Abstract
Mice with disruptions of the red blood cell (RBC) cytoskeleton provide severe hemolytic anemia models in which to study multiorgan thrombosis and infarction. The incidence of cerebral infarction ranges from 70% to 100% in mice with α-spectrin deficiency. To determine whether mutant RBCs abnormally bind adhesive vascular components, we measured adhesion of mouse and human RBCs to immobilized human thrombospondin (TSP) and laminin (LM) under controlled flow conditions. Mutant RBCs had at least 10-fold higher adhesion to TSP compared with normal RBCs (P < .006). Mutant relative to unaffected RBC adhesion to LM was significantly (P < .01) increased as well. Treatment of RBCs with the anionic polysaccharide dextran sulfate inhibited mutant RBC adhesion to TSP (P < .001). Treatment of RBCs with antibodies to CD47 or the CD47-binding TSP peptide 4N1K did not inhibit TSP adhesion of RBCs. Previously, we have shown that infarcts in α-spectrin–deficient sph/sph mice become histologically evident beginning at 6 weeks of age. TSP adhesion of RBCs from 3- to 4- and 6- to 8-week-old sph/sph mice was significantly higher than RBCs from adult mice (> 12 weeks old; P < .005). While the mechanism of infarction in these mice is unknown, we speculate that changes in RBC adhesive characteristics contribute to this pathology.
Introduction
Pathologic complications of heritable hemolytic anemia in humans include vaso-occlusion, thrombosis, and stroke. In sickle cell disease (SS-D), 20% to 30% of patients have either clinical or subclinical (neuroimaging) evidence of cerebrovascular disease, and all patients experience microvascular obstruction outside the central nervous system (CNS).1,2 Several mouse models of SS-D exist but the overall prevalence of thrombosis and stroke is, as yet, unpublished.3-8 Sickle RBCs from humans and mice exhibit increased exposure of the aminophospholipid phosphatidylserine (PS) on the outer surface of the sickle RBC.9,10 The increased PS exposure correlates with increased activity of the coagulation cascade as well as with increased RBC adhesion in humans with SS-D.11-13
Severe hereditary spherocytosis (HS) and hereditary elliptocytosis (HE) in laboratory mice (Mus) are attributed to spontaneous autosomal recessive mutations in erythroid cytoskeletal proteins that normally provide the RBC with mechanical strength and deformability.14-16 While disease severity in human HS/HE is quite variable,16,17 thrombosis and stroke have been documented in a small number of cases.18-21 Mice with deficiencies in erythroid α-spectrin have the highest incidences of thrombosis and stroke (62%-100% of adult mice) described to date.15,22 α-spectrin normally binds β-spectrin to form the backbone of the membrane skeleton.16 There are 4 murine α-spectrin mutations with different genetic alterations. The sph/sph and sph2BC/sph2BC mice with severe HS are essentially α-spectrin nulls due to premature termination of the α-spectrin protein prior to the region required for dimerization with β-spectrin.23,24 Severe HS in sphJ/sphJ mice is due to a nonsense mutation that truncates 13 amino acids from the C-terminal end of α-spectrin; α-spectrin is produced but is not stably bound to the cytoskeleton.24 The sphDem/sphDem mice manifest with severe HE rather than HS, due to generation of a protein unable to tetramerize with other α/β spectrin dimers, results in a weakened cytoskeleton.15
All α-spectrin mutant mice develop a severe hemolytic anemia within hours of birth. The misshapen erythrocytes are extremely short-lived (1 day vs 48 days in normal mice), and reticulocytes comprise 50% to 95% of the peripheral hemoglobinized cells.14 As in humans and mice with SS-D, there is increased exposure of PS on the outer leaflet of the RBC membrane.22 In addition to thrombosis and stroke, the pathophysiologic effects of murine HS/HE include cardiomegaly, hepatomegaly, splenomegaly, deposition of iron in the liver and kidney, and premature death.25
The propensity for the development of cerebral infarction combined with the advantage of working with inbred animals on genetically identical backgrounds make α-spectrin–deficient mice good models in which to identify mechanisms and molecules that trigger stroke in hemolytic anemia. The majority of strokes in these mice are subclinical and do not result in death, although they sometimes affect large areas of the brain. Furthermore, cerebral infarction and thrombosis are not pathologically evident in sph/sph mice younger than 6 weeks of age, yet affect 100% of sph/sph mice by 12 weeks of age, providing a window for critical analyses.26
Sickle RBCs from both mice and humans exhibit increased adhesion to endothelial cells and components of the subendothelial matrix under both static and flow conditions.12,27,28 In the current paper, we determined whether murine and human HS/HE RBCs were abnormally adhesive to components of the subendothelial matrix under controlled flow conditions and explored potential relationships between RBC adhesion and pathologic cerebral infarction.
Materials and methods
Mice
With the exception of sphDem/sphDem mice, normal and mutant mice were maintained on both the WB/ReJ (WB) and C57BL/6J (B6) backgrounds. F1 hybrid (WBB6F1), mutant (sph/sph, sph2BC/sph2BC, sphJ/sphJ), and normal (+/+, sph/+) mice were generated by mating WB and B6 heterozygotes and were genetically identical except at the mutated locus. F1 hybrid mice survive longer than mutant mice on inbred strain backgrounds. The sphDem/sphDem mice with HE and unaffected (+/+, sphDem/+) littermates were maintained by inbreeding heterozygotes on the CcS3/Dem recombinant congenic background. “Hi-retic” mice were generated by daily retroorbital phlebotomy of 100 to 150 μL of blood for 7 days until reticulocyte percentage reached 30% as determined by flow cytometry with thiazole orange stain (Becton Dickinson, San Jose, CA). Mice were housed and cared for according to Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) specifications. All experimental groups contained similar numbers of males and females.
Reagents
High–molecular weight (MW; average MW 500 kDa) dextran sulfate was purchased from Sigma (St Louis, MO), chondroitin sulfate A was from CalBiochem (San Diego, CA), human laminin (LM) was from Invitrogen/GIBCO-BRL (Gaithersburg, MD), and annexin V was from Celsus (Cincinnati, OH). Thrombospondin-1 (TSP) was purified from human platelets as previously described.27,29 Rat monoclonal antimouse CD47 (clone miap301) and murine monoclonal antihuman CD47 (clone B6H12) were purchased from Neomarkers/Labvision (Fremont, CA). Murine monoclonal antihuman CD47 clone 1F7 was a gift from Dr Eric Brown (University of California San Francisco). TSP peptides 4N1K (KRFYVVMWKK) containing the TSP binding site for CD47 and its scrambled counterpart SCR (VKMKWKYVRF) were synthesized on an Applied Biosystems (Foster City, CA) 433A Peptide Synthesizer by the Peptide Core at the Blood Research Institute.
RBC preparation
Whole blood from mice was collected from the retroorbital sinus vein into either 3.8% sodium citrate or 40 U/mL sodium heparin. For studies using RBCs from humans with HS, blood samples were collected into 3.8% sodium citrate (1:9 citrate-blood volume) after obtaining informed consent. Approval for these studies was obtained from the institutional review board of the Children's Hospital of Wisconsin. RBCs were separated from platelet-rich plasma by centrifugation (180g, 20 minutes, 22°C), washed 3 times with a citrate/glucose/saline buffer, and resuspended at a 2% hematocrit in M199 serum-free cell culture medium (Sigma) containing 0.2% bovine serum albumin (SFM-BSA) for flow adhesion studies.
Dynamic flow adhesion assay
RBC-adhesive ligand interactions were assayed using a parallel plate perfusion chamber as previously described.27 Flow experiments were performed at 37°C using an air curtain incubator and an inverted phase microscope. In brief, the purified adhesive protein (TSP or LM) was coated on a 35-mm2 tissue culture plate at 2 μg/cm2 at 37°C for 30 minutes. After a rinse with SFM-BSA that served to block the plate, washed RBCs suspended at a 2% hematocrit in SFM-BSA were perfused through the flow chamber under controlled shear forces of 1 dyne/cm2 for 5 minutes. Following an additional 6-minute rinse period, the number of adherent RBCs per unit surface area was quantified in 4 to 5 areas in duplicate wells. For inhibition experiments, the inhibitor (high-MW dextran sulfate; chondroitin sulfate A; annexin V; TSP peptides 4N1K and SCR; antibodies miap301, B6H12, and 1F7) was incubated at a final concentration of 500 μg/mL (dextran sulfate, chondroitin sulfate A), 10 μg/mL (annexin V), 200 μM (peptides 4N1K and SCR), or 2 μg/mL (antibodies miap301, B6H12, and 1F7) with the RBC suspension for 30 minutes at 37°C prior to the flow experiment. For assessment of the percentage of adherent cells that were reticulocytes, after quantification of adherent RBCs the 35-mm2 tissue culture plate containing adherent RBCs was removed from the flow chamber. Thiazole orange stain (ReticCount; BD Biosciences, San Jose, CA) was added to coat the wells and the plate was incubated in the dark for 1 to 2 hours. Reticulocytes, which take up the fluorescent stain, as well as mature RBCs, which do not take up the stain, were enumerated under both bright field and fluorescein isothiocyanate (FITC) fluorescent filters on a Nikon Eclipse TE200 microscope (Nikon, Japan). The number of reticulocytes versus the number of total cells was quantified in 4 areas in duplicate wells.
Statistics
Statistical values were generated with statistical analysis packages in Microsoft Excel and SAS. Two-tailed Student t tests assuming equal variances were used for comparisons of sample groups in Figures 1 and 4. Paired Student t tests of square root adjusted data were used to assess inhibitor effects in Figure 3. A P value of less than .05 was considered significant.
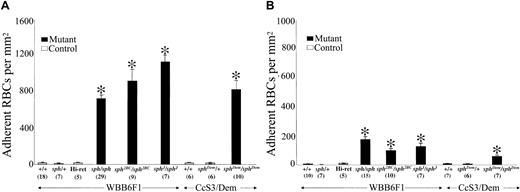
Increased adhesion of murine HS/HE RBCs to thrombospondin (TSP) and laminin (LM). Washed RBCs from control (WBB6F1-+/+ and -sph/+; CcS3/Dem-+/+ and -sphDem/+), elevated reticulocyte control (WBB6F1 Hi-ret), and mutant (severe HS: WBB6F1-sph/sph, -sph2BC/sph2BC, and -sphJ/sphJ; severe HE: CcS3/Dem-sphDem/sphDem) mice were perfused through flow chambers previously coated (2 μg/cm2) with human TSP (A) or human LM (B) at a wall shear stress of 1 dyne/cm2. Adherent RBCs per unit area were counted by direct microscopic visualization as described in “Materials and methods.” Group/genotype is listed on x-axis just below bars; numbers of measurements per group are indicated in parentheses below genotype/group. Strain background for each group is indicated between horizontal arrows. Adherent RBCs/mm2 are depicted as mean ± SEM. *P < .001 versus +/+ values.
Increased adhesion of murine HS/HE RBCs to thrombospondin (TSP) and laminin (LM). Washed RBCs from control (WBB6F1-+/+ and -sph/+; CcS3/Dem-+/+ and -sphDem/+), elevated reticulocyte control (WBB6F1 Hi-ret), and mutant (severe HS: WBB6F1-sph/sph, -sph2BC/sph2BC, and -sphJ/sphJ; severe HE: CcS3/Dem-sphDem/sphDem) mice were perfused through flow chambers previously coated (2 μg/cm2) with human TSP (A) or human LM (B) at a wall shear stress of 1 dyne/cm2. Adherent RBCs per unit area were counted by direct microscopic visualization as described in “Materials and methods.” Group/genotype is listed on x-axis just below bars; numbers of measurements per group are indicated in parentheses below genotype/group. Strain background for each group is indicated between horizontal arrows. Adherent RBCs/mm2 are depicted as mean ± SEM. *P < .001 versus +/+ values.
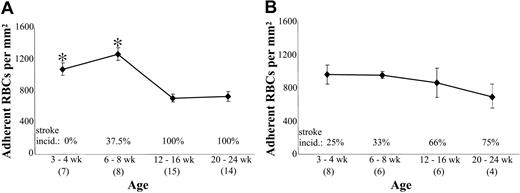
Adhesion of RBCs from younger versus older sph/sph and sphDem/sphDem mice to TSP. Washed RBCs from sph/sph (A) and sphDem/sphDem (B) mice in 4 different age groups (3-4 weeks, 6-8 weeks, 12-16 weeks, and 20-24 weeks old) were perfused through flow chambers coated with human TSP as described for Figure 1. Adherent RBCs/mm2 are depicted as mean ± SEM for each age group. Stroke incidence for the same groups of mice used for adhesion measurements is indicated above the x-axis of each graph. Numbers of measurements for RBC adhesion to TSP in each age group are indicated in parentheses below age group label on x-axis. *P < .005 versus 12 to 16 weeks and/or 20 to 24 weeks.
Adhesion of RBCs from younger versus older sph/sph and sphDem/sphDem mice to TSP. Washed RBCs from sph/sph (A) and sphDem/sphDem (B) mice in 4 different age groups (3-4 weeks, 6-8 weeks, 12-16 weeks, and 20-24 weeks old) were perfused through flow chambers coated with human TSP as described for Figure 1. Adherent RBCs/mm2 are depicted as mean ± SEM for each age group. Stroke incidence for the same groups of mice used for adhesion measurements is indicated above the x-axis of each graph. Numbers of measurements for RBC adhesion to TSP in each age group are indicated in parentheses below age group label on x-axis. *P < .005 versus 12 to 16 weeks and/or 20 to 24 weeks.
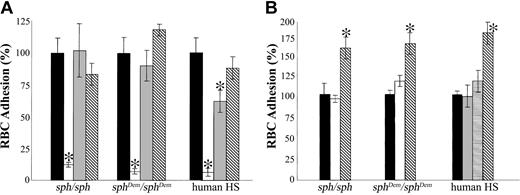
Effect of potential inhibitors on adhesion of murine HS/HE RBCs to human TSP. (A) Washed RBCs from mice and humans with HS or HE (as described in Figures 1-2) were preincubated with control buffer (▪), 500 μg/mL high–molecular weight dextran sulfate (□), 500 μg/mL chondroitin sulfate A (▤), or 10 μg/mL phosphatidylserine-binding annexin V (▧) at 37 °C for 30 minutes prior to perfusion through flow chambers coated with human TSP. (B) Washed RBCs from mice and humans with HS or HE (as described in Figures 1-2) were preincubated with control buffer (▪), antimouse CD47 clone miap301 (□, 2 μg/mL), antihuman CD47 clone B6H12 (▤, 2 μg/mL), antihuman CD47 clone 1F7 (▦,2 μg/mL), or 200 μM TSP peptide 4N1K (▧) at 37°C for 30 minutes prior to perfusion through flow chambers coated with human TSP. Adherent RBCs/mm2 in the presence of potential inhibitors are normalized to adherent RBCs/mm2 in control buffer. Results are mean ± SEM for n = 3 samples; *P < .001 versus control buffer for panel A and P < .05 versus control buffer for panel B.
Effect of potential inhibitors on adhesion of murine HS/HE RBCs to human TSP. (A) Washed RBCs from mice and humans with HS or HE (as described in Figures 1-2) were preincubated with control buffer (▪), 500 μg/mL high–molecular weight dextran sulfate (□), 500 μg/mL chondroitin sulfate A (▤), or 10 μg/mL phosphatidylserine-binding annexin V (▧) at 37 °C for 30 minutes prior to perfusion through flow chambers coated with human TSP. (B) Washed RBCs from mice and humans with HS or HE (as described in Figures 1-2) were preincubated with control buffer (▪), antimouse CD47 clone miap301 (□, 2 μg/mL), antihuman CD47 clone B6H12 (▤, 2 μg/mL), antihuman CD47 clone 1F7 (▦,2 μg/mL), or 200 μM TSP peptide 4N1K (▧) at 37°C for 30 minutes prior to perfusion through flow chambers coated with human TSP. Adherent RBCs/mm2 in the presence of potential inhibitors are normalized to adherent RBCs/mm2 in control buffer. Results are mean ± SEM for n = 3 samples; *P < .001 versus control buffer for panel A and P < .05 versus control buffer for panel B.
Results
Increased adhesion of murine HS/HE RBCs to TSP and LM
Initially, we wished to determine whether RBCs from mice with severe HS or HE had increased adhesion to components of the subendothelial matrix. We measured adhesion of washed RBCs to immobilized human TSP and LM under controlled flow conditions at a wall shear stress of 1 dyne/cm2. RBC adhesion for adult mice 12 weeks of age or older was compared between α-spectrin (severe HS: sph/sph, sph2BC/sph2BC, sphJ/sphJ; severe HE: sphDem/sphDem) mutant mice and wild-type (+/+) or heterozygous (sph/+, sphDem/+) mice.
As shown in Figure 1A, adhesion of RBCs from HS and HE mutant mice to immobilized human TSP (▪) was 40- to 60-fold higher than adhesion of RBCs from unaffected (+/+ and heterozygote) mice to TSP (□; P < .001). Adhesion of RBCs from HS and HE mutant mice to immobilized human LM was less robust (Figure 1B; P < .001). However, compared with adhesion of RBCs from unaffected (+/+ and heterozygote) mice, adhesion of HS/HE RBCs was still 12- to 55-fold higher. It was possible that the increased RBC adhesion to TSP and LM in the mutant mice is related to the increased level of reticulocytes, which have increased levels of cell adhesion molecules.30-32 To address this possibility, wild-type (+/+) mice were induced to 30% reticulocytosis by repeated phlebotomy. RBCs from these mice did not exhibit increased adhesion to TSP or LM (Figure 1A-B; Hi-ret). Finally, thiazole orange staining in hi-retic wild-type (+/+), sph/sph, and sphDem/sphDem mice indicated that the percentage of TSP-adherent RBCs that were reticulocytes was 15% to 20% less than the percentage of reticulocytes enumerated in whole blood by thiazole orange staining and flow cytometry analysis (data not shown). These data suggest that the increased RBC adhesion in HS/HE mice was not solely caused by reticulocytosis.
Table 1 summarizes the data on adhesion of murine HS/HE RBCs to human TSP and LM. Analyses of the data on adult mice failed to find significant correlations between RBC adhesion to TSP and pathologic parameters such as reticulocytosis, thrombotic incidence, or stroke incidence in the 4 groups of mutant mice. Similarly, no significant predictive trends were found between RBC adhesion to LM and the above pathologic parameters. In addition, there were no differences between RBC adhesion to TSP or LM in male versus female mice in the groups studied (data not shown).
Stroke incidence, reticulocytosis, and RBC adhesion to TSP and LM
Age and genotype . | Retic %* . | % Cerebral infarction† . | RBC adhesion to TSP, cells/mm2‡ . | RBC adhesion to LM, cells/mm2‡ . |
|---|---|---|---|---|
| Adult mice, older than 12 wk old | ||||
| ++, wild-type§ | 2 | 0 | 19.0 ± 4.0 (18) | 3.1 ± 1.4 (10) |
| Hi-retic +/+ | 30 | 0 | 18.5 ± 3.5 (5) | 7.4 ± 1.6 (5) |
| sphJ/sphJ | 95 | 75 | 1105.8 ± 67.5 (7) | 122.3 ± 23.1 (7) |
| sph2BC/sph2BC | 90 | 90 | 899.0 ± 127.9 (9) | 94.9 ± 14.9 (10) |
| sph/sph, 12-24 wk | 95 | 100 | 708.7 ± 38.6 (29) | 170.2 ± 20.9 (15) |
| sph/sph, 12-16 wk | 95 | 100 | 696.4 ± 49.9 (15) | 172.5 ± 33.2 (7) |
| sph/sph, 20-24 wk | 95 | 100 | 721.8 ± 61.9 (14) | 168.1 ± 29.6 (8) |
| sphDem/sphDem, 12-24 wk | 50 | 70 | 798.9 ± 113.3 (10) | 53.4 ± 11.0 (7) |
| sphDem/sphDem, 12-16 wk | 50 | 66 | 859.9 ± 177.7 (6) | 46.8 ± 8.2 (4) |
| sphDem/sphDem, 20-24 wk | 50 | 75 | 707.4 ± 134.9 (4) | 62.3 ± 25.3 (3) |
| Young mice (3-8 wk old) | ||||
| sph/sph, 3-4 wk | ND | 0 | 1069.6 ± 79.7 (7) | ND |
| sph/sph, 6-8 wk | ND | 37.5 | 1261.6 ± 79.8 (8) | ND |
| sphDem/sphDem, 3-4 wk | ND | 25 | 959.7 ± 115.6 (8) | ND |
| sphDem/sphDem, 6-8 wk | ND | 33 | 954.3 ± 43.7 (6) | ND |
Age and genotype . | Retic %* . | % Cerebral infarction† . | RBC adhesion to TSP, cells/mm2‡ . | RBC adhesion to LM, cells/mm2‡ . |
|---|---|---|---|---|
| Adult mice, older than 12 wk old | ||||
| ++, wild-type§ | 2 | 0 | 19.0 ± 4.0 (18) | 3.1 ± 1.4 (10) |
| Hi-retic +/+ | 30 | 0 | 18.5 ± 3.5 (5) | 7.4 ± 1.6 (5) |
| sphJ/sphJ | 95 | 75 | 1105.8 ± 67.5 (7) | 122.3 ± 23.1 (7) |
| sph2BC/sph2BC | 90 | 90 | 899.0 ± 127.9 (9) | 94.9 ± 14.9 (10) |
| sph/sph, 12-24 wk | 95 | 100 | 708.7 ± 38.6 (29) | 170.2 ± 20.9 (15) |
| sph/sph, 12-16 wk | 95 | 100 | 696.4 ± 49.9 (15) | 172.5 ± 33.2 (7) |
| sph/sph, 20-24 wk | 95 | 100 | 721.8 ± 61.9 (14) | 168.1 ± 29.6 (8) |
| sphDem/sphDem, 12-24 wk | 50 | 70 | 798.9 ± 113.3 (10) | 53.4 ± 11.0 (7) |
| sphDem/sphDem, 12-16 wk | 50 | 66 | 859.9 ± 177.7 (6) | 46.8 ± 8.2 (4) |
| sphDem/sphDem, 20-24 wk | 50 | 75 | 707.4 ± 134.9 (4) | 62.3 ± 25.3 (3) |
| Young mice (3-8 wk old) | ||||
| sph/sph, 3-4 wk | ND | 0 | 1069.6 ± 79.7 (7) | ND |
| sph/sph, 6-8 wk | ND | 37.5 | 1261.6 ± 79.8 (8) | ND |
| sphDem/sphDem, 3-4 wk | ND | 25 | 959.7 ± 115.6 (8) | ND |
| sphDem/sphDem, 6-8 wk | ND | 33 | 954.3 ± 43.7 (6) | ND |
Retic indicates reticulocyte; and ND, not done.
Percent cerebral infarction derived from a separate cohort of mice for sphJ/sphJ and sph2BC/sph2BC but from the same cohort of mice used in adhesion experiments in this study for all other groups.
Mean ± SEM; numbers in parentheses indicate number of measurements; P values are found in Figure 1.
WBB6F1-+/+ mice; data is similar for WBB6F1-sph/+, and CcS3/Dem-+/+ and -sphDem/+ mice.
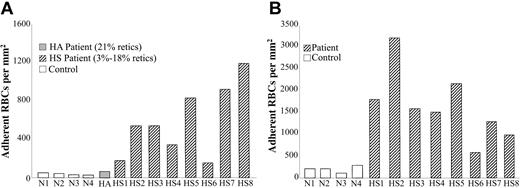
To determine whether increased RBC adhesion is also a characteristic of human HS/HE RBCs, we assessed the adhesion of RBCs from 9 HS patients (4%-18% reticulocytes) to TSP and LM 9 (Table 2). The adhesion of human HS RBCs to TSP was at or below the level of murine HS/HE RBCs (Figure 2A). In comparison, TSP adhesion of RBCs from a patient with hemolytic anemia of unknown etiology and 21% reticulocytes was not significantly increased over that of normal RBCs. Thiazole orange enumeration of reticulocytes in patient HS5 did not indicate an increase in the percentage of reticulocytes present in TSP-adherent RBCs versus whole blood (data not shown). These data again suggest that reticulocytes do not preferentially bind TSP under these flow conditions.
Hematologic parameters in humans with HS
ID number (age) . | Hemoglobin, g/L . | MCHC, g/dL . | Reticulocytes, % . | Splenectomy, yes/no . |
|---|---|---|---|---|
| HS1 (8 y) | 104 | 34.4 | 10.7 | No |
| HS2 (10 y) | 99 | 36.2 | 15.9 | No |
| HS3 (11 y) | 131 | 35.8 | 4.3 | No |
| HS4 (4 y) | 131 | 35.8 | 14.5 | No |
| HS5 (adult) | 169 | 37.0 | 3.3 | Yes |
| HS6 (5 y) | 124 | 34.6 | 4.1 | No |
| HS7 (2 y) | 90 | 36.3 | 12.7 | No |
| HS8 (4 y) | 88 | 36.4 | 18.1 | No |
| HA (16 y) | 55 | 33.5 | 21.1 | Yes |
| Normal | 115-150 | 32.0-36.0 | <2.2 | NA |
ID number (age) . | Hemoglobin, g/L . | MCHC, g/dL . | Reticulocytes, % . | Splenectomy, yes/no . |
|---|---|---|---|---|
| HS1 (8 y) | 104 | 34.4 | 10.7 | No |
| HS2 (10 y) | 99 | 36.2 | 15.9 | No |
| HS3 (11 y) | 131 | 35.8 | 4.3 | No |
| HS4 (4 y) | 131 | 35.8 | 14.5 | No |
| HS5 (adult) | 169 | 37.0 | 3.3 | Yes |
| HS6 (5 y) | 124 | 34.6 | 4.1 | No |
| HS7 (2 y) | 90 | 36.3 | 12.7 | No |
| HS8 (4 y) | 88 | 36.4 | 18.1 | No |
| HA (16 y) | 55 | 33.5 | 21.1 | Yes |
| Normal | 115-150 | 32.0-36.0 | <2.2 | NA |
HS3 and HS4 are half-siblings. HS5 and HS6 are father and daughter. HS7 and HS8 are siblings. HA has a nonspherocytic hemolytic anemia of unknown etiology.
MCHC indicates mean corpuscular hemoglobin concentration; ND, not done; and NA, not applicable.
Increased adhesion of human HS RBCs to TSP and LM. Washed RBCs from human controls (□,N1 through N4), a patient with nonspherocytic hemolytic anemia of unknown etiology (▤, HA), and patients with HS (▧, HS1 through HS8) were perfused through flow chambers coated with TSP (A) or LM (B), as described for Figure 1. Adherent RBCs/mm2 are depicted as the mean of duplicate measurements from the same blood sample.
Increased adhesion of human HS RBCs to TSP and LM. Washed RBCs from human controls (□,N1 through N4), a patient with nonspherocytic hemolytic anemia of unknown etiology (▤, HA), and patients with HS (▧, HS1 through HS8) were perfused through flow chambers coated with TSP (A) or LM (B), as described for Figure 1. Adherent RBCs/mm2 are depicted as the mean of duplicate measurements from the same blood sample.
In contrast to adhesion to TSP, adhesion of human HS RBCs to human LM is approximately 10-fold higher than adhesion of murine HS/HE RBCs to human LM (Figure 2B). Adhesion of human HS RBCs to LM is generally higher than adhesion to TSP, similar to our previous observations with human sickle RBCs.27,33 Since adhesion of murine HS/HE RBCs to human LM was significantly lower than adhesion of human HS RBCs to human LM, we focused our additional studies on the adhesion of murine and human HS/HE RBCs to human TSP.
HS/HE RBC adhesion to TSP is inhibited by dextran sulfate
Previous studies in our laboratory have shown that adherence of human sickle RBCs to TSP can be blocked by preincubation of the RBCs with the anionic polysaccharides high–molecular weight dextran sulfate (HMW DS) and chondroitin sulfate A (CSA), which interfere with potential interactions between TSP and sulfated glycolipids on the RBC surface.27 Similarly, adherence of murine HS (sph/sph) or HE (sphDem/sphDem) RBCs to immobilized TSP was also blocked by preincubation of the RBCs with HMW DS (Figure 3A; P < .001). Adherence of human HS RBCs to TSP was also blocked by preincubation with HMW DS (Figure 3A). Inhibition of TSP adhesion of mouse and human HS/HE RBCs by preincubation with HMW DS was lost if RBCs were washed once with SFM-BSA immediately prior to flow (data not shown), suggesting that high–molecular weight dextran sulfate does not bind with high affinity to the RBC surface. In contrast to our data with human sickle RBCs,27 adherence of murine HS/HE RBCs to TSP was not blocked by preincubation of the RBCs with CSA (Figure 3A). Adhesion of human HS RBCs to TSP was modestly but significantly inhibited by preincubation with CSA (Figure 3A; P < .001).
Murine HS/HE RBCs have increased exposure of phosphatidylserine (PS) on the outer surface (3- to 20-fold higher than normal RBCs).22 To investigate whether PS contributed to mouse or human HS/HE RBC adhesion to TSP, RBCs were preincubated with annexin V to block exposed PS prior to addition to the flow chamber. As shown in Figure 3A, binding of exposed PS with annexin V did not significantly alter the adhesive characteristics of murine HS and HE RBCs, suggesting that adherence of the mutant RBCs to TSP was not dependent on PS exposure. Preincubation of human HS RBCs with annexin V also did not significantly affect adhesion to TSP (Figure 3A).
Previous studies have suggested a role for integrin-associated protein (IAP; CD47) in the adhesion of human sickle RBCs to TSP under flow conditions.34,35 To investigate a potential role for IAP in the adhesion of HS/HE RBCs to TSP, mutant RBCs were preincubated either with antibodies to CD47 or with peptides containing the intact or scrambled TSP binding site for IAP. Preincubation of mouse HS (sph/sph)orHE(sphDem/sphDem) RBCs with monoclonal antibody miap301 against murine CD47 did not inhibit adhesion to TSP (Figure 3B). Likewise, pretreatment of human HS RBCs with the IAP ligand- and function-blocking monoclonal antibodies B6H12 and 1F7 against human CD47 did not affect TSP adhesion of the mutant RBCs (Figure 3B), although noticeable RBC clumping was observed in the 1F7-treated samples (data not shown). Pretreatment of mouse and human HS/HE RBCs with the IAP-specific agonist peptide 4N1K, which contains the intact TSP binding site for IAP, did not inhibit but rather increased the TSP adhesion of the mutant RBCs by 1.5- to 2-fold (Figure 3B; P < .05), whereas preincubation with the scrambled peptide SCR had no effect on RBC adhesion to TSP (data not shown).
Age-dependent variation in the TSP adherence of HS RBCs
Our initial analyses have found that thrombi and infarcts in α-spectrin–deficient sph/sph mice with HS are not histologically evident prior to 6 weeks of age, affect 17% to 50% of sph/sph mice between 6 and 8 weeks of age, and are present in all sph/sph mice by 12 weeks of age.26 We assessed the TSP adhesion of RBCs from “prethrombotic” 3- to 4-week-old, “perithrombotic” 6- to 8-week-old, and “postthrombotic” 12- to 16- and 20- to 24-week-old sph/sph mice. As shown in Figure 4A, the TSP adhesion of RBCs from 3- to 4- and 6- to 8-week-old sph/sph mice is 1.5- and 1.8-fold higher, respectively, than adult (> 12 weeks old) RBC adhesion (P < .005). These data suggest that sph/sph RBCs have an enhanced adhesive phenotype immediately prior to and during a high-risk period for development of cerebral infarction. As a comparison, we assessed the TSP adhesion of RBCs from similar age groups of sphDem/sphDem mice. These mice have HE, and, in contrast to sph/sph mice with HS, exhibit neonatal thrombosis and infarction.15 As shown in Figure 4B, we found stable levels of TSP adhesion of RBCs from sphDem/sphDem mice among all ages tested. Finally, analysis of sph/sph and sphDem/sphDem data from Figures 4A and 4B did not show any differences between RBC adhesion to TSP in male versus female mice in any of the age groups studied, suggesting a lack of influence of sex on RBC adhesion to TSP (data not shown).
Discussion
There are 6 major conclusions from the data presented in this paper. (1) Adhesion of murine HS and HE RBCs to human TSP and human LM is significantly increased compared with adhesion of normal murine RBCs to TSP and LM. (2) RBCs from humans with HS also exhibit increased adhesion to TSP and LM under flow conditions. (3) Adhesion of mouse HS/HE RBCs to TSP is inhibited by preincubation of the RBCs with high–molecular weight dextran sulfate but not by chondroitin sulfate A. (4) Adhesion of mouse and human HS/HE RBCs to TSP under our in vitro flow conditions is not dependent on PS exposure. (5) Adhesion of mouse and human HS/HE RBCs to TSP is not inhibited by preincubation with several antibodies against CD47 and is increased by preincubation with TSP peptide 4N1K. (6) The TSP adhesion of RBCs from prethrombotic and perithrombotic sph/sph mice is significantly higher than the TSP adhesion of RBCs from postthrombotic sph/sph mice.
As is seen with HS/HE mice, humans with heritable hemolytic anemias suffer from a number of pathologic complications including vaso-occlusion, thrombosis, and stroke. Disease severity in human HS is variable; most patients exhibit only mild to moderate anemia.16,17 Nevertheless, both venous and arterial thrombosis, including stroke, have been documented in patients with HS.18-21 Approximately 8% of children with sickle cell disease (SS-D) develop clinical evidence of stroke; an additional 10% to 20% have subclinical cerebrovascular disease documented by magnetic resonance imaging.1,2 Both human sickle and HS RBCs exhibit abnormal cation homeostasis and decreased deformability.16,36 Sickle RBCs exhibit increased adhesion to endothelial cells and components of the subendothelial matrix under both static and flow conditions. Here, we present the first report that human HS RBCs exhibit increased adhesion to TSP and LM under flow conditions. Similar to adhesion of human sickle RBCs, adhesion of human HS RBCs to TSP and LM is variable from patient to patient. Overall, human HS RBCs tend to have lower adhesion to TSP and LM than is seen with human sickle RBCs.27,33
Animal models that duplicate the pathology of human diseases allow more comprehensive analyses of pathophysiologic mechanisms than is possible in humans. Several mouse models of SS-D exist.5,6,37-39 While spontaneous stroke has been reported,8 the overall prevalence of thrombosis and stroke in these mouse models of SS-D is, as yet, unpublished. Mice with spontaneous mutations in cytoskeletal genes present with a very severe HS or HE that is rarely observed in human HS/HE patients. HS/HE mice also have a much higher incidence of thrombosis and stroke than has been documented in human HS/HE. The high incidence of stroke in the HS/HE mice make these excellent model systems in which to define the contribution of hemolytic RBCs to the development of vascular pathology. In addition, the highly inbred genetic background on which the murine cytoskeletal gene mutations are maintained minimizes the phenotypic variability that can confound experimental analyses.
The severe hemolytic anemia of the α-spectrin mutant mice raises the question of whether the increased RBC adhesion to TSP and LM is due to the concomitant and compensatory reticulocytosis. However, there was no significant increase in RBC adhesion to TSP or LM in +/+ mice induced to 30% reticulocytosis (Figure 1A-B; Hi-ret). It is possible that a certain “threshold” of reticulocytosis must be reached before reticulocytes express adhesive molecules. This seems unlikely, since the human HS samples presented here have lower reticulocyte counts, and higher adhesion to TSP and LM, than either the hi-retic mice or the human hemolytic anemia patient with 21% reticulocytes. Alternatively, it is possible that the reticulocytes generated by repeated phlebotomy of +/+ mice may not be the “stress” reticulocytes that are typically seen in chronic hemolytic anemia and which have been shown in human sickle cell disease to express unique subsets of cell adhesion molecules.30-32 The low TSP adhesion of RBCs from the hemolytic anemia patient with 21% reticulocytes also argues against this possibility. Finally, quantification of the percent reticulocytes among TSP-adherent RBCs in both mouse and human suggests a distinct lack of preferential binding of reticulocytes to TSP under our flow conditions.
Adhesion of murine HS/HE RBCs to human LM is approximately 10-fold lower than adhesion of human HS or SS RBCs to human LM (Figure 2B; Punzalan et al8 and Hillery and colleagues27,33 ). Lutheran (Lu) blood group glycoproteins are likely to be a major receptor for laminin on red blood cells.40,41 Our results are consistent with the 10-fold lower affinity documented by Parsons and colleagues42 of mouse Lu compared with human Lu for human LM. This suggests that the adhesive component of RBCs that binds laminin may significantly differ between murine and human erythrocytes. Commercially available mouse LMs are not derived from placenta, a unique source in humans of the laminin 10/11 isoform, which contains the α5 chain shown to be critical to the binding of human RBCs.42 The lower affinity of mutant mouse RBCs for human LM, combined with the lack of commercially available murine laminin 10/11, makes an assessment of the role of the mouse Lu receptor in pathogenic adhesion of murine RBCs unfeasible under our current assay conditions. For this reason, we have focused our studies on the interaction of murine HS/HE RBCs with human TSP.
The C-terminal domain of TSP, previously shown to be critical to the binding of human sickle RBCs to TSP,43 is 90% homologous between mouse and human44,45 ; this likely explains why both murine HS and human sickle RBCs bind at similar levels to human TSP.8 The lack of inhibition of murine HS/HE RBC adhesion to TSP by annexin V in our assay system indicates that PS exposure on the murine HS/HE RBC is not essential for binding to TSP. The level of inhibition of RBC adhesion to TSP by HMW dextran sulfate is similar between mouse and human HS/HE RBCs and human sickle RBCs.27 In contrast, human sickle27 and human HS/HE RBC adhesion to TSP is inhibited by chondroitin sulfate A, whereas murine HS/HE RBC adhesion to TSP is not. Since HMW DS is a much more highly charged molecule than CSA, these data suggest that “charged” interactions, such as acidic RBC membrane glycolipids, influence erythrocyte adhesion to TSP differently in mice and humans. While we hypothesize that there are common mechanisms involved in the adhesion of HS/HE and sickle RBCs, identification of the precise molecules involved is necessary before accurate comparisons can be made.
Similar to previous studies by our laboratory43 as well as Brittain and colleagues34,35 with human sickle RBCs, adhesion of murine and human HS/HE RBCs to TSP is increased by preincubation with the TSP peptide 4N1K. Since this peptide contains the binding site on TSP for CD47/integrin associated protein (IAP), these data suggest that binding of TSP by CD47 on HS/HE RBCs further enhances RBC adhesion to immobilized TSP. However, preincubation with ligand- and function-blocking antibodies to CD47 did not inhibit murine and human HS/HE RBC adhesion to TSP. These results imply that adhesion of HS/HE RBCs to immobilized TSP may involve molecules other than CD47. Identification of additional molecules involved in the adhesion of HS/HE RBCs to immobilized TSP will aid in the delineation of the contribution(s) of CD47 to pathologic HS/HE RBC adhesion.
Analyses of the data on adult mice (Table 1) failed to find significant correlations between stroke incidence and RBC adhesion to TSP. In contrast, our results from young sph/sph mice (Figure 4; Table 1) indicate that the mutant RBCs exhibit enhanced adhesion to TSP immediately prior to and during a high-risk period for initiation of cerebral infarction.26 The lack of correlation between RBC adhesion to TSP and stroke incidence in adult mice with HS/HE may reflect the assessment of RBC adhesion at an age after which strokes have already occurred. This theory is strengthened by the lack of increased adhesion of RBCs from young compared with adult sphDem/sphDem mice, in which thrombosis and infarction are already present in neonates. Based on this hypothesis, we would predict that increased RBC adhesion would be seen prenatally or in 0- to 1-week-old sphDem/sphDem mice. While the mechanism of thrombosis and infarction in HS/HE mice is not known, a critical environment for the development of stroke may exist in physically maturing sph/sph mice. The data presented herein indicate that sph/sph RBCs have enhanced adhesion to TSP immediately prior to and during a high-risk period for stroke development in these animals; therefore, we speculate that changes in the adhesive characteristics of the RBC membrane may contribute to this pathology. However, it is probable that changes in adhesive molecule expression on nonerythroid cells, particularly vascular endothelial cells in the brain, also influence the precipitation of cerebral infarction in maturing animals. The vascular endothelium is diverse; brain microvascular endothelial cells (ECs) likely express different complements of adhesion molecules than nonbrain microvessel and large vein endothelial cells.46 In addition, vascular endothelial cells from neonates may express different amounts and combinations of adhesion molecules at different developmental time points.47 Therefore, a complete understanding of the mechanism(s) involved in stroke in sph/sph mice must address the particular complement(s) of adhesion molecules that are exposed on both erythroid and nonerythroid (eg, cerebrovascular endothelial) cells at different ages.
Prepublished online as Blood First Edition Paper, August 28, 2003; DOI 10.1182/blood-2003-02-0492.
Supported by National Institutes of Health (NIH) grants HL29305 (J.E.B.) and HL70981 (C.A.H.), NIH NRSA DK09482 (N.J.W.), American Heart Association (AHA) grants 0050467N (C.A.H.) and 0265250Z (N.J.W.), and Clinical Research Center Grant RR00058 from the NIH.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Paul Scott and Sandy Steffes, RN, for assistance with coordination of HS patient sample collection and Nyama Sillah and Terri Besch for technical assistance with flow adhesion assays.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal