Abstract
Intravenously applied normal human immunoglobulin G (IgG) has anti-inflammatory effects in the treatment of autoimmune diseases. Systemic inflammation can originate from an overreacting amplification loop of the complement system. In blood, C3b2-containing complexes maintain complement amplification much better than the extremely short-lived C3b. Therefore, in patients with the complement-dependent autoimmune disease, dermatomyositis, we studied whether intravenously applied normal human IgG (IVIG) stimulated in vivo inactivation of these complexes. In the course of IVIG treatment, clinically effective in 6 of 8 patients, the concentration of C3b2-containing complexes dropped to 37% ± 14% (n = 6) of the pretreatment level when having infused 0.5 g IgG/kg body weight, increased marginally and in parallel to factor Bb thereafter until full-dose IgG was infused. By day 14 following infusion of 2 g IgG/kg body weight the concentration of C3b2-containing complexes was 66% ± 19%. The plasma concentration of C3 remained constant in myopathic or increased by 15% to 20% in amyopathic patients. In contrast to this, IVIG infusion was associated with consumption of up to 40% of plasma C4 at day 1 to 2 after completion of IVIG infusion. Thus, IVIG had an immediate and long-lasting attenuating effect on complement amplification in vivo, despite the fact that it induced classical complement pathway activation.
Introduction
Intravenously applied human immunoglobulin G (IgG) has an immunomodulatory potential.1 This effect might in part be due to modulation of phagocytic cells either by altering autocrine or paracrine cytokines through the action of anticytokine antibodies2,3 or alterations of cell receptor functions4 or expression.5,6 Intravenously applied human IgG (IVIG) is, however, similarly beneficial in autoimmune diseases that are associated with excessive complement activation via the classical pathway and the amplification loop.7-9 Such complement activation is proinflammatory by generating C3b in excess. C3b, the active form of C3, binds covalently to targets and promotes generation of C3 convertases, which, in turn, consume C3 and allow assembly of C5 convertase. Elevated C3/C5 convertase activity10 produces locally proinflammatory anaphylatoxins, C3a/C5a, and generates C5b that can initiate formation of the membrane attack complex on target cells. Finally, insertion of C5b to 7 and C9 into membranes of nucleated cells can result in activation of the arachidonic acid pathway that forms mediators of inflammation.11
IVIG at high doses is able to displace nascent C3b from tissue-bound immune complexes to the fluid phase12,13 by serving as a preferential binding site for C3b.14 Although this process reduces local complement activation, it does not stop systemic complement amplification. Indeed, nascent C3b deposited to fluid-phase IgG primarily forms C3b2-IgG complexes.14 These complexes represent potent activators of the amplification loop and are, on a molar basis, more efficient activators than immobilized C3b.15,16 Hence, displacement of nascent C3b to fluid-phase IgG may aggravate rather than attenuate systemic complement amplification. This would not be compatible with the acknowledged anti-inflammatory effect of IVIG and has directed our interest toward the possibility that IVIG may also have an inhibitory effect on fluid phase complement activation. Indeed, IVIG has a complement-attenuating effect in vitro because IgG concentrations, comparable to those reached during an IVIG treatment, lowered the half-life of C3b2-IgG complexes in 20% serum from 3 to 4 minutes to 1 to 2 minutes.14 Because this complement-attenuating effect of IVIG might represent an important therapeutic effect in clinical applications where complement is involved in the pathogenic process, we addressed the question whether IVIG stimulated inactivation of C3b-containing complexes in vivo. C3b-containing complexes comprise a population of ester-linked complexes14,17-22 of which the most abundant ones carry 2 C3b and another plasma protein, primarily IgG.14,17,21
We selected dermatomyositis (DM) for our studies because it is associated with complement activation beyond C3, with deposition of C5b to 9 to endomysial capillaries,13 and patients may profit from high-dose IVIG treatment.7,8 DM is an inflammatory myopathy with muscular pain and weakness, elevated serum levels of muscle enzymes, and characteristic, often intensely pruritic skin manifestations.23 A particular entity is amyopathic dermatomyositis (ADM), which is characterized by the cutaneous manifestations of dermatomyositis without muscle involvement for the duration of at least 2 years.24
Patients, materials, and methods
Patients and patients' plasma
This study on patients having DM or ADM was reviewed by the ethics committee of the University Hospital of Zurich and before any intervention, informed written consent was obtained from each patient. We studied 8 patients affected by DM or ADM (Table 1). Diagnosis was dependent on clinical findings, sustained by skin or muscle biopsy (or both), including immune histology, electromyography, and immune serology. None of the patients had detectable signs of associated malignancy during the study. The patients were assigned to receive IVIG infusions in monthly intervals. All patients with ADM and 3 patients with myopathic DM (patients F, H, and L) received 2 g/kg body weight in 2 days per cycle, whereas 2 patients were treated with 1 g/kg body weight. At admission, routine blood chemistry as well as serum muscle enzymes (creatine kinase [CK], normal values < 150 U/L) and aldolase (Ald; data not shown) were measured. Blood samples for determination of C3b-containing complexes were drawn 1 hour before IVIG treatment, then following infusion of 10%, 25%, 50%, 75%, and 100% of the IVIG and at days 4, 7, 14, 21, and 28 after the start of IVIG infusion. On days 0, 1, 7, 14, 21, and 28 serum muscle enzyme levels (CK and Ald) were determined together with a clinical evaluation by a neuromuscular symptom score25 to assess the severity of muscle impairment. Table 1 summarizes the clinical findings at admission to the study and lists the outcome of the IVIG treatment. The data compiled in this study were from the first cycle of IVIG treatment, except for data in Figure 4, which also include additional treatment cycles.
Clinical characterization of patients
. | Age, y/sex . | . | Duration, y . | Before IVIG, muscle involvement . | . | Before IVIG, skin involvement . | . | IVIG cycles . | Final outcome, muscle involvement . | . | Final outcome, skin involvement . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | . | Diagnosis . | . | Score* . | CK level . | Extent† . | Pruritus‡ . | . | Score* . | CK level . | Extent† . | Pruritus‡ . | ||||
| A | 34/F | ADM | 5 | 60 | 57 | + | +++ | 2 × 2 | 60 | 51 | 0 | + | ||||
| B | 16/F | ADM | 10 | 60 | 82 | + | 0 | 1 × 2 | 60 | 101 | + | 0 | ||||
| G | 50/F | ADM | 14 | 60 | 56 | + | +++ | 1 × 2 | 60 | 64 | + | ++ | ||||
| F | 35/M | DM | 3 | 52 | 302 | ++ | ++ | 2 × 2 | 57 | 103 | + | 0 | ||||
| H | 40/F | DM | 2 | 34 | 1140 | ++ | + | 7 × 2 | 34 | 344 | + | 0 | ||||
| I | 71/F | DM | < 1 | 25 | 1620 | ++ | ++ | 4 × 1 | 51 | 46 | + | ++ | ||||
| K | 54/M | DM | 3 | 46 | 343 | + | 0 | 4 × 1 | 45 | 283 | 0 | 0 | ||||
| L | 53/F | DM | < 1 | 54 | 490 | ++ | + | 4 × 2 | 57 | 65 | ++ | + | ||||
. | Age, y/sex . | . | Duration, y . | Before IVIG, muscle involvement . | . | Before IVIG, skin involvement . | . | IVIG cycles . | Final outcome, muscle involvement . | . | Final outcome, skin involvement . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | . | Diagnosis . | . | Score* . | CK level . | Extent† . | Pruritus‡ . | . | Score* . | CK level . | Extent† . | Pruritus‡ . | ||||
| A | 34/F | ADM | 5 | 60 | 57 | + | +++ | 2 × 2 | 60 | 51 | 0 | + | ||||
| B | 16/F | ADM | 10 | 60 | 82 | + | 0 | 1 × 2 | 60 | 101 | + | 0 | ||||
| G | 50/F | ADM | 14 | 60 | 56 | + | +++ | 1 × 2 | 60 | 64 | + | ++ | ||||
| F | 35/M | DM | 3 | 52 | 302 | ++ | ++ | 2 × 2 | 57 | 103 | + | 0 | ||||
| H | 40/F | DM | 2 | 34 | 1140 | ++ | + | 7 × 2 | 34 | 344 | + | 0 | ||||
| I | 71/F | DM | < 1 | 25 | 1620 | ++ | ++ | 4 × 1 | 51 | 46 | + | ++ | ||||
| K | 54/M | DM | 3 | 46 | 343 | + | 0 | 4 × 1 | 45 | 283 | 0 | 0 | ||||
| L | 53/F | DM | < 1 | 54 | 490 | ++ | + | 4 × 2 | 57 | 65 | ++ | + | ||||
Score determined according to Dalakas et al.25
0 = none; + = localized; ++ = extensive.
0 = none; + = slight; ++ = moderate; +++ = severe.
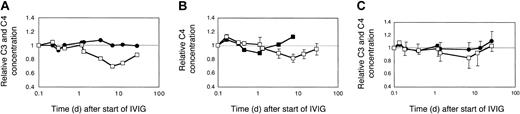
Effect of dose and brand of IVIG on complement levels in myopathic patients. (A) Two myopathic DM patients (H and L) were treated with 2 g IVIG/kg body weight for the first time. The averaged relative C3 (•) and C4 (□) concentrations were plotted. (B) C4 consumption following injection of 2 different brands of IVIG. Patient H with DM was treated sequentially, at 2 g IgG/kg body weight, twice with Sandoglobulin, once with Endobulin, and 2 more times with Sandoglobulin. The time course of the relative C4 concentrations is illustrated for the treatment with Endobulin (▪) in comparison to that averaged (± SD) from all 4 treatments with Sandoglobulin (□). (C) C3 and C4 concentrations following treatment with 1 g IVIG/kg body weight. Two previously untreated myopathic DM patients (I and K) received 4 cycles of 1g IVIG/kg body weight. The averaged relative C3 (•) and C4 (□) concentrations were plotted. Minus or plus SD are shown for 4 to 8 determinations.
Effect of dose and brand of IVIG on complement levels in myopathic patients. (A) Two myopathic DM patients (H and L) were treated with 2 g IVIG/kg body weight for the first time. The averaged relative C3 (•) and C4 (□) concentrations were plotted. (B) C4 consumption following injection of 2 different brands of IVIG. Patient H with DM was treated sequentially, at 2 g IgG/kg body weight, twice with Sandoglobulin, once with Endobulin, and 2 more times with Sandoglobulin. The time course of the relative C4 concentrations is illustrated for the treatment with Endobulin (▪) in comparison to that averaged (± SD) from all 4 treatments with Sandoglobulin (□). (C) C3 and C4 concentrations following treatment with 1 g IVIG/kg body weight. Two previously untreated myopathic DM patients (I and K) received 4 cycles of 1g IVIG/kg body weight. The averaged relative C3 (•) and C4 (□) concentrations were plotted. Minus or plus SD are shown for 4 to 8 determinations.
Materials
Freshly drawn whole blood from regular blood donors was obtained from the Blood Transfusion Service of the Swiss Red Cross (SRC), Zurich. Sera from these donations were stored at –70°C. Sera from a factor I–deficient Swiss patient were available from earlier studies.26 As whole human IgG, we used Sandoglobulin (manufactured by the Central Laboratory, Blood Transfusion Service [now ZLB Bioplasma], Berne, Switzerland). Where indicated, patient H was once treated with Endobulin (Baxter Immuno, Vienna, Austria). Sodium 125I iodide was from Amersham (Buckinghamshire, United Kingdom); human complement C3 was purified,27 stored, and depleted of inactivated (iC3)28 as described.14,16 Monoclonal antibody (mAb) H206, a monoclonal IgG antibody against the C-terminal peptide of human αC3 was purified from culture supernates on recombinant protein G (Pharmacia, Uppsala, Sweden) and stored frozen in aliquots. H206 was 125I-iodinated as outlined elsewhere.21
Patients' plasma samples
Rapidly denatured plasma. To preserve the short-lived C3b-containing complexes, 2 mL blood was collected in an EDTA (ethylenediaminetetraacetic acid)–containing Vacutainer. The tube was removed from the needle, the material mixed, an aliquot transferred to an Eppendorf tube, and centrifuged for 20 seconds or less at bedside. An aliquot of 350 μL of the supernatant plasma was denatured by immediately mixing with 150 μL electrophoresis sample buffer yielding a final concentration of 60 mM dithiothreitol and was placed into a boiling water bath within less than 75 seconds from blood collection. In case the limit was missed, the first sample was discarded and a second, newly collected sample was processed accordingly. The samples were boiled for 3 minutes and 225 μL 250 mM N-ethylmaleimide was added within 5 minutes at room temperature. This material was aliquoted, put on dry ice, and stored at –70°C.
Freshly frozen plasma. Using the same needle in place, a 10-mL EDTA Vacutainer was filled. This material was centrifuged within 10 minutes from collection. The plasma was aliquoted, put on dry ice within 15 minutes from blood collection, and later used for determination of plasma proteins.
Plasma proteins
Analyses on samples were performed in a Good Laboratory Practice (GLP) compliant laboratory. Complement C3 and C4 and α2-macroglobulin were determined by nephelometric methods using monospecific antisera or purified antibodies. Complement factor Bb was determined by using an enzyme-linked immunosorbent assay (ELISA) kit from Quidel (Santa Clara, CA). Complement peptide C3a[desArg] was quantified using the ABICAP method (Biognosis, Jülich, Germany). The plasma was passed over a column with monoclonal anti-C3a[desArg]. After extensive washing of the columns, a second, fluorescein-labeled anti-C3a[desArg] antibody was fixed to the immobilized material. After washing, the complexes were eluted and the fluorescence intensity was measured and compared to a standard curve. C1q was quantified by radial immunodiffusion.
In vitro generation of C3b-containing complexes
Normal sera were diluted to a final concentration of 20% with Veronal-buffered saline (VBS), supplemented with 0.15 mM Ca2+ and 0.5 mM Mg2+, and the given concentration of IgG. When factor I–deficient sera were used, reaction mixtures were supplemented with 200 μg/mL C3 and 40 μg/mL factor B (Advanced Research Technologies, San Diego, CA). Heat-aggregated IgG (100 μg/mL) was added and the samples incubated at 37°C for the given times. Reactions were stopped by adding electrophoresis sample buffer to achieve 2% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol, and 10 mM EDTA (pH 6.8). Each sample was immediately heated for 3 minutes at 100°C and then supplemented with an excess of N-ethylmaleimide. Aliquots of the denatured samples were either frozen at –70°C or used immediately for SDS–polyacrylamide gel electrophoresis (SDS-PAGE). In some experiments complexes were generated in the presence of 125I-labeled C3 exactly as outlined elsewhere.14
Electrophoresis
SDS-PAGE was performed according to a modified Neville gel system,29 using 5%, 6%, or 8% acrylamide in the separating gel as indicated. Gels containing labeled material were stained with Coomassie blue, dried, and exposed to PhosphorImager screens (Molecular Dynamics, Sunnyvale, CA). Rapidly denatured plasma samples from patients were thawed once. For one-dimensional mini-SDS-PAGE, samples were diluted 1:10 with prediluted electrophoresis sample buffer, lacking reducing agent. For 2-dimensional SDS-PAGE on 2-mm gels, the denatured samples were warmed up for 10 minutes at 37°C but not diluted. The amount of material loaded per lane was 15 μL for both types of gels for the pretreatment samples. The amount added of all subsequent samples from a particular patient was corrected for blood volume changes. Corrections were made on the basis of the concentration of the largest known plasma protein, α2-macroglobulin. The range of corrections did not exceed ± 3 μL and was necessary primarily for samples taken during the 2 days of IVIG infusion. Two-dimensional SDS-PAGE including a hydroxylamine treatment between the first and second dimension was carried out as described elsewhere.14 Briefly, reduced and alkylated polypeptides were electrophoresed on SDS-PAGE (6% acrylamide). Strips from these gels extending from the beginning of the running gel to the migration edge of the αC3 polypeptide were treated twice for 20 minutes at 37°C with 1 M hydroxylamine (pH 10) and washed with water and several times with stacking gel buffer before running in the second dimension at 8% acrylamide.
Immunoblotting
One- and 2-dimensional gels were blotted onto Immobilon P (Millipore, Bedford, MA) according to the manufacturer's procedure. Blots were incubated overnight with 125I-iodinated mAb H206 (0.5 × 106 cpm/mL) in blotting buffer containing 0.9% NaCl, 20 mM Tris (tris(hydroxymethyl)aminomethane), 0.08% sodium azide, 50 μg/mL phenylmethylsulfonyl fluoride, 1% gelatin, and 0.04% Triton X-100 (pH 7.4).30 Blots were washed 3 times with blotting buffer containing detergent and gelatin and without these additions. Dried blots were exposed to PhosphorImager screens. Digitized autoradiographs were obtained with a PhosphorImager (Molecular Dynamics) by using identical linear gray tone ranges for blots to be compared. Immunoblots were quantified from these autoradiographs with Image Quant (Molecular Dynamics). Samples that were compared were run on the same gel/blot (3 samples for 2-dimensional gels). Two to 3 blots were run on different occasions for each patient analyzed. In case the results of the first 2 blots deviated by more than 25%, a third blot was run and data were averaged from the 2 runs giving similar results.
Assessment of C3b-containing complexes in human plasma
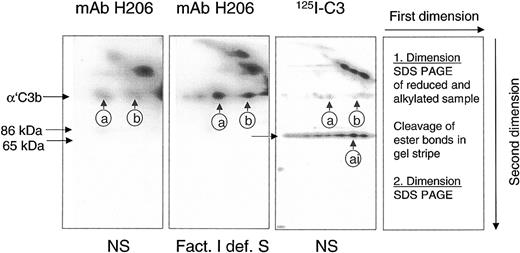
A reliable method had to be developed to detect and quantify C3b-containing complexes in rapidly denatured plasma from patients to assess the potential of a sample to activate C3 via the alternative complement pathway. The detection system had to differentiate between C3b-containing complexes, capable of activating complement amplification, and those already inactivated (iC3b-containing). To achieve this goal, we used mAb H206 generated against the C-terminal portion of αC3. Its binding properties were studied on blots from 2-dimensional SDS-PAGE on serum samples in which the classical pathway of complement was activated. Samples were run in the first dimension. Separated polypeptides were re-electrophoresed in the second dimension after cleavage of ester bonds (Figure 1).
mAb H206 recognized α′C3b released by hydroxylamine from C3b-containing complexes. Complement was activated for 3 minutes in either normal serum (NS) or factor I–deficient serum (Fact. I def. S), or in NS supplemented with labeled C3 (125I-C3). Denatured sera were subjected to 2-dimensional SDS-PAGE with a hydroxylamine treatment between the dimensions. Blots from 2-dimensional SDS-PAGE were either incubated with 125I-iodinated mAb H206 (mAb H206) or stained, when originating from samples supplemented with 125I-labeled C3 (125I-C3). Autoradiographs are shown. Conditions and arrangement of the 2-dimensional SDS-PAGE are given at the right-hand side. The circled a and b mark the position of the reduced form of known C3b-containing complexes: complex b represents α′C3b2, originating from dimeric C3b and complex a has earlier been identified as α′C3b2-HC, originating from a C3b2-IgG complex14 ; ai denotes the inactivated form of complex a. The position of α′C3b and 2 of its fragments (86 kDa and 65 kDa) are marked.
mAb H206 recognized α′C3b released by hydroxylamine from C3b-containing complexes. Complement was activated for 3 minutes in either normal serum (NS) or factor I–deficient serum (Fact. I def. S), or in NS supplemented with labeled C3 (125I-C3). Denatured sera were subjected to 2-dimensional SDS-PAGE with a hydroxylamine treatment between the dimensions. Blots from 2-dimensional SDS-PAGE were either incubated with 125I-iodinated mAb H206 (mAb H206) or stained, when originating from samples supplemented with 125I-labeled C3 (125I-C3). Autoradiographs are shown. Conditions and arrangement of the 2-dimensional SDS-PAGE are given at the right-hand side. The circled a and b mark the position of the reduced form of known C3b-containing complexes: complex b represents α′C3b2, originating from dimeric C3b and complex a has earlier been identified as α′C3b2-HC, originating from a C3b2-IgG complex14 ; ai denotes the inactivated form of complex a. The position of α′C3b and 2 of its fragments (86 kDa and 65 kDa) are marked.
mAb H206 bound to α′C3b that was released off-diagonal from high–molecular-weight C3b-containing complexes (Figure 1, mAb H206). The amount of α′C3b off-diagonal was low in normal serum (Figure 1, NS and mAb H206), but high in serum from a factor I–deficient patient, incapable of inactivating C3b (Figure 1, Fact.I def. S and mAb H206). Corresponding autoradiographs from NS samples supplemented with labeled C3 revealed very little α′C3b (Figure 1, NS and 125I-C3). The majority of label was in the 65-kDa fragment of α′C3b and therefore originated from C3b-containing complexes that were inactivated to iC3b-containining complexes within 3 minutes. Thus, mAb H206 bound exclusively to intact α′C3b subunit, but not to its inactivated form, the 65-kDa fragment, although the fragment was present in comparable amounts as indicated by analogous samples incubated with 125I-labeled C3 (Figure 1, 125I-C3 and NS). Based on these data we concluded that mAb H206 specifically detected non-inactivated C3b-containing complexes and thus allowed their quantification. The binding of mAb H206 to chemically liberated α′C3b on blots from 2-dimensional gels was a measure of ester-bonded complexes because the amide-bonded complexes are not cleavable by hydroxylamine and as a consequence remained in the diagonal. When mAb H206 was applied to blots of one-dimensional SDS-PAGE (Figure 2), it revealed the sum of C3b-containing complexes, including ester- and amide-bonded material of which the latter contained in part C4b, as judged from immunoblots incubated with anti-C4 antibodies (not shown).
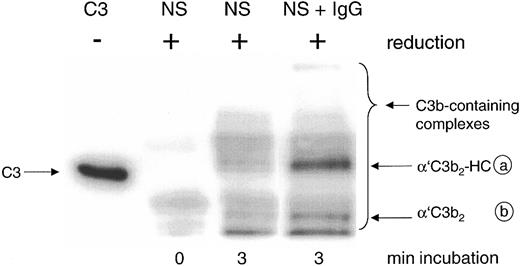
Detection by mAb H206 of C3b-containing complexes formed in normal serum on complement activation in vitro. Complement was activated for the given times in 20% normal serum (NS) or NS supplemented with 10 mg/mL IgG (NS + IgG). Samples were denatured and run along with unreduced C3 (C3, MW 185 kDa) on SDS-PAGE (6% acrylamide) and a blot from this gel was incubated with 125I-iodinated mAb H206. An autoradiograph of the blot is shown, revealing the α′C3b-containing protein complexes migrating above α′C3b (MW 112 kDa) as indicated by the bracket, referring to samples run in reduced form. For an explanation of the circled letters, see Figure 1 legend.
Detection by mAb H206 of C3b-containing complexes formed in normal serum on complement activation in vitro. Complement was activated for the given times in 20% normal serum (NS) or NS supplemented with 10 mg/mL IgG (NS + IgG). Samples were denatured and run along with unreduced C3 (C3, MW 185 kDa) on SDS-PAGE (6% acrylamide) and a blot from this gel was incubated with 125I-iodinated mAb H206. An autoradiograph of the blot is shown, revealing the α′C3b-containing protein complexes migrating above α′C3b (MW 112 kDa) as indicated by the bracket, referring to samples run in reduced form. For an explanation of the circled letters, see Figure 1 legend.
The major mAb H206-detectable complex formed in the presence of exogenous IgG represented C3b2-IgG. It appeared on reduction as a band with an apparent molecular weight (MW) exceeding that of unreduced C3 (MW 185 kDa) and was composed of 2 α′C3b subunits and one IgG heavy chain (α′C3b2-HC; complex a; Figure 2, NS + IgG), as verified previously using labeled C3.14 The second most abundant complex represents C3b2 and appears as a band composed of 2 α′C3b subunits (complex b).31 Both complexes were almost fully cleavable by hydroxylamine and released α′C3b (Figure 1). Among the minor, yet uncharacterized complexes with MW exceeding that of complex a, none contained significant amounts of IgG (not shown).
Results
High-dose IVIG induced consumption of C4 but not of C3 in patients with DM
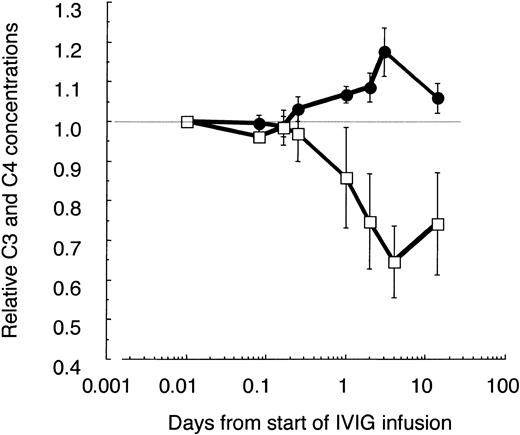
It is known that IVIG activates classical complement pathway most likely due to formation of complexes in vivo.32 Indeed, the C4 concentration decreased by 30% to 40% until day 4 and slowly recovered thereafter (Figure 3). Surprisingly, in parallel the C3 concentration increased significantly by 15% to 20% during the first IVIG treatment in ADM patients and patient F with DM, but low CK activity (Table 1). This increase of C3 in patients with low or normal CK values and a pretreatment concentration of C3 of about 1 mg/mL was not due to false recognition of elevated C3/C3a[desArg] concentrations by the detecting polyclonal antibody. The concentration of C3a increased only from 128 ± 76 to 331 ± 85 ng/mL by day 4, as determined in 3 of these patients compared with 4 controls with an average concentration of 73 ± 47 ng/mL. These results imply that an IVIG treatment with 2 g/kg body weight transiently activated the classical complement pathway, but at the same time attenuated complement amplification, whereby the C3 concentration increased.
Relative changes of C3 and C4 concentrations in plasma of IVIG-treated patients with ADM. C3, C4, and α2-macroglobulin (α2M) were determined at the given time points in freshly frozen plasma drawn from 3 amyopathic patients and patient F with DM, having low CK activity. All patients received the first course of IVIG treatment (2 g IgG/kg body weight during the first 2 days). The C3 and C4 concentrations were corrected for blood volume changes by calculating ratios (C3/α2M; C4/α2M) and normalized to the pretreatment values. Averaged values are shown with ± 1 SD for C3 (•) and C4 (□). Data are plotted in a logarithmic time course with the pretreatment values set to 0.01 days.
Relative changes of C3 and C4 concentrations in plasma of IVIG-treated patients with ADM. C3, C4, and α2-macroglobulin (α2M) were determined at the given time points in freshly frozen plasma drawn from 3 amyopathic patients and patient F with DM, having low CK activity. All patients received the first course of IVIG treatment (2 g IgG/kg body weight during the first 2 days). The C3 and C4 concentrations were corrected for blood volume changes by calculating ratios (C3/α2M; C4/α2M) and normalized to the pretreatment values. Averaged values are shown with ± 1 SD for C3 (•) and C4 (□). Data are plotted in a logarithmic time course with the pretreatment values set to 0.01 days.
C4 consumption was similar in myopathic patients with moderate to high CK activities when treated with 2 g IVIG/kg body weight (Figure 4A, averaged data from 2 patients treated for the first time). The C3 concentrations, however, remained at initial levels and did not significantly increase. Nevertheless, the absolute C3 concentration increased by about 20% by the end of the third cycle in patient H, when treated sequentially with high-dose IVIG (not shown).
The IVIG-induced C4 consumption observed with 2 g IgG/kg body weight was only partially due to the presence of IgG oligomers in the IVIG preparation, because a preparation treated with immobilized proteases and devoid of IgG3 (Endobulin) lowered and shortened the phase of C4 consumption, but did not eliminate C4 consumption as compared to 4 cycles of treatment of patient H with Sandoglobulin (Figure 4B). An IVIG treatment of myopathic patients at a dose of 1g IgG/kg body weight did not cause a significant C4 consumption as was found in 2 patients each treated 4 times sequentially (Figure 4C). Hence, classical complement pathway activation was dependent on the dose of IVIG. At a high dose, IVIG activated classical complement pathway with consumption of C4 and 16% to 41% of C1q (not shown), but inhibited C3 consumption via the amplification loop.
Attenuation of complement amplification by lowering the concentration of C3 convertase precursors
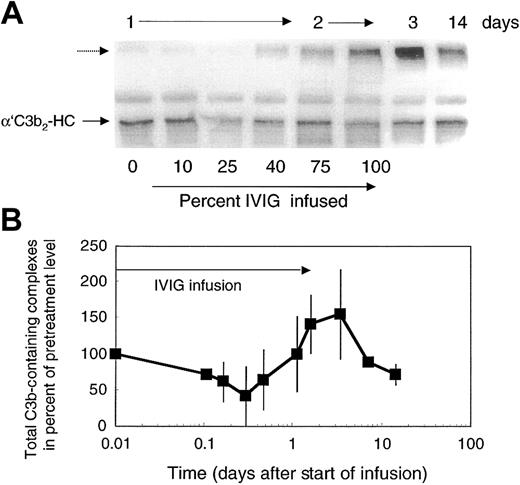
Attenuation of complement amplification by IVIG suggested that it might interfere with C3 convertase precursors. To address this question, we studied the concentration of the short-lived C3b-containing complexes in patients' plasma. C3b-containing complexes were visualized by mAb H206, as outlined in “Patients, materials, and methods,” on samples derived from freshly drawn blood processed at bedside to denature plasma within 75 seconds. Patients' pretreatment plasma revealed C3b-containing complexes with the major band, α′C3b2-HC, originating from C3b2-IgG complexes (Figure 5A). The total amount of C3b-containing complexes in pretreatment plasma was 1.8 to 3.1 times that in control plasma (n = 3, with 4 controls: 1 ± 0.21). In the course of the first IVIG treatment of each patient with DM, we noticed 4 changes in the level of C3b-complexes: (1) During infusion of up to 25% of total IVIG, which corresponded to an infused dose of 0.5 g/kg body weight, the total amount of C3b-containing complexes decreased (Figure 5A-B) to 55% ± 14% (n = 6). (2) Beyond that dose, a new, high–molecular-weight C3b-containing complex appeared and reached a peak in concentration by day 3 to 4 (Figure 5A dashed arrow). Correspondingly, the total amount of mAb H206-reactive material increased and reached a maximum (Figure 5B). (3) Thereafter, the total amount of C3b-containing complexes dropped below the pretreatment level. (4) The major species of complexes suspected to sustain inflammation, the C3b2-IgG complex (migrating as α′C3b2-HC and indicated by an arrow) decreased rapidly after the start of infusion (Figure 5A). Then these complexes increased close to pretreatment levels during infusion of the full dose and dropped again to reach by day 14 a level still lower than at the beginning of treatment.
Total C3b-containing complexes in plasma of ADM and DM patients treated with 2 g IVIG/kg body weight. Plasma samples were drawn during IVIG infusion (percent infused IgG is listed during day 1 and 2) and at the days indicated. Plasma samples were denatured within 75 seconds from collection. Plasma samples were electrophoresed, blotted, and incubated with labeled mAb H206. Autoradiographs from blots are shown (A) and were quantified from the first cycle of treatment of 4 patients (B). (A) The major band in pretreatment plasma—α′C3b2-HC, representing the reduced form of C3b2-IgG—is marked. The unlabeled dashed arrow points to the high–molecular-weight C3b-containing complexes appearing after infusion of IVIG. (B) Total C3b-containing complexes comprised label in all complexes above α′C3 to the beginning of the stacking gel. Data are expressed in percent of the pretreatment values (mean ± 1 SD).
Total C3b-containing complexes in plasma of ADM and DM patients treated with 2 g IVIG/kg body weight. Plasma samples were drawn during IVIG infusion (percent infused IgG is listed during day 1 and 2) and at the days indicated. Plasma samples were denatured within 75 seconds from collection. Plasma samples were electrophoresed, blotted, and incubated with labeled mAb H206. Autoradiographs from blots are shown (A) and were quantified from the first cycle of treatment of 4 patients (B). (A) The major band in pretreatment plasma—α′C3b2-HC, representing the reduced form of C3b2-IgG—is marked. The unlabeled dashed arrow points to the high–molecular-weight C3b-containing complexes appearing after infusion of IVIG. (B) Total C3b-containing complexes comprised label in all complexes above α′C3 to the beginning of the stacking gel. Data are expressed in percent of the pretreatment values (mean ± 1 SD).
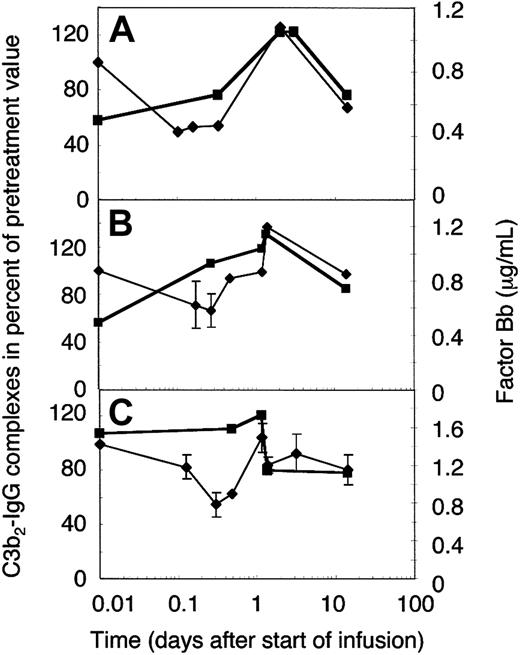
To establish whether the extent of complement amplification correlated with the relative concentration of its major precursor, C3b2-IgG (Figure 5A), or the total mAb H206-reactive material (Figure 5B), we determined the concentration of the activated factor B (factor Bb). The normal concentration range of factor Bb is 0.4 to 1.0 μg/mL,31 the mean 0.65 ± 0.23 μg/mL,33 and high levels were found in septic shock (3.7 ± 2.1 μg/mL34 ). Pretreatment levels of factor Bb were within the normal range in 2 amyopathic patients (Figure 6A-B) and twice as high in one of the myopathic patients studied (Figure 6C). The concentration of factor Bb, with a presumed half-life of a few hours, remained constant early during IgG infusion in 2 of 3 cases, whereas that of C3b2-IgG complexes dropped. Afterward, the concentration of factor Bb correlated well with the relative concentration of the C3b2-IgG complexes. Any increase in the C3b2-IgG concentration during the first 2 days was immediately followed by a rise of the factor Bb concentration to not more than 1.5 times the physiologic level. Thereafter, factor Bb decreased to physiologic levels and the C3b2-IgG concentration dropped below pretreatment levels. In fact, the 2 components decreased within a day following infusion of the full dose as has been observed on plasma of DM patient F who was studied in more detail (Figure 6C).
Factor Bb and the relative concentration of C3b2-IgG complexes in plasma of ADM and DM patients treated with 2 g IVIG/kg body weight. Plasma samples were drawn from 2 amyopathic (A-B) and one myopathic patient (C) during the first IVIG treatment at the times indicated. IVIG infusion was completed by 33 hours in patient G (B) and patient F (C), and by 48 hours in patient B (A). Plasma samples were either freshly frozen for factor Bb determination (▪) or denatured within 75 seconds from collection for quantification of C3b2-IgG complexes (♦, thin line). C3b2-IgG complexes were quantified from one-dimensional immunoblots incubated with labeled mAb H206. Immunoblotting data were averaged from 2 or more (with SD) blots and normalized to the pretreatment value.
Factor Bb and the relative concentration of C3b2-IgG complexes in plasma of ADM and DM patients treated with 2 g IVIG/kg body weight. Plasma samples were drawn from 2 amyopathic (A-B) and one myopathic patient (C) during the first IVIG treatment at the times indicated. IVIG infusion was completed by 33 hours in patient G (B) and patient F (C), and by 48 hours in patient B (A). Plasma samples were either freshly frozen for factor Bb determination (▪) or denatured within 75 seconds from collection for quantification of C3b2-IgG complexes (♦, thin line). C3b2-IgG complexes were quantified from one-dimensional immunoblots incubated with labeled mAb H206. Immunoblotting data were averaged from 2 or more (with SD) blots and normalized to the pretreatment value.
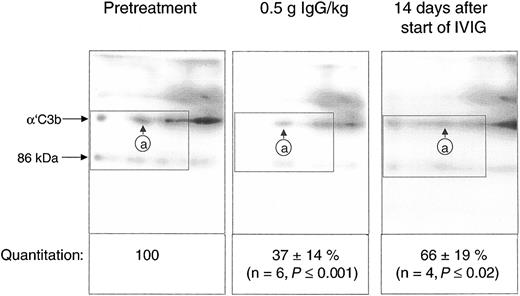
Quantification of the C3b2-IgG complexes in Figure 6 was based on immunoblots from one-dimensional SDS-PAGE. Such data do not yet provide conclusive evidence that IVIG attenuated complement amplification by stimulating inactivation of C3 convertase precursors, namely, ester-bonded complexes with 2 C3b molecules (C3b2-IgG and C3b2). To verify this, we quantified binding of mAb H206 to α′C3b released from such complexes by hydroxylamine as outlined in “Patients, materials, and methods” (Figure 1). Figure 7 shows immunoblots from 2-dimensional SDS-PAGE run with rapidly denatured plasma of a patient before treatment, after infusing 0.5 g IgG/kg body weight (6.5 hours from start), and after 14 days. IVIG significantly diminished the amount of mAb H206-reactive, off-diagonally migrating α′C3b fragments to 37% ± 14% of the pretreatment level following infusion of 0.5 g IgG/kg body weight. This initial decrease of C3b2-containing complexes was statistically significant and observed in all ADM and DM patients treated with IVIG (Figure 7). It was not due to infusion-induced volume changes, because gel loadings were corrected for variations in the concentration of α2-macroglobulin, a protein unlikely to extravasate (see “Patients, materials, and methods”). By day 14 the C3b2-containing complexes represented 66% ± 19% of the pretreatment level, when determined from the amount of released α′C3b. By days 28 to 30 the values were no longer significantly different from pretreatment levels (not shown). Thus, IVIG rapidly stimulated inactivation of C3 convertase precursors and maintained them below pretreatment values at least until day 14, despite a transient rise at the end of infusion.
IgG infusion induced a rapid early decline of ester-bonded C3b2-containing complexes in patients. Plasma samples (rapidly denatured plasma) were drawn 1 hour before infusion, after infusion of 0.5 g IgG/kg body weight, and 14 days after the start of the infusion and aliquots were subjected to 2-dimensional electrophoresis. Gels were blotted and α′C3b released from ester-linked complexes was quantified using labeled mAb H206. A blot from consecutive samples of an amyopathic patient is shown. The circled letter a refers to the position of α′C3b2-HC in the first dimension. The dotted rectangle indicates the area comprising the mAb H206-reactive polypeptides α′C3b and a fragment of α′C3b with an apparent MW of 86 kDa. The 86-kDa fragment originated from cleavage of C3bn-containing complexes while plasma was yet in contact with cells. It was not found in serum activated by exogenous immune complexes and was also not identical with the N-terminal 65-kDa fragment, because H206 bound exclusively to the C-terminal portion of α′C3b (Figure 1 H206). The 86-kDa fragment most likely represented the large, C-terminal fragment of α′C3b, generated by factor I and CR135 before plasma was separated from cells. For quantification of released α′C3b label within rectangles was determined and normalized to pretreatment values. Results are given as means ± SD. Data taken at 0.5 g IgG/kg body weight were from 2 amyopathic (B and G) and 4 myopathic (F, H, I, and L) patients, those at 14 days from the 3 amyopathic and the myopapthic patient H treated for the first time with 2 g/kg body weight within 2 days.
IgG infusion induced a rapid early decline of ester-bonded C3b2-containing complexes in patients. Plasma samples (rapidly denatured plasma) were drawn 1 hour before infusion, after infusion of 0.5 g IgG/kg body weight, and 14 days after the start of the infusion and aliquots were subjected to 2-dimensional electrophoresis. Gels were blotted and α′C3b released from ester-linked complexes was quantified using labeled mAb H206. A blot from consecutive samples of an amyopathic patient is shown. The circled letter a refers to the position of α′C3b2-HC in the first dimension. The dotted rectangle indicates the area comprising the mAb H206-reactive polypeptides α′C3b and a fragment of α′C3b with an apparent MW of 86 kDa. The 86-kDa fragment originated from cleavage of C3bn-containing complexes while plasma was yet in contact with cells. It was not found in serum activated by exogenous immune complexes and was also not identical with the N-terminal 65-kDa fragment, because H206 bound exclusively to the C-terminal portion of α′C3b (Figure 1 H206). The 86-kDa fragment most likely represented the large, C-terminal fragment of α′C3b, generated by factor I and CR135 before plasma was separated from cells. For quantification of released α′C3b label within rectangles was determined and normalized to pretreatment values. Results are given as means ± SD. Data taken at 0.5 g IgG/kg body weight were from 2 amyopathic (B and G) and 4 myopathic (F, H, I, and L) patients, those at 14 days from the 3 amyopathic and the myopapthic patient H treated for the first time with 2 g/kg body weight within 2 days.
Factor Bb generation followed the relative concentration of C3b2-containing complexes (Figure 6) rather than that of the total mAb H206-reactive material (Figure 5B). The total mAb H206-reactive material also included high–molecular-weight material that peaked at day 3 to 4. This high–molecular-weight material migrated diffusely at the beginning of SDS-PAGE. It contained some C3b, but was contaminated by C3 SS linked to other proteins that may have been generated subsequent to reduction. The C3-containing portion released αC3 rather than α′C3b (not shown). Alkylation might have been incomplete in samples having an extremely high plasma protein concentration due to the infused IgG. The amount of high–molecular-weight material was low in early samples and those beyond day 7. It released α′C3b after cleavage with hydroxylamine and decreased in its amount on infusion of 0.5 g/kg IgG (Figure 7).
Clinical outcome of IVIG treatment
The rapid decrease in C3b2-containing complexes during infusion of IgG preceded the first clinical sign of improvement, namely, a transient disappearance of pruritus starting on day 2 in patients who suffered from pruritus. On the other hand, the postinfusion C4 consumption preceded an unusual fatigue lasting until day 7 in all patients receiving 2 g IgG/kg body weight. The final outcome of IVIG treatment is summarized in Table 1. In 2 of 3 patients with ADM, IVIG had a moderate to sustained effect on pruritus. In all patients with DM muscular disease activity was considerably reduced, but with varying impact on the muscle score. Skin lesions and pruritus showed a variable, mostly transient response.
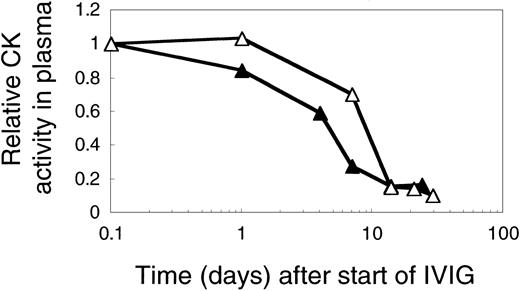
The overall effect of IVIG on plasma CK activity was comparable at 1 or 2 g IgG/kg and independent of the IVIG-induced C4 consumption as is illustrated by comparing results from 2 previously untreated DM patients (Figure 8). In both patients plasma CK activity was less than 20% of pretreatment values at day 14. The kinetics of CK activity, however, appeared to be IVIG dose dependent with a drop to 50% of the pretreatment activity by 5 days instead of 10 days at the higher dose (Figure 8).
CK activity in plasma of 2 myopathic patients treated with 1 or 2 g IVIG/kg body weight in the first cycle. CK activities in plasma of patient L (▵), treated with 2 g, and of patient I (▴) treated with 1 g IVIG/kg body weight. The figure shows the relative CK activity profiles.
CK activity in plasma of 2 myopathic patients treated with 1 or 2 g IVIG/kg body weight in the first cycle. CK activities in plasma of patient L (▵), treated with 2 g, and of patient I (▴) treated with 1 g IVIG/kg body weight. The figure shows the relative CK activity profiles.
Discussion
The results of this study show that IVIG has a potential to attenuate complement amplification in vivo. High-dose IVIG stopped further C3 activation via the amplification loop, despite the fact that it induced classical complement pathway activation. It attenuated complement amplification sufficiently to stop excess C3 consumption, to compensate for the extra amounts of C3 activated by IVIG through the classical pathway, and even to induce a slight increase of the C3 concentration in patients with ADM. IVIG appeared to attenuate complement amplification by stimulating inactivation of ester-linked C3b2-containing complexes that are intrinsically 7 to 10 times more effective C3 convertase precursors than immobilized C3b.16 Thus, the in vivo effect of IVIG was similar to that of high-dose IgG on C3b-containing complexes in vitro.14
Infusion of 2 g IgG/kg body weight elicited a transient 30% to 40% consumption of C4 in ADM and DM patients, but only a marginal rise of C3 convertase precursors and of factor Bb. The concentration of factor Bb never exceed 1.5 times the upper limit of the physiologic range during treatment with 2 g IgG/kg body weight. The factor Bb concentration did not increase at all in 2 of 3 cases up to a dose of 0.5 g IgG/kg body weight. The same low dose infused in the form of a pasteurized IgG preparation increased the factor Bb concentration from 0.66 to 1.66 μg/mL.32 Thus, the concentration of factor Bb and that of the C3 convertase precursors remained low after infusion of Sandoglobulin at 2 g/kg body weight. If IVIG did not contain substances that stimulated inactivation of C3 convertase precursors, a dose of 2 g/kg body weight would have substantially raised factor Bb concentration and caused a C3 consumption at least proportional to that of C4. This would have resulted in an estimated decrease of C3 by 10% to 15% (based on the relative concentrations of C4 and C3). Instead, we found a 15% to 20% increase of C3 in ADM and a constant level of C3 in DM patients with severe disease-mediated complement activation, in whom C3 pretreatment levels were in the lower normal range (not shown). It is unlikely that the increase in C3 levels was due to an IVIG-induced up-regulation of C3 synthesis because it appeared immediately after infusion of IgG and peaked between day 2 and 4.
Attenuation of complement amplification by IVIG was most likely induced by its ability to stimulate inactivation of C3 convertase precursors. This is best seen by the decrease of C3b2-containing complexes early during IgG infusion, when C4 levels were yet unchanged. Ester-bonded C3b2-containing complexes comprising C3b2-IgG and C3b2 complexes decreased to 37% as verified by 2-dimensional SDS-PAGE. This potential of IVIG to attenuate complement amplification further increased with the full dose, but was transiently used to prevent additional amplification during maximum classical pathway activation. The potential persisted beyond full-dose infusion whereby C3b2-containing complex concentrations decreased below pretreatment values with factor Bb being within the physiologic range.
It is known that high-dose IgG activates the classical pathway in vitro36 and in vivo,32 presumably because IVIGs may contain minute amounts of aggregates or because immune complexes are formed in vivo. A substantial portion of classical pathway activation apparently occurred by immune complex formation with components from the patient because C4 consumption was not abrogated by injecting an IVIG preparation that was treated with solid-phase proteases. Activation of the classical pathway by IVIG in patients' blood may itself represent a beneficial effect of IVIG in autoimmune diseases, related to immune complex formation between idiotypes and anti-idiotypes.37-41 Despite classical pathway activation, IVIG treatments are usually well tolerated,8 except that most patients treated with 2 g IgG/kg in this study complained about fatigue from day 3 to 7 after the infusion.
The complement-attenuating substance within IVIG apparently does not constitute an impurity in pooled human IgG. The active constituent appears to have the half-life of IgG,42 based on the kinetics of the IVIG effect. It retained activity by day 14, but lost it by 30 days. Preliminary data suggest that the active principle resides in naturally occurring autoantibodies that retain their complement-attenuating activity as F(ab′)2.43 Others have indeed observed that F(ab′)2 rather than Fc fragments from IVIG had a complement-attenuating effect in delaying hyperacute xenograft rejection.44
IVIG has recently been shown to have an anti-inflammatory effect by scavenging the anaphylatoxins C3a and C5a via a low-affinity site within the constant region of F(ab′)2.45 The ability of IVIG to attenuate complement amplification by stimulating inactivation of C3 convertase precursors introduces yet another anti-inflammatory property that interferes with complement prior to generation of anaphylatoxins. The 2 anti-inflammatory potentials not only differ at the level of interference with the complement system, but also with regard to how they are mediated. Although a common structural element conveys binding to anaphylatoxins, we hypothesize that highly specific, naturally occurring antibodies may stimulate factors H- and I-dependent inactivation of C3b2-containing complexes by interfering with these C3 convertase precursors before or during C3 convertase assembly.
The potential of IVIG to attenuate complement amplification appears crucial because it might help to prevent immune complex–like diseases induced by interactions of IVIG with endogenous immunoglobulins in patients treated with IVIG. In addition, attenuation of complement amplification is beneficial in patients with complement involvement and may not only occur on fluid-phase C3 convertase precursors, but also on those in tissues in contact with IVIG. Hence, identification of the ability of IVIG to attenuate complement amplification in vivo may open up means to isolate this physiologic regulator for specific applications.
Prepublished online as Blood First Edition Paper, September 25, 2003; DOI 10.1182/blood-2003-05-1530.
Supported by KTI grants nos. 3189.1 and 3772.1 of ZLB Central Laboratory, Blood Transfusion Services of the Swiss Red Cross, Berne, and the Swiss government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the patients who participated in this study. We thank Roland Zehnder at the ZLB Bioplasma AG for his excellent technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal