Abstract
Imatinib mesylate (STI571) is a competitive Bcr-Abl tyrosine kinase inhibitor and has yielded encouraging results in treatment of chronic myelogenous leukemia (CML) and gastrointestinal stroma tumors (GISTs). Apart from inhibition of the Abl protein tyrosine kinases, it also shows activity against platelet-derived growth factor receptor (PDGF-R), c-Kit, Abl-related gene (ARG), and their fusion proteins while sparing other kinases. In vitro studies have revealed that imatinib mesylate can inhibit growth of cell lines and primitive malignant progenitor cells in CML expressing Bcr-Abl. However, little is known about the effects of imatinib mesylate on nonmalignant hematopoietic cells. In the current study we demonstrate that in vitro exposure of mobilized human CD34+ progenitors to therapeutic concentrations of imatinib mesylate (1-5 μM) inhibits their differentiation into dendritic cells (DCs). DCs obtained after 10 to 16 days of culture in the presence of imatinib mesylate showed concentration-dependent reduced expression levels of CD1a and costimulatory molecules such as CD80 and CD40. Furthermore, exposure to imatinib mesylate inhibited the induction of primary cytotoxic T-lymphocyte (CTL) responses. The inhibitory effects of imatinib mesylate were accompanied by down-regulation of nuclear localized RelB protein. Our results demonstrate that imatinib mesylate can act on normal hematopoietic cells and inhibits the differentiation and function of DCs, which is in part mediated via the nuclear factor κB signal transduction pathway.
Introduction
Imatinib mesylate, also known as STI571 or Glivec (Novartis, Basel, Switzerland), is a promising new treatment for chronic myelogenous leukemia (CML). Imatinib mesylate is a 2-phenylaminopyrimidine derivate that was designed as a selective competitive inhibitor of the Abl protein tyrosine kinases (v-Abl, Bcr-Abl, and c-Abl).1-5 It also has strong activity against the platelet-derived growth-factor receptor (PDGF-R), c-Kit, ARG, and their fusion proteins Tel-Abl and Tel-PDGF-R, but does not affect other kinases.6-9
Recent clinical trials of imatinib mesylate in the treatment of chronic-phase CML have demonstrated that the drug is well tolerated with only few adverse effects and can induce complete hematologic and cytogenetic responses in a significant proportion of patients.2,3,10-12 Moreover, activity of imatinib mesylate against more advanced, accelerated-phase blast crises and in patients with relapsed or refractory Philadelphia chromosome–positive (Ph+) acute lymphoid leukemias was reported.13,14 Furthermore, in patients with gastrointestinal stroma tumors (GISTs), where activating mutations of c-Kit are likely responsible for the pathogenetic events, imatinib mesylate yielded encouraging results.6,7,15,16 Because this tumor has so far been highly refractory to chemotherapy, imatinib mesylate is emerging as an important new therapeutic agent.
However, the effects of imatinib mesylate on normal, nonmalignant hematopoietic cells have not been extensively evaluated so far. It is not clear whether some side effects like cytopenias that occur during treatment with imatinib mesylate may result from suppression of normal progenitor growth and differentiation. Recently, it was demonstrated that imatinib mesylate reduces the number of colony-forming cells in peripheral blood or bone marrow (BM) from patients with CML, with minimal effect on normal cells.17
Dendritic cells (DCs) are recognized as the most powerful antigen-presenting cells (APCs) with the unique ability to initiate and maintain primary immune responses. They originate from BM-derived progenitor cells, spread via the bloodstream, and can be found in almost every organ as the sentinels of the immune system. In vitro, DCs can be generated from peripheral blood monocytes using granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). The differentiation of DCs from CD34+ progenitor cells can be mediated by different cytokines like GM-CSF, tumor necrosis factor α (TNF-α), IL-4, and FMS-like tyrosine kinase 3 (FLT3) ligand and stem cell factor (SCF).18-25
Because SCF has been shown to play an important role in DC development, we here explored a potential effect of imatinib mesylate on the development of mobilized human CD34+ peripheral blood progenitor cells (PBPCs) into DCs. We show that in vitro exposure of CD34+ PBPCs to therapeutic concentrations of imatinib mesylate (1-5 μM) affects the differentiation and functional properties of generated DCs via inhibition of the nuclear factor κB (NF-κB) family member RelB.
Materials and methods
Cells and reagents
All cells were cultured in RPMI 1640 with glutamax-I, supplemented with 10% inactivated fetal calf serum (FCS), 50 μM 2-mercaptoethanol, and antibiotics (Invitrogen, Karlsruhe, Germany). Imatinib mesylate was a kind gift of Novartis Pharmaceuticals (Basel, Switzerland). The cell lines used in the experiments were A498 (renal cell carcinoma cell line, Her-2/neu+, HLA-A2+; DSMZ, Braunschweig, Germany), Croft (Epstein-Barr virus [EBV]–immortalized B-cell line, Her-2/neu–, HLA-A2+, kindly provided by O. J. Finn, Pittsburgh, PA), SK-OV-3 (ovary adenocarcinoma cell line, HLA-A3+, kindly provided by O. J. Finn). The CML cell line K562 was used to determine the activity of natural killer (NK) cells.
DC generation
DCs were generated from human CD34+ PBPCs mobilized with granulocyte colony-stimulating factor (G-CSF) as described previously.26 In brief, cryopreserved CD34+ cells were cultured for 10 to 16 days with a combination of different cytokines (GM-CSF [100 ng/mL; Leucomax, Novartis], IL-4 [20 ng/mL; R&D Systems, Wiesbaden, Germany), TNF-α [10 ng/mL; R&D Systems], and FLT3 ligand [FLT3L; 100 ng/mL; R&D Systems]). Cytokines were added to differentiating DCs every 2 to 3 days. Imatinib mesylate was dissolved in dimethyl sulfoxide (DMSO) and added to the culture medium starting from the first day of culture together with GM-CSF, IL-4, TNF-α, and FLT3L in concentrations varying from 1 to 5 μM. Equal amounts of DMSO were added to the control cells to exclude any effects of the solvent. In some experiments, DC maturation was induced by adding soluble CD40 ligand (CD40L, 500 ng/mL; Bender MedSystems, Vienna, Austria) and interferon γ (IFN-γ; 100 U/mL; R&D Systems) 24 hours before harvesting the cells. For blocking experiments, monoclonal antibodies against SCF (1 μg/mL; R&D Systems) and its receptor c-Kit (10 μg/mL; Sigma, Deisenhofen, Germany) were added to the cells together with the cytokines. Mouse IgG (Dianova, Hamburg, Germany) was added to control cells. DCs were enumerated by flow cytometry as lineage (CD14, CD3, CD19) negative and HLA-DR bright. Furthermore, analysis of the expression levels of the DC markers CD1a and CD83 and costimulatory molecules CD80, CD86, and CD40 was performed.
Immunostaining
Cells were stained using fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–conjugated mouse monoclonal antibodies against CD14, CD80, HLA-DR, CD3, CD19, CD54, CD11c (Becton Dickinson, Heidelberg, Germany); CD40, CD86, CD33 (PharMingen, Hamburg, Germany); CD1a, CD11a (Dako Diagnostika, Hamburg, Germany); CD83 (Immunotech, Marseille, France); and mouse IgG isotype control (Becton Dickinson). Cells were analyzed on a FACSCalibur cytometer (Becton Dickinson). To calculate the percentage of positive cells, a proportion of 1% false-positive events was accepted in the negative control samples throughout.
Detection of apoptosis
For detection of apoptosis, the annexin V–Fluos staining kit (Roche Diagnostics, Mannheim, Germany) was used according to the instructions of the manufacturer. In addition, leakage of fragmented DNA from apoptotic nuclei was measured as described previously.27,28 In short, DC nuclei were isolated using a hypotonic lysis buffer (0.1% sodium citrate, 0.1% Triton X-100, 50 mg/mL propidium iodide) and immediately analyzed by flow cytometry.
Induction of antigen-specific CTL responses
The induction of Her-2/neu and influenza matrix protein (IMP)–specific CTL was performed as described previously.29-31 The HLA-A2–binding peptides derived from Her-2/neu E75 (amino acids 369-377: KIGSFLAFL), IMP peptide (amino acids 58-66: GILGFVFTL), and HIV (pol HIV-1 reverse transcriptase peptide; amino acids 476-484: ILKEPVHGV) were synthesized using standard F-moc chemistry on a peptide synthesizer (432A; Applied Biosystems, Weiterstadt, Germany) and analyzed by reversed-phase high-performance liquid chromatography and mass spectrometry. For induction of peptide-specific cytotoxic T lymphocytes (CTLs), 5 × 105 DCs were pulsed with 50 μg/mL E75 or IMP peptide for 2 hours, washed, and incubated with 3 × 106 peripheral blood mononuclear cells (PBMNCs) without imatinib mesylate. After 7 days of culture, cells were restimulated with autologous peptide-pulsed PBMNCs, and 2 ng/mL IL-2 (R&D Systems) was added on days 1, 3, and 5. The cytolytic activity of induced CTLs was analyzed on day 5 after the last restimulation in a standard 51Cr-release assay.
CTL assay
The standard 51Cr-release assay was performed as described previously.29-31 Target cells were pulsed with 50 μg/mL peptide for 2 hours and labeled with [51Cr]-sodium chromate for 1 hour at 37°C. Then, 1 × 104 cells were transferred to a well of a round-bottom 96-well plate. Varying numbers of CTLs were added to a final volume of 200 μL and incubated for 4 hours at 37°C. At the end of the assay, supernatants (50 μL/well) were harvested and counted in a β-plate counter. The percentage of specific lysis was calculated as: 100 × (experimental release – spontaneous release/maximal release – spontaneous release). Spontaneous and maximal releases were determined in the presence of either medium or 2% Triton X-100, respectively.
For antibody-blocking experiments, cells were incubated for 30 minutes with 10 μg/mL monoclonal antibody BB7.2 against HLA-A2 (kindly provided by Stefan Stevanovic, Tübingen, Germany) and mouse IgG, respectively, before seeding in 96-well plates.
RT-PCR
Preparation of nuclear extracts
Nuclear extracts were prepared from DCs as described previously.34 Briefly, 5 × 105 cells were washed with phosphate-buffered saline (PBS) and resuspended in 400 μL cold buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.9; 10 mM KCl; 0.1 mM EDTA [ethylenediaminetetraacetic acid]; 0.1 mM EGTA [ethylene glycol tetraacetic acid]; 1 mM dithiothreitol [DTT]; 0.5 mM phenylmethylsulfonyl fluoride [PMSF]). After an incubation of 15 minutes on ice, 25 μL of a cold 10% Igepal solution was added and the tubes were vortexed for 10 seconds. The homogenate was centrifuged for 30 seconds in a microfuge and the supernatant containing cytoplasmatic proteins was transferred to a new tube. The nuclear pellet was resuspended in 50 μL ice-cold buffer C (20 mM HEPES, pH 7.9; 0.4 M NaCl; 1 mM EDTA; 1 mM EGTA; 1 mM DTT; 1 mM PMSF) and incubated on ice for 15 minutes with vigorous shaking every 2 to 3 minutes. After 5 minutes of centrifugation, the supernatants were stored at –80°C.
Polyacrylamide gel electrophoresis and Western blotting
Protein concentrations of nuclear extracts were determined using a bicinchoninic acid (BCA) assay (Pierce, Perbio Science, Bonn, Germany). Approximately 20 μg nuclear extracts were separated by 10% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). The blot was probed with a polyclonal rabbit RelB antibody (C-19; Santa Cruz Biotechnology, Santa Cruz, CA), a monoclonal mouse c-Rel antibody (B-6; Santa Cruz Biotechnology), or a polyclonal rabbit RelA antibody (Upstate Biotechnology, Lake Placid, NY) followed by incubation with a horseradish peroxidase–conjugated secondary antibody. Bands were visualized by enhanced chemiluminescence (ECL) staining (Amersham Biosciences Europe, Freiburg, Germany).
Results
Exposure of mobilized CD34+ PBPCs to imatinib mesylate inhibits their differentiation into DCs
SCF and its receptor were shown to play a critical role during the development of DCs from CD34+ progenitor cells.19-21,23,24 Because imatinib mesylate is a selective inhibitor of the Abl tyrosine kinases with equipotent activity against PDGF-R and c-Kit–associated tyrosine kinases,1,6,8,9 we determined the effect of imatinib mesylate on the normal development of DCs by monitoring the acquisition of DC morphology and phenotype after 10 to 16 days in culture. Purified human CD34+ PBPCs from healthy donors mobilized with G-CSF were cultured with GM-CSF and TNF-α.18 The cells differentiated into large, round, loosely adherent cells showing the typical cell protrusions in the form of veils or dendrites. Phenotypic analysis of GM-CSF/TNF-α–generated DCs demonstrated acquisition of a DC phenotype characterized by the expression of CD1a, CD83, HLA-DR, and costimulatory molecules such as CD80, CD86, and CD40. Addition of 1.25 μM imatinib mesylate together with GM-CSF and TNF-α from the first day of culture did not substantially affect the morphology but severely altered DC phenotype, characterized by reduction of CD1a, CD83, HLA-DR, CD80, and CD40 expression (Figure 1A), while not affecting CD86 and CD54 levels.
Exposure of CD34+cells to imatinib mesylate inhibits their differentiation into DCs in a concentration-dependent manner. (A) Mobilized human CD34+ PBPCs were cultured in the presence of GM-CSF and TNF-α for 10 to 16 days with or without imatinib mesylate. (B) CD34+ PBPCs were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate. Cells were analyzed by flow cytometry for expression of the DC markers CD1a and CD83 and costimulatory molecules CD80, CD86, and CD40. The level of surface expression is indicated as mean fluorescence intensity. (C) Mobilized human CD34+ progenitors were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate in different concentrations (1-5 μM). Fluorescence-activated cell sorting (FACS) analyses were performed to determine the phenotype of the generated cell populations. The level of cell surface expression is presented as mean fluorescence intensity.
Exposure of CD34+cells to imatinib mesylate inhibits their differentiation into DCs in a concentration-dependent manner. (A) Mobilized human CD34+ PBPCs were cultured in the presence of GM-CSF and TNF-α for 10 to 16 days with or without imatinib mesylate. (B) CD34+ PBPCs were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate. Cells were analyzed by flow cytometry for expression of the DC markers CD1a and CD83 and costimulatory molecules CD80, CD86, and CD40. The level of surface expression is indicated as mean fluorescence intensity. (C) Mobilized human CD34+ progenitors were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate in different concentrations (1-5 μM). Fluorescence-activated cell sorting (FACS) analyses were performed to determine the phenotype of the generated cell populations. The level of cell surface expression is presented as mean fluorescence intensity.
In the next set of experiments we evaluated whether the inhibitory effects of imatinib mesylate can be antagonized using a different cytokine combination including the ligand of the receptor tyrosine kinase FLT3. However, cells cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L together with 1.25 μM imatinib mesylate demonstrated a similar phenotype as the one observed in previous experiments (Figure 1B).
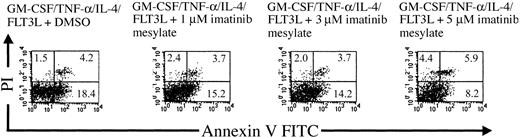
Moreover, the inhibitory effects of imatinib mesylate were found to be concentration dependent, as monitored by evaluating cell phenotypes after incubation with different imatinib mesylate concentrations (1 μM, 3 μM, 5 μM; Figure 1C), whereas no differences in the expression of CD33, CD11a, and CD11c were detected (data not shown). Importantly, the observed effects of imatinib mesylate were not mediated by the induction of cell death. When checking the viability of the cell populations and rate of apoptosis induction by annexin V and propidium iodide cell staining, we did not detect any increase in the rate of dead cells following exposure to the compound (Figure 2). These results were further confirmed by analyzing the leakage of fragmented DNA from apoptotic nuclei as described by Nicoletti et al27 (data not shown).
The effects of imatinib mesylate are not mediated by induction of apoptosis. Mobilized human CD34+ progenitors were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate in concentrations varying from 1 to 5 μM. The viability of the cell populations and rate of apoptosis induction were analyzed by annexin V FITC and propidium iodide staining in a flow cytometer. The percentages of necrotic or apoptotic cells are indicated in the corresponding quadrants.
The effects of imatinib mesylate are not mediated by induction of apoptosis. Mobilized human CD34+ progenitors were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate in concentrations varying from 1 to 5 μM. The viability of the cell populations and rate of apoptosis induction were analyzed by annexin V FITC and propidium iodide staining in a flow cytometer. The percentages of necrotic or apoptotic cells are indicated in the corresponding quadrants.
To determine the possible role of c-Kit in the observed inhibition of DC development, we incubated CD34+ cells with blocking antibodies against SCF and its receptor c-Kit. However, no effect on DC development could be detected (Figure 3), indicating that imatinib mesylate acts by inhibition of other tyrosine kinases.
The inhibitory effect of imatinib mesylate on DC differentiation is not mediated by c-Kit. Mobilized human CD34+ PBPCs were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without blocking antibodies against SCF (anti-SCF) and c-Kit (anti–c-Kit). Mouse IgG was added to control cells. DC surface marker expression was measured by FACS analyses. The surface expression is indicated as mean fluorescence intensity. Shaded histograms represent the isotype control; open histograms, the indicated antibody.
The inhibitory effect of imatinib mesylate on DC differentiation is not mediated by c-Kit. Mobilized human CD34+ PBPCs were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without blocking antibodies against SCF (anti-SCF) and c-Kit (anti–c-Kit). Mouse IgG was added to control cells. DC surface marker expression was measured by FACS analyses. The surface expression is indicated as mean fluorescence intensity. Shaded histograms represent the isotype control; open histograms, the indicated antibody.
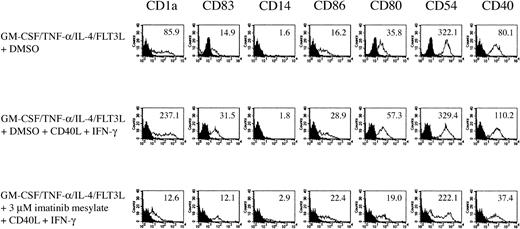
To further evaluate the responsiveness of DCs generated in the presence of imatinib mesylate to a maturation stimulus, we added soluble CD40L and IFN-γ 24 hours before harvesting the cells. As shown in Figure 4, the inhibitory effects of imatinib mesylate on DC surface expression of CD1a, CD83, CD80, and CD40 could not be antagonized by these maturation stimuli.
Imatinib mesylate inhibits activation of
DCs. DCs generated from CD34+ cells with GM-CSF, TNF-α, IL-4, and FLT3L in the presence of 3 μM imatinib mesylate were incubated with soluble CD40L and IFN-γ as a maturation stimulus 24 hours before harvesting the cells. The effect of imatinib mesylate on DC phenotype was analyzed by FACS. The surface expression is indicated as mean fluorescence intensity.
Imatinib mesylate inhibits activation of
DCs. DCs generated from CD34+ cells with GM-CSF, TNF-α, IL-4, and FLT3L in the presence of 3 μM imatinib mesylate were incubated with soluble CD40L and IFN-γ as a maturation stimulus 24 hours before harvesting the cells. The effect of imatinib mesylate on DC phenotype was analyzed by FACS. The surface expression is indicated as mean fluorescence intensity.
Exposure to imatinib mesylate reduces the capacity of DCs to activate lymphocyte responses
Initiation of primary immune responses is a unique feature of DCs. We therefore analyzed the ability of the DCs generated from CD34+ progenitor cells in the presence of GM-CSF, TNF-α, IL-4, and FLT-3L with or without imatinib mesylate to induce antigen-specific T cells against the Her-2/neu antigen. For the induction of a primary CTL response, DCs generated from CD34+ cells were pulsed with the synthetic HLA-A2–binding peptide E75 derived from Her-2/neu tumor-associated antigen and used as APCs. After 2 weekly restimulations, the CTL lines showed peptide-specific and HLA-A2–restricted killing of tumor cell lines pulsed with the cognate peptide or constitutively expressing the Her-2/neu antigen (Figure 5A). DCs generated in the presence of 3 μM imatinib mesylate failed to activate antigen-specific CTL responses (Figure 5B). Similar results were observed when a recall antigen (IMP peptide) was used for the induction of antigen-specific CTLs. CTL lines generated with DCs grown with GM-CSF/TNF-α/IL-4/FLT3L showed peptide-specific killing of tumor cell lines pulsed with the IMP peptide (Figure 5C), whereas DCs generated in the presence of 3 μM imatinib mesylate were unable to elicit a CTL response (Figure 5D).
Induction of CTL responses by peptide-pulsed DCs is impaired by addition of imatinib mesylate. Mobilized human CD34+ PBPCs were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate (3 μM) and used for the induction of primary Her-2/neu–specific CTL (A-B) or to elicit a CTL response against a recall antigen (IMP; C-D). (A) DCs were pulsed with the synthetic HLA-A2–binding peptide E75 derived from Her-2/neu tumor-associated antigen and used as APCs to induce a CTL response. The cytotoxic activity was determined after 2 restimulations in a standard 51Cr-release assay using Croft cells (HLA-A2+, Her-2/neu–) pulsed with E75 (▪) or HIV peptide (□), SK-OV-3 cells (HLA-A3+, Her-2/neu–; ▴), K 562 cells (♦) and A498 cells (HLA-A2+, Her-2/neu+) with (○) or without (•) blocking HLA-A2 antibody as target cells. (B) DCs generated with 3 μM imatinib mesylate were used as APCs in the setting described in panel A. (C) DCs were pulsed with the IMP peptide and used as APCs for CTL induction. The cytotoxic activity was determined after 2 restimulations in a standard 51Cr-release assay using Croft cells pulsed with IMP peptide (▪) or HIV peptide (□) and K 562 cells (♦). (D) DCs generated with 3 μM imatinib mesylate were used as APCs in the setting described in panel C. E/T indicates effector-target ratio.
Induction of CTL responses by peptide-pulsed DCs is impaired by addition of imatinib mesylate. Mobilized human CD34+ PBPCs were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate (3 μM) and used for the induction of primary Her-2/neu–specific CTL (A-B) or to elicit a CTL response against a recall antigen (IMP; C-D). (A) DCs were pulsed with the synthetic HLA-A2–binding peptide E75 derived from Her-2/neu tumor-associated antigen and used as APCs to induce a CTL response. The cytotoxic activity was determined after 2 restimulations in a standard 51Cr-release assay using Croft cells (HLA-A2+, Her-2/neu–) pulsed with E75 (▪) or HIV peptide (□), SK-OV-3 cells (HLA-A3+, Her-2/neu–; ▴), K 562 cells (♦) and A498 cells (HLA-A2+, Her-2/neu+) with (○) or without (•) blocking HLA-A2 antibody as target cells. (B) DCs generated with 3 μM imatinib mesylate were used as APCs in the setting described in panel A. (C) DCs were pulsed with the IMP peptide and used as APCs for CTL induction. The cytotoxic activity was determined after 2 restimulations in a standard 51Cr-release assay using Croft cells pulsed with IMP peptide (▪) or HIV peptide (□) and K 562 cells (♦). (D) DCs generated with 3 μM imatinib mesylate were used as APCs in the setting described in panel C. E/T indicates effector-target ratio.
The inhibitory effects of imatinib mesylate on the stimulatory capacity of DCs were not due to an increased secretion of IL-10 as analyzed by a commercially available enzyme-linked immunosorbent assay (data not shown).
Imatinib mesylate modulates chemokine mRNA level in DCs
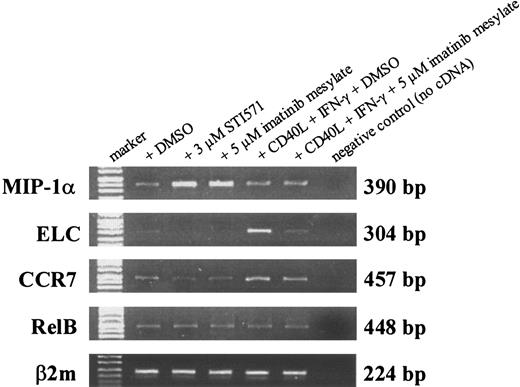
Transcription of chemokines known to be important for DC function was analyzed by RT-PCR. On treatment with imatinib mesylate in different concentrations, mRNA level of MIP-1α was up-regulated (Figure 6), whereas no differences in the transcription of TARC (CCL17) and the chemokine receptor CCR6 were observed (data not shown). In contrast, mRNA levels of ELC (CCL19) and the corresponding receptor CCR7 were reduced in the presence of imatinib mesylate (Figure 6).
Imatinib mesylate modulates mRNA level of MIP-1α, ELC, and CCR7 in DCs. RNA was extracted from DCs generated from mobilized human CD34+ PBPCs that were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate (3 μM and 5 μM). For activation of DCs, cells were incubated with soluble CD40L and IFN-γ 24 hours before harvesting the cells. Transcription of MIP-1α, ELC, CCR7, and RelB was analyzed by RT-PCR. Amplification of β2microglobulin (β2m) served as internal control to ensure the use of equal amounts of cDNA.
Imatinib mesylate modulates mRNA level of MIP-1α, ELC, and CCR7 in DCs. RNA was extracted from DCs generated from mobilized human CD34+ PBPCs that were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate (3 μM and 5 μM). For activation of DCs, cells were incubated with soluble CD40L and IFN-γ 24 hours before harvesting the cells. Transcription of MIP-1α, ELC, CCR7, and RelB was analyzed by RT-PCR. Amplification of β2microglobulin (β2m) served as internal control to ensure the use of equal amounts of cDNA.
Imatinib mesylate down-regulates nuclear RelB transcription factor but not c-Rel and RelA
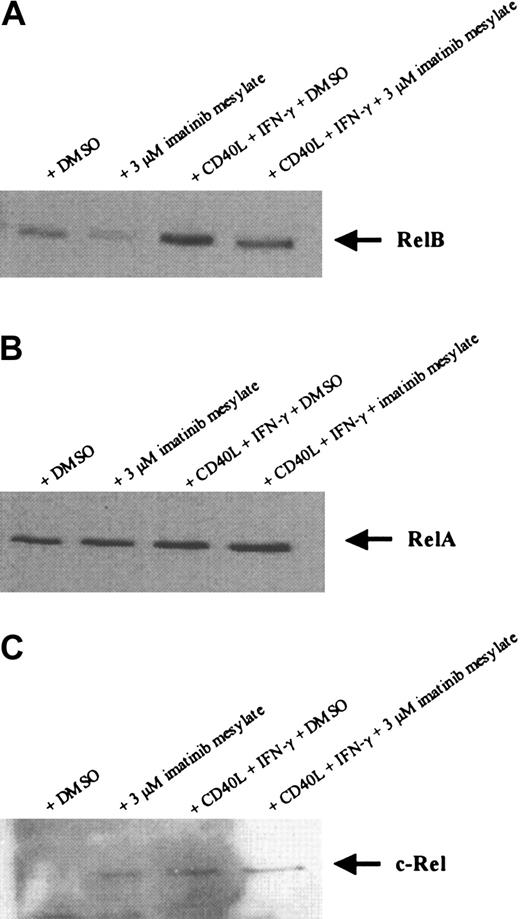
Recently it was shown that members of the NF-κB family of transcription factors are important for the differentiation and function of DCs.35-41 Because we did not detect any effect of imatinib mesylate on the level of RelB mRNA expression by RT-PCR (Figure 6), we further evaluated the nuclear localization of RelB, RelA, and c-Rel proteins in the generated DC populations. As shown in Figure 7, Western blot analysis revealed that the amount of nuclear-localized RelB and its induction by maturation stimuli is reduced in DCs generated in the presence of 3 μM imatinib mesylate, whereas the expression of RelA and c-Rel proteins was not affected. These results suggest that the effects of imatinib mesylate are mediated at least in part by inhibition of NF-κB signaling pathways.
Imatinib mesylate down-regulates the expression of nuclear localized RelB protein but not RelA and c-Rel in DCs. Mobilized human CD34+ PBPCs were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate (3 μM). For activation of DCs, cells were incubated with soluble CD40L and IFN-γ 24 hours before preparing nuclear extracts. Nuclear localized RelB (A), RelA (B), and c-Rel (C) protein were detected by SDS-PAGE and Western blot.
Imatinib mesylate down-regulates the expression of nuclear localized RelB protein but not RelA and c-Rel in DCs. Mobilized human CD34+ PBPCs were cultured in the presence of GM-CSF, TNF-α, IL-4, and FLT3L with or without imatinib mesylate (3 μM). For activation of DCs, cells were incubated with soluble CD40L and IFN-γ 24 hours before preparing nuclear extracts. Nuclear localized RelB (A), RelA (B), and c-Rel (C) protein were detected by SDS-PAGE and Western blot.
Discussion
The causative event in the initiation of CML is the formation of the Bcr-Abl oncogene, which codes for a constitutively activated tyrosine kinase as a result of the reciprocal translocation t(9;22). Therefore, a specific inhibitor of the Bcr-Abl kinase was thought to be effective in the therapy of patients with CML.
Imatinib mesylate is an adenosine triphosphate (ATP)–competitive inhibitor of the Abl tyrosine kinases (v-Abl, Bcr-Abl, c-Abl)1-4 and was also shown to inhibit c-Kit, ARG, PDGF-R, and their fusion proteins, but has no significant activity against other kinases.1,3,42,43 Moreover, in vitro studies revealed that the antiproliferative activity of imatinib mesylate occurred only in cells that expressed activated forms of Abl (Bcr-Abl, Tel-Abl, v-Abl, c-Abl), mutated forms of c-Kit, and activated PDGF-R (Tel-PDGF-R, v-sis-activated PDGF-R).3,6,9,44,45 In recent clinical trials, the tyrosine kinase inhibitor imatinib mesylate has shown promising results in the treatment of CML and also demonstrated activity in patients with myeloproliferative disorders including activating mutations in the gene encoding PDGF-Rβ46 as well as in patients with GISTs involving activating mutations of the c-Kit gene.6,7,15,16
Recently, it could be demonstrated that imatinib mesylate inhibits the proliferation of CML primitive precursors rather than to induce apoptosis, resulting in removal of the proliferative advantage of Bcr-Abl progenitors but not in elimination of these cells.47 In addition, it was shown that nondividing, quiescent Ph+ hematopoietic stem cells are insensitive to imatinib mesylate in vitro.48 In contrast to Ph+ hematopoiesis, little is known about the effect of imatinib mesylate on growth and differentiation of normal, nonmalignant hematopoietic stem and progenitor as well as differentiated cells. Previous studies revealed that imatinib mesylate reduces the number of colony-forming cells in peripheral blood or BM from patients with CML while not affecting normal hematopoiesis17 and has no significant effects on hematopoietic recovery in mice in vivo.49
Here we demonstrate that imatinib mesylate can directly act on mobilized human CD34+ progenitor cells and inhibits their differentiation into DCs. This finding might be of major clinical importance because imatinib mesylate is given continuously so far to prevent disease relapse. Furthermore, because CML in particular is considered to be an “immunogenic” disease, an impaired immune response due to treatment-related side effects could alter the immune surveillance by the organism, thereby potentially impairing immune-mediated long-term disease control. Recent data emphasize this aspect by showing that treatment of patients with the immune stimulatory cytokine IFN-α led to up-regulation of myeloblastin (MBN) expression and to detection of MBN-specific CTL in all patients studied. In contrast, in only a minority of patients treated with imatinib mesylate were MBN-specific CTLs detected.50
DCs are professional APCs that are important for the initiation of primary immune responses.51 They acquire antigens in the periphery that they process to peptide fragments and present in the context of major histocompatibility complex (MHC) molecules. On activation, they migrate to secondary lymphoid organs where the peptide-MHC complexes are presented to and recognized by antigen-specific T lymphocytes via the T-cell receptor.51-53 DCs residing in tissues originate from BM-derived circulating precursors that home in peripheral tissues and are able to further develop into antigen-capturing DCs.51,52 It was shown that CD34+ progenitor cells can be induced to differentiate into DCs in vitro using different cytokine combinations, including SCF.18,19,23,54 We therefore analyzed the effect of imatinib mesylate on the differentiation and function of DCs generated ex vivo from human CD34+ PBPCs that were mobilized with G-CSF.
The presence of imatinib mesylate in cell cultures in concentrations varying from 1 to 5 μM, serum levels typically achieved in patients, resulted in a severely altered DC phenotype characterized by reduction of DC surface markers CD1a and CD83 and costimulatory molecules CD80 and CD40 in a concentration-dependent manner. These effects were not mediated by induction of apoptosis, because the analysis of cell viability, which was examined by annexin V and propidium iodide staining, did not reveal a higher rate of apoptosis induction in the cell culture in the presence of imatinib mesylate.
In the next set of experiments we analyzed the possible effects of different cytokine combinations including FLT3L on the inhibition of DC differentiation by imatinib mesylate. FLT3L is an important growth and differentiation factor for DCs and was shown to expand different DC populations in vitro and in vivo in mice and humans.55-58 FLT3L is the ligand for the FLT3 that is a member of the PDGF-R tyrosine kinase subfamily and shares the structural features of KIT, FMS, and PDGF-R.59 On binding, FLT3L induces dimerization of the receptor leading to the activation of the tyrosine kinase domain, which results in autophosphorylation. Downstream signaling proteins of FLT3 include RAS-GAP, PLC-γ, PI3K, STAT5, and ERK1/2. This signal transduction pathway of FLT3L converges with the pathway of c-Kit activation. In our experiments, however, the use of a cytokine combination containing GM-CSF, TNF-α, IL-4, and FLT3L could not antagonize the inhibiting effects mediated by imatinib mesylate.
Interestingly, we could not mimic the changes in the DC phenotype observed on the incubation of cells with imatinib mesylate using blocking antibodies specific for SCF and c-Kit, because culturing CD34+ PBPCs in the presence of these antibodies resulted in a normal DC phenotype suggesting that other mechanisms might be active and are not mediated by inhibition of c-Kit receptor signaling.
In line with the altered DC phenotype we found that the capacity of DCs generated in the presence of imatinib mesylate to induce antigen-specific T-cell responses against tumor-associated antigens (Her-2/neu) or a recall antigen (IMP), was impaired. This effect was most likely due to the decreased expression of costimulatory molecules including CD80 and CD40. This inhibition was not mediated by IL-10, which was shown to be immunosuppressive by inhibition of antigen presentation in APCs and induction of T-cell tolerance.60-63
The Rel/NF-κB proteins p50, p52, p65, c-Rel, and RelB constitute a family of transcription factors involved in the regulation of a variety of genes during the immune response.35-41 NF-κB activation occurs by nuclear translocation caused by the inducible phosphorylation of inhibiting IκB proteins by the IκB kinase complex. Targeted disruption of RelB gene in mice as well as in in vitro studies revealed that it plays a critical role in DC function and differentiation. RelB–/– mice produce no apparent mature myeloid DCs,41 and BM chimeras have shown that it is due to a direct effect of RelB on stem cell development.39
We therefore analyzed the expression of RelB by RT-PCR and Western blot analysis in cells generated in the presence of imatinib mesylate. Whereas the mRNA level of RelB was not affected by imatinib mesylate, the nuclear-localized RelB protein was down-regulated in DCs treated with imatinib mesylate and could not be induced on maturation with CD40L and IFN-γ. In contrast, expression of RelA and c-Rel was not affected. These results suggest that the effects of imatinib mesylate are mediated at least in part via inhibition of RelB signaling, and therefore represent a novel signal transduction way of imatinib mesylate.
Prepublished online as Blood First Edition Paper, September 22, 2003; DOI 10.1182/blood-2003-03-0975.
Supported by a grant from Deutsche Forschungsgemeinschaft (SFB 510).
S.A. and A.M.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Sylvia Stephan, Bruni Schuster, and Tina Wiesner for the excellent technical assistance, Gerburg Stein for her help with the annexin V staining, as well as Reinhild Klein and Hans-Georg Rammensee, Tübingen, Germany, for critical reading of the manuscript.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal