, DP thymocytes fail to develop from immature SP thymocytes,27 although in these animals unlike in ApcMin/+ mice, T-cell maturation deteriorates progressively from birth and halts completely around 6 months of age.27,28 Mice lacking both Tcf-1 and Lef-1 have a complete block in T lymphopoiesis.52 Lef-1-/- animals die 2 weeks after birth and Lef-1-/- pro-B cells show reduced cell proliferation and increased apoptosis both in vitro and in vivo.24 In contrast, Wnt signaling in stromal cells has been shown to inhibit B-cell lymphopoiesis. Yamane et al25 showed in vitro that B-cell cultures supplemented with Wnt 3a completely inhibited B-cell development and that this effect was mediated through stromal cells.
The ApcMin/+ T- and B-cell defects described here at first sight resembled those described for Lef-1-/- and double knockout Lef-1-/-/Tcf-/- cells, showing a similar stage-specific block in development. However, in ApcMin/+ mice there was no obvious defect in T- and B-cell progenitors prior to 83 days of age. Using BM transplantation experiments, we showed that there was no apparent intrinsic defect in ApcMin/+ BM stem cells and progenitors as they are capable of long-term T- and B-cell reconstitution in wt mice. This suggests that normal Wnt/Lef-1/Tcf signaling pathways are active in these populations. In contrast, Tcf-1-deficient BM was unable to reconstitute lethally irradiated recipients.28 Furthermore, 100-day ApcMin/+ BM grafts taken from animals at an age when lymphodepletion was advanced could also support long-term lymphoid reconstitution. This shows that hematopoietic stem cell populations as well as T- and B-progenitor cells capable of short-term reconstitution were present in the ApcMin/+ BM at this time and in a wt environment could proliferate and differentiate normally. This suggests that the ApcMin/+ allele does not disrupt Lef-1/Tcf action in hematopoietic progenitor and stem cell populations and that lymphodepletion may therefore be related to changes in the BM microenvironment.
Hematopoiesis is regulated by the complex interplay between the BM microenvironment and stem and progenitor cells. BM stroma contains a heterogeneous population of cells that provides the required cytokines, adhesion molecules, and matrix molecules for stem cell populations to self-renew and differentiate appropriately.47 Transplantation of wt BM into ApcMin/+ animals was performed to determine if the ApcMin/+ hemato-lymphoid microenvironment was capable of supporting reconstitution. As short-term reconstitution of T and B cells was achieved, this suggested that the microenvironment could initially support seeding of transplanted BM cells and sustain lymphoid development. However, by 143 days of age ApcMin/+ animals grafted with wt BM of a similar age showed the same pattern of lymphodepletion with loss of T- and B-cell progenitors in thymi, spleen, and BM. This suggests that the BM microenvironment in ApcMin/+ animals cannot sustain lymphopoiesis.
BM stromal cells originating from MPCs play a major role in supporting hematopoiesis in the BM. Surprisingly, we observed that the number of colony-forming MPCs was markedly reduced in the BM of ApcMin/+ mice compared with wt animals. This reduction was already evident in young animals (36 days of age) at a time when the lymphocyte compartment appeared normal. There was no change in the number of CFU-Fs with age in ApcMin/+ mice. This raises the possibility that the ApcMin/+ genotype exerts an intrinsic effect on the development of mesenchymal progenitors in BM. This could arise from a dominant-negative effect of the truncated protein expressed from the ApcMin allele, from haploinsufficiency for full-length Apc protein, or by loss of Apc function via somatic mutation of the wt Apc allele. All 3 mechanisms have been suggested to deregulate Wnt signaling. However, our own preliminary data (S. Holwell, personal oral communication, June 2003) suggest that there is no loss of the wt Apc allele in cultured MPCs.
The reduction in MPCs in ApcMin/+ mice raises the possibility that Apc and/or Wnt signaling regulate proliferation and/or differentiation of mesenchymal progenitors. BM stromal cells express frizzled receptors and respond to WNT proteins25 and a number of Wnt proteins are found in BM.24,53 In this context, it is interesting to note that TCF-4-/- animals have reduced numbers of epithelial stem cells in the intestine54 showing Wnt involvement in the regulation of stem cell compartments. Furthermore, in the hematopoietic system, stimulation of HSCs and progenitors with soluble Wnt proteins or downstream activators of the Wnt signaling pathway leads to their expansion. Reduction of the MPC population in ApcMin/+ mice suggest that Wnt signaling may play a similar role in the expansion/differentiation of MPCs and that failure to expand the MPC population may underlie the lymphodepletion in these animals.
It is not yet clear why lymphodepletion in ApcMin/+ mice starts at around 80 days of age while prior to then, T-, B-, and NK-cell development appears normal. The timing of onset may reflect a cumulative effect of a reduced MPC population over time, or alternatively that secondary factors may be involved. Physiologic states including stress and nutritional deficiency may trigger lymphodepletion by exerting an additional effect on a fragile BM microenvironment. At 80 to 90 days of age at the onset of lymphodepletion, ApcMin/+ mice have macroscopically visible adenomas in the intestine, which equates to a diameter larger than 1 mm in size. From this stage onwards, these animals exhibit progressive physiologic stress associated with tumor burden. It is possible that this factor may contribute to lymphodepletion. BM stromal cells have been shown to protect hematopoietic precursors from corticosteroid-induced apoptosis,55 suggesting a possible relationship between tumor burden and an altered BM compartment in ApcMin/+ mice.
It is not known if the MPC population is altered or if lymphodepletion as observed in ApcMin mice occurs in individuals with FAP who carry germ line mutations in the APC gene. Lymphopenia in patients with sporadic colorectal cancer has, however, been described and changes in cytokine levels were documented.35,36 The ApcMin/+ mouse provides a unique model to investigate regulation of proliferation and differentiation of BM MPCs and to investigate the relationship between the BM stroma, lymphodepletion, and immune suppression relating to tumorigenesis in vivo.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-03-0707.
Supported by the Medical Research Council and Yorkshire Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Lars Nitschke for critical reading of this manuscript and David Brooke, Lyndsey Williams, and Andrew Horner for expert technical assistance.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal