Abstract
Erythropoietin (EPO) is required for cell survival during differentiation and for progenitor expansion during stress erythropoiesis. Although signaling pathways may couple directly to docking sites on the EPO receptor (EpoR), additional docking molecules expand the signaling platform of the receptor. We studied the roles of the docking molecules Grb2-associated binder-1 (Gab1) and Gab2 in EPO-induced signal transduction and erythropoiesis. Inhibitors of phosphatidylinositide 3-kinase and Src kinases suppressed EPO-dependent phosphorylation of Gab2. In contrast, Gab1 activation depends on recruitment and phosphorylation by the tyrosine kinase receptor RON, with which it is constitutively associated. RON activation induces the phosphorylation of Gab1, mitogen-activated protein kinase (MAPK), and protein kinase B (PKB) but not of signal transducer and activator of transcription 5 (Stat5). RON activation was sufficient to replace EPO in progenitor expansion but not in differentiation. In conclusion, we elucidated a novel mechanism specifically involved in the expansion of erythroblasts involving RON as a downstream target of the EpoR. (Blood. 2004;103:4457-4465)
Introduction
Erythropoietin (EPO) is required for the survival, proliferation, and differentiation of erythroblasts. It acts with other growth factors and hormones to balance the expansion and terminal differentiation of the erythroid compartment. For instance, stem cell factor (SCF), a ligand of c-Kit, cooperates with EPO to delay differentiation and to enhance progenitor numbers.1,2 The EPO receptor (EpoR) is a homodimer constitutively associated with Janus tyrosine kinase 2 (Jak2). Ligand binding induces a conformational change of the EpoR dimer,3,4 resulting in the activation of Jak2, the phosphorylation of the EpoR on 8 tyrosine residues, and the recruitment of multiple signaling molecules.5-7 Mice deficient in EPO, EpoR, or Jak2 lack definitive erythropoiesis.8-11 Although this demonstrates a crucial role for EPO-induced Jak2 activation in erythropoiesis, the contribution of individual downstream signaling pathways to erythroblast proliferation, differentiation, and survival is less clear. Notably, mice expressing a truncated EpoR, lacking all tyrosine residues, are not anemic.12 This may implicate that additional proteins, such as docking molecules, can form a complex with the EpoR to create a signaling platform with sufficient specificity to regulate the balance of erythroid expansion and differentiation.
Candidates for this function are the Grb2-associated binder (Gab) family members. At present, 3 Gab family members have been identified—Gab1, Gab2, and Gab3.13,14 Gab1 is ubiquitously expressed, whereas Gab2 and Gab3 expression are tissue specific. In the hematopoietic system, Gab3 is expressed in B cells and T cells and in the myeloid compartment, whereas Gab2 is expressed in all hematopoietic cells except T cells. Gab1 deficiency is embryonically lethal because of placental defects; Gab1-/-embryos are also characterized by reduced liver size and migration defects of muscle precursor cells.15,16 In contrast, Gab2 deficiency results only in mild defects in mast cell and macrophage responses,17,18 and Gab3-deficient mice show normal hematopoiesis.19 Gab2 and Gab3 lack the c-Met binding sequence (MBS) of Gab1 and, therefore, may not complement Gab1 in c-Met signaling. Furthermore, the high homology between family members suggests that complementation by the ubiquitously expressed Gab1 may occur in Gab2/3 knockouts. On tyrosine phosphorylation, Gab proteins recruit signaling intermediates, among them Shc (Src homology-containing protein), Shp2 (Src-homology domain containing phosphatase), p85 subunit of phosphatidylinositol 3-kinase (PI3K), and phospholipase C γ1/2 (PLCγ1/2).20,21 Gab1 is phosphorylated after the stimulation of the EpoR.22 However, it has not been determined whether Jak2 is directly involved in this process or whether kinases other than Jak2 could be required for Gab1 phosphorylation.
Gab1 is a direct substrate of the receptor tyrosine kinase c-Met.23 RON (Stk), a close homologue of c-Met, is expressed in erythroblasts as full length (RONfl) and short form (RONsf); RONsf lacks the extracellular domain. Notably, susceptibility for Friend virus-induced murine erythroid leukemia is fully dependent on RONsf expression.24 Friend virus induces EPO-independent erythroblast growth through a mechanism that has not been completely determined but that involves binding of the viral protein gp55 to EpoR and RONsf.25,26 RON and c-Met are highly homologous at the multiple docking site with which Gab1 interacts, suggesting a role for Gab1 in RON signaling. RON activation results in activation of PI3K and of the mitogen-activated protein kinase (MAPK) route,27,28 pathways implicated in the expansion of erythroblasts29 and downstream effectors of Gab proteins.22
We examined how Gab proteins function in EPO-induced signaling. We show that Gab2 is phosphorylated in response to EPO by a mechanism requiring a Src-like kinase and PI3K. In contrast, EPO-induced Gab1 phosphorylation occurs through a mechanism involving EPO-dependent activation of RON. Direct activation of RON, using a TrkA-RON fusion receptor, is sufficient to substitute EPO in progenitor expansion, but it cannot substitute for EPO to sustain differentiation to mature erythrocytes.
Materials and methods
Antibodies and growth factors
Rabbit antisera recognizing Jak2 or Gab1 and a mouse monoclonal antibody recognizing phospho-signal transducer and activator of transduction 5 (Stat5) (Y694-Stat5A/Y699-Stat5B; 05-495) were obtained from Upstate Biotechnology (Lake Placid, NY); rabbit antisera recognizing the mouse EpoR, RON, TrkA, or p44/p42 Erk1/2 and the antiphosphotyrosine mouse monoclonal antibody PY99 were from Santa Cruz Biotechnology (Santa Cruz, CA; sc-697, sc-322, sc-14024, sc-94, sc-7020, respectively); and rabbit antisera recognizing phospho-protein kinase B (PKB) (Ser473; 9271) and mouse monoclonal antibodies recognizing phospho-p44/p42 Erk1/2 (Thr202/Tyr204; 9101) were from Cell Signaling Technology (Beverly, MA). Rabbit antiserum recognizing Gab2 was a kind gift from Dr T. Hirano (Osaka University, Japan). Human macrophage-stimulating protein (MSP) was obtained from R&D Systems (Minneapolis, MN), recombinant human EPO was a gift from Ortho-Biotech (Tilburg, The Netherlands), dexamethasone (Dex) was purchased from Sigma (Zwijndrecht, The Netherlands), and SCF was obtained from the supernatant of producer Chinese hamster ovary (CHO) cells.
Plasmids and oligonucleotides
The complete open reading frame of RONfl (a kind gift of Dr Breathnach, Institut de Biologie-CHR, Nantes, France), Tec, Jak2, Lyn, EpoR, Gab1, and Gab2 were cloned into the mammalian expression vector pSG5 (Stratagene, La Jolla, CA) and the retroviral vector pBabe-puro when indicated. The kinase dead form of RON, K1114M, was generated using the Quickchange Site-directed Mutagenesis Kit (Stratagene) with the oligonucleotides 5′-CCA ATG TGC CAT CGA TTC ACT AAG TCG CA-3 and 5′-GCT CTC CAG CGC CGT CCA CTT CAC A-3′. To construct TrkA-RON, the extracellular domain of the TrkA receptor was expanded using polymerase chain reaction (PCR) (from TrkA-Met30 ) using forward primer 5′-GCG AAT TCA TGC TGC GAG GCG GAC GGC GC-3′ and reverse primer 5′-GCG ATA TCC TTC TTC TCC ACC GGG TCT CC-3′, after which the fragment was cut with EcoRI and EcoRV. The RON transmembrane/intracellular domain PCR product was amplified using forward primer 5′-GCG ATA TCC TCC TTG GTA TCC TGC TGC CT-3′ and reverse primer 5′-CGC TCG AGC TAA GCA GGT CCA GCC CAA GA-3′ and was digested with EcoRV and XhoI. Both fragments were simultaneously ligated into the EcoRI/SalI site of the retroviral vector pBabe-puro, creating the TrkA-RON fusion receptor (Figure 5A). The prey plasmids VP16-Gab1, VP16-Gab2, and VP16-Gab2+MBS have been described.23
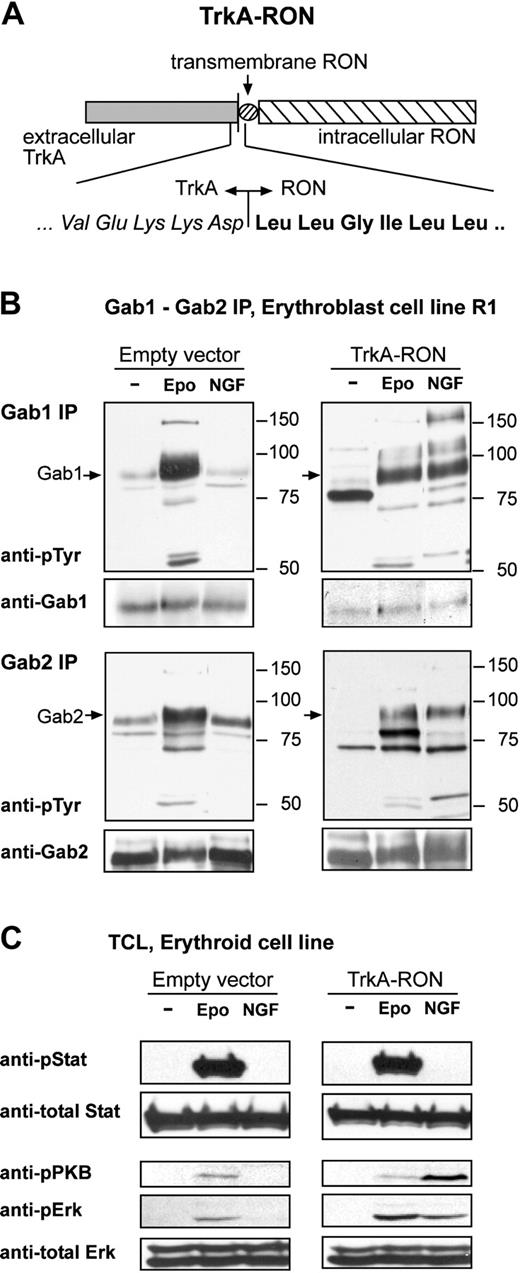
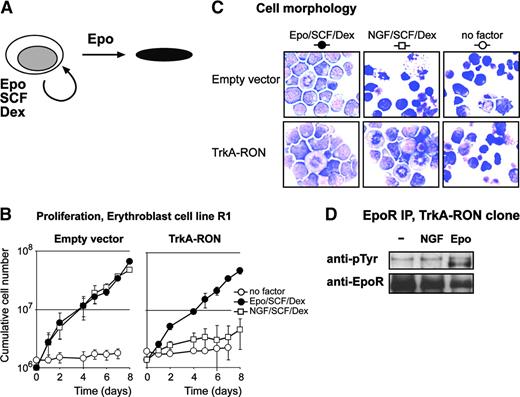
Activation of TrkA-RON induces phosphorylation of multiple downstream targets. (A) Representation of the TrkA-RON fusion protein transduced into erythroblasts. The extracellular portion of TrkA (italicized amino acids) was fused to the total transmembrane domain and intracellular tail of RON (boldface amino acids). (B-C) TrkA-RON-expressing and empty vector control cells were factor deprived and restimulated with EPO (5 U/mL; 10 minutes) or NGF (100 ng/mL; 10 minutes), as indicated. (B) Western blots containing Gab1 and Gab2 immunoprecipitates were stained with antiphosphotyrosine antibodies and antibodies against Gab1 and Gab2. Positions of Gab1 and Gab2 are indicated by arrows; positions of size markers are indicated in kDa. (C) Western blots containing whole-cell lysates were stained for Tyr694/699-phosphorylated Stat5, Ser473-phosphorylated PKB, and Thr202/Tyr204-phosphorylated ERK1/2. To control for equal loading, the blot was restained with anti-Stat5 and anti-ERK1/2.
Activation of TrkA-RON induces phosphorylation of multiple downstream targets. (A) Representation of the TrkA-RON fusion protein transduced into erythroblasts. The extracellular portion of TrkA (italicized amino acids) was fused to the total transmembrane domain and intracellular tail of RON (boldface amino acids). (B-C) TrkA-RON-expressing and empty vector control cells were factor deprived and restimulated with EPO (5 U/mL; 10 minutes) or NGF (100 ng/mL; 10 minutes), as indicated. (B) Western blots containing Gab1 and Gab2 immunoprecipitates were stained with antiphosphotyrosine antibodies and antibodies against Gab1 and Gab2. Positions of Gab1 and Gab2 are indicated by arrows; positions of size markers are indicated in kDa. (C) Western blots containing whole-cell lysates were stained for Tyr694/699-phosphorylated Stat5, Ser473-phosphorylated PKB, and Thr202/Tyr204-phosphorylated ERK1/2. To control for equal loading, the blot was restained with anti-Stat5 and anti-ERK1/2.
Yeast 2-hybrid analysis
The yeast strain L40 was used for the yeast-2-hybrid (Y2H) transformation assays.31 It contains his3 and lacZ as reporter genes. Bait plasmids BTM-RON and BTM-RONkd code for the kinase-active and kinase-inactive cytoplasmic portions of the human receptor tyrosine kinase RON, respectively. This cytoplasmic portion was fused to the LexA DNA-binding and dimerization domain. Sequences encoding amino acids 983 to 1400 of wild-type (wt) RON or RON K1114M were amplified by PCR (using 5′-GGA ATT CCG GAG GAA GCA GCT AGT TCT TCC-3′ and 5′-AAA AAA GTC GAC TCA AGT GGG CCG AGG AGG CTC-3′) and were cloned into pBMT as EcoRI/SalI fragments. The β-galactosidase assay measures lacZ reporter gene activity to quantify the interactions between bait and prey proteins. Yeast was grown in liquid culture, and optical density was measured at λ = 600 nm, pelleted, and cracked open using a freeze-thaw technique in liquid nitrogen. β-galactosidase activity was determined colorimetrically using the compound ONPG (o-nitrophenyl-β-D-galactopyranoside) as a substrate (λ = 420 nm). The following formula was used to calculate relative β-galactosidase activity: 1000 × OD 420/(t × V × OD 600), where OD indicates optical density, t indicates time (minute), and V indicates volume (mL).
Cell lines, primary cells, transfections, and viral transductions
COS, HEK293, and ecotropic Phoenix (φE) cells were maintained in Dulbecco modified Eagle medium (DMEM; Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum (FCS; Life Technologies). For COS and HEK293 transfection experiments, 1 million cells were seeded in 60-mm2 dishes and, after 3 to 4 hours (COS cells) and 24 hours (293 cells), were transfected with 12 μg DNA, as previously described.32 After 24 hours the cells were washed once with phosphate-buffered saline (PBS), and new medium was added. Cells were harvested 48 hours after transfection. Primary fetal liver erythroblasts and the murine erythroblast cell line R1 (generated as described before)29 were cultured in StemPro-34 medium (Life Technologies) supplemented with EPO (0.5 U/mL), SCF (100 ng/mL), and Dex (50 μM). Cell cultures were maintained at 1.5 to 3 × 106 cells/mL. Viral transduction of the erythroblast cell line R1 was performed as described.33 To obtain single cell-derived clones, cells were seeded in semisolid Stempro-34 medium supplemented with EPO, SCF, and Dex, as described, plus puromycin (2 μg/mL). To induce differentiation, erythroid cells were washed 2 times with PBS and were reseeded in StemPro-34 medium supplemented with 5 U/mL EPO and 0.5 mg/mL iron-saturated human transferrin (Intergen, Purchase, NY). During differentiation, cells were kept at 2 to 5 × 106 cells/mL.
Hemoglobin content determination and cell morphology
To measure the hemoglobin content in cells, 3 50-μL aliquots of the cultures were taken and processed for photometric determination of hemoglobin, as described.33 To analyze cell morphology, 50-μL samples were taken from culture, cytocentrifuged onto slides, and stained with histologic dyes and neutral benzidine for hemoglobin.34 Images were taken with a charge-coupled device (CCD) camera and were processed using Adobe Photoshop.
Immunoprecipitations and Western blotting
Primary erythroid fetal liver cells and the erythroblast cell line R1 were growth factor deprived for 4 hours in plain Iscove modified Dulbecco medium (IMDM; Life Technology), and HEK293 cells were serum deprived for 24 hours in plain DMEM. Erythroblasts (40-80 × 106/mL) were stimulated at 37°C with SCF (1 μg/mL; 5 minutes), EPO (5 U/mL; 10 minutes), MSP (100 ng/mL; 10 minutes), or NGF (100 ng/mL; 10 minutes), as indicated, and HEK293 cells and COS cells were stimulated for 10 minutes with 100 ng/mL MSP or EPO (5 U/mL). Reactions were stopped with excess ice-cold PBS. Cells were washed twice with ice-cold PBS, lysed in ice-cold lysis buffer,35 incubated on ice for 10 minutes, frozen in nitrogen, and stored at -80°C until use. Lysates were thawed on ice and cleared by centrifugation at 4°C at 15 000 rpm. Immunoprecipitation, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and Western blot analysis were performed as described previously.35 Membranes were stripped in 63 mM Tris-HCl pH 6.1, 2% SDS, and 100 mM β-mercaptoethanol for 30 minutes at 50°C, after which they were reblocked and restained.
Results
EPO-induced phosphorylation of Gab1 and Gab2 is differentially dependent on PI3K activity
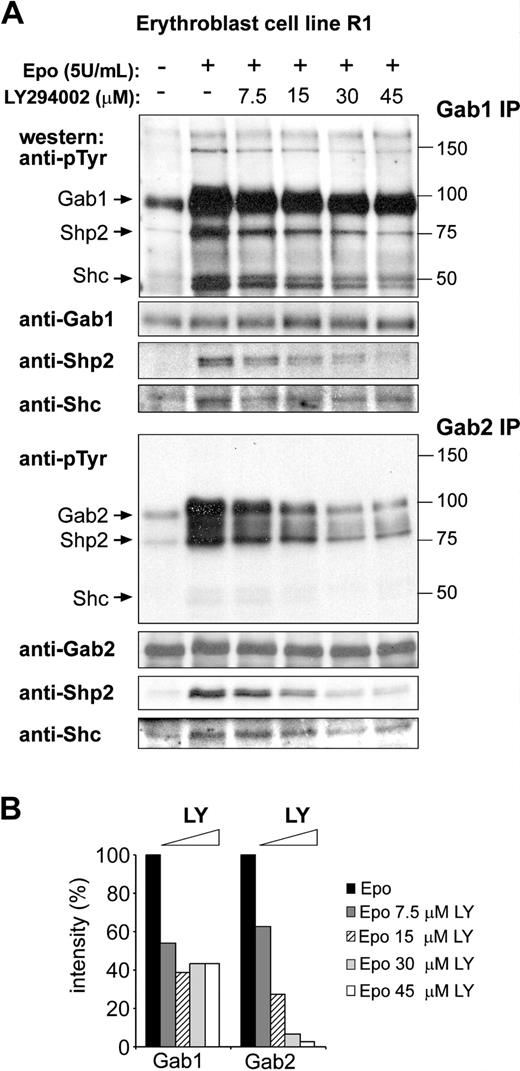
In accordance with previous observations, Gab1 and Gab2 are tyrosine phosphorylated in response to EPO in the erythroid cell line R1 (Figure 1A).20,21 Several phosphorylated proteins coimmunoprecipitated with Gab1 and Gab2. Reprobing the same blots with specific antibodies identified the adaptor protein Shc (50 kDa) and the tyrosine phosphatase Shp2 (75 kDa; Figure 1A). EPO-induced Gab1 and Gab2 phosphorylation were also detected in erythroblasts cultured from primary bone marrow and from fetal liver (data not shown). Because an N-terminal pleckstrin homology (PH) domain characterizes Gab proteins, their recruitment and phosphorylation are expected to be dependent on PI3K activity. R1 cells were factor-deprived and restimulated with EPO in the presence of increasing concentrations of the PI3K inhibitor LY294002 (7.5-45 μM; Figure 1A). Although EPO-induced Gab2 phosphorylation was dose-dependently inhibited in the presence of LY294002, Gab1 phosphorylation was, to a large extent (> 40%), resistant to inhibition by LY294002 (Figure 1A-B). Similarly, the coimmunoprecipitation of Shc with Gab2, but not with Gab1, was fully inhibited by LY294002. The level of phosphorylated Shp2 detected in Gab1 immunoprecipitates was reduced at increasing concentrations of LY294002, which might have been because of the phosphorylation of Shp2 by LY294002-sensitive kinases. Together the data suggest that alternative mechanisms for Gab1 and Gab2 phosphorylation may exist.
EPO-induced phosphorylation of Gab2, but not Gab1, is dependent on PI3K activity. (A) Erythroblast cell line R1 was factor deprived (4 hours), left untreated (lane 1), or restimulated with EPO (5 U/mL; 10 minutes) in the presence of increasing amounts of the PI3K inhibitor LY294002. Gab1 and Gab2 were immunoprecipitated from cell lysates and analyzed for tyrosine phosphorylation on Western blot. The same blots were stained with anti-Gab1 and anti-Gab2 to control equal loading. Coimmunoprecipitated Shp2 and Shc were detected by specific antibodies. Size markers (kDa) and coimmunoprecipitating proteins are indicated next to the panels. (B) Tyrosine phosphorylation of Gab1 and Gab2 in the presence of increasing concentrations of LY294002 was quantified by densitometric analysis as shown in panel A. Intensity in absence of LY294002 was set at 100%.
EPO-induced phosphorylation of Gab2, but not Gab1, is dependent on PI3K activity. (A) Erythroblast cell line R1 was factor deprived (4 hours), left untreated (lane 1), or restimulated with EPO (5 U/mL; 10 minutes) in the presence of increasing amounts of the PI3K inhibitor LY294002. Gab1 and Gab2 were immunoprecipitated from cell lysates and analyzed for tyrosine phosphorylation on Western blot. The same blots were stained with anti-Gab1 and anti-Gab2 to control equal loading. Coimmunoprecipitated Shp2 and Shc were detected by specific antibodies. Size markers (kDa) and coimmunoprecipitating proteins are indicated next to the panels. (B) Tyrosine phosphorylation of Gab1 and Gab2 in the presence of increasing concentrations of LY294002 was quantified by densitometric analysis as shown in panel A. Intensity in absence of LY294002 was set at 100%.
Gab1 is a good substrate of RON and Lyn, Gab2 of Lyn only
To investigate what kinases are involved in the phosphorylation of Gab1 and Gab2, we expressed Gab1 or Gab2 in COS cells with various kinases involved in EpoR and c-Kit signaling, among them Jak2, Tec, Btk, Lyn, RONfl, and RONsf. Expression of the various kinases was checked on a phosphotyrosine Western blot (Figure 2A). Next, Gab1 and Gab2 were immunoprecipitated and analyzed for phosphorylation (oFigure 2B-C). Expression of the Src kinase family member Lyn resulted in the phosphorylation of Gab1 and Gab2. In addition, Gab1, but not Gab2, appeared to be a good substrate for RON. Moreover, RONfl and RONsf coimmunoprecipitated with Gab1, but not with Gab2. Jak2, Tec, and Btk did not efficiently phosphorylate Gab1 or Gab2, though the absence of Gab phosphorylation by Tec might have been the result of its low autophosphorylation signal (Figure 2A). To investigate whether Src kinases are required for Gab1/2 phosphorylation in erythroblasts, we analyzed EPO-induced phosphorylation of Gab1 and Gab2 in immortal (R1) and primary erythroblasts (cultured for 5 days from fetal livers) preincubated with the Src kinase inhibitor PP2 (5 μM). EPO-induced Gab1 phosphorylation appeared resistant to the Src kinase inhibitor PP2, whereas EPO-induced Gab2 phosphorylation and coimmunoprecipitation of Gab2-associated proteins were completely inhibited (Figure 2D-E).
Distinct regulation of Gab1 and Gab2 tyrosine phosphorylation. (A-C) COS cells were transfected with pSG5-based expression constructs encoding Gab1, Gab2, and various kinases, as indicated. After 48 hours, cells were harvested. (A) Phosphorylation status of transfected kinases was detected on Western blots using antiphosphotyrosine antibodies (PY99). Similarly, Gab1 (B) and Gab2 (C) immunoprecipitates were analyzed for tyrosine phosphorylation. Arrows indicate the positions of Gab1, Gab2, RONsf, and RONfl. Size markers are indicated in kDa. Blots were restained with anti-Gab1 or anti-Gab2, as indicated. (D-E) R1 erythroblasts (D) and fetal liver-derived erythroblasts (E) were factor deprived for 4 hours in the absence or presence of the Src kinase family inhibitor PP2 (5 μM), as indicated. Cells were left unstimulated or were stimulated with EPO (5 U/mL; 10 minutes). EPO-induced Gab1 or Gab2 phosphorylation was assayed on immunoblots using PY99, and equal loading was controlled by staining with anti-Gab1 or anti-Gab2. Size markers are indicated in kDa. (F) Gab 1 associated directly with RON in Y2H assays. Gab proteins were coexpressed with RON, RONkd, and Grb2 in the yeast reporter strain L40. Gab2+MBS encodes Gab2 protein with an insertion of MBS. Associations were quantified by β-galactosidase liquid assay.
Distinct regulation of Gab1 and Gab2 tyrosine phosphorylation. (A-C) COS cells were transfected with pSG5-based expression constructs encoding Gab1, Gab2, and various kinases, as indicated. After 48 hours, cells were harvested. (A) Phosphorylation status of transfected kinases was detected on Western blots using antiphosphotyrosine antibodies (PY99). Similarly, Gab1 (B) and Gab2 (C) immunoprecipitates were analyzed for tyrosine phosphorylation. Arrows indicate the positions of Gab1, Gab2, RONsf, and RONfl. Size markers are indicated in kDa. Blots were restained with anti-Gab1 or anti-Gab2, as indicated. (D-E) R1 erythroblasts (D) and fetal liver-derived erythroblasts (E) were factor deprived for 4 hours in the absence or presence of the Src kinase family inhibitor PP2 (5 μM), as indicated. Cells were left unstimulated or were stimulated with EPO (5 U/mL; 10 minutes). EPO-induced Gab1 or Gab2 phosphorylation was assayed on immunoblots using PY99, and equal loading was controlled by staining with anti-Gab1 or anti-Gab2. Size markers are indicated in kDa. (F) Gab 1 associated directly with RON in Y2H assays. Gab proteins were coexpressed with RON, RONkd, and Grb2 in the yeast reporter strain L40. Gab2+MBS encodes Gab2 protein with an insertion of MBS. Associations were quantified by β-galactosidase liquid assay.
The insensitivity of EPO-induced Gab1 phosphorylation to inhibitors of Src kinases and PI3K may result from the observed association of Gab1 and RON and the phosphorylation of Gab1 by RON. To investigate whether Gab1 can interact with RON as it does with the RON family member c-MET, we carried out Y2H interaction experiments.23 The cytoplasmic domain of kinase-active RON or kinase-dead RON receptor (RONkd; K1114M) was expressed as bait and full-size Gab1 or Gab2 as prey vector. Gab1, but not Gab2, associated efficiently with activated RON, whereas both Gab1 and Gab2 bound the known interaction partner Grb2 (Figure 2F). The association with RON in yeast was phosphorylation-dependent; we did not detect the binding with kinase-dead bait. A 13-amino acid MBS is responsible for the direct association of Gab1 with the c-Met receptor.23 A Gab2 construct in which the MBS of Gab1 is inserted interacted efficiently with RON, indicating that the direct association of Gab1 with activated RON is mediated through the MBS of Gab1. In conclusion, RON is able to phosphorylate Gab1, whereas Src-like kinases are able to phosphorylate Gab1 and Gab2. The ability of RON to recruit and phosphorylate Gab1 may be responsible for PI3K and Src family-independent phosphorylation of Gab1 in erythroblasts.
EPO induces tyrosine phosphorylation of RON
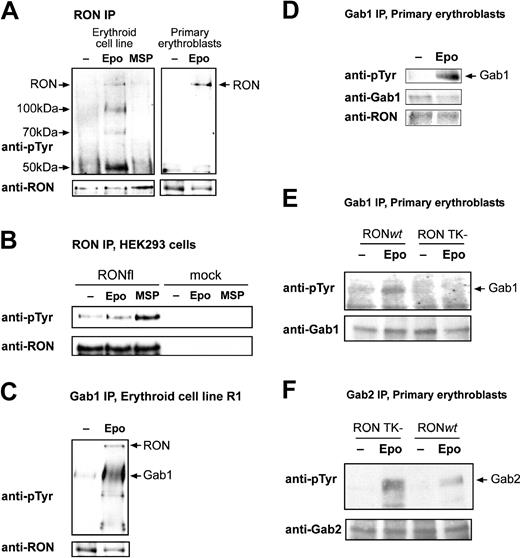
If RON mediates EPO-induced Gab1 phosphorylation, it is expected to be a downstream target of EPO signaling. The natural ligand for RON is MSP, though ligand-independent phosphorylation and activation have also been described (see “Discussion”). To investigate factor-dependent RON tyrosine phosphorylation, R1 erythroblasts were factor-deprived and stimulated with MSP or EPO. RON immunoprecipitates were assayed for tyrosine-phosphorylated proteins. MSP failed to induce tyrosine phosphorylation of RON (Figure 3A). In contrast, RON was tyrosine phosphorylated in response to EPO. Tyrosine-phosphorylated proteins of 100 kDa, 70 kDa, and 50 kDa were detected in the RON immunoprecipitates from EPO-stimulated cells. The lack of response to MSP was not attributed to an inactive batch of MSP because MSP induced the phosphorylation of transiently expressed RONfl in HEK293 cells (Figure 3B). Note that EPO did not induce RON phosphorylation in cells lacking endogenous EpoR.
Gab1 is an interaction partner and a substrate of RON in erythroblasts. (A) Immortalized (R1) and primary fetal liver-derived erythroblasts were factor deprived and subsequently stimulated with EPO (5 U/mL; 10 minutes) or MSP (100 ng/mL; 10 minutes). Anti-RON immunoprecipitates were analyzed for proteins phosphorylated on tyrosine using monoclonal PY99. Blots were stained with anti-RON to control phosphorylated protein comigration with RON and to control loading. Arrows indicate the position of RON and the position plus molecular weight of phosphorylated coimmunoprecipitated proteins. Anti-RON did not stain a 150-kDa protein in immunoprecipitates obtained with unrelated antibodies (eg, anti-Btk; data not shown), indicating that the 150-kDa band is specific for the anti-RON immunoprecipitate. (B) HEK293 cells were mock transfected or transfected with pSG5-RONfl, serum deprived, and stimulated with EPO (5 U/mL; 10 minutes) or MSP (100 ng/mL; 10 minutes). Anti-RON immunoprecipitates were tested for RON phosphorylation, as described for panel A. (C-D) Erythroblast cell line R1 (C) and fetal liver-derived erythroblasts (D) were factor deprived and restimulated with EPO (5 U/mL; 10 minutes). Gab1 was immunoprecipitated, and blots were stained with antiphosphotyrosine antibodies (PY99) and antibodies against Gab1 and RON, as indicated. (E-F) Fetal liver-derived erythroblasts derived from RON TK-/-embryos and wt littermates were factor deprived and restimulated with EPO (5 U/mL; 10 minutes). Western blots containing Gab1 and Gab2 immunoprecipitates were stained with antiphosphotyrosine and with anti-Gab1 or anti-Gab2 to check equal loading.
Gab1 is an interaction partner and a substrate of RON in erythroblasts. (A) Immortalized (R1) and primary fetal liver-derived erythroblasts were factor deprived and subsequently stimulated with EPO (5 U/mL; 10 minutes) or MSP (100 ng/mL; 10 minutes). Anti-RON immunoprecipitates were analyzed for proteins phosphorylated on tyrosine using monoclonal PY99. Blots were stained with anti-RON to control phosphorylated protein comigration with RON and to control loading. Arrows indicate the position of RON and the position plus molecular weight of phosphorylated coimmunoprecipitated proteins. Anti-RON did not stain a 150-kDa protein in immunoprecipitates obtained with unrelated antibodies (eg, anti-Btk; data not shown), indicating that the 150-kDa band is specific for the anti-RON immunoprecipitate. (B) HEK293 cells were mock transfected or transfected with pSG5-RONfl, serum deprived, and stimulated with EPO (5 U/mL; 10 minutes) or MSP (100 ng/mL; 10 minutes). Anti-RON immunoprecipitates were tested for RON phosphorylation, as described for panel A. (C-D) Erythroblast cell line R1 (C) and fetal liver-derived erythroblasts (D) were factor deprived and restimulated with EPO (5 U/mL; 10 minutes). Gab1 was immunoprecipitated, and blots were stained with antiphosphotyrosine antibodies (PY99) and antibodies against Gab1 and RON, as indicated. (E-F) Fetal liver-derived erythroblasts derived from RON TK-/-embryos and wt littermates were factor deprived and restimulated with EPO (5 U/mL; 10 minutes). Western blots containing Gab1 and Gab2 immunoprecipitates were stained with antiphosphotyrosine and with anti-Gab1 or anti-Gab2 to check equal loading.
To analyze factor-dependent recruitment of Gab1 to RON in erythroblasts, Gab1 was immunoprecipitated from EPO-stimulated and nonstimulated lysates of primary and immortalized (R1) erythroblasts (Figure 3C-D). In contrast to the data obtained by Y2H, RON coimmunoprecipitated with Gab1 in all conditions tested (see “Discussion”). We were unable to detect Gab1 in RON immunoprecipitates because a strong background band interfered with specific detection of Gab1 (data not shown). To check whether Gab1 is dependent on RON for its phosphorylation, we analyzed EPO-dependent Gab1 and Gab2 phosphorylation in erythroblasts expanded from embryonic day 13.5 (E13.5) fetal livers of mice in which the intracellular portion of RON, including the kinase domain and Gab1-docking domain, was deleted (RON TK-/-).36 Although Gab1 is phosphorylated in response to EPO in wt erythroblasts, it is not phosphorylated in RON TK-/- cells, despite the fact that Gab1 expression in RON TK-/- cells is comparable to that in wt cells (Figure 3E). In contrast, EPO-dependent phosphorylation of Gab2 is increased in RON TK-/- erythroblasts compared with wt cells (Figure 3F). In conclusion, the tyrosine kinase receptor RON is a target of EpoR signaling and is the exclusive kinase required for the recruitment and phosphorylation of Gab1.
RON is phosphorylated by Jak2 and associates with the EpoR
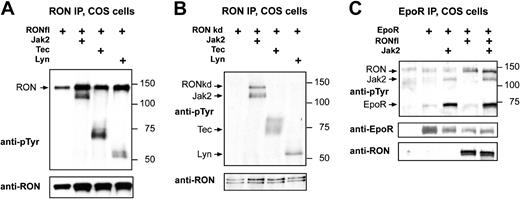
The EpoR activates several tyrosine kinases, including Jak2 and Lyn.37,38 To identify kinases with the potential to tyrosine phosphorylate RON, we expressed RONkd in COS cells, together with the tyrosine kinases Lyn, Tec, and Jak2. Lyn, Tec, and Jak2 all coimmunoprecipitated with wt RONfl (Figure 4A), and RON-specific antibodies did not immunoprecipitate these kinases from COS cells when RON was not coexpressed (data not shown). Kinase-dead RONfl was only phosphorylated in the presence of Jak2 (Figure 4B). Taking all other experiments into account, this suggests, but does not necessarily imply, that Jak2 also activates RON.
RON forms a complex with the EpoR and is a substrate of Jak2. (A-B) COS cells were transfected with pSG5-based expression constructs encoding RONwt (A) or RONkd (B), together with Lyn, Tec, and Jak2. Cells were harvested without factor stimulation. (C) COS cells were transfected with RONwt, together with EpoR and Jak2, and were stimulated with EPO (5 U/mL) 10 minutes before cell harvest. (A-C) Two days after transfection, COS cells were lysed, and RON (A-B) or EpoR (C) was immunoprecipitated. Immunoprecipitated proteins were subjected to SDS-PAGE and immunoblotting. Size markers are indicated in kDa. Upper panels represent blots stained with antiphosphotyrosine antibodies (PY99), and lower panels represent blots stained with anti-RON, anti-EpoR, or both, when appropriate. Arrows indicate the positions of the expressed proteins.
RON forms a complex with the EpoR and is a substrate of Jak2. (A-B) COS cells were transfected with pSG5-based expression constructs encoding RONwt (A) or RONkd (B), together with Lyn, Tec, and Jak2. Cells were harvested without factor stimulation. (C) COS cells were transfected with RONwt, together with EpoR and Jak2, and were stimulated with EPO (5 U/mL) 10 minutes before cell harvest. (A-C) Two days after transfection, COS cells were lysed, and RON (A-B) or EpoR (C) was immunoprecipitated. Immunoprecipitated proteins were subjected to SDS-PAGE and immunoblotting. Size markers are indicated in kDa. Upper panels represent blots stained with antiphosphotyrosine antibodies (PY99), and lower panels represent blots stained with anti-RON, anti-EpoR, or both, when appropriate. Arrows indicate the positions of the expressed proteins.
It has been shown that RONsf associates with the EpoR, but no data were published for RONfl.39 Therefore, EpoR, RONfl, and Jak2 were expressed in COS cells, which were stimulated with EPO before harvesting. Both Jak2 and RON coimmunoprecipitated with EpoR in the presence and the absence of EPO stimulation (Figure 4C; data not shown). However, Jak2, but not RON, induced EPO-dependent EpoR phosphorylation. Coexpression of RON and Jak2 did not affect the phosphorylation of EpoR by Jak2 or the association of RON and Jak2 with the EpoR.
Activation of RON results in phosphorylation of Gab1, PKB, and Erk1/2, but not Stat5
We next examined the role of RON and Gab1 in the expansion, differentiation, and survival of erythroblasts. Given that RON was not activated by MSP in erythroid cells, we constructed a nerve growth factor (NGF)-responsive TrkA-RON fusion receptor (Figure 5A) that was expressed in immortalized erythroblasts (R1). TrkA-RON was equally well expressed in a large number of clones, and NGF stimulation resulted in the phosphorylation of TrkA-RON (data not shown).
Although Gab1, but not Gab2, was a substrate of RON in COS cells, NGF-induced activation of TrkA-RON in R1 cells resulted in Gab1 and Gab2 tyrosine phosphorylation. NGF did not induce Gab1/2 phosphorylation in control clones, but EPO induced Gab1 and Gab2 phosphorylation in all cultures (Figure 5B). This implies that, in the natural context of erythroblasts, Gab1 and Gab2 are downstream targets of RON signaling. Because RON was able to associate with Lyn (Figure 4A-B) and because the inhibition of Src kinases abrogated EPO-induced Gab2 phosphorylation in erythroblasts (Figure 2D-E), it is possible that RON-mediated Gab2 phosphorylation occurs through Lyn. The RON homologue c-Met was similarly shown to associate with Lyn on activation by NGF.40 Both NGF and EPO induced the phosphorylation and association of 120- and 150-kDa unknown proteins with Gab1 in TrkA-RON-expressing cells (Figure 5B). Finally, we tested whether the activation of TrkA-RON induced some of the major signaling pathways activated by EpoR. NGF, like EPO, induced the phosphorylation of Erk1/2 and PKB. In contrast, NGF failed to induce Stat5 (Figure 5C) or Stat3 tyrosine phosphorylation (data not shown).
RON activation can substitute EPO in inducing progenitor expansion
EPO, SCF, and Dex are required for erythroblast expansion, including the erythroblast cell line R1, whereas EPO induces differentiation into mature erythrocytes in the absence of SCF and Dex (Figure 6A). Erythroblast exposure to SCF and Dex in the absence of EPO cannot sustain renewal divisions.29 To examine the role of RON signaling in EPO-dependent expansion and differentiation of erythroblasts, TrkA-RON-expressing erythroblasts and control erythroblasts were seeded in medium supplemented with EPO, SCF, and Dex, in medium supplemented with NGF, SCF, and Dex, or in medium without factors. Vector-transduced control clones cultured in the presence of NGF, SCF, and Dex did not proliferate but became pyknotic, indicating that NGF does not exert any biologic effect on control erythroblasts (Figure 6B-C). In contrast, TrkA-RON-expressing erythroblasts proliferated equally well in the presence of NGF, SCF, and Dex and in the presence of EPO, SCF, and Dex (Figure 6B), and they retained a blast morphology under each condition (Figure 6C). Both the TrkARON and the empty vector clones are still factor dependent; the absence of factors resulted in no growth and in rapid cell death (Figure 6B-C). This showed that RON activation can replace the ligand-activated EpoR in its function to enhance the expansion of erythroblasts. Although we showed that RON can associate with EpoR (Figure 4), the EPO-independent proliferation of TrkA-RON-expressing progenitors is not caused by NGF-induced phosphorylation of EpoR (Figure 6D).
TrkA-RON activation can replace EPO in erythroblast expansion. (A) Erythroblasts undergo renewal division in the presence of EPO, SCF, and Dex, but they differentiate to mature erythroblasts in the presence of EPO. (B) Empty vector (EV) and TrkA-RON-transduced erythroblasts were seeded in medium containing no factors (○) or SCF and Dex supplemented with either EPO (2 U/mL; •) or NGF (10 ng/mL; □). Cells were counted daily, and cell densities were maintained between 1.5 and 3 × 106 cells/mL. Cumulative cell numbers were calculated. Error bars indicate SD of 3 independent experiments. (C) Cell morphology in the cultures described for panel B was examined on day 6. Cytospins were stained with histologic dyes and neutral benzidine to stain hemoglobin and were photographed with 60× magnification. Both cultures contained proliferating blasts in the presence of EPO/SCF/Dex. In the presence of NGF/SCF/Dex, control cultures (empty vector) contained pyknotic cells, whereas TrkA-RON-expressing cultures contained proliferating blasts. In the absence of factors, both cultures contained pyknotic cells. (D) TrkA-RON-expressing cells were factor deprived (-) and stimulated with NGF (100 ng/mL) or EPO (5 U/mL). EpoR was immunoprecipitated from cell lysates and was analyzed on Western blot with antiphosphotyrosine antibodies or specific antibodies to check for equal loading.
TrkA-RON activation can replace EPO in erythroblast expansion. (A) Erythroblasts undergo renewal division in the presence of EPO, SCF, and Dex, but they differentiate to mature erythroblasts in the presence of EPO. (B) Empty vector (EV) and TrkA-RON-transduced erythroblasts were seeded in medium containing no factors (○) or SCF and Dex supplemented with either EPO (2 U/mL; •) or NGF (10 ng/mL; □). Cells were counted daily, and cell densities were maintained between 1.5 and 3 × 106 cells/mL. Cumulative cell numbers were calculated. Error bars indicate SD of 3 independent experiments. (C) Cell morphology in the cultures described for panel B was examined on day 6. Cytospins were stained with histologic dyes and neutral benzidine to stain hemoglobin and were photographed with 60× magnification. Both cultures contained proliferating blasts in the presence of EPO/SCF/Dex. In the presence of NGF/SCF/Dex, control cultures (empty vector) contained pyknotic cells, whereas TrkA-RON-expressing cultures contained proliferating blasts. In the absence of factors, both cultures contained pyknotic cells. (D) TrkA-RON-expressing cells were factor deprived (-) and stimulated with NGF (100 ng/mL) or EPO (5 U/mL). EpoR was immunoprecipitated from cell lysates and was analyzed on Western blot with antiphosphotyrosine antibodies or specific antibodies to check for equal loading.
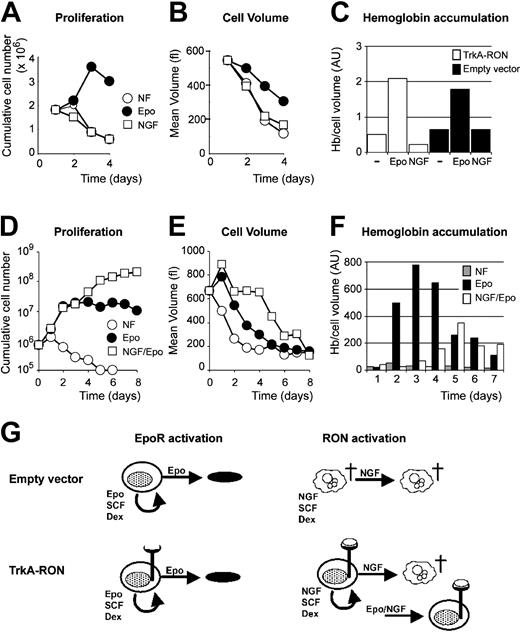
RON activation delays EPO-induced differentiation
We next examined whether RON activation is able to support erythroid differentiation. TrkA-RON-expressing and control clones were seeded in differentiation medium without factors, supplemented with EPO (2 U/mL) or with NGF (100 ng/mL). Cumulative cell number, cell size, hemoglobin accumulation, and cell morphology were determined at regular intervals. Although EPO induced transient proliferation, gradual decreases in cell size, and accumulations of hemoglobin, NGF completely failed to do any of these either in the control or in the TrkA-RON-expressing cells (Figure 7A-C). TrkA-RON-expressing cells rapidly died in the presence and in the absence of NGF (data not shown). Thus, RON activation could not replace EPO in differentiation. Given that TrkA-RON activation can replace EPO during self-renewal, we examined how the proliferation signals from TrkA-RON compared with EPO-induced differentiation signals. We cultured TrkA-RON clones with EPO or with EPO and NGF and assayed proliferation, cell size, and hemoglobinization. Exposing TrkA-RON-expressing erythroblasts to NGF and EPO caused prolonged proliferation, delayed hemoglobinization, and sustained blastoid cell size during the first 4 days of culture (Figure 7D-F). The data indicate that signals from TrkA-RON dominantly promoted erythroid renewal division but failed to sustain erythroblast differentiation (Figure 7G). Finally, we examined whether the tyrosine kinase activity of RON and the activation of Gab1 are essential for the expansion of erythroblasts. Erythroid progenitors were derived from E13.5 fetal livers of RON TK-/-36 and Gab1-deficient16 mouse embryos and control littermates and were seeded in medium supplemented with EPO, SCF, and Dex. All cultures expanded with similar kinetics for 14 days, as previously shown for primary cells.29,41 Progenitors cultured for 5 days from RON TK-/- livers and wt littermates, or from Gab-deficient livers and wt littermates, were seeded in differentiation conditions (5 U/mL EPO; no SCF or Dex), and cell number, cell size, and hemoglobin accumulation were determined at 8-hour intervals. In all cultures, cells were enucleated in 48 hours, as previously described,41 and no differences in cell proliferation, cell size, or hemoglobin accumulation were detected (data not shown). Thus, RON and Gab1 activation seemed not to be crucial, which may be explained by the increased EPO-induced Gab2 phosphorylation observed in these cells (Figure 3F and data not shown).
Activation of TrkA-RON fails to support erythroid differentiation and delays differentiation in the presence of EPO. (A-B) TrkA-RON-expressing erythroblasts were cultured in medium containing iron-loaded transferrin without additional factor (NF; ○), supplemented with EPO (2 U/mL; •) or with NGF (10 ng/mL; □). Total cell numbers (A) and mean cell volume (B; fl) are depicted. (C) On day 4 of the experiment, hemoglobin content was measured (Hb/cell volume in arbitrary units) in empty vector-transduced cultures and TrkA-RON-expressing cultures seeded in the absence of factor (-) or in the presence of EPO or NGF. (D-F) TrkA-RON-expressing erythroblasts were seeded in differentiation medium lacking growth factor (NF; ○) or in medium supplemented with EPO (2 U/mL; •) or EPO (2 U/mL) plus NGF (10 ng/mL; □). Cumulative cell number (D), cell volume (E; fl), and hemoglobin accumulation (F; Hb/cell volume, in arbitrary units) were determined at daily intervals. (G) Schematic representation of the results. Empty vector-transduced cultures and TrkA-RON-expressing cultures could be expanded in the presence of EPO/SCF/Dex and differentiated in the presence of EPO. When NGF was substituted for EPO, TrkA-RON-expressing cultures were expanded, whereas control cells became pyknotic. However, NGF failed to support the differentiation of TrkA-RON-expressing erythroblasts. In combination with EPO, NGF delayed differentiation of the TrkA-RON-expressing erythroblasts.
Activation of TrkA-RON fails to support erythroid differentiation and delays differentiation in the presence of EPO. (A-B) TrkA-RON-expressing erythroblasts were cultured in medium containing iron-loaded transferrin without additional factor (NF; ○), supplemented with EPO (2 U/mL; •) or with NGF (10 ng/mL; □). Total cell numbers (A) and mean cell volume (B; fl) are depicted. (C) On day 4 of the experiment, hemoglobin content was measured (Hb/cell volume in arbitrary units) in empty vector-transduced cultures and TrkA-RON-expressing cultures seeded in the absence of factor (-) or in the presence of EPO or NGF. (D-F) TrkA-RON-expressing erythroblasts were seeded in differentiation medium lacking growth factor (NF; ○) or in medium supplemented with EPO (2 U/mL; •) or EPO (2 U/mL) plus NGF (10 ng/mL; □). Cumulative cell number (D), cell volume (E; fl), and hemoglobin accumulation (F; Hb/cell volume, in arbitrary units) were determined at daily intervals. (G) Schematic representation of the results. Empty vector-transduced cultures and TrkA-RON-expressing cultures could be expanded in the presence of EPO/SCF/Dex and differentiated in the presence of EPO. When NGF was substituted for EPO, TrkA-RON-expressing cultures were expanded, whereas control cells became pyknotic. However, NGF failed to support the differentiation of TrkA-RON-expressing erythroblasts. In combination with EPO, NGF delayed differentiation of the TrkA-RON-expressing erythroblasts.
Discussion
In the present study, we identified Gab1 and Gab2 as important players in EPO-induced progenitor-expansion signaling, each recruited and phosphorylated by a distinct mechanism. Recruitment and phosphorylation of Gab2 were dependent on the activation of PI3K and Src-like kinase, respectively, whereas Gab1 phosphorylation was fully dependent on the tyrosine kinase receptor RON, which appeared to be a specific target of EpoR signaling.
Gab docking proteins are activated by distinct mechanisms
As can be expected from proteins carrying a PH domain, Gab2 is recruited to the phosphatidylinositol-3,4,5-triphosphate (PIP3)-rich pockets in the membrane around the activated receptor. Gab2 can interact with Grb2, which in turn associates with tyrosine residue Y464 of the EpoR.42 However, the requirement for Grb2 to recruit Gab2 to the EpoR has not been analyzed. Possibly this interaction facilitates the localization of Gab2 close to Lyn (reported to associate with tyrosine residues 464 and 479 of the EpoR43 ). The observation that the Src kinase inhibitor PP2 suppresses Gab2 phosphorylation and the reported role for Lyn in EpoR signaling44,45 make Lyn a likely kinase responsible for Gab2 phosphorylation. In contrast, Gab1 phosphorylation was only partially affected by inhibitors of PI3K or Src kinase, and Gab1 appeared to be recruited by the tyrosine kinase receptor RON. The observation that EPO-induced Gab1 phosphorylation was absent in erythroblasts expressing RON lacking the intracellular portion indicated that RON is absolutely required for EPO-induced Gab1 tyrosine phosphorylation. The partial dependence of Gab1 phosphorylation on PI3K activity suggests that recruitment of Gab1 to RON can be facilitated by the interaction between the PH domain of Gab1 with local PIP3. Alternatively, RON may phosphorylate only part of the Gab1 tyrosines, whereas other tyrosine residues may be phosphorylated by PI3K-dependent kinases such as Tec and Btk. We did observe some discrepancies in the interaction between RON and Gab molecules in erythroblasts and in overexpression systems. First, the interaction between RON and Gab1 required RON kinase activity in Y2H experiments, but the interaction appeared constitutive in erythroblasts. Second, Gab2 was no substrate for RON on overexpression in COS cells, whereas Gab2 was phosphorylated in response to TrkA-RON activation. To us, these data represent the intricacy of the EpoR-signaling complex. RON can interact with Lyn (Figure 4A). In erythroblasts Lyn is abundantly expressed and present in the EpoR complex. Apparently, Gab2 can be phosphorylated by Lyn, associated with either the EpoR or RON. RON homologue c-MET also associates with Src kinases, and its activation similarly induces the phosphorylation of Gab1 and Gab2.46 In addition, the interaction between RON and Gab1 may not only depend on interactions between phosphorylated tyrosine residues with the MBS and Grb2, it may be stabilized by additional scaffolding proteins.
The question arises whether the distinct regulation of EPO-induced Gab1 and Gab2 is associated with a specific function. Gab1-deficient mice die at days 14 to 16 of development because of placental defects.16 At this time the liver is smaller than wt livers. No obvious erythroid defect can be detected in Gab1-/-mice, but the liver defects exclude in vivo assessment of normal adult and stress erythropoiesis. We observed no difference in the in vitro expansion and differentiation capacity of erythroblasts derived from E13 Gab1-/-fetal livers or in erythroblasts from RON kinase-deficient mice that did not phosphorylate Gab1 (data not shown). However, Gab1-/- and RON TK-/-erythroblasts did show enhanced EPO-induced tyrosine phosphorylation of Gab2. In all other cells, Gab proteins can complement each other, resulting in healthy mice lacking Gab2 and Gab3. Thus, the EpoR appears to have 2 mechanisms that ensure phosphorylation of either Gab1 or Gab2 and that may be tuned to ascertain a certain level of signaling activity.
RON is part of the EpoR signaling complex
Although RON can be activated through its ligand (MSP), increasing evidence suggests that RON can also be activated by MSP-independent mechanisms through heterodimerization. RON has been shown to interact with various receptors, such as c-Met, integrins, and the common β chain of the interleukin-5 (IL-5), IL-3, and granulocyte macrophage-colony-stimulating factor (GM-CSF) receptor, leading to cross-activation.24,25,47-49 Furthermore, hyaluronoglucosaminidase-2 (HYAL2) was found to be a functional inhibitor of RON. HYAL2 sequestering by the viral envelope protein of Jaagsiekte sheep retrovirus results in constitutive RON activity in the absence of ligand.50 In this study, we observed that MSP did not induce RONfl phosphorylation or downstream signaling in erythroblasts but that EPO stimulation efficiently induced RON phosphorylation in the same cells. This occurred in 2 independent cell lines derived from distinct genetic backgrounds and in erythroblasts expanded from wt fetal livers of either CBA or 129P mice (data not shown). Thus, ligand binding is apparently not a prerequisite for RON activation in erythroblasts. In addition, on the overexpression of RONfl in the erythroblast cell line R1, tyrosine phosphorylation of RON was induced by EPO but not by MSP (data not shown), suggesting that an inhibitory mechanism might have been active in these cells, as previously described for epithelial cells.50
Our data show that Jak2 can phosphorylate RON in COS cells. The dependence of Gab1 phosphorylation on EPO stimulation in erythroid cells and on RON kinase activity in COS and erythroid cells suggests that EPO-induced Jak2 activation not only leads to phosphorylation of RON but also to its activation. Furthermore, the data suggest that the EpoR, Jak2, and RON are part of a single complex because RON is a substrate of Jak2. Although mice deficient in EPO, EpoR, and the associated kinase Jak2 die at day 13 of gestation because of a lack of erythrocytes, mice in which the EpoR lacks all tyrosine-docking sites in the EpoR C-terminal tail develop normally without major aberrations in erythropoiesis.12 Previously it was suggested that signaling intermediates such as Shc, Shp2, and PI3K could dock to Jak2 on deletion of the C-terminal tail of EpoR containing the 8 phosphorylated tyrosine residues. However, because EPO-induced activation of Jak2 results in RON activation, which subsequently results in the phosphorylation of Gab1 (directly) and Gab2 (through Src kinases), these docking molecules may be responsible for recruiting several signaling intermediates such as Shc, Ship2, and PI3K, independently of an intact EpoR C-terminus.
RON is involved in renewal induction
The ability of ligand-activated TrkA-RON to replace EPO stimulation in progenitor expansion, but not differentiation, is in line with the role of the truncated avian homologue v-SEA, which also induces the expansion, but not the differentiation, of erythroblasts.51 However, though v-SEA expression is sufficient to induce renewal division in avian erythroblasts, signals emanating from activated RON in mouse erythroblasts necessitate cooperative signaling by c-Kit and the glucocorticoid receptor to induce expansion. Because RON is a downstream target of EpoR signaling, the mechanism of cooperation between NGF, SCF, and Dex will most likely be similar to cooperative signaling between EPO, SCF, and Dex, but this remains to be elucidated. TrkA-RON-expressing erythroblasts exposed to NGF alone rapidly die, indicating that RON signal transduction through Gab1/2, PI3K, PKB, and the MAPK route is insufficient for the survival of these cells. Exogenous (over)expression of a kinase always warrants a careful interpretation of the results because its regulation may not be subject to normal controls. However, the fact that activation of TrkA-RON alone is insufficient for the survival or proliferation of erythroblasts, but requires cooperation with SCF, suggests that its function closely mimics that of endogenous RON.
Although RON activation can substitute EpoR activation to maintain renewal divisions in cooperation with SCF and Dex, it also transiently sustains renewal division under differentiation conditions in the presence of EPO. Thus, the EpoR apparently activates renewal- and differentiation-specific signaling pathways, which need careful balancing for maintenance of the proper number of red blood cells. When this balance is disrupted by, for example, increased RON activity, a polycythemic phenotype may arise (it occurs in SFFV-infected mice). As shown, EPO induces several alternative routes to activate common targets, such as PI3K and MAPK. Thus far it is unclear how these complementary pathways contribute to EpoR signaling and how cell fate is affected by change in the balance of these integrated cascades.
Our data are consistent with the observation that RONsf is required for SFFV to induce erythroleukemia in mice. The gp55 protein targets RONsf,39 resulting in constitutive phosphorylation and activation of RON and EPO-independent expansion of progenitors. As do the TrkA-RON clones, SFFV-induced erythroleukemia still requires SCF for renewal induction52 and it permits EPO-induced differentiation to functional erythrocytes. One of the major EPO-induced signaling molecules not activated by RON is Stat5. The inability of ligand-activated TrkA-RON to support in vitro terminal differentiation of erythroblasts may be attributed to a lack of Stat5 phosphorylation. Stat5-deficient erythroblasts, cultured in vitro in serum-free medium, are impaired in EPO-induced differentiation because of the inability to activate the antiapoptotic protein Bcl-XL.41 Accordingly, mice deficient in Stat5 are impaired in their response to hypoxia. On hypoxia induction, Stat5-deficient mice experience anemia accompanied by erythroblast apoptosis.53 Yet these mice have enlarged spleens and expanded erythroblasts in the spleen, suggesting impaired differentiation rather than a defect in expansion. These data suggest that Stat5 activation is essential for erythroid differentiation but not for expansion. The fact that RON does not activate Stat5 may be reflected in its inability to induce differentiation and survival during the onset of differentiation.
Prepublished online as Blood First Edition Paper, February 24, 2004; DOI 10.1182/blood-2003-08-2713.
Supported by grants from the Dutch Cancer Society (EUR 1999-2064), the European Union (HPRN-CT-2000-00083), the Netherlands Organisation for Scientific Research (NWO 901-08-338), and the National Institutes of Health (SEW HD36888) and by fellowships from the Erasmus University Rotterdam (T.v.D.) and from the Dutch Academy of Arts and Sciences (M.v.L.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr I. P. Touw for many critical discussions regarding this work. We thank Dr R. Breathnach (Nantes, France) for the RON cDNA construct, Dr J. Borst (Amsterdam, Netherlands) for the Btk cDNA construct, and Dr T. Hirano (Osaka, Japan) for the Gab2 antisera. We thank Dr A. Danilkovitch-Miagkova (Frederick, MD) for advice regarding the use of MSP.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal