Abstract
Mutations that lead to a loss of the copper-containing plasma enzyme ceruloplasmin disrupt mammalian iron homeostasis. The mechanism by which ceruloplasmin mobilizes iron from cell stores has been controversial. We demonstrate that injection of a soluble copper-containing yeast protein Fet3p can restore iron homeostasis in phlebotomized mice with a deletion of the ceruloplasmin gene. These results show the conservation of function of copper-containing proteins in eukaryotic iron metabolism. (Blood. 2004;103:4672-4673)
Introduction
Ceruloplasmin is a member of the multicopper oxidase family, an evolutionarily conserved group of proteins that utilize copper to couple substrate oxidation with the 4-electron reduction of oxygen to water.1 Although multicopper oxidases may accept a variety of substrates, 3 members of this group of enzymes function as essential ferroxidases, oxidizing Fe2+ to Fe3+, which is then bound to an acceptor or transporter. Enzymatic iron oxidation by these ferroxidases ensures a sufficient rate of Fe3+ formation while preventing the generation of reactive oxygen intermediates that occurs with spontaneous iron oxidation. Although there is a report that ceruloplasmin may permit iron uptake in cultured cells,2 the preponderance of data shows that ceruloplasmin, and the related multicopper oxidase hephaestin, are involved in exporting iron from tissues.1,3 In fungi, the multicopper oxidase Fet3p is required for cellular iron acquisition.4 Aceruloplasminemia is a disorder of iron homeostasis resulting from inherited loss-of-function mutations in the ceruloplasmin gene.5 Here we demonstrate that a soluble form of Fet3p can functionally replace ceruloplasmin in restoring iron homeostasis in phlebotomized mice with a targeted deletion in the ceruloplasmin gene.
Study design
A soluble Fet3p lacking the transmembrane and cytosolic domains and containing a carboxyl terminal FLAG epitope was generated as described by Hassett et al.6 The engineered FET3 was placed on a plasmid under the control of the FET3 promoter. The plasmid was transformed into yeast that had an AFT1-1up allele, permitting constitutive expression of the soluble Fet3p. Transformed yeast were grown in synthetic drop-out media without uracil (CM-ura) medium containing 50 mM Tris (tris(hydroxymethyl) aminomethane)-acetate, pH 6.5, and supplemented with 200 μM CuSO4. Fet3p was isolated from culture media using anion chromatography; the complete method can be found in the online data supplement (available at the Blood website, see the Supplemental Document link at the top of the online article). Procedures for assaying Fet3p activity are described by de Silva et al7 and Davis-Kaplan et al.8 Isolated Fet3p was deglycosylated by addition of endoglycosidase F as per manufacturer's instructions (New England BioLabs, Beverly, MA).
Fet3p activity was measured in terms of o-dianisidine activity relative to purified human ceruloplasmin (Vital Products, Delray Beach, CA): 1.0 μg purified Fet3p is equal to 6 μg purified ceruloplasmin enzymatic activity. Each mouse was given an amount of Fet3p equivalent to 30 μg ceruloplasmin per 100 μL blood volume. Fet3p was injected intravenously through the tail vein. This amount of Fet3p was calculated to provide a transient circulating serum ceruloplasmin (Cp) of 3.0 g/L (30 mg/dL), which is the normal Cp value for humans.
Mice (5 Cp+/Cp+ and 5 Cp-/Cp-) were bled via daily phlebotomy, and serum iron was measured. The mice used were 129/SvJ × BSW (outbred strain) F4, 10-week-old males. Descriptions of the mice as well as procedures for measuring plasma iron and transferrin saturation in normal and aceruloplasminemic mice (Cp-/Cp-) are provided by Harris et al.9 Blood was drawn by retro-orbital puncture, removing 120 μL per day. This was calculated to remove 10% of the circulating blood volume. Circulating blood volume was calculated to be 1.2 mL for a 20 g mouse.
Results and discussion
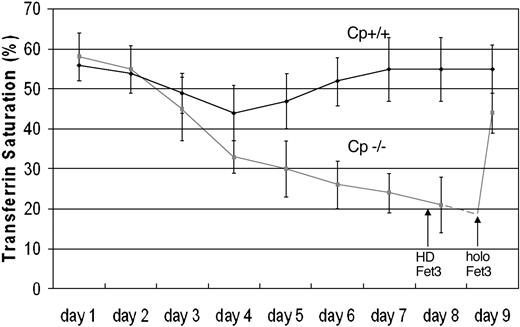
Transferrin is the iron-binding transport protein that functions as the Fe3+ acceptor in mammalian plasma. Phlebotomy results in a rapid reduction in transferrin-bound iron, which gradually increases again over time as iron is mobilized from storage sites. Mice homozygous for a targeted deletion of the ceruloplasmin gene demonstrate a marked impairment in tissue iron efflux and are unable to restore normal transferrin iron saturation following phlebotomy.9 As anticipated, plasma transferrin saturation is restored to normal in phlebotomized mice following injection of holoceruloplasmin.
We previously demonstrated that detergent-solubilized Fet3p is capable of loading iron onto transferrin in vitro.7 To determine if Fet3p could function in a similar fashion in vivo, aceruloplasminemic mice were injected with purified Fet3p, which had been modified to remove the transmembrane domain,6 and then deglycosylated with endoglycosidase F to prevent rapid hepatic clearance. Turnover studies following injection revealed that this modified Fet3p was stable and persisted in plasma for hours. Analysis of plasma utilizing a diaminobenzidine oxidation assay revealed the presence of enzymatically active Fet3p in the plasma of aceruloplasminemic mice following injection (data not shown). Injection of Fet3p resulted in a dramatic and rapid restoration of normal iron homeostasis with return of plasma transferrin saturations to control values within 1 hour (Figure 1). As shown in Table 1, the change in plasma transferrin saturation results from an increase in plasma iron. Enzymatically inactive Fet3p, prepared by treatment of modified Fet3p at 100°C for 7 minutes, failed to alter the low transferrin saturation in phlebotomized aceruloplasminemic mice.
Injection of soluble Fet3p increases transferrin saturation in aceruloplasminemic mice. Both normal mice (Cp+/Cp+) and mice with a targeted deletion in the ceruloplasmin gene (Cp-/Cp-) were bled via daily phlebotomy, and plasma iron and transferrin saturation was measured. Aceruloplasminemic (Cp-/Cp-) mice were injected on day 8 with heat-denatured Fet3p (HD Fet3). On day 9, the same mice were injected with active Fet3p, and blood samples were obtained 1 hour later and transferrin saturation determined (mean and standard deviation).
Injection of soluble Fet3p increases transferrin saturation in aceruloplasminemic mice. Both normal mice (Cp+/Cp+) and mice with a targeted deletion in the ceruloplasmin gene (Cp-/Cp-) were bled via daily phlebotomy, and plasma iron and transferrin saturation was measured. Aceruloplasminemic (Cp-/Cp-) mice were injected on day 8 with heat-denatured Fet3p (HD Fet3). On day 9, the same mice were injected with active Fet3p, and blood samples were obtained 1 hour later and transferrin saturation determined (mean and standard deviation).
Injection of soluble Fet3p increases plasma iron levels in phlebotomized Cp−/− mice
Day . | Cp+/+ . | Cp−/− . |
|---|---|---|
| 1 | 416 ± 24 | 354 ± 31 |
| 4 | 330 ± 47 | 229 ± 20 |
| 6 | 387 ± 30 | 183 ± 58 |
| 7 | 405 ± 28 | 168 ± 31 |
| 8 | 403 ± 32 | 150 ± 43 |
| 9 | 407 ± 40 | 305 ± 41 (+Fet3p) |
Day . | Cp+/+ . | Cp−/− . |
|---|---|---|
| 1 | 416 ± 24 | 354 ± 31 |
| 4 | 330 ± 47 | 229 ± 20 |
| 6 | 387 ± 30 | 183 ± 58 |
| 7 | 405 ± 28 | 168 ± 31 |
| 8 | 403 ± 32 | 150 ± 43 |
| 9 | 407 ± 40 | 305 ± 41 (+Fet3p) |
Samples of plasma from mice, treated as described in Figure 1, were assayed for iron. The data are presented as micrograms per deciliter (mean ± standard deviation).
These experiments demonstrate that a soluble fungal ferroxidase can restore iron homeostasis in aceruloplasminemic mice by replacing the function of ceruloplasmin and promoting iron release from mobilizable stores. The presence of tissue ceruloplasmin receptors has been suggested; hephaestin is membrane bound, and a membrane-bound glycosylphosphatidylinositol (GPI)-ceruloplasmin is found in brain and other tissues.10 Jeong and David11 reported that GPI-linked ceruloplasmin is bound to ferroportin/IREG-1 and suggested that this binding may be important for iron export. Our current findings argue strongly that membrane binding of ceruloplasmin is not required to mediate iron oxidation and release, because Fet3p contains little homology to ceruloplasmin beyond the amino acids that ligate copper and mammalian receptors for fungal Fet3p are unlikely. The transfer of Fe3+ to transferrin does not require a physical interaction among Fet3p, cell membranes, and transferrin and is therefore entirely diffusion limited. We speculate that the ferroxidase-mediated loading of plasma iron onto transferrin prevents the uptake of iron by Fe2+ transport systems, which is present on most somatic cell types, ensuring the delivery of iron to cells expressing transferrin receptors. The facilitation of iron export in aceruloplasminemic mice by soluble Fet3p, a ferroxidase required for iron acquisition in fungi, reveals a striking evolutionary conservation of ferroxidase function and indicates that the direction of iron movement is not specific to the multicopper oxidase but rather is determined by the acceptor of the ferroxidase reaction.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-11-4060.
Supported by funds from the National Institutes of Health (DK58086 [Z.L.H.], DK61763 [J.D.G], DK30534 [J.K.]).
The online version of the article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal