Abstract
The novel immunosuppressant FTY720 activates sphingosine 1-phosphate receptors (S1PRs) that affect responsiveness of lymphocytes to chemokines such as stromal cell-derived factor 1 (SDF-1), resulting in increased lymphocyte homing to secondary lymphoid organs. Since SDF-1 and its receptor CXCR4 are also involved in bone marrow (BM) homing of hematopoietic stem and progenitor cells (HPCs), we analyzed expression of S1PRs and the influence of FTY720 on SDF-1/CXCR4-mediated effects in human HPCs. By reverse transcriptase-polymerase chain reaction (RT-PCR), S1PRs were expressed in mobilized CD34+ HPCs, particularly in primitive CD34+/CD38- cells. Incubation of HPCs with FTY720 resulted in prolonged SDF-1-induced calcium mobilization and actin polymerization, and substantially increased SDF-1-dependent in vitro transendothelial migration, without affecting VLA-4, VLA-5, and CXCR4 expression. In nonobese diabetic-severe combined immunodeficient (NOD/SCID) mice, the number of CD34+/CD38- cells that homed to the BM after 18 hours was significantly raised by pretreatment of animals and cells with FTY720, tending to result in improved engraftment. In addition, in vitro growth of HPCs (week-5 cobblestone area-forming cells [CAFCs]) was 2.4-fold increased. We conclude that activation of S1PRs by FTY720 increases CXCR4 function in HPCs both in vitro and in vivo, supporting homing and proliferation of HPCs. In the hematopoietic microenvironment, S1PRs are involved in migration and maintenance of HPCs by modulating the effects of SDF-1. (Blood. 2004;103:4478-4486)
Introduction
Homing of hematopoietic stem and progenitor cells (HPCs) to the bone marrow is an active and rapid process that takes place less than one day after transplantation, as demonstrated in the nonobese diabetic-severe combined immunodeficient (NOD/SCID) mouse model1 as well as in recent in vivo tracking experiments using a bioluminescence technique.2 In addition to adhesion molecules, proteolytic enzymes, and cytokines, especially chemokines such as stromal cell-derived factor 1 (SDF-1, CXCL12) are involved in HPC trafficking. CXCR4, the receptor for SDF-1, is expressed on HPCs and plays a central role in both progenitor cell homing and mobilization.3-9 Blocking of CXCR4 with monoclonal antibodies has been shown to reduce homing of transplanted human progenitors to the bone marrow of NOD/SCID mice.3 In the adult human bone marrow, SDF-1 was found to be expressed particularly by endothelial cells and along the endosteum region, suggesting SDF-1 mediates both early steps of HPC homing and final lodgement of stem cells in the niches of the bone marrow microenvironment.10 Stimulation of CXCR4 by SDF-1 also activates adhesion molecules (eg, VLA-4) important for the homing process and may modulate proliferation of progenitors and stem cells.11-13 In addition, there is indirect evidence that the SDF-1/CXCR4 interaction is important for stem cell homing after transplantation in humans, since the migratory capacity of human progenitor cells in response to SDF-1 is correlated with hematopoietic recovery after stem cell transplantation,14 and the expression level of CXCR4 predicts the speed of platelet recovery.15,16 A lower expression level of CXCR4 on circulating progenitors and leukemic cells compared with their counterparts in the bone marrow further supports the idea that SDF-1 and CXCR4 are involved in the tropism of progenitors and immature malignant hematopoietic cells to the bone marrow.5,17,18
However, stem cell homing does not appear to depend exclusively on the interaction of CXCR4 and SDF-1, as the homing capacity of CXCR4null stem cells is impaired, but not completely lost.19,20 These findings suggest that other chemotactic receptors and their ligands also play a role in stem cell migration. For instance, we have recently shown that cysLT1, a receptor for certain chemotactic lipid mediators of the leukotriene family, is highly expressed on CD34+ hematopoietic progenitor cells and activates similar signal transduction pathways as CXCR4.21
There are several reports suggesting that modulation of CXCR4 expression and also of CXCR4 function may be useful to increase the homing capacity of HPCs. Expression of CXCR4 on mobilized progenitors has been shown to correlate with engraftment.16 Up-regulation of CXCR4 expression might be achieved by specific in vitro culture conditions, such as incubation with cytokines for several days.22 In the mentioned study, enhanced expression of CXCR4 resulted in both increased SDF-1-induced migration in vitro and homing capacity in vivo. However, culturing of HPCs requires extensive ex vivo manipulation of the graft and is potentially associated with the loss of stem cell activity and long-term engraftment.23 Direct, rapid pharmacologic modulation of CXCR4 function without the need of culturing therefore represents a more attractive approach to optimize the homing of HPCs.
Recent studies have shown that the novel drug FTY720 (2-amino-2-(2-[4-octyphenyl]ethyl)-1,3-propanediol hydrochloride) used as an immunosuppressant after organ transplantation increases the effects of chemokines on lymphocytes.24 FTY720 is a chemical derivative of myriocin, a substance isolated from the ascomycete Isaria sinclairii, which exhibits immunosuppressive activity in vivo.25,26 For a long time, mechanisms responsible for its immunosuppressive effect have been unknown. The observation that FTY720 results in increased homing of circulating lymphocytes to lymph nodes and augments the effects of chemokines on cell migration raised the possibility that FTY720 activates elements of the signal transduction cascade of G protein-coupled receptors.24,26,27 This idea was supported by the finding that in contrast to other immunosuppressants, FTY720 was not antiproliferative for lymphocytes at moderate doses.26,28
Recently, FTY720 was identified as an agonist of sphingosine 1-phosphate receptors29,30 (S1PRs; formerly termed endothelial differentiation gene [EDG] receptors), a novel class of 5 G protein-coupled 7-transmembrane receptors (S1P1-S1P5)31 associated with modulation of cell migration. Several S1PRs appear to be expressed on murine hematopoietic progenitor cells, suggesting a role of the lipid mediator S1P in the hematopoietic microenvironment.32,33 While some S1PRs, particularly those with high affinity for FTY720, support cell migration, others may also be inhibitory, as demonstrated for S1P2.34
In the present study, we analyzed the effects of the S1P receptor agonist FTY720 on CXCR4-mediated effects both in vitro and in vivo to further characterize the role of S1PRs in human hematopoiesis, and to explore potential effects of FTY720 on the homing capacity of progenitor cells when used as an immunosuppressant in bone marrow transplantation.
Materials and methods
Isolation of human cells
After informed consent, peripheral blood (PB) mononuclear cells (MNCs) were obtained from patients with nonhematologic malignancies or healthy stem cell donors during peripheral blood progenitor cell mobilization with granulocyte colony-stimulating factor (G-CSF). MNCs were separated by Ficoll density gradient centrifugation. Lineage-depleted (Lin-) HPCs were prepared by immunomagnetic negative selection (StemSep; StemCell Technologies, Vancouver, BC, Canada) with a cocktail of monoclonal antibodies (mAbs) for lineage-specific antigens (CD2, CD3, CD14, CD16, CD19, CD24, CD56, CD66b, and glycophorin A). CD34+ cells were isolated using immunomagnetic microbeads (MACS system; Miltenyi Biotec, Bergisch Gladbach, Germany). Natural killer (NK) cells, used as a positive control for reverse transcriptase-polymerase chain reaction (RTPCR) analysis of S1P receptor expression, were isolated from the CD34- fraction by incubation of the cell suspension with a CD56-fluoresceine isothiocyanate (FITC) antibody (clone NCAM16.2; BD Biosciences, Heidelberg, Germany) and anti-FITC microbeads (Miltenyi Biotec). Human umbilical vein endothelial cells (HUVECs) were isolated and grown as described previously.35 As determined by flow cytometry, at least 90% of the Lin- cells were CD45low/CD34+. The purity of PB CD34+ isolated by positive selection was more than 95%.
Cell lines
The 2 murine bone marrow stromal cell lines, MS-5 (obtained from German Collection of Microorganisms and Cell Cultures [DSMZ], Braunschweig, Germany) and FBMD-1 (kindly provided by Dr R. E. Ploemacher, Institute of Hematology, Erasmus University, Rotterdam, The Netherlands) were maintained in α minumum essential medium (αMEM; Invitrogen, Karlsruhe, Germany) containing 10% heat-inactivated fetal calf serum (FCS; PAA, Cölbe, Germany). The human bone marrow microvascular endothelial cell line BMEC-1 (kindly provided by Dr S. Rafii, New York, NY) was cultured in Medium199 (Biochrom; Berlin, Germany) supplemented with 20% FCS. Cells were incubated at 37°C with 5% CO2.
Flow cytometry analysis and cell sorting
Cells (2 to 5 × 105) were incubated for 30 minutes at 4°C with the FITC-, phycoerythrin (PE)-, or PE-cyanine-5 (PC5)-conjugated mAbs: CD34-FITC/PE (BD Biosciences), CD38-FITC/PE (Beckman Coulter, Hialeah, FL), CD45-FITC (BD Biosciences), CD45-PC5 (Beckman Coulter), CXCR4-PE (clone 12G5; BD Biosciences), VLA-4(CD49d)-FITC, and VLA-5(CD49e)-FITC. Isotype-identical antibodies served as controls (IgG1 and IgG2, FITC/PE-conjugated; BD Biosciences). Cells were analyzed using a FACScalibur flow cytometer (BD Biosciences). To calculate the percentage of positive cells, a proportion of less than or equal to 1% false-positive events was accepted in the negative control sample. Isolated progenitor cells (5 to 10 × 106) were stained with CD34-FITC and CD38-PE, and the CD34+/CD38- and CD34+/CD38+ subpopulations were sorted using a FACSvantage cell sorter (BD Biosciences).
RT-PCR
Total RNA from CD34+, CD34+/CD38-, and CD34+/CD38+ progenitors; NK cells (positive control for S1P1, S1P4, and S1P5)36 ; and HUVECs (positive control for S1P1 and S1P3)37 was isolated using RNeasy Mini Kit (Qiagen, Hilden, Germany) including digestion with DNase I. First-strand cDNA was synthesized with the use of MuLV reverse transcriptase and random hexamers (Applied Biosystems, Foster City, CA). Subsequent PCR analysis was performed with gene-specific primers based on the human receptor sequences as follows: S1P1: forward 5′-TCATCGTCCGGCATTACAACTA-3′ and reverse 5′-GAGTGAGCTTGTAGGTGGTG-3′ (product size, 270 base pairs [bp]); S1P2: forward 5′-ATGGGCAGCTTGTACTCGGAG-3′ and reverse 5′-CAGCCAGCAGACGATAAAGAC-3′ (product size, 741 bp); S1P3: forward 5′-CTTGGTCATCTGCAGCTTCATC-3′ and reverse 5′-TGCTGATGCAGAAGGCAATGTA-3′ (product size, 470 bp); S1P4: forward 5′-TGAACATCACGCTGAGTGACCT-3′ and reverse 5′-GATCATCAGCACCGTCTTCAGC-3′ (product size, 512 bp); S1P5: forward 5′-TGATTCCAGTGACAAACGACA-3′ and reverse 5′-GCTTCTATGGCTCCCACCTC-3′ (product size, 328 bp). β-actin forward 5′-TCATGTTTGAGACCTTCAA-3′ and reverse 5′-GTCTTTGCGGATGTCCACG-3′ (product size, 512 bp) was taken as an internal standard. A pre-PCR heat-step (5 minutes, 95°C) provided a PCR Hot Start by partially activating AmpliTaq Gold DNA Polymerase (Applied Biosystems, Foster City, CA). For analysis of S1PR expression, 35 cycles were run, and for analysis of β-actin, 28 amplification cycles were run, each consisting of denaturation for 15 seconds at 95°C, annealing for 30 seconds at 57°C (β-actin, S1P5) or 59°C (S1P1,2,3,4), and elongation for 90 seconds at 72°C followed by a final extension step of 10 minutes at 72°C. PCR products were separated by horizontal agarose gel electrophoresis with subsequent ethidium bromide staining. In order to detect possible contaminations of genomic DNA in the RNA sample each reaction was performed without previous reverse transcription.
In vivo homing and engraftment assay
Female NOD/SCID mice were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained in individually ventilated cages under specific pathogen-free conditions at the animal research facility of the University Hospital in Tübingen. All experiments were approved by the animal care committee of the University of Tübingen. The 15-week-old mice were irradiated with a sublethal dose of 300 cGy from a 137Cesium source (Gammacell 1000 Elite; MDS Nordion, Freiburg, Germany). After 24 hours, human CD34+ HPCs (2 × 106 cells per mouse for analysis of homing and 1 × 106 cells for analysis of engraftment) were injected into the tail vein in 315 μL X-VIVO 20 medium (Biowhittaker, Verviers, Belgium). To assess for a potential effect of FTY720 on progenitor cell homing, HPCs were transplanted after systemic pretreatment of animals (n = 10) with FTY720 (0.3 mg/kg; Novartis Pharma, Basel, Switzerland; injected intraperitoneally one hour prior to transplantation) and in vitro incubation of the cells with the immunomodulator (100 nM, one hour at 37°C). This treatment schedule was chosen from the pharmacokinetics of FTY720 and ensured that the effects on the progenitors were present immediately from the time point of injection for 6 hours and longer. HPCs from untreated animals (n = 10) served as controls. Cells were recovered from the bone marrow of mice that had received transplants after 18 hours and analyzed for the presence of human cells by flow cytometry using CD34-PE, CD38-FITC, and CD45-PC5 mAbs. Cells obtained from mice that had not received transplants and isotope control mAbs were used to exclude false-positive cells. Human engraftment was assessed in the second month after transplantation by measuring the percentage of CD45+ human hematopoietic cells. Only a distinct population of cells (more than 10 events) in a human CD45-PE/mouse CD45-FITC dot plot was considered as human engraftment. The percentage of engraftment was calculated as human CD45+ cells/(human CD45+ cells + mouse CD45+ cells) × 100%.
Transendothelial migration assay
Migration across bone marrow endothelial cells in vitro was analyzed as described previously.5 In brief, BMEC-1 monolayers were grown to confluency on transwell inserts (3.0 μm pore size; BD Biosciences) that were subsequently placed in a fresh 24-well tissue-culture plate (BD Biosciences). To assess the effect of SDF-1 on transendothelial migration in vitro, MS-5-conditioned medium was added to the lower chamber, while the upper chamber contained a total of 1 × 105 Lin- cells in αMEM/10% FCS, preincubated or not with FTY720 at the indicated concentrations for 3 hours at 37°C. To assess the neutralizing effects of anti-CXCR4 mAb (clone 12G5; R&D Systems, Wiesbaden, Germany) and pertussis toxin (PTX; Calbiochem, Darmstadt, Germany) on SDF-1-induced transendothelial migration, cells were preincubated with anti-CXCR4 mAb or normal mouse IgG (negative control; Serotec, Oxford, United Kingdom) for 30 minutes and with PTX for 90 minutes, respectively, prior to the migration assay. Transmigrated cells were recovered from the lower chamber after 12 to 14 hours of incubation at 37°C/5% CO2 for further characterization (phenotypic analysis, clonal cell culture, and long-term culture). Enumeration of migrated cells was accomplished by staining cells with anti-CD45-PC5 and anti-CD34-PE followed by addition of a fixed number of FITC-CaliBRITE beads (BD Biosciences) per sample and subsequent FACS analysis. The gate for data acquisition was established for low forward scatter (FSC) and high FL-1, which is specific for FITC beads. A dual-parameter dot diagram displaying PC5 (CD45) and PE (CD34) was also generated from the gated events of intermediate FSC and low side scatter (SSC) for Lin- cells. The number of transmigrating CD45low/CD34+ hematopoietic progenitor cells was calculated from the ratio of HPCs per bead multiplied by the number of beads added.
Integrin (VLA-4, VLA-5) and CXCR4 expression
To assess potential effects of FTY720 on VLA-4, VLA-5, and CXCR4 expression, CD34+ cells were cultured in αMEM/10% FCS with or without 100 nM FTY720. After 1 hour, 6 hours, and 18 hours, cells were recovered and expression of integrins and CXCR4 was measured by flow cytometry.
Measurement of intracellular calcium mobilization
Measurement of intracellular free Ca2+ was performed as described previously21 with some modifications. Briefly, HPCs were incubated in Hanks balanced salt solution (HBSS; Sigma, St Louis, MO) containing 10 μM Fluo-3 (Molecular Probes, Leiden, The Netherlands) for 30 minutes at 37°C. After a 1:5 dilution in HBSS/1% FCS followed by incubation for 40 minutes, the cells were washed 3 times, resuspended in HEPES-buffered saline, and incubated for at least 10 minutes at 37°C. During all steps of preparation and in the final reaction tube, FTY720 (100 nM) was either present or absent. After stimulation with SDF-1 (final concentration 100 ng/mL, SDF-1β; R&D Systems), the FL-1 fluorescence was analyzed using a FACSCalibur (BD Biosciences) in 5-second acquisition intervals.
Measurement of polymerized F-actin
Cells were suspended in X-VIVO 20 medium (Biowhittaker) with or without 100 nM FTY720. After 90 minutes of incubation at 37°C, SDF-1 (final concentration 10 ng/mL) or carrier (PBS/0.1% BSA) was added. By adding one volume of FITC-phalloidine (Sigma) working solution (18% formaldehyde, 250 μg/mL lysophosphatidylcholine, 0.4 μM FITC-conjugated phalloidine) to 4 volumes of cell suspension, polymerized F-actin was fluorescence labeled, and the fixed cells were analyzed by flow cytometry (FACSCalibur) as described previously.38,39
Hematopoietic colony assay
Colony-forming efficiency of Lin- cells and transmigrating cells was analyzed by standard semisolid culture. For this, cells were cultured at 37°C/5% CO2 in methylcellulose medium (MethoCult H4230; StemCell Technologies) supplemented with recombinant human (rh) SCF, rh interleukin 3 (IL-3), rh granulocyte-macrophage colony-stimulating factor (GMCSF) (each 20 ng/mL; PeproTech, London, England), rhG-CSF (50 ng/mL; Amgen, Thousand Oaks, CA), rhEPO (epoetin-alpha, 6 U/mL; Jannsen-Cilag, Neuss, Germany), and the designated concentrations of FTY720. Morphologic features of the generated colonies were scored after 12 to 14 days of incubation.
Long-term culture
To detect primitive hematopoietic progenitors in vitro in both Lin- cells and transmigrating cells, long-term cultures were performed. A total of 5 × 103 Lin- cells were cultured with 1 × 104 single MS-5 or FBMD-1 cells in 35-mm Lux suspension culture dishes (Greiner, Frickenhausen, Germany) containing 2 mL methylcellulose medium (MethoCult H4230) with or without 100 nM FTY720. In transendothelial migration experiments, transmigrating cells were directly transfered onto confluent FBMD-1 monolayers prepared in wells of fibronectin-coated 24-well tissue plates (BIOCOAT Cellware; BD Biosciences) in one mL αMEM/10% FCS, which was after 7 days of incubation carefully replaced by one mL of methylcellulose medium. Dishes and plates were incubated at 37°C/5% CO2. Cultures were demi-depopulated by removal of half the volume with replacement by fresh methylcellulose medium on days 14, 21, and 28 of cocultivation. The number of cobblestone areas was directly counted in situ on days 34 to 36.
Statistical analysis
Data are expressed as the average plus or minus standard error of the mean (SEM). Differences between groups were compared by Student t test and were considered significant at a P value of less than .05. For the in vivo studies, the paired t test was applied after calculating the average proportion of human cells in the mouse bone marrow for each donor in the presence or absence of FTY720. To compare the frequency of animals with detectable human hematopoiesis (independent from the percentage of human CD45+ cells) in the treatment versus control groups, the Fisher exact test was applied.
Results
S1PRs are expressed in human PB CD34+ progenitor cells
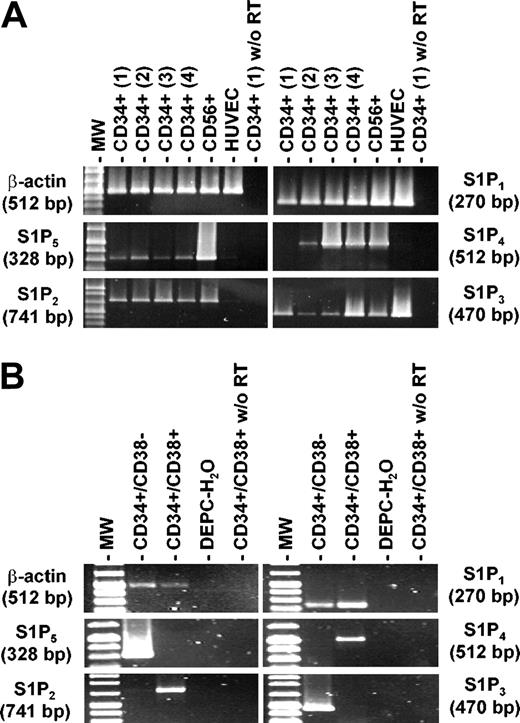
Recent reports have demonstrated that FTY720 targets G protein-coupled receptors of the S1P receptor family, whose natural ligand is sphingosine 1-phosphate.29,30 In receptor affinity studies, S1P1, S1P4, and S1P5 avidly bind FTY720, while S1P2 and S1P3 are less likely to be involved in FTY720-induced cellular responses. Therefore, expression of S1P receptor mRNA in CD34+ cells derived from peripheral blood of 4 different donors was analyzed by RT-PCR. HUVECs and NK cells served as a positive control. As shown in Figure 1A, all samples of PB CD34+ progenitor cells brightly expressed S1P1 mRNA, while the other S1PRs were only weakly or less consistently positive. S1P1, rather than S1P4 or S1P5, is therefore most likely to mediate the observed effects of FTY720 on hematopoietic progenitor cells. Of note, S1P1 is the receptor with the greatest affinity for FTY720.29 Analysis of S1PR expression in sorted primitive CD34+/CD38- and more committed CD34+/CD38+ cells (Figure 1B) revealed that primitive progenitors expressed both receptors (S1P1 and S1P5) which bind FTY720 most avidly and stimulate migration, while S1P2, which in contrast to other S1PRs has been shown to inhibit migration,34 was only expressed in CD34+/CD38+ cells. From these findings, one might predict that particularly primitive progenitors respond to FTY720 (and S1P) with increased chemokine-induced migration.
Expression of S1P receptor mRNAs in CD34+ hematopoietic progenitors. (A) RT-PCR analysis of S1P1, S1P2, S1P3, S1P4, S1P5, and β-actin expression was carried out with RNA isolated from mobilized PB CD34+ cells originating from 4 different donors as described in “Materials and methods.” CD56+ cells and HUVECs served as positive controls for S1PR expression. Control experiments in the absence of RT are also shown. All samples of PB CD34+ progenitors highly and constantly expressed mRNA for S1P1, the receptor with the highest affinity for FTY720 (in this figure, S1PRs are arranged according to the affinity for FTY720 from high to low). (B) The expression pattern of S1PRs in FACS-sorted CD34+/CD38- and CD34+/CD38+ progenitors was compared. While S1P1 was expressed in both subsets, S1P3 and S1P5 were positive only in CD34+/CD38- progenitors. In contrast, S1P2 and S1P4 mRNA was found only in CD34+/CD38+ cells.
Expression of S1P receptor mRNAs in CD34+ hematopoietic progenitors. (A) RT-PCR analysis of S1P1, S1P2, S1P3, S1P4, S1P5, and β-actin expression was carried out with RNA isolated from mobilized PB CD34+ cells originating from 4 different donors as described in “Materials and methods.” CD56+ cells and HUVECs served as positive controls for S1PR expression. Control experiments in the absence of RT are also shown. All samples of PB CD34+ progenitors highly and constantly expressed mRNA for S1P1, the receptor with the highest affinity for FTY720 (in this figure, S1PRs are arranged according to the affinity for FTY720 from high to low). (B) The expression pattern of S1PRs in FACS-sorted CD34+/CD38- and CD34+/CD38+ progenitors was compared. While S1P1 was expressed in both subsets, S1P3 and S1P5 were positive only in CD34+/CD38- progenitors. In contrast, S1P2 and S1P4 mRNA was found only in CD34+/CD38+ cells.
FTY720 stimulates CXCR4-dependent in vitro migration of HPCs across bone marrow endothelium without affecting integrin (VLA-4, VLA-5) and CXCR4 expression
The data available to date on the immunosuppressive effect of FTY720 suggest that FTY720 functions by triggering the chemotactic response of lymphocytes to chemokines, resulting in increased homing and trapping of lymphocytes in the lymph nodes. Given the fact that CXCR4, one of the key receptors in HPC homing, also is activated in lymphocytes by FTY720, we addressed the question of how far FTY720 might influence CXCR4-mediated hematopoietic progenitor cell trafficking.
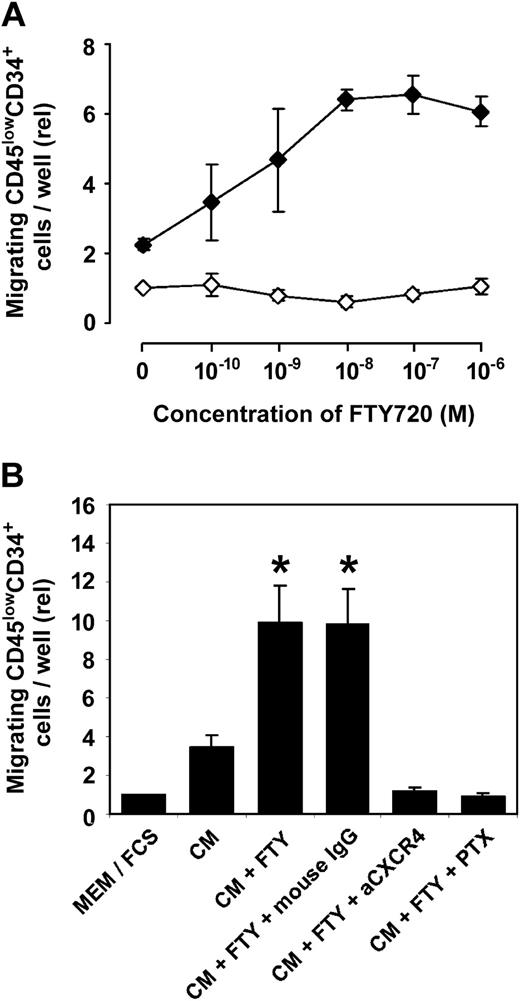
While FTY720 did not affect spontaneous migration of isolated, G-CSF-mobilized Lin- peripheral blood progenitor cells across BMEC-1 cells in vitro, a dose-dependent increase of SDF-1-induced transmigration was observed in the presence of FTY720 when SDF-1-containing conditioned medium (CM; MS-5 cell line) was added to the lower chamber of the transmigration system (Figure 2A). The SDF-1-induced, FTY720-supported transmigration could be blocked completely by addition of anti-CXCR4 mAb or pertussis toxin (PTX), indicating that CXCR4 is the only chemokine receptor involved in the observed effects (Figure 2B).
FTY720 enhances CXCR4-dependent transmigration of Lin- cells through bone marrow endothelium in vitro. (A) In an in vitro assay of transendothelial migration across bone marrow endothelium,5 FTY720 did not affect spontaneous migration of Lin- cells isolated from peripheral blood, whereas SDF-1-induced migration (MS-5 conditioned medium) was enhanced by FTY720 in a dose-dependent manner after preincubation of cells for 3 hours with the indicated concentrations of FTY720. Data represent the mean ± SEM from 3 independent experiments. Spontaneous migration: ⋄; SDF-1-induced migration: ♦. (B). The triggering effect of FTY720 on SDF-1-driven transmigration (MS-5 conditioned medium; CM) could be completely blocked by preincubation of Lin- cells with an anti-CXCR4 mAb or pertussis toxin (PTX), indicating that CXCR4 is the only receptor involved in this system. Data represent the mean ± SEM from 5 independent experiments. *P < .05 versus CM.
FTY720 enhances CXCR4-dependent transmigration of Lin- cells through bone marrow endothelium in vitro. (A) In an in vitro assay of transendothelial migration across bone marrow endothelium,5 FTY720 did not affect spontaneous migration of Lin- cells isolated from peripheral blood, whereas SDF-1-induced migration (MS-5 conditioned medium) was enhanced by FTY720 in a dose-dependent manner after preincubation of cells for 3 hours with the indicated concentrations of FTY720. Data represent the mean ± SEM from 3 independent experiments. Spontaneous migration: ⋄; SDF-1-induced migration: ♦. (B). The triggering effect of FTY720 on SDF-1-driven transmigration (MS-5 conditioned medium; CM) could be completely blocked by preincubation of Lin- cells with an anti-CXCR4 mAb or pertussis toxin (PTX), indicating that CXCR4 is the only receptor involved in this system. Data represent the mean ± SEM from 5 independent experiments. *P < .05 versus CM.
Rather than affecting chemotaxis directly, effects of FTY720 on integrin or CXCR4 expression could be responsible for the increased rate of migration. However, when CD34+ hematopoietic progenitors were incubated with 100 nM FTY720 for 1, 6, or 18 hours, the expression of VLA-4, VLA-5, and CXCR4 was unchanged compared with the control without FTY720. The difference in the mean fluorescence (arbitrary units) calculated from the fluorescence histograms was below 5% for all conditions tested (FTY720 vs control, data not shown).
Primitive and committed progenitors respond to FTY720
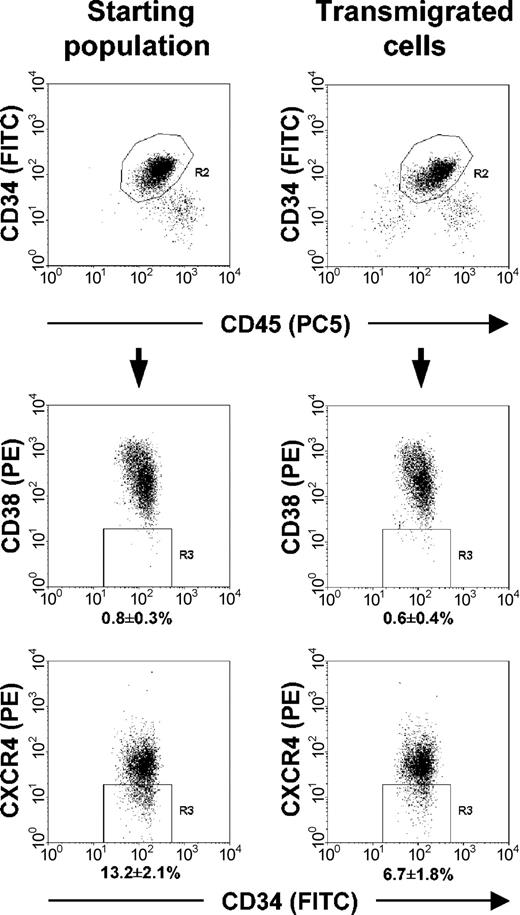
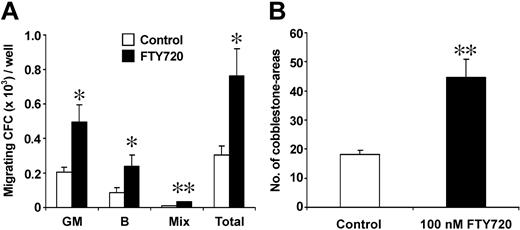
Phenotypic and functional analyses of cells recovered from the transmigration assays demonstrated that progenitors migrating in response to SDF-1 and FTY720 showed a similar percentage of CD34+/CD38- cells, only the proportion of CD34+/CXCR4- cells tended to be decreased compared with analysis of the starting cell population before migration (Figure 3). Evaluation of the clonogenic potential of transmigrated cells revealed that SDF-1-induced migration of lineage-committed progenitors was significantly increased after preincubation of HPCs with FTY720 (100 nM, 3 hours) prior to the transmigration assay (Figure 4A). In addition, a more than 2-fold increase in SDF-1-driven transmigration of pluripotent week-5 cobblestone-area forming cells (CAFCs) was observed in the presence of 100 nM FTY720 (Figure 4B), indicating a substantial effect of FTY720 on migration of both primitive and committed progenitor cell subsets.
Phenotypic analysis of transmigrating cells compared with the untreated starting cell population before migration. Flow cytometric analysis of Lin- progenitors (CD34+ cells, gated = R2) migrating in response to SDF-1 (MS-5 conditioned medium) after preincubation with 100 nM FTY720 showed a similar percentage of CD34+/CD38- cells (gate R3, middle panels), only the proportion of CD34+/CXCR4- cells (gate R3, lower panels) was lower compared with the starting population, demonstrating the migratory capabilities of more immature progenitors in the employed in vitro assay and additionally underlining the CXCR4 dependency of the transmigration process, as already observed in the blocking experiments shown in Figure 2B. Transmigrating cells, right panels; untreated cell population before migration, left panels.
Phenotypic analysis of transmigrating cells compared with the untreated starting cell population before migration. Flow cytometric analysis of Lin- progenitors (CD34+ cells, gated = R2) migrating in response to SDF-1 (MS-5 conditioned medium) after preincubation with 100 nM FTY720 showed a similar percentage of CD34+/CD38- cells (gate R3, middle panels), only the proportion of CD34+/CXCR4- cells (gate R3, lower panels) was lower compared with the starting population, demonstrating the migratory capabilities of more immature progenitors in the employed in vitro assay and additionally underlining the CXCR4 dependency of the transmigration process, as already observed in the blocking experiments shown in Figure 2B. Transmigrating cells, right panels; untreated cell population before migration, left panels.
Effect of FTY720 on in vitro transmigration of lineage-committed and pluripotent progenitors. (A) Lin- cells transmigrating in response to SDF-1 (MS-5 conditioned medium) were analyzed for committed progenitors using a methylcellulose colony assay. When preincubated with FTY720 (100 nM every 3 hours) prior to transmigration, a significantly increased number of committed progenitors of both granulopoietic and erythroid lineages transmigrated (expressed as CFCs per well in the transwell assay). GM: granulopoietic colonies (CFU-GMs); B: erythroid colonies (BFU-Es); Mix: multilineage colonies (CFU-GEMMs); Total: total number of colonies. *P < .05; **P < .01; n = 4. (B) Total numbers of cobblestone areas of transmigrated Lin- cells pretreated or not with FTY720 (100 nM every 3 hours) on FBMD-1 stromal feeders in fibronectin-coated 24-well plates. The number of cobblestone areas was scored after 5 weeks of incubation. **P < .01, n = 4.
Effect of FTY720 on in vitro transmigration of lineage-committed and pluripotent progenitors. (A) Lin- cells transmigrating in response to SDF-1 (MS-5 conditioned medium) were analyzed for committed progenitors using a methylcellulose colony assay. When preincubated with FTY720 (100 nM every 3 hours) prior to transmigration, a significantly increased number of committed progenitors of both granulopoietic and erythroid lineages transmigrated (expressed as CFCs per well in the transwell assay). GM: granulopoietic colonies (CFU-GMs); B: erythroid colonies (BFU-Es); Mix: multilineage colonies (CFU-GEMMs); Total: total number of colonies. *P < .05; **P < .01; n = 4. (B) Total numbers of cobblestone areas of transmigrated Lin- cells pretreated or not with FTY720 (100 nM every 3 hours) on FBMD-1 stromal feeders in fibronectin-coated 24-well plates. The number of cobblestone areas was scored after 5 weeks of incubation. **P < .01, n = 4.
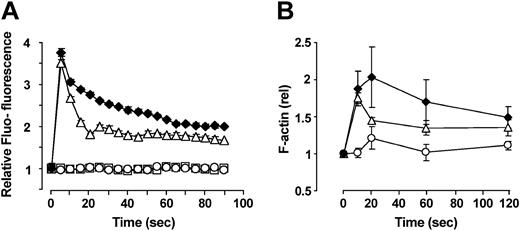
SDF-1-triggered calcium mobilization and actin-polymerization are up-regulated by FTY720
To further characterize the influence of FTY720 on CXCR4-mediated effects, SDF-1-triggered calcium mobilization and actin polymerization as initial steps in cellular locomotion were analyzed after treatment of cells with FTY720 (Figure 5). Preincubation of PB CD34+ progenitors with 100 nM FTY720 for 90 minutes prior to stimulation with SDF-1 (100 ng/mL for calcium mobilization and 10 ng/mL for actin polymerization) resulted in sustained calcium fluxes and had a stabilizing effect on the cytoskeleton. The maximum effect of FTY720 on SDF-1-stimulated calcium mobilization was observed 20 seconds after addition of the chemokine. Interestingly, the effect of preincubation with FTY720 on actin polymerization was highest at the 20-second time point with a more pronounced increase in total filamentous actin and delayed rate of depolymerization as compared with SDF-1 stimulation alone.
SDF-1-triggered calcium mobilization and actin-polymerization are up-regulated by FTY720. (A) Effect of FTY720 on SDF-1-excited calcium mobilization. Isolated PB CD34+ cells were loaded with the fluorescent dye Fluo-3 in the presence or absence of 100 nM FTY720 as indicated. After stimulation with 100 ng/mL SDF-1, the FL-1 fluorescence was analyzed in 5-second acquisition intervals by flow cytometry. Preincubation of cells with FTY720 lead to a transient increase in the amount of intracellular free Ca2+, with the maximum effect of FTY720 on SDF-1-induced calcium mobilization being observed 20 seconds after stimulation with the chemokine. ♦: FTY720 plus SDF-1; ▵: SDF-1 alone; □: FTY720 alone; ○: Control (carrier). Data represent the mean ± SEM of 4 independent experiments. (B) Effect of FTY720 on SDF-1-induced formation of filamentous actin. Isolated PB CD34+ progenitor cells were preincubated or not for 90 minutes with 100 nM FTY720 and subsequently stimulated with 10 ng/mL SDF-1 or carrier (PBS/1% BSA). Formation of filamentous actin was analyzed flow cytometrically by fixation of cells at the indicated time points following permeabilization and staining with FITC-conjugated phalloidin as described in “Materials and methods.” Preincubation of cells with FTY720 had a sustained stabilizing effect on the cytoskeleton. As observed in the calcium mobilization experiments, the effect of FTY720 was highest at the 20-second time point. Data represent the mean ± SEM of 3 independent experiments. ♦: FTY720 plus SDF-1; ▵: SDF-1 alone; ○: FTY720 plus PBS/BSA.
SDF-1-triggered calcium mobilization and actin-polymerization are up-regulated by FTY720. (A) Effect of FTY720 on SDF-1-excited calcium mobilization. Isolated PB CD34+ cells were loaded with the fluorescent dye Fluo-3 in the presence or absence of 100 nM FTY720 as indicated. After stimulation with 100 ng/mL SDF-1, the FL-1 fluorescence was analyzed in 5-second acquisition intervals by flow cytometry. Preincubation of cells with FTY720 lead to a transient increase in the amount of intracellular free Ca2+, with the maximum effect of FTY720 on SDF-1-induced calcium mobilization being observed 20 seconds after stimulation with the chemokine. ♦: FTY720 plus SDF-1; ▵: SDF-1 alone; □: FTY720 alone; ○: Control (carrier). Data represent the mean ± SEM of 4 independent experiments. (B) Effect of FTY720 on SDF-1-induced formation of filamentous actin. Isolated PB CD34+ progenitor cells were preincubated or not for 90 minutes with 100 nM FTY720 and subsequently stimulated with 10 ng/mL SDF-1 or carrier (PBS/1% BSA). Formation of filamentous actin was analyzed flow cytometrically by fixation of cells at the indicated time points following permeabilization and staining with FITC-conjugated phalloidin as described in “Materials and methods.” Preincubation of cells with FTY720 had a sustained stabilizing effect on the cytoskeleton. As observed in the calcium mobilization experiments, the effect of FTY720 was highest at the 20-second time point. Data represent the mean ± SEM of 3 independent experiments. ♦: FTY720 plus SDF-1; ▵: SDF-1 alone; ○: FTY720 plus PBS/BSA.
FTY720 increases bone marrow homing of CD34+/CD38- progenitor cells in vivo, tending to result in improved engraftment
In order to further assess the observed effects of FTY720 on progenitor cell transmigration in an in vivo system, NOD/SCID mice (n = 10 in the FTY720 group and n = 10 in the control group) received a transplant of 2 × 106 mobilized PB CD34+ cells/mouse originating from 4 different donor sources. Bone marrow cell suspensions from mice that received transplants were analyzed by flow cytometry for the presence of human cells 18 hours after transplantation.
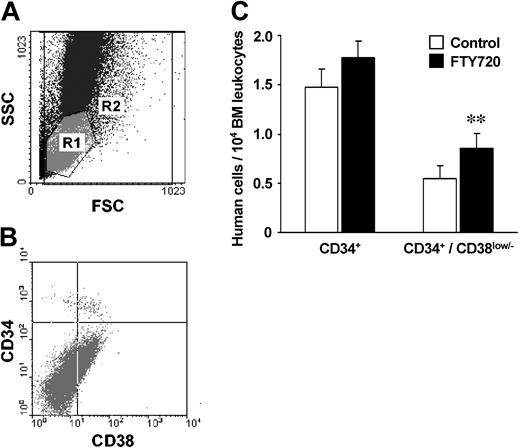
Systemic pretreatment of animals and CD34+ cells with FTY720 resulted in a rapid and significant increase of the frequency of more primitive, CD34+/CD38- cells recovered from the bone marrow (8.5 ± 1.1 vs 5.4 ± 1.4 per 104 bone marrow cells; P < .05), while the total number of human CD34+ HPCs was moderately enhanced (17.9 ± 1.6 vs 14.7 ± 2.3; not significant; Figure 6A-C). Together, these results underline the effects of FTY720, especially on the migratory capabilities of more primitive progenitor cell subsets that were seen in the in vitro experiments. Our findings are consistent with previous reports showing the rapid homing kinetics of CD34+/CD38low/- HPC subpopulations in the NOD/SCID mouse model.1
FTY720 up-regulates bone marrow homing of immature hematopoietic progenitors in vivo. PB CD34+ cells (2 × 106) were injected into NOD/SCID mice, either untreated or after systemic pretreatment of animals and cells with FTY720 as indicated. (A) Representative flow cytometry analysis of cells recovered from the bone marrow of NOD/SCID mice 18 hours after transplantation. R1 = gate for human CD34+ cells according to the FSC/SSC characteristics; R2 = total bone marrow leukocytes. (B) Gated on R1; CD34/CD38 staining of cells recovered from the bone marrow of NOD/SCID mice that received transplantations of CD34+-enriched cells. (C) Homing of human progenitor cells in the bone marrow of NOD/SCID mice 18 hours after transplantation, depicted as human cells per 104 total bone marrow leukocytes. Systemic pretreatment of animals and cells with FTY720 resulted in a significant increase in the number of more immature, CD34+/CD38- cells recovered from the bone marrow, while the total number of human CD34+ HPCs was only moderately enhanced. **P < .01.
FTY720 up-regulates bone marrow homing of immature hematopoietic progenitors in vivo. PB CD34+ cells (2 × 106) were injected into NOD/SCID mice, either untreated or after systemic pretreatment of animals and cells with FTY720 as indicated. (A) Representative flow cytometry analysis of cells recovered from the bone marrow of NOD/SCID mice 18 hours after transplantation. R1 = gate for human CD34+ cells according to the FSC/SSC characteristics; R2 = total bone marrow leukocytes. (B) Gated on R1; CD34/CD38 staining of cells recovered from the bone marrow of NOD/SCID mice that received transplantations of CD34+-enriched cells. (C) Homing of human progenitor cells in the bone marrow of NOD/SCID mice 18 hours after transplantation, depicted as human cells per 104 total bone marrow leukocytes. Systemic pretreatment of animals and cells with FTY720 resulted in a significant increase in the number of more immature, CD34+/CD38- cells recovered from the bone marrow, while the total number of human CD34+ HPCs was only moderately enhanced. **P < .01.
Engraftment of human hematopoietic cells (CD45+) was analyzed between week 5 and week 8 (median: week 7) after transplantation of 1 × 106 human CD34+ cells (close to the limit required for long-term engraftment in this model) with (n = 15) or without (n = 18) FTY720 pretreatment. There was a trend that more frequent engraftment was observed after pretreatment with FTY720 compared with control (9 of 15 vs 5 of 18), but the difference was not statistically significant. In addition, the greatest proportion of human CD45+ cells in the bone marrow was found in the treatment group (34% human cells). In contrast to the homing data, however, there was a high variability in the percentage of engrafted human CD45+ cells, resulting in a lack of statistical significance (control: 2.0% ± 2.0% human CD45+ cells; FTY720: 5.6% ± 5.5%). Mainly B-lymphoid (CD19+) and myeloid (CD33+) engraftment could be detected in the bone marrow of mice engrafted with human CD45+ hematopoietic cells, independent of the treatment with FTY720 during the initial homing. In addition, a small proportion of CD56+ cells was also present.
FTY720 supports in vitro growth of pluripotent HPCs
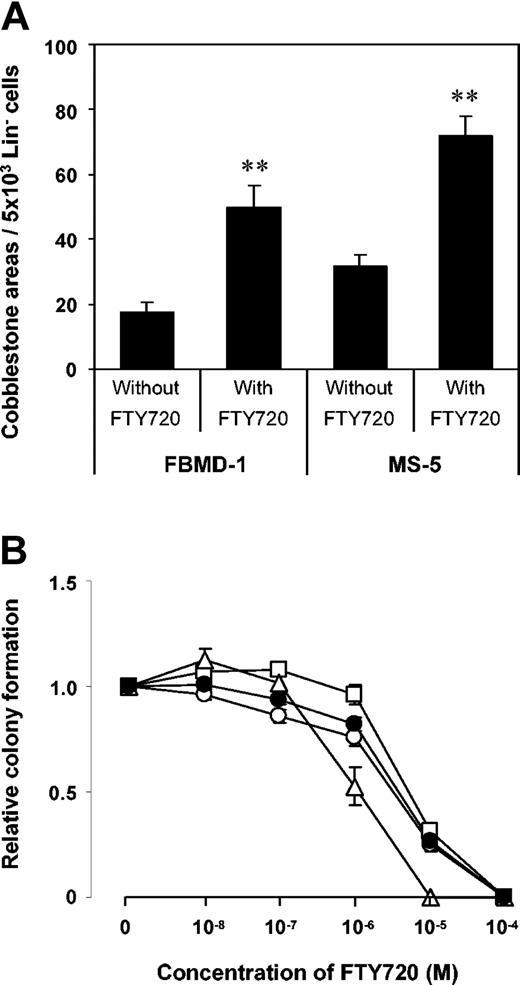
As FTY720 might be associated with increased apoptosis,40,41 we investigated the effect of FTY720 on proliferation and survival of both primitive and lineage-committed HPC subpopulations. Long-term cultures and methylcellulose assays were set up with Lin- cells in the presence or absence of FTY720. Here, generation of week-5 cobblestone areas was significantly increased in the presence of 100 nM FTY720, suggesting even a positive effect on survival and proliferation of more pluripotent HPC subsets. The highest number of cobblestone areas was observed when Lin- cells were cocultured with MS-5 stroma (Figure 7A), which is known to produce large amounts of SDF-1.42
Proliferation of pluripotent and committed progenitors in the presence of FTY720. (A) Effect of FTY720 on week-5 cobblestone area formation by mobilized Lin- progenitor cells. Lin-cells (5 × 103) were cultured with 1 × 104 single MS-5 or FBMD-1 cells with or without 100 nM FTY720. The effect of the immunomodulator on proliferation of multipotent progenitors was highest in cultures containing MS-5 cells, which are known to produce large amounts of SDF-1. Data represent the mean plus or minus SEM of 4 independent experiments. **P < .01. (B) Effect of FTY720 on colony formation by mobilized Lin- progenitor cells. Colony-forming efficiency was analyzed by standard semisolid culture in methylcellulose medium supplemented with growth factors and the designated concentrations of FTY720. Only at concentrations of FTY720 more than or equal to one μM was the proliferative capacity of lineage-committed progenitors impaired. Data represent the mean plus or minus SEM of 3 independent experiments. □: BFU-E; ▵: CFU-Mix; ○: CFU-GM; •: total colonies.
Proliferation of pluripotent and committed progenitors in the presence of FTY720. (A) Effect of FTY720 on week-5 cobblestone area formation by mobilized Lin- progenitor cells. Lin-cells (5 × 103) were cultured with 1 × 104 single MS-5 or FBMD-1 cells with or without 100 nM FTY720. The effect of the immunomodulator on proliferation of multipotent progenitors was highest in cultures containing MS-5 cells, which are known to produce large amounts of SDF-1. Data represent the mean plus or minus SEM of 4 independent experiments. **P < .01. (B) Effect of FTY720 on colony formation by mobilized Lin- progenitor cells. Colony-forming efficiency was analyzed by standard semisolid culture in methylcellulose medium supplemented with growth factors and the designated concentrations of FTY720. Only at concentrations of FTY720 more than or equal to one μM was the proliferative capacity of lineage-committed progenitors impaired. Data represent the mean plus or minus SEM of 3 independent experiments. □: BFU-E; ▵: CFU-Mix; ○: CFU-GM; •: total colonies.
Colony formation (total CFU) from Lin- cells was not affected by FTY720 at concentrations of up to 100 nM (Figure 7B). Only at concentrations more than or equal to one μM did the number of CFUs decrease in a dose-dependent manner, an effect that has been reported in a similar way in lymphocytes and is probably due to increased apoptosis.40,41
Discussion
In this study, we demonstrate that mobilized, human CD34+ progenitor cells express G protein-coupled 7-transmembrane receptors of the S1P receptor family. Furthermore, we show that activation of S1PRs with the agonist FTY720 augments SDF-1-mediated effects, which may allow us to pharmacologically manipulate the migratory behavior of HPCs. CXCR4-dependent transendothelial progenitor cell migration in vitro and bone marrow homing in vivo were significantly increased by FTY720. However, albeit significant, the difference in CD34+/CD38- progenitor cell homing in vivo was moderate, and did not result in statistically significant improvement of long-term engraftment. In regard to the latter, S1P and its receptors may therefore represent only one of probably several mechanisms that modulate CXCR4-dependent migration in vivo.
The most striking feature of FTY720 as an immunosuppressant is its ability to decrease the number of circulating B and T cells by indirect alteration of lymphocyte migration and homing.27,43 Recent reports have elucidated the mode of action, in that FTY720 is rapidly phosphorylated in vivo, and that FTY720-phosphate (FTY720-P) is a high-affinity agonist at G protein-coupled receptors for S1P.29,30 Expression of S1PRs has been found in different types of mammalian cells. In the human, S1PRs are expressed among others on endothelial and epithelial cells, as well as on blood lymphocytes.44-46 Sphingosine 1-phosphate (S1P) has been demonstrated to induce the chemotaxis of human natural killer cells and immature dendritic cells,36,47 and to stimulate the migratory abilitity of HUVECs.37
In murine bone marrow cultures, the migratory behavior of hematopoietic progenitors also has been shown to depend on the presence of S1P. In this system, more primitive progenitors lost their ability to migrate underneath stromal layers after withdrawal of S1P present in the serum of the culture media.32 Notably, migration of progenitors as well as of malignant cells underneath a stromal layer has been shown to depend on the SDF-1/CXCR4 system,32,48,49 which is in accordance with the finding that S1P increased the migratory capacity of murine HPCs,33 and with our results that S1P receptor agonists augmented the effects of SDF-1 in human HPCs. Interestingly, the pattern of S1PR expression was different in primitive (CD34+/CD38-) and more committed (CD34+/CD38+) progenitors. S1P2, which in contrast to other S1PRs has been shown to inhibit migration,34 was only expressed in CD34+/CD38+ cells. This could contribute to the more efficient homing of primitive CD34+/CD38- progenitors in the NOD/SCID mouse model,1 provided that S1P indeed contributes to the bone marrow homing, as suggested by our results. S1P1 and S1P5, which bind FTY720 (and FTY720-P) most avidly, were expressed particularly by primitive CD34+/CD38- progenitors, which could also explain that significant improvement of bone marrow homing was only observed in the subpopulation of CD34+/CD38- cells.
Our functional studies regarding calcium mobilization and actin polymerization clearly demonstrate that activation of S1PRs directly triggers CXCR4-dependent signaling in human HPCs. The observation that preincubation of HPCs with FTY720, but not direct stimulation of cells with the immunosuppressant (data not shown), did increase the amount of intracellular free Ca2+ in response to SDF-1 is consistent with the observation that FTY720 needs to be phosphorylated to evoke an agonistic response at S1PRs.30 Formation of filamentous actin (an early step in cellular locomotion) was markedly raised by FTY720 even when cells were stimulated with low doses of SDF-1 (10 ng/mL), underlining the observed potent effect of the S1P agonist on HPC migration. S1PRs expressed on hematopoietic progenitors therefore appear to modulate the effect of SDF-1, and continuous presence of S1P receptor ligands in the hematopoietic microenvironment might be important for SDF-1-dependent lodgement and proliferation of hematopoietic progenitors. Notably, we observed a positive effect of FTY720 on the development of cobblestone areas in long-term cultures, which is consistent with previous reports demonstrating the capability of S1P to significantly increase the proliferation rate of human cells.50-52 Taken together, these findings suggest a regulatory role of physiologic S1P receptor ligands such as the lipid mediator S1P in normal hematopoiesis.
The exact molecular events that link S1PRs with chemokine receptors (eg, CXCR4) are not exactly known. In mouse hematopoietic progenitor cells, Rac, Rho, and Cdc42 G proteins mediate the increased migratory response due to S1PR activation.33 Furthermore, PI3 kinase and the hematopoietic-specific guanyl nucleotide exchange factor Vav 1 are involved in S1PR signaling.33 On the other hand, recent findings even suggest that the mechanism by which FTY720 activates chemokine receptors might be more complex than initially thought, at least in T cells,53 and involves in addition to S1PRs also transporter proteins and other lipid mediators (eg, cysteinyl-leukotrienes). According to this study, FTY720 is rapidly phosphorylated and first activates the sphingosine transporter Abcb1 (Mdr1), which transports FTY720-P as well as S1P from the cytosol to the extracellular space where they bind to S1PRs in an autocrine fashion. Stimulated S1PRs then activate 5-lipoxygenase, the key enzyme of leukotriene synthesis, resulting in increased cysteinyl-leukotriene C4 (LTC4) production. After being transported to the extracellular space by the leukotriene C4 transporter Abcc1 (Mrp1), LTC4 also activates its receptor (cysLT1), which finally leads to increased responsiveness of chemokine receptors to their ligands. The fact that we previously found both 5-lipoxygenase and cysLT1 highly expressed in CD34+ cells21 supports the hypothesis that also in hematopoietic progenitor cells, a similar complex mechanism of indirect chemokine receptor activation by FTY720 and by endogenously produced S1P could exist.
Our results further suggest that stimulation of CXCR4 signaling by S1P receptor agonists could support bone marrow homing also in vivo. Increased SDF-1-mediated effects resulting in increased bone marrow homing have also been observed after prestimulation of HPCs with SDF-1 itself.11,54,55 Since CXCR4, S1PRs, and also cysLT1 belong to the family of G protein-coupled 7-transmembrane receptors, this observation indicates that activation of identical signal transduction elements either by S1PRs/cysLT1 or by CXCR4 itself is sufficient to subsequently increase SDF-1-mediated effects. Instead of using prestimulation with SDF-1, activation of CXCR4-mediated signaling by a pharmacologic agonist of a receptor other than CXCR4 represents an alternative approach to support HPC homing. In ongoing in vivo studies, the effect of FTY720 as an immunosuppressant in hematopoietic stem cell transplantation is being explored.56-58 Our findings encourage treatment regimens that involve administration of FTY720 closely before or during stem cell transplantation. As S1PRs only modulate CXCR4-dependent homing, it is uncertain whether administration of FTY720 can be used to significantly improve also long-term engraftment which is influenced by many different factors. In contrast to other immunosuppressants, however, it appears that FTY720 is rather beneficial than adverse for the development of hematopoiesis after bone marrow transplantation.
At high micromolar concentrations, FTY720 has been reported to induce apoptosis in several human cell lines and primary lymphocytes.40,41,59 Induction of apoptosis could therefore be the reason for our observation that colony formation by PB-derived Lin- cells is suppressed in the presence of FTY720 at doses more than or equal to one μM. However, at therapeutic concentrations (low nanomolar), proliferation of lineage-committed progenitors was not impaired. More important, the proliferation of primitive progenitors was raised in the presence of both SDF-1 and FTY720, as demonstrated in the cobblestone assays. It appears that induction of apoptosis by high concentrations of FTY720 is not mediated by S1PRs and does not reflect a physiologic effect of S1P.30
S1P can be released by several cell types including hematopoietic cells and endothelium.32,60 As cytokine stimulation is a major trigger of S1P production in, for example, endothelial cells,61 it is conceivable that the level of S1P in the bone marrow microenvironment might be related to the hematopoietic activity, which also depends on the presence of cytokines. However, regulation of S1P and related lipid mediators involves complex metabolic pathways that are only partially understood. Intracellular S1P additionally functions as second messenger, as the balance between S1P and its precursor ceramide regulates the balance between apoptosis and proliferation.60 Our results suggest that in addition to its role within the cell, in the hematopoietic system extracellular S1P also regulates migration and spatial distribution of hematopoietic progenitor and stem cells by modulating the effects of the chemokine SDF-1.
Prepublished online as Blood First Edition Paper, February 26, 2004; DOI 10.1182/blood-2003-03-0875.
Supported by grants from Deutsche Forschungsgemeinschaft (SFB 510).
V.B. is an employee of Novartis, whose potential product (FTY720) was studied in the present work.
T.K. and A.M.B. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Christine Zimmermann for excellent technical assistance, Hans-Jörg Bühring for operating the cell sorter, as well as Stefan Scheding and Max Topp for carefully reading the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal