Abstract
CD26 is a T-cell activation antigen that contains dipeptidyl peptidase IV activity and binds adenosine deaminase. Recent work showed that specialized membrane microdomains, also known as lipid rafts, play a key role in T-cell signaling. In this study, we investigate the role of CD26 in cord blood T-cell activation and signal transduction. We demonstrated that different expression levels of CD26 were observed between cord blood T cells (CBTCs) and peripheral blood T cells (PBTCs) and that CD26+CD45RA+ CBTCs were different compared with CD26+CD45RA+ PBTCs. Moreover, the comitogenic effect of CD26 was not as pronounced in CBTCs as in PBTCs. We also showed that CD26 cross-linking induced less phosphorylation of T-cell receptor-signaling molecules, lymphoid T-cell protein tyrosine kinase (Lck), zeta-associated protein 70 (ZAP-70), T-cell receptor ζ (TCRζ), and linker for activator of T cells (LAT) in CBTCs than in PBTCs. Furthermore, CD26 molecules associated with CD45RA molecules outside lipid rafts in CBTCs. Our results suggest that strong physical linkage of CD26 with CD45RA outside lipid rafts may be responsible for the attenuation of T-cell activation signaling through CD26, which may be responsible for immature immune response and the low incidence of severe graft-versus-host disease in cord blood transplantation. (Blood. 2004;103:1002-1010)
Introduction
CD26 is a 110-kDa cell surface glycoprotein that possesses dipeptidyl peptidase IV activity in its extracellular domain1,2 and has an important role in T-cell activation.3 Although constitutively expressed in liver, intestine, and kidney, CD26 expression level is tightly regulated on T cells and its density is markedly enhanced after T-cell activation.4 In the resting state of human T lymphocytes, CD26 is preferentially expressed on a subset of CD4+ memory T cells, and this CD4+CD26high T-cell population has been shown to respond maximally to recall antigens.5 In fact, CD26 is involved in the processes of T-cell signal transduction, as are costimulatory molecules like CD28 with T-cell receptor (TCR) complex stimulation.6,7
Intensive work over the past several years has characterized the T-cell surface molecule CD26 as a receptor capable of generating T-cell costimulatory signals.8,9 Cross-linking of CD26 and CD3 with immobilized monoclonal antibodies (mAbs) induced T-cell activation and interleukin-2 (IL-2) production.5 Moreover, anti-CD26 antibody treatment of T cells led to decreased surface expression of CD26 via its internalization, and this modulation of CD26 resulted in an enhanced proliferative response to anti-CD3 or anti-CD2 stimulation.9 Cross-linking of CD26 by the anti-CD26 mAb 1F7 induced increased tyrosine phosphorylation of an array of intracellular proteins involved in TCR/CD3-mediated signal transduction, including lymphoid T-cell protein tyrosine kinase (Lck), zeta-associated protein 70 (ZAP-70), TCRζ, mitogen-activated protein kinase (MAP kinase, or extracellular-regulated kinase [ERK1/2]), and phospholipase Cγ (PLCγ).10 However, the exact mechanisms responsible for CD26-mediated tyrosine phosphorylation and costimulation are still unclear, particularly in view of the fact that CD26 has a short cytoplasmic domain consisting of only 6 amino acid residues, without intrinsic protein tyrosine kinase function or known binding motifs for tyrosine kinases.5 Antibody-induced modulation of CD26 on T cells resulted in a concurrent decrease in CD45RO expression, enhanced phosphorylation of the TCRζ, and increased Lck activity.11 CD26 is therefore involved in essential T-cell signaling events through its physical and functional association with structures with key roles in T-cell activation.
Recent studies demonstrated that T-cell activation induced by anti-CD3 stimulation led to a redistribution of the detergent-insoluble glycolipid-enriched domains (DIGs; also called lipid rafts or membrane microdomains),12-15 and the raft-resident costimulatory molecules strengthened TCR-mediated signaling when coligated with CD3 by inducing aggregation of rafts as well as enhancing association of TCR/CD3 and rafts. We have recently shown that CD26 molecules are present in membrane rafts. Importantly, binding of the CD26 molecules by the anti-CD26 mAb 1F7 led to the recruitment of additional CD26 molecules to the lipid rafts and subsequent interaction of CD26 with CD45 tyrosine phosphatase in the raft fraction, especially CD45RO.16
Contradictory evidence has been reported concerning the functional differences between human neonatal and adult immune cells. In general, cord blood T cells (CBTCs) show decreased levels of certain cell surface molecules and display an inability to secrete cytokines relative to adult peripheral blood T cells (PBTCs).17-20 It has been described that CBTCs consist of a large population of CD45RA+-expressing cells and a very small population of CD45RO+-expressing cells when compared with PBTCs.21,22 Although such differences are associated with the well-known immunologic immaturity of newborns, the mechanisms underlying the physiologic immature immune response of neonatal T cells are poorly defined. Furthermore, human cord blood stem cell transplantation becomes increasingly important because of its potential use in allogeneic bone marrow reconstitution with low incidence of graft-versus-host disease (GVHD).23-28 The phenomenon is also closely associated with functional immaturity of neonatal immune cells. For instance, T-cell proliferation after secondary stimulation was significantly reduced and alloantigen-specific cytotoxicity was decreased in CBTCs. Moreover, production of the cytokines IL-2, IL-4, interferon γ (IFN-γ), and tumor necrosis factor α (TNF-α) in activated CBTCs was significantly reduced compared with PBTCs.29-33
Since the CD26 molecule plays an important role in T-cell differentiation as well as in T-cell activation, we attempted to define the role of CD26 in cord blood T-cell activation and signal transduction. We demonstrated that CD26+CD45RA+ CBTCs were different compared with CD26+CD45RA+ PBTCs and the comitogenic effect of CD26 was not as pronounced in CBTCs as in PBTCs. Furthermore, we showed that CD26 cross-linking induced less phosphorylation of Lck, ZAP-70, and linker of activated T cells (LAT) in CBTCs than in PBTCs, and CD26 molecules comodulated with CD45RA molecules outside lipid rafts in CBTCs. Our results suggest that strong physical linkage of CD26 with CD45RA outside lipid rafts may be responsible for the attenuation of T-cell activation signaling through CD26, which may be responsible for immature immune response and the reported low incidence of severe GVHD in cord blood transplantation.
Materials and methods
Cells
Samples of peripheral blood, umbilical cord blood, and the buffy coat were obtained according to institutional guidelines with an informed consent from all healthy volunteers. T cells were purified and cultured as described previously.34 For preparation of CD45RA+ and CD45RO+ cells, PBTCs and CBTCs were negatively selected with anti-CD45RA mAb and anti-CD45RO mAb (2H4 and UCHL-1) in combination with goat antimouse immunoglobulin G (IgG) mAb-conjugated immunomagnetic beads (Dynal, Oslo, Norway). The purities of these preparations were more than 98% by flow cytometric analysis with fluorescein isothiocyanate (FITC)-conjugated anti-CD45RA mAb and phycoerythrin (PE)-conjugated anti-CD45RO mAb (all from Beckman-Coulter, Miami, FL).
Antibodies and reagents
Anti-CD3 mAb OKT-3, anti-CD26 mAb 1F7, 5F8 (as previously reported 1F7 and 5F8 mAbs defined distinct epitopes of CD26), anti-CD28 mAb 4B10, anti-CD45RA mAb 2H4, and anti-CD45RO mAb UCHL-1 were previously described.1,11 The following antibodies were purchased: mouse monoclonal anti-Lck (BD-PharMingen, San Diego, CA); mouse monoclonal anti-TCRζ (Beckman-Coulter); mouse monoclonal phosphotyrosine (4G10), rabbit anti-phosphor-ZAP-70 (Y319), rabbit anti-LAT (Upstate Biotechnology, Lake Placid, NY); rabbit anti-ZAP-70 (Santa Cruz Biotechnology, Santa Cruz, CA); goat antimouse IgG (H+L; Jackson Immuno-Research, West Grove, PA); horseradish peroxidase (HRP)-labeled antimouse and antirabbit Ig (Amersham Pharmacia, Piscataway, NJ); FITC-conjugated cholera toxin B subunit (CTB), Texas red-conjugated streptavidin, and methyl-β-cyclodextrin (MβCD; all from Sigma-Aldrich, St Louis, MO); and enhanced chemiluminescence (ECL) reagents (PerkinElmer Life Sciences, Boston, MA).
Flow cytometry
T cells were treated with 0.5% mouse serum (Dako, Glostrup, Denmark) for 15 minutes at 4°C to block the Fc receptor and stained with FITC-conjugated anti-CD3 mAb (Becton Dickinson, San Jose, CA) and phycoerythrin (PE)-conjugated anti-CD26 (Ta1), PE-conjugated anti-CD28, PE-conjugated CD45RA, or PE-conjugated CD45RO. Cells were also stained with the corresponding FITC- and PE-conjugated isotype-matched mAbs (Beckman-Coulter). Analysis of fluorescence staining was performed with FACS Calibur flow cytometer and CELLQuest Software (Becton Dickinson).
Proliferation assays
PBTCs and CBTCs were cultured in 96-well flat-bottom plates (Corning Life Sciences, Acton, MA) at a density of 1 × 105 cells and were costimulated with a combination of immobilized mAbs to CD3 (OKT-3) and CD26 (1F7) or CD3 and CD28 (4B10). To assay for proliferation, after 72 hours of incubation, cells were pulsed with 1 μCi (0.037 MBq) of [3H] thymidine (PerkinElmer) for the last 18 hours. Cells were then harvested and incorporation was determined by scintillation spectrometry.
Comodulation study
For antibody-induced modulation studies, cells were incubated for various periods of time at 37°C in Iscove modified Dulbecco medium (IMDM) containing 2 μg/mL anti-CD26 mAb (1F7). Subsequently, surface expression of CD26, CD45RA, and CD45RO after modulation on PBTCs and CBTCs was analyzed by flow cytometry.
T-cell stimulation
An equivalent number of PBTCs and CBTCs was used to analyze tyrosine protein phosphorylation as described previously.10 Upon lipid rafts disruption, PBTCs and CBTCs were resuspended (5 × 106) in IMDM with or without 10 mM MβCD and incubated at 37°C for 5 minutes, following 1F7 (10 μg/mL) for 30 minutes at 4°C. Cells were washed once with cold IMDM and then incubated with 200 μL of medium containing goat antimouse IgG (5 μg/mL) for various periods of time at 37°C. The reactions were terminated by the addition of 1 mL of ice-cold phosphate-buffered saline (PBS) containing 5 mM ethylenediaminetetraacetic acid (EDTA), 10 mM NaF, 10 mM Na pyrophosphate, 0.4 mM Na3VO4. Cell lysates were prepared and Western blotting was performed after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), as described previously.16 Specific antigens and phosphorylated tyrosine were probed by the corresponding mAbs and antiphosphotyrosine mAb 4G10, respectively, followed by an antimouse or antirabbit horseradish peroxidase-conjugated IgG. The proteins were revealed by enhanced chemiluminescence (ECL).
Isolation of raft fractions
To obtain the DIG/raft membrane fraction, PBTCs and CBTCs (1 × 108) were stimulated for 2 minutes with anti-CD3 mAb or anti-CD26 mAb. Fractionated lysates were obtained as described previously.16 Equal amounts of cell lysates were separated on a 12% SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membrane for immunoblotting.
Membrane patching and immunofluorescence confocal laser microscopy
For all fluorescence microscopy experiments, cells (5 × 105 cells/slip) were attached to TESPA-coated coverslips (3-aminopropyltri-ethoxysilane; MATSUNAMI GLASS, Tokyo, Japan) by incubation for 10 minutes at room temperature. For observation of colocalization of CD26 and CD45RA, cells were incubated with biotinylated anti-CD26 mAb (5 μg/mL) on ice for 30 minutes. Following washing twice with cold PBS, cells were incubated with Texas red-conjugated streptavidin at 37°C for 20 minutes to induce patching. For fixation, T cells were treated with 3.7% paraformaldehyde in PBS for 8 minutes at room temperature. After being blocked with 3% bovine serum albumin (BSA)/PBS, T cells were incubated with FITC-conjugated anti-CD45RA mAb (2H4, 5 μg/mL). For observation of CD26 in lipid rafts, after patching of CD26 was achieved in the same manner, T cells were incubated with CTB-FITC for 30 minutes at room temperature again after blocking with 3% BSA/PBS. Confocal laser microscopy was performed with an Olympus IX70 confocal microscope with 60 objective lenses (Olympus, Tokyo, Japan) using laser excitation at 488 and 590 nm. The widths of the FITC and Texas red emission channels were set to maximize specificity.
Cell surface cross-linking and immunoprecipitation
PBTCs and CBTCs (1 × 108) were incubated with anti-CD26 mAb (1F7 mAb, 10 μg/mL) in IMDM for 30 minutes at 4°C. After washing once with cold IMDM, T cells were then incubated with goat antimouse IgG (5 μg/mL) at 37°C for 30 minutes. Cells were washed twice with cold PBS and incubated with cross-linker solution DTSSP (3,3′-Dithiobis [sulfosuccinimidylpropionate]; Pierce, Rockford, IL) in PBS at room temperature for 30 minutes. The reaction was quenched by the addition of Tris-HCl at pH 7.5 to a final concentration of 20 mM and incubation for 15 minutes. T cells were then washed 3 times in PBS and lysed at 1 × 108 cells per mL in 4°C lysis buffer (10 mM Tris-HCl at pH 7.5, 150 mM NaCl, 1% 3-[(3-Cholamidopropyl)dimethylammonio]-1-propane-sulfonate [Sigma-Aldrich]) and a mixture of proteinase inhibitor (Sigma-Aldrich). After 30 minutes at 4°C, lysates were prepared by a 15-minute centrifugation at 4°C and 15 000 g and were then immunoprecipitated by protein Sepharose-A beads (Amersham Pharmacia). After a 2-hour incubation period at 4°C under constant agitation, the beads were washed 4 times in lysis buffer. The precipitated proteins were separated by SDS-PAGE on a 6% gel under reducing conditions and transferred to PVDF membrane. Blots were probed with anti-CD45RA mAb (2H4) and anti-CD26 mAb (5F8) and detected with HRP-conjugated secondary antibody, using ECL.
Results
Differences in expression level of CD26 between PBTCs and CBTCs
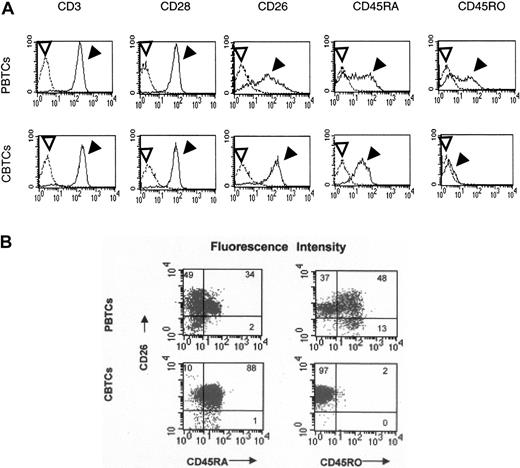
It is well known that cord lymphocytes have immunologic defects compared with adult lymphocytes.17-20 Since CD26 is involved in memory T-cell function as well as in T-cell costimulation,4,15 we defined the role of CD26 in the activation of cord blood T cells by first examining cell surface expression levels of CD3, CD26, CD28, CD45RA, and CD45RO on PBTCs and CBTCs by flow cytometry with the respective mAbs. Flow cytometric analysis revealed similar expression levels of CD3 and CD28 between PBTCs and CBTCs, and the percentages of the cell subpopulations expressing CD3 and/or CD28 were not different in these T-cell subsets. PBTCs exhibited similar ratios of CD45RA+CD45RO- T cells and CD45RA-CD45RO+ T cells, while CBTCs expressed a large population of CD45RA+CD45RO- and a smaller population of CD45RA-CD45RO+. The expression level of CD26 in PBTCs is composed of 3 peaks as previously described5 ; in contrast, the expression level of CD26 in CBTCs is composed of 1 peak (Figure 1A). We next examined the percentage of the cell subpopulations expressing CD26+CD45RA+ or CD26+CD45RO+ in PBTCs and CBTCs. The percentage of T-cell subpopulation expressing CD26+CD45RA+ was 34% and that of CD26+CD45RO+ was 48% in PBTCs in this representative donor. Moreover, CD26high T cells expressed CD45RO antigen. In contrast, the percentage of T-cell subpopulation-expressing CD26+CD45RA+ was more than 88% in CBTCs and that of CD26+CD45RO+ was less than 2% in these representative CBTCs (Figure 1B). In additional donors, similar findings were observed. These results showed that the expression pattern of CD26 was different between PBTCs and CBTCs.
Cell surface expressions of CD3, CD26, CD28, CD45RA, and CD45RO on PBTCs and CBTCs. Data are represented by histograms in which cells were stained with stated mAbs (filled arrowheads) or isotype-matched mAbs (open arrowheads) (A) and by dot plots (B). The data are representative of 20 different donors.
Cell surface expressions of CD3, CD26, CD28, CD45RA, and CD45RO on PBTCs and CBTCs. Data are represented by histograms in which cells were stained with stated mAbs (filled arrowheads) or isotype-matched mAbs (open arrowheads) (A) and by dot plots (B). The data are representative of 20 different donors.
Inability of CD26 to deliver efficient comitogenic signal to CBTCs
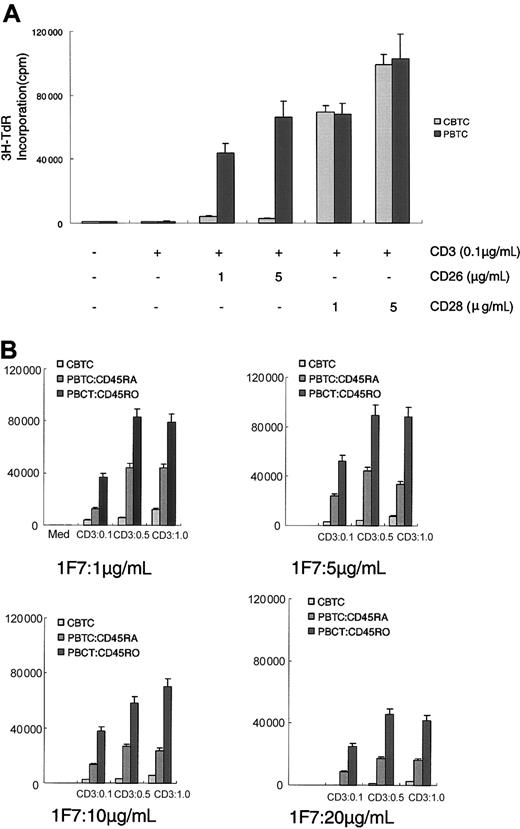
CD26 is a costimulatory molecule via the CD3 pathway of activation in mature T cells.5 To investigate the comitogenic effect of CD26 and CD28 on proliferation of PBTCs and CBTCs, those cells were stimulated with mAbs to CD3 and CD26 or CD3 and CD28. Dual stimulation with mAbs to CD3 and CD28 induced enhanced proliferative activity of PBTCs and CBTCs. On the other hand, anti-CD26 mAb (1F7) and submitogenic doses of anti-CD3 mAb led to an intense increase in proliferative activity of PBTCs. However, anti-CD26 mAb was not sufficient to induce proliferation of CBTCs in the presence of submitogenic dose of anti-CD3 (Figure 2A). As the CD26-expressing T-cell subset of CBTCs expressed a high level of CD45RA, we next investigated the proliferative activity of CBTCs induced by CD26 compared with the PBTC subpopulation expressing CD45RA or CD45RO. The addition of anti-CD26 mAb up to 10 μg/mL had a preferentially comitogenic effect on anti-CD3-mediated proliferation of PBTCs expressing CD45RA or CD45RO. An anti-CD26 mAb dose of 5 μg/mL mediated proliferation that was greater in magnitude than any other dose of anti-CD26 mAb. On the other hand, an anti-CD26 mAb dose of 20 μg/mL did not have a fully comitogenic effect in PBTCs. However, the addition of anti-CD26 mAb did not result in any significant increase in CBTC proliferation (Figure 2B). These results therefore suggest that the comitogenic effect of CD26 is not as efficient in CBTCs as in PBTCs, although a high level of CD26 is found on CBTCs.
Comitogenic effect of CD26 on CD3-dependent proliferation of CBTCs compared with PBTCs, CD45RA+ PBTCs, and CD45RO+ PBTCs. (A) Proliferation assays were performed as described in “Materials and methods” with PBTCs and CBTCs incubated in the presence of submitogenic doses of solid-phase immobilized anti-CD3 (OKT-3) and solid-phase immobilized anti-CD26 (1F7) at the doses indicated or anti-CD3 and anti-CD28 (4B10). (B) Proliferation assays data of CBTCs compared with PBTCs expressing CD45RA+ or CD45RO+. The data are expressed as mean ± standard error for 10 different donors.
Comitogenic effect of CD26 on CD3-dependent proliferation of CBTCs compared with PBTCs, CD45RA+ PBTCs, and CD45RO+ PBTCs. (A) Proliferation assays were performed as described in “Materials and methods” with PBTCs and CBTCs incubated in the presence of submitogenic doses of solid-phase immobilized anti-CD3 (OKT-3) and solid-phase immobilized anti-CD26 (1F7) at the doses indicated or anti-CD3 and anti-CD28 (4B10). (B) Proliferation assays data of CBTCs compared with PBTCs expressing CD45RA+ or CD45RO+. The data are expressed as mean ± standard error for 10 different donors.
Comodulation of CD26 with CD45RA on CBTCs
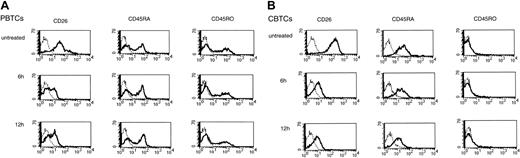
We have previously shown that anti-CD26 antibody treatment of T cells led to decreased cell surface expression of CD26 via its internalization on PBTCs, and this modulation of CD26 resulted in an enhanced proliferative response to anti-CD3 stimulation.9 More recently, we have demonstrated that CD26-mediated signaling for PBTC activation occurred through its association with CD45RO.16 To determine whether a physical linkage between CD26 and CD45 exists in CBTCs, we examined potential CD26 comodulation with CD45 following anti-CD26 mAb treatment by flow cytometry. PBTCs and CBTCs were analyzed for CD26, CD45RA, and CD45RO expressions prior to antibody-induced modulation and following incubation for 6 hours and 12 hours at 37°C with anti-CD26 mAb. Incubation with anti-CD26 mAb led to a significant reduction in CD26 expression in PBTCs and CBTCs. In PBTCs, as previously reported,5,11 the expression level of CD45RO was reduced and the expression level of CD45RA was slightly increased (Figure 3A), while the expression level of CD45RA was markedly reduced in CBTCs (Figure 3B). Indeed, the percentage and mean fluorescence intensity of CD45RA following CD26 modulation were gradually decreased over time in CBTCs (Tables 1 and 2). These results therefore suggest that a physical linkage between CD26 and CD45RA exists in CBTCs in contrast with the linkage between CD26 and CD45RO in PBTCs.
Comodulation of CD26 with CD45RA in CBTCs. CD26, CD45RA, and CD45RO surface expression was determined by immunofluorescence as described in “Materials and methods.” PBTCs (A) and CBTCs (B) were analyzed for CD26, CD45RA, and CD45RO expression prior to and following incubation for the time periods indicated at 37°C with anti-CD26 mAb. Data are represented by histograms in which cells were stained with stated mAbs (thick lines) or isotype-matched mAbs (thin lines). The data are representative of 10 different donors.
Comodulation of CD26 with CD45RA in CBTCs. CD26, CD45RA, and CD45RO surface expression was determined by immunofluorescence as described in “Materials and methods.” PBTCs (A) and CBTCs (B) were analyzed for CD26, CD45RA, and CD45RO expression prior to and following incubation for the time periods indicated at 37°C with anti-CD26 mAb. Data are represented by histograms in which cells were stained with stated mAbs (thick lines) or isotype-matched mAbs (thin lines). The data are representative of 10 different donors.
Percentage of cells expressing surface molecules comodulated by CD26 in PBTCs and CBTCs
Cell surface marker . | Untreated, % . | 6 h, % . | 12 h, % . |
|---|---|---|---|
| PBTCs | |||
| CD26 | 77.7 | 16.4 | 12.5 |
| CD45RA | 48.4 | 53.8 | 56.9 |
| CD45RO | 38.6 | 31.9 | 29.1 |
| CBTCs | |||
| CD26 | 96.2 | 24.3 | 22.8 |
| CD45RA | 91.2 | 68.7 | 32.8 |
| CD45RO | 3.6 | 3.6 | 4.1 |
Cell surface marker . | Untreated, % . | 6 h, % . | 12 h, % . |
|---|---|---|---|
| PBTCs | |||
| CD26 | 77.7 | 16.4 | 12.5 |
| CD45RA | 48.4 | 53.8 | 56.9 |
| CD45RO | 38.6 | 31.9 | 29.1 |
| CBTCs | |||
| CD26 | 96.2 | 24.3 | 22.8 |
| CD45RA | 91.2 | 68.7 | 32.8 |
| CD45RO | 3.6 | 3.6 | 4.1 |
Data are from Figure 3.
Mean fluorescence intensity of cells expressing cell surface markers
Cell surface marker . | Untreated . | 6 h . | 12 h . |
|---|---|---|---|
| PBTCs | |||
| CD26 | 76.2 | 17.4 | 17.1 |
| CD45RA | 46.7 | 49.6 | 56.7 |
| CD45RO | 72.1 | 62.1 | 57.6 |
| CBTCs | |||
| CD26 | 149.2 | 15.2 | 15.4 |
| CD45RA | 50.1 | 31.9 | 22.7 |
| CD45RO | 22.9 | 24.4 | 24.4 |
Cell surface marker . | Untreated . | 6 h . | 12 h . |
|---|---|---|---|
| PBTCs | |||
| CD26 | 76.2 | 17.4 | 17.1 |
| CD45RA | 46.7 | 49.6 | 56.7 |
| CD45RO | 72.1 | 62.1 | 57.6 |
| CBTCs | |||
| CD26 | 149.2 | 15.2 | 15.4 |
| CD45RA | 50.1 | 31.9 | 22.7 |
| CD45RO | 22.9 | 24.4 | 24.4 |
Data are from Figure 3.
Attenuation of TCR/CD3 signal transduction following CD26 cross-linking in CBTCs
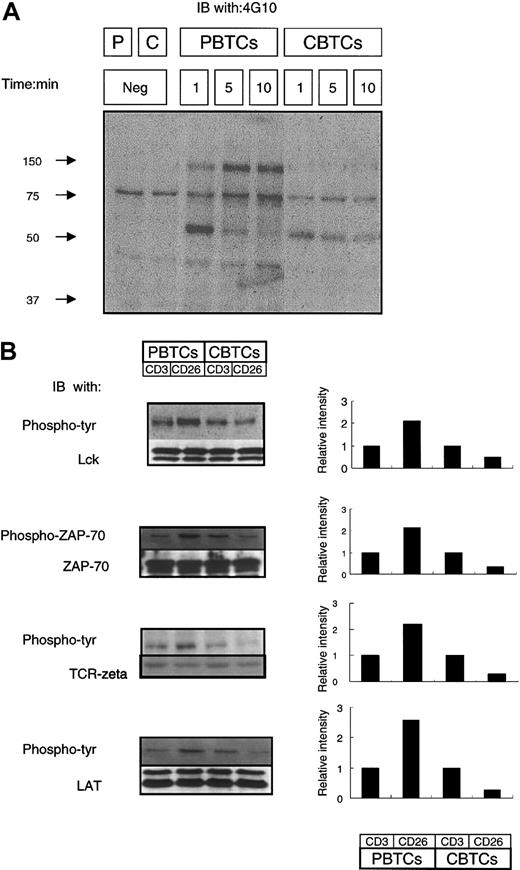
CD26 is costimulatory for CD3-induced signal transduction since co-cross-linking of CD26 and CD3 amplified the CD3-induced intensity of tyrosine phosphorylation of Lck, TCRζ, ZAP-70, ERK, and PLCγ and enhanced CD3-induced IL-2 production.10,16 We examined the efficiency of TCR/CD3-dependent signal transduction in the presence of CD26 cross-linking in CBTCs. We first analyzed tyrosine phosphorylation of intracellular proteins after cross-linking of CD26 in PBTCs and CBTCs. PBTCs and CBTCs were stimulated with anti-CD26 mAb, and whole-cell lysates were analyzed by immunoblotting with antiphosphotyrosine mAb (Figure 4). Tyrosine phosphorylation of intracellular proteins was induced by CD26 cross-linking in PBTCs and CBTCs. In PBTCs, tyrosine phosphorylation of the 50- to 60-kDa protein was intensely induced at the 1-minute time point following activation mediated by CD26 cross-linking, and tyrosine phosphorylation of 37-kDa, 40-kDa 70-kDa, and 140-kDa proteins were detected at the 1-minute time point and amplified both at the 5-minute and 10-minute time points. However, at the 1-minute time point in CBTCs, tyrosine phosphorylation of the 50- to 60-kDa protein was also clearly induced but at a reduced level compared with PBTCs. Tyrosine phosphorylation of the other 4 proteins in CBTCs, which were tyrosine phosphorylated by CD26 cross-linking in PBTCs, was significantly decreased at any of the evaluated time points (Figure 4A). We did not detect any other tyrosine-phosphorylated proteins following 60 minutes of CD26 cross-linking (data not shown). Cross-linking of CD26 induced increased tyrosine phosphorylation of an array of intracellular proteins involved in TCR/CD3-mediated signal transduction. We next examined the potential differences in CD3- or CD26-induced intensity of tyrosine phosphorylation, specifically of Lck at the 1-minute time point and ZAP-70, TCRζ, and LAT at the 5-minute time point by immunoblotting with antiphosphotyrosine antibody or anti-phosphor-ZAP-70 antibody. The blot was subsequently stripped and reprobed with the indicated antibodies. Differences in the intensities of tyrosine phosphorylation of Lck, ZAP-70, TCRζ, and LAT were assayed using densitometry and expressed graphically (Figure 4B). Lck, ZAP-70, TCRζ, and LAT were more heavily phosphorylated with cross-linking of CD26 than cross-linking of CD3 in PBTCs, whereas those proteins were less phosphorylated with cross-linking of CD26 than cross-linking of CD3 in CBTCs.
Attenuation of TCR/CD3 signal transduction following CD26 stimulation in CBTCs. (A) CD26-activated protein tyrosine phosphorylation. PBTCs and CBTCs (5 × 106) were incubated in media alone or stimulated with CD26 cross-linking (1F7; 10 μg/mL) for the indicated time periods at 37°C. Equal amounts of total protein loaded from samples were analyzed by immunoblotting with antiphosphotyrosine Ab (4G10). Molecular weight markers are indicated. Results are representative of 10 different donors. (B) Reduced phosphorylation of Lck, ZAP-70, and LAT stimulated with anti-CD26 compared with anti-CD3. PBTCs and CBTCs (5 × 106) were stimulated with anti-CD3 (OKT-3; 10 μg/mL) or anti-CD26 (1F7; 10 μg/mL) for 2 minutes at 37°C. Equal amounts of total protein loaded from samples were analyzed by immunoblotting with antiphosphotyrosine (4G10) or anti-phosphor-Y319 ZAP-70. The blots were stripped and reprobed for Lck, ZAP-70, and LAT. Differences in the intensity of the phosphorylation proteins were assessed using densitometric scanning and expressed graphically. These data are representative of 10 different donors.
Attenuation of TCR/CD3 signal transduction following CD26 stimulation in CBTCs. (A) CD26-activated protein tyrosine phosphorylation. PBTCs and CBTCs (5 × 106) were incubated in media alone or stimulated with CD26 cross-linking (1F7; 10 μg/mL) for the indicated time periods at 37°C. Equal amounts of total protein loaded from samples were analyzed by immunoblotting with antiphosphotyrosine Ab (4G10). Molecular weight markers are indicated. Results are representative of 10 different donors. (B) Reduced phosphorylation of Lck, ZAP-70, and LAT stimulated with anti-CD26 compared with anti-CD3. PBTCs and CBTCs (5 × 106) were stimulated with anti-CD3 (OKT-3; 10 μg/mL) or anti-CD26 (1F7; 10 μg/mL) for 2 minutes at 37°C. Equal amounts of total protein loaded from samples were analyzed by immunoblotting with antiphosphotyrosine (4G10) or anti-phosphor-Y319 ZAP-70. The blots were stripped and reprobed for Lck, ZAP-70, and LAT. Differences in the intensity of the phosphorylation proteins were assessed using densitometric scanning and expressed graphically. These data are representative of 10 different donors.
Lack of CD26 recruitment into lipid rafts following antibody binding
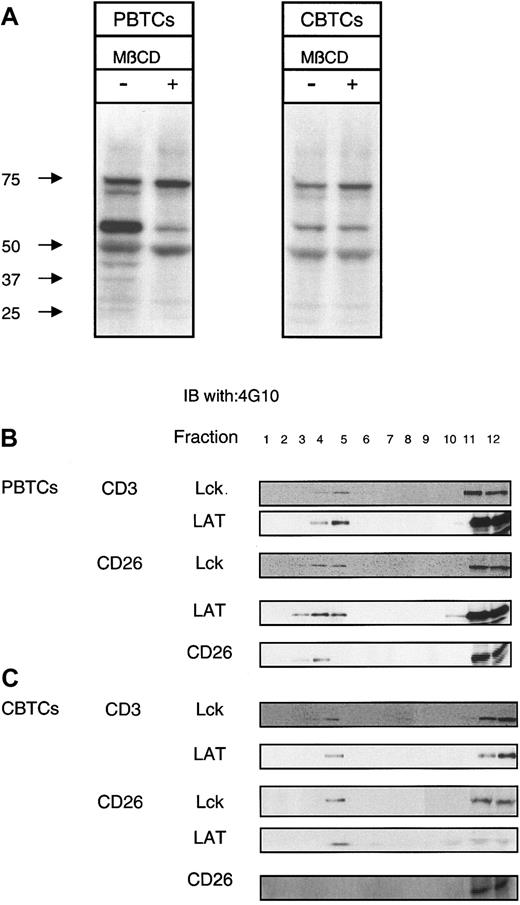
Recent studies have shown that lipid rafts play an important role in the generation of TCR-related signaling with various types of costimulatory molecules (eg, CD2, CD5, CD9, CD44, and CD81).35,36 We have demonstrated that CD26 is costimulatory for CD3-induced signal transduction and targeting of CD26 to lipid rafts is necessary for signaling events through CD26.16 We first investigated whether disruption of lipid rafts' integrity via cholesterol extraction with MβCD would inhibit CD26-induced signal transduction. As expected, when PBTCs were stimulated via CD26 cross-linking for 2 minutes at 37°C there was a pronounced increase in total protein tyrosine phosphorylation. This robust induction of the TCR signaling cascade was strongly inhibited when the PBTCs were pretreated with 10 mM MβCD for 5 minutes at 37°C before stimulation. However, CBTCs exhibited a very low level of protein tyrosine phosphorylation when cultured either in media alone or in media containing 10 mM MβCD prior to stimulation (Figure 5A). These results suggest that CD26 is not likely to be involved in raft-mediated T-cell activation signaling through CD26 in CBTCs, in contrast with PBTCs. To confirm the recruitment of CD26 to lipid rafts after cross-linking of CD26 in CBTCs, lysates were subjected to sucrose gradient centrifugation to separate lipid raft fractions from Triton X-100-soluble fractions. Consistent with previous published data, LAT was constitutively present in the raft fractions, and Lck was also present in the raft fractions following cross-linking of CD3 or CD26 in PBTCs. CD26 was recruited to lipid rafts following cross-linking of CD26 in PBTCs (Figure 5B). Although LAT and Lck were present in the raft fractions of CBTCs after cross-linking of CD3 or CD26, translocation of CD26 to lipid rafts was not detectable following cross-linking of CD26 (Figure 5C). These results therefore suggest that insufficiency of CD26 recruitment to lipid rafts has a specific inhibitory effect on TCR-mediated signaling events in CBTCs following cross-linking of CD26.
CD26-mediated redistribution of signaling molecules. (A) PBTCs and CBTCs (5 × 106) were left untreated or treated with 10 mM MβCD and incubated at 37°C for 5 minutes. Cells were stimulated via anti-CD26 mAb (1F7; 10 μg/mL) for 30 minutes. Samples were analyzed by SDS-PAGE under reducing conditions and Western blotting for antiphosphotyrosine mAb (4G10). Molecular weight markers are indicated. These results are representative of 10 different donors. (B) PBTCs and CBTCs (1 × 108) were lysed with alpha-methylnorepinephrine (MNE) buffer containing 1% Triton X-100, and the lysates were subjected to equilibrium gradient centrifugation. Electrophoresis of an aliquot of each fraction was performed under reducing conditions, and immunoblotting was performed with anti-Lck, anti-LAT, and anti-CD26 (5F8). (C) Distribution of phospho-Lck in PBTCs and CBTCs was analyzed with immunoblot for phosphotyrosine residues (4G10). The data are representative of 5 different donors.
CD26-mediated redistribution of signaling molecules. (A) PBTCs and CBTCs (5 × 106) were left untreated or treated with 10 mM MβCD and incubated at 37°C for 5 minutes. Cells were stimulated via anti-CD26 mAb (1F7; 10 μg/mL) for 30 minutes. Samples were analyzed by SDS-PAGE under reducing conditions and Western blotting for antiphosphotyrosine mAb (4G10). Molecular weight markers are indicated. These results are representative of 10 different donors. (B) PBTCs and CBTCs (1 × 108) were lysed with alpha-methylnorepinephrine (MNE) buffer containing 1% Triton X-100, and the lysates were subjected to equilibrium gradient centrifugation. Electrophoresis of an aliquot of each fraction was performed under reducing conditions, and immunoblotting was performed with anti-Lck, anti-LAT, and anti-CD26 (5F8). (C) Distribution of phospho-Lck in PBTCs and CBTCs was analyzed with immunoblot for phosphotyrosine residues (4G10). The data are representative of 5 different donors.
Colocalization of CD45RA with CD26 outside of lipid rafts following CD26 cross-linking in CBTCs
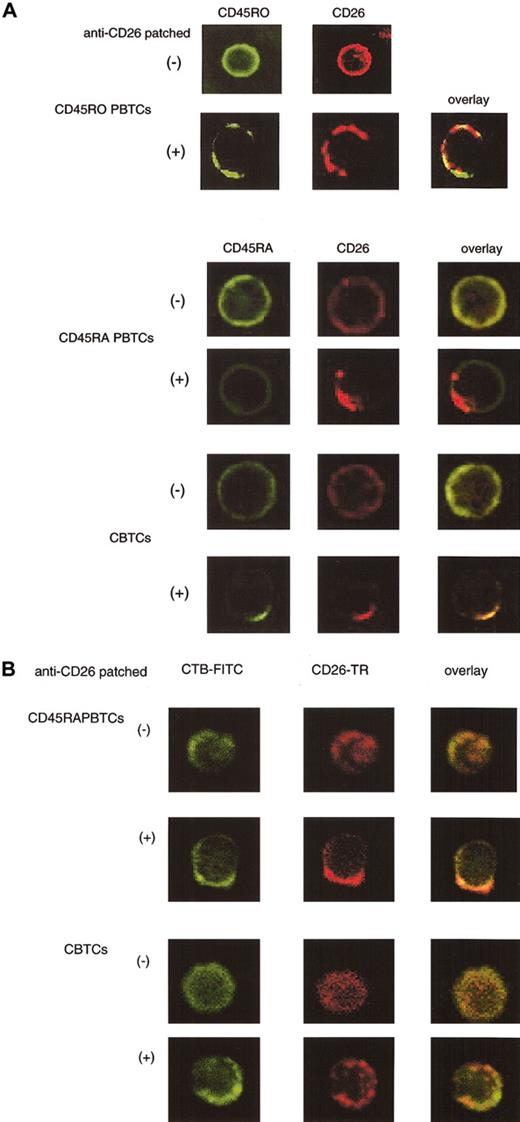
Since CD26 was comodulated with CD45RA following cross-linking of CD26 in CBTCs, we investigated whether CD45RA was colocalized with CD26 through the use of confocal laser microscopy. CD45RA+ or CD45RO+ PBTCs and CBTCs were incubated either in media alone or in media containing anti-CD26 mAb, and they were then analyzed for potential colocalization of CD26, CD45RO, or CD45RA. As we reported previously,16 CD26 in PBTCs was colocalized with CD45RO after CD26 patching (Figure 6A). On the other hand, CD26 and CD45RA were uniformly distributed at the plasma membrane in CD45RA+ PBTCs and CBTCs (Figure 6A). Cross-linking of CD26 induced its patching at the plasma membrane in PBTCs and CBTCs. Although cross-linking of CD26 had no effect on CD45RA distribution in CD45RA+ PBTCs, copatching of CD26 and CD45RA was observed in CBTCs (Figure 6A). Since CD26 was recruited into lipid rafts after cross-linking with anti-CD26 mAb in PBTCs, we next examined whether CD26 was recruited to lipid rafts in CBTCs. Membrane rafts were stained with CTB-FITC after cross-linking of CD26. CD26 molecules were colocalized in lipid rafts in CD45RA+ PBTCs (Figure 6B). Similarly, as reported previously,37 CD45RA molecules were not colocalized in lipid rafts (data not shown). However, CD26 molecules were not colocalized in lipid rafts in CBTCs (Figure 6B). These results hence suggest that CD26 is colocalized with CD45RA outside lipid rafts in CBTCs, which may explain the attenuation of TCR/CD3 signal transduction following CD26 cross-linking.
Colocalization of CD26 and CD45RA outside lipid rafts after cross-linking of CD26 in CTBCs. For staining CD45RO+ PBTCs, cells were incubated with biotinylated anti-CD26 mAb (1F7) and cross-linked with Texas red-conjugate streptavidin for patching, as described in “Materials and methods.” After fixation, cells were stained with anti-CD45RO-FITC (UCHL-1). For analysis of CD45RA and CD26, CD45RA+ PBTCs and CBTCs were incubated with biotinylated anti-CD26 mAb (1F7) and cross-linked with Texas red-conjugate streptavidin, as described in “Materials and methods.” Cells were fixed and stained with FITC-conjugated anti-CD45RA mAb (2H4) (A) and CTB-FITC (B) and visualized by confocal microscopy (original magnification, × 400).
Colocalization of CD26 and CD45RA outside lipid rafts after cross-linking of CD26 in CTBCs. For staining CD45RO+ PBTCs, cells were incubated with biotinylated anti-CD26 mAb (1F7) and cross-linked with Texas red-conjugate streptavidin for patching, as described in “Materials and methods.” After fixation, cells were stained with anti-CD45RO-FITC (UCHL-1). For analysis of CD45RA and CD26, CD45RA+ PBTCs and CBTCs were incubated with biotinylated anti-CD26 mAb (1F7) and cross-linked with Texas red-conjugate streptavidin, as described in “Materials and methods.” Cells were fixed and stained with FITC-conjugated anti-CD45RA mAb (2H4) (A) and CTB-FITC (B) and visualized by confocal microscopy (original magnification, × 400).
Association of CD26 with CD45RA on the surface of CBTCs
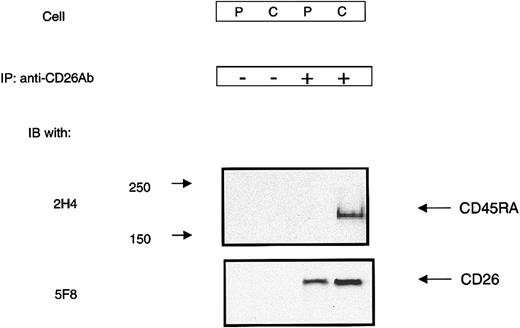
Immunofluorescence analysis provided evidence of colocalization of CD26 and CD45RA in CBTCs. We next examined the possibility of an association between CD26 and CD45RA in CBTCs through a biochemical approach. For this purpose, CBTCs were treated with the homobifunctional cross-linker DTSSP. DTSSP is membrane insoluble and therefore only cross-links surface molecules on live cells. Following incubation with CD26 mAb (1F7) and subsequent cross-linking with goat antimouse IgG, PBTCs and CBTCs were treated with DTSSP and lysed. Whole-cell lysates were immunoprecipitated and analyzed by Western blotting with CD45RA mAb (2H4) and CD26 mAb (5F8). CD45RA and CD26 were detected in CBTCs after cross-linking of CD26. In contrast, only CD26 was detected in PBTCs after the same treatment (Figure 7). These results suggest that CD26 is likely to be involved in a low-affinity or transient interaction with CD45RA in CBTCs, and comodulation of CD26 with CD45RA results in inefficient CD26-mediated activation of CBTCs.
Association between CD26 and CD45RA in CBTCs. PBTCs (P) and CBTCs (C) were incubated with anti-CD26 mAb (1F7) and then cross-linked with secondary antibodies followed by cross-linking with the cleavable cross-linker DTSSP. CD26 and its cross-linked complex were immunoprecipitated as described in “Materials and methods.” Immunoprecipitated materials were analyzed by SDS-PAGE under reducing conditions and then immunoblotted with anti-CD26 and anti-CD45RA mAb.
Association between CD26 and CD45RA in CBTCs. PBTCs (P) and CBTCs (C) were incubated with anti-CD26 mAb (1F7) and then cross-linked with secondary antibodies followed by cross-linking with the cleavable cross-linker DTSSP. CD26 and its cross-linked complex were immunoprecipitated as described in “Materials and methods.” Immunoprecipitated materials were analyzed by SDS-PAGE under reducing conditions and then immunoblotted with anti-CD26 and anti-CD45RA mAb.
Discussion
Although the clinical use of umbilical cord blood as a source of stem cells for bone marrow reconstitution has been characterized by an apparent lower incidence of severe graft-versus-host disease compared with adult bone marrow, little is known regarding the precise mechanism for this phenomenon. Furthermore, the molecular mechanisms underlying the physiologically immature immune response of cord blood T cells are poorly defined. In this study, we have demonstrated that T-cell activation through CD26 is inefficient in cord blood T cells compared with adult peripheral blood T cells. The deficiency in CD26 recruitment into lipid rafts was independently confirmed by biochemical and confocal analyses.
Allogeneic bone marrow transplantation (BMT) is indicated for selected hematologic diseases and for hematopoietic reconstitution in cases of iatrogenic or accidental ablation of bone marrow. Human leukocyte antigen (HLA)-identical sibling donors offer the best graft compatibility, but HLA-identical unrelated donors may also provide acceptable graft. However, the successful outcome of allogeneic BMT is significantly limited by the risk of GVHD leading to morbidity and mortality.22,25,26 On the other hand, umbilical cord blood, as an alternative source of hematopoietic stem cells for BM reconstitution, has recently been shown to yield successful HLA-identical or HLA-disparate sibling and unrelated donor CB grafts in children and adults.25,26 A remarkable attribute of CB as donor tissue for BM replacement has been a lesser incidence of GVHD.25,26 Thus, CB has the potential to overcome some of the limitations for sibling and unrelated BMT, and there is increasing clinical interest in CB transplantation (CBT) as an alternative new therapeutic protocol for BM reconstitution. Although the use of CB has been characterized by an apparent lower incidence of severe GVHD, the scientific basis for this lower incidence of GVHD remains unclear.
We have previously shown that CD26 was comodulated with CD45, particularly CD45RO, after CD26 cross-linking and that anti-CD26 mAb was capable of coprecipitating CD45RO from T-cell lysates.11 Moreover, we showed that antibody-mediated modulation of CD26 on the T-cell surface resulted in an enhanced proliferation to anti-CD3 or anti-CD2-mediated stimulation.9-11 CBTCs are known to contain primarily “unprimed” T cells expressing the CD45RA phenotype,21-23 which was confirmed in this study. Upon primary T-cell receptor stimulation, PBTCs and CBTCs proliferated to the same extent, as measured by thymidine incorporation.38 Therefore, a possible explanation for the lower proliferative response of CBTCs to dual stimulation with mAbs to CD3 and CD26 is the low proportion of CD45RO T cells present in CBTCs. However, the results presented here showed that despite anti-CD26 mAb-mediated modulation on CBTCs, anti-CD26 mAb treatment was not sufficient to induce proliferation of CBTCs. Furthermore, we have shown that the CD26 molecules comodulated with CD45RA after CD26 cross-linking in CBTCs, but CD26 molecules did not comodulate with CD45 in CD45RA+ PBTCs. These observations suggest that CD26+CD45RA+ CBTCs are different compared with CD26+CD45RA+ PBTCs.
Recent reports have shown that several functions are impaired in CBTCs following various forms of stimulation. For example, immature immune response of cord blood T cells may be associated with inefficient PLC activation and decreased Lck expression, defective activation of Ras, and reduced nuclear factor of activated T cells 1 (NFAT1) protein expression after secondary stimulation compared with PBTCs.32,39,40 Cross-linking of CD26 by antibody caused increased tyrosine phosphorylation of T-cell signaling molecules such as Lck, ZAP-70, TCRζ, and ERK1/2 in PBTCs.10,16 Lck is crucial for the initiation of the tyrosine kinase cascade in T-cell activation signaling.41-45 However, the intensity of tyrosine phosphorylation of Lck induced by cross-linking of CD26 was significantly reduced in CBTCs compared with PBTCs. This development suggests that attenuated tyrosine phosphorylation of Lck induced by CD26 cross-linking leads to the incomplete activation of ZAP-70 and LAT, which eventually results in a defect in T-cell proliferation via CD26 in CBTCs.
Specialized membrane microdomains, also known as lipid rafts, play an important role in T-cell signaling. After engagement of TCR, the immunologic synapse, which consists of clusters of adhesion/costimulatory molecules and complexes of intracellular signaling molecules, forms at the contact area.46,47 This synapse is believed to sustain TCR engagement and T-cell signaling. It is still unclear how the synapse forms or is maintained, but rearrangement of the actin cytoskeleton and membrane rafts toward this contact area is one possible mechanism.48,49 We recently demonstrated that CD26 localized into lipid rafts in PBTCs and targeting of CD26 to lipid rafts was necessary for signaling events through CD26, thereby enhancing protein tyrosine phosphorylation of various signaling molecules and subsequent interleukin-2 production.5,16 We have demonstrated that CD26 was not detectable in the raft fractions following cross-linking of CD26 in CBTCs by biochemical analyses. Furthermore, CD26 patching induced by CD26 mAb was sufficient to drive lipid raft aggregation in PBTCs, while in CBTCs CD26 molecules were not colocalized with CTB patches by confocal analyses. These data suggest that deficiency in recruitment of CD26 to lipid rafts in CBTCs results in incomplete T-cell activation through CD26.
The receptor-like protein tyrosine phosphatase CD45 is essential for TCR signal transduction. In T cells it has been estimated that CD45 comprises up to 10% of the cell surface area. Substrates of CD45 include the protein tyrosine kinases Lck and Fyn.37,50,51 Despite intensive efforts by many laboratories, no physiologic ligand for CD45 has been definitively identified. Most studies on the localization of CD45 showed that it is absent in membrane lipid rafts and the central region of the interface between the T cell and the antigen-presenting cell (APC).37 The CD45RA isoform may be activated by monomers.52 The function of CD45 may also be modulated through its interactions with other proteins such as CD26. CD45 associates with molecules both on the cell surface and inside the cell, although the functional significance of these interactions is unclear at the moment. We have shown that in CBTCs, CD26 molecules comodulated with CD45RA gradually after cross-linking of CD26 by flow cytometry analysis and colocalized with CD45RA outside lipid rafts after cross-linking of CD26 by confocal microscopy. Furthermore, experiments via the chemical cross-linker method showed that CD26 molecules coprecipitated with CD45RA in CBTCs. These results suggest that the association of CD26 with CD45RA leads to a deficiency in cord blood T-cell activation via CD26, although our observation does not necessarily exclude other means of CD45 regulation.
We propose that comodulation of CD26 with CD45RA outside lipid rafts attenuates T-cell signaling via CD26 in CBTCs. These observations imply that insufficiency of CBTCs in proliferative response and T-cell activation signal via CD26 may partially account for the lower incidence and severity of acute or extensive chronic GVHD of CBT when compared with BM transplantation as well as PB stem cell transplantation.
Our data suggest that CBTCs are different from CD45RA+ PBTCs and that comodulation of CD26 with CD45RA outside lipid rafts may lead to reduced activation of CBTCs through CD26. Our results provide a possible reason for the observed advantage of CB as a donor tissue in allogeneic CBT in terms of the low incidence of severe GVHD. Although our findings suggest that insufficiency of T-cell activation via CD26 as well as other functional deficiencies in CBTCs may contribute to the low incidence of severe GVHD in CBT, mechanisms involving other cells, such as B cells and natural killer (NK) cells, may also participate in these phenomena. Defining the precise underlying mechanisms of the immunologic properties of CB T cells is of crucial importance in understanding the apparent reduced incidence and severity of GVHD in CB transplantation.
Prepublished online as Blood First Edition Paper, October 2, 2003; DOI 10.1182/blood-2003-08-2691.
Supported by National Institutes of Health grant AR33713 and by Grant-in-Aid from Ministry of Education, Science, Sports and Culture, and Ministry of Health, Labor, and Welfare, Japan (C.M.). K.O. is a recipient of a grant for the fellows of the Japan Society for the Promotion of Science. N.H.D. is supported by the MD Anderson Physician-Scientist Program, the V Foundation, and the Gillson Longenbaugh Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Ms Fumiki Nojima for excellent secretarial assistance and Ms Akiko Kuribara for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal