Abstract
In the current study, we tested whether higher numbers of hematopoietic stem cells correlate with the speed of immune reconstitution in a congenic transplantation model (C57BL/Ka, CD45.1, Thy1.1→C57BL/6, CD45.2, Thy1.2) using purified hematopoietic stem cells (c-Kit+Thy1.1lowLin-/lowSca-1+). There were 3 different doses of stem cells used (400, 1000, and 5000). Phenotypic analyses in peripheral blood and spleen demonstrated that higher numbers of infused stem cells are associated with more rapid regeneration of T cells (CD4+, CD8+, naive CD4+, naive CD8+) and B cells at early time points. The numbers of T and B cells eventually became equivalent between different dose groups at late time points. Production of interleukin-2 and inter-feron-γ per T cell was similar regardless of stem cell dose even when tested at the time when there were significant differences in peripheral T-cell counts. The improved immune recovery was attributed to a more rapid regeneration of donor-type immune cells. Higher numbers of total thymocytes and signal joint T-cell receptor excision circles were observed in the higher dose stem cell recipients, suggesting that accelerated regeneration of T cells was due to enhanced thymopoiesis. (Blood. 2004;103:4344-4352)
Introduction
Infections represent a major obstacle to the success of marrow,1-4 peripheral blood,5 and umbilical cord blood transplantation.6-8 The susceptibility to opportunistic infections after hematopoietic cell transplantation is mainly due to the prolonged time required for the donor-derived immune system to develop.5 This delay is especially true in the adult recipients of umbilical cord blood, in whom limited numbers of stem cells are infused.8-10 The use of mobilized peripheral blood as an alternative stem cell source has dramatically reduced the time required for hematopoietic engraftment, suggesting that increased numbers of hematopoietic stem and/or progenitor cells result in the reduction of time required for hematopoietic recovery.11 Indeed, it has been demonstrated that purified stem cells can dramatically shorten time for hematologic engraftment when given in higher doses in both syngeneic and allogeneic settings.12 This observation raises the question whether immune reconstitution (lymphoid engraftment and function) can also be accelerated when larger numbers of stem cells are given. Although it has been reported that an improved immune reconstitution was associated with peripheral blood stem cell transplantation compared with bone marrow transplantation in both autologus13,14 and allogeneic settings,15-17 this question has not yet been systematically analyzed.
The purpose of this study is to determine whether hematopoietic stem cell dose correlates with the speed of immune reconstitution. Graded numbers of purified hematopoietic stem cells were transplanted into lethally irradiated recipients. Phenotypic and functional immune reconstitution was then followed at various time points in the course of hematologic recovery following transplantation, with the focus on T-cell recovery. The positive correlation between stem cell dose and the speed of immune reconstitution may provide preclinical evidence that in the appropriate clinical settings, more stem cells should be infused in hematopoietic cell transplantation. These higher cell doses will lead to a shorter amount of time required for the development of the new immune system and to a decrease in the infection-related morbidity and mortality.
Materials and methods
Animals
BALB/c (H2d), C3H/HeJ (H2k), and C57BL/6, CD45.2, Thy1.2 (H2b, termed “CD45.2 mice”) mice were purchased from The Jackson Laboratories (Bar Harbor, ME). C57BL/Ka, CD45.1, Thy1.1 mice (H2b, termed “CD45.1 mice”) were bred in our animal facility. All animals except the heart donors were female and used when they were 8 to 12 weeks old. Animals were housed in sterile microisolator cages in which they received autoclaved food and autoclaved acidified drinking water in a specific pathogen-free facility throughout the study. Studies were performed in accordance with Duke University Institutional Animal Care and Use Committee-approved procedures.
Antibodies
All antibodies except those used for purification of hematopoietic stem cells are listed here. These antibodies included biotin-conjugated fluorescein isothiocyanate (FITC)-conjugated anti-CD11b (clone M1/70) and anti-CD62L (clone MEL-14); R-phycoerythrin (PE)-conjugated anti-CD45.1 (clone A20); Cy-Chrome-conjugated anti-CD3 (clone 145-2C11), anti-CD44 (clone IM7), and their isotype controls from BD Pharmingen (San Diego, CA); FITC-conjugated anti-B220 (clone RA3-6B2), anti-CD3 (clone 500-A2), anti-CD62L (clone MEL-14), anti-H-2Db (clone CTDb), anti-interleukin-2 (anti-IL-2; clone JES6-5H3), and anti-interferon-γ (anti-IFN-γ; clone XMG1.2); PE-conjugated anti-CD4 (clone CT-CD4) and anti-B220 (clone RA3-6B2); PE-Texas Red-conjugated anti-CD4 (clone RM4-5) and anti-CD8 (clone 5H10); Tricolor-conjugated anti-CD4 (clone CT-CD4), anti-CD8α (clone CT-CD8α), anti-CD45 (clone YW62.3), and their isotype controls from Caltag (South San Francisco, CA).
T-cell depletion from bone marrow
Bone marrow cells flushed out from femurs, tibias, and humeri obtained from the donor mice were first strained through a 70-μm cell strainer (Becton Dickinson labware, Franklin Lakes, NJ). There were 2 different protocols used to deplete T cells from bone marrow. For bone marrow from CD45.2 mice, cells were resuspended in Cytotoxicity Medium (Cedarlane, Hornby, ON, Canada) at a concentration of 1 × 107/mL. Anti-Thy1.2 monoclonal antibody (0.1 μg per 1 × 107 cells, clone 30H12; BD Pharmingen) was added and mixed. After a 60-minute incubation on ice, the cells were washed once and then suspended in Cytotoxicity Medium (Cedarlane) containing 1:10 Low-Tox-M Rabbit Complement (Cedarlane). The cells were incubated at 37°C for 60 minutes and washed twice before use. The final product using this protocol contained less than 0.08% mature T cells. For the bone marrow from CD45.1 mice, T cells were depleted using anti-CD3, anti-CD4, anti-CD5, and anti-CD8 microbeads (Miltenyi Biotec, Auburn, CA). The residual mature T cells were less than 0.002% after T-cell depletion using this protocol.
Purification of hematopoietic stem cells
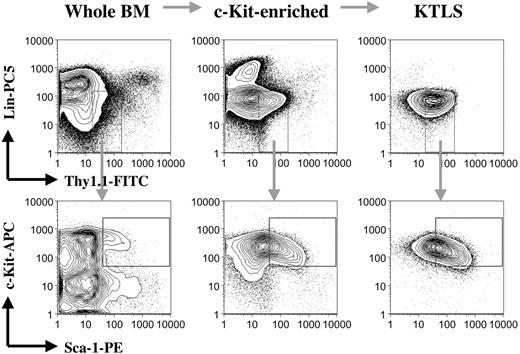
Whole bone marrow cells prepared from the CD45.1 mice were first depleted of red blood cells using ACK solution (0.15 M ammonium and 0.01 M potassium carbonate). Red cell-depleted bone marrow cells were then enriched for c-Kit+ cells by positive selection using purified anti-c-Kit antibody (clone ACK45; BD Pharmingen) followed by incubation with goat antirat magnetic beads from Miltenyi Biotec. c-Kit-enriched bone marrow cells were subsequently stained with a cocktail of primary monoclonal antibodies consisting of biotin-conjugated anti-CD3 (clone 145-2C11), anti-CD4 (clone RM4-5), anti-CD5 (clone 53-7.3), anti-CD8a (clone 53-6.7), anti-CD11b (clone M1/70), anti-B220 (clone RA3-6B2), anti-Gr-1 (clone RB6-8C5), and anti-TER-119 (clone TER-119); FITC-conjugated anti-Thy1.1 (clone OX-7); PE-conjugated anti-Sca-1 (clone E13-161.7); and allophycocyanin-conjugated anti-c-Kit (clone 2B8); followed by secondary staining with streptavidin-Cy-Chrome (all purchased from BD Pharmingen). Propidium iodide (1 μg/mL; Sigma, St Louis, MO) was added to the cell suspension to exclude dead cells before cell sorting. The stained samples were sorted for c-Kit+Thy1.1lowLin-/lowSca-1+ (KTLS) cells (purified hematopoietic stem cells18-20 ) using a dual laser fluorescence activated cell sorter (FACS) Vantage SE (Becton Dickinson, San Jose, CA). The sorted KTLS cells were always resorted before use to ensure purity and accuracy of cell counts. As few as 50 sorted KTLS cells were able to reconstitute all blood lineages in a competitive transplantation assay18 (data not shown). The procedures and quality of samples for each step are illustrated in Figure 1. KTLS cells represent about 1 per 2000 bone marrow cells.
Purification of hematopoietic stem cells. Bone marrow cells from the CD45.1 mice were first depleted of red blood cells. Red blood cell-lysed bone marrow cells were then enriched for c-Kit+ cells by positive selection using magnetic beads. c-Kit+ bone marrow cells were subsequently stained with a cocktail of monoclonal antibodies, including anti-c-Kit, anti-Thy1.1, anti-Sca-1, and anti-lineage cell markers (CD3, CD4, CD5, CD8a, CD11b, B220, Gr-1, and TER-119). The stained samples were finally sorted for c-Kit+Thy1.1lowLin-/lowSca-1+ (KTLS) cells (purified hematopoietic stem cells) using a FACS machine. The figure shows the procedures and quality of samples for each step. BM indicates bone marrow; KTLS, c-Kit+Thy1.1lowLin-/lowSca-1+ hematopoietic stem cells; Lin, lineage; APC, antigen-presenting cell; and CD45.1 mice, C57BL/Ka, CD45.1, Thy1.1 mice.
Purification of hematopoietic stem cells. Bone marrow cells from the CD45.1 mice were first depleted of red blood cells. Red blood cell-lysed bone marrow cells were then enriched for c-Kit+ cells by positive selection using magnetic beads. c-Kit+ bone marrow cells were subsequently stained with a cocktail of monoclonal antibodies, including anti-c-Kit, anti-Thy1.1, anti-Sca-1, and anti-lineage cell markers (CD3, CD4, CD5, CD8a, CD11b, B220, Gr-1, and TER-119). The stained samples were finally sorted for c-Kit+Thy1.1lowLin-/lowSca-1+ (KTLS) cells (purified hematopoietic stem cells) using a FACS machine. The figure shows the procedures and quality of samples for each step. BM indicates bone marrow; KTLS, c-Kit+Thy1.1lowLin-/lowSca-1+ hematopoietic stem cells; Lin, lineage; APC, antigen-presenting cell; and CD45.1 mice, C57BL/Ka, CD45.1, Thy1.1 mice.
Hematopoietic cell transplantation
Recipient mice were lethally irradiated (BALB/c, 8.5 Gy; CD45.2, 10.5 Gy) using a cesium irradiator. Within 4 hours after irradiation, the mice were injected intravenously via tail vein with either T-cell-depleted bone marrow or sorted KTLS cells. Mortality was recorded daily. Body weight was obtained weekly. The mice were monitored daily for clinical graft-versus-host disease (GVHD) by body weight and clinical signs such as skin changes (hair loss and erythema), diarrhea, and posture.21
Absolute count of lymphocyte and lymphocyte subsets
Heparinized peripheral blood (50 μL) was stained with monoclonal antibodies for 15 minutes at room temperature. The stained whole-blood samples were then processed in a Multi-Q-Prep (Coulter, Miami, FL) to lyse red blood cells. Flow-Count fluorospheres (50 μL; Coulter) were added before flow cytometric analysis. The stained cells were analyzed using Coulter EPICS XL equipped with System II software. The absolute counts were calculated automatically by the System II software using the following formula: Absolute count (cells/μL blood) = (Total number of cells counted/Total number of fluorospheres counted) × Flow-Count fluorosphere concentration.
Detection of donor chimerism
The donor chimerism in both allogeneic and congenic models was detected by flow cytometry. In the allogeneic model (C57BL/6→BALB/c), anti-H-2Db antibody that recognized donor but not recipient-type cells was used.22 In the congenic model (CD45.1→CD45.2), anti-CD45.1 antibody was used. Preliminary studies demonstrated that it is appropriate to consider donor-negative cells as recipient-type cells in these 2 models.
Quantification of mouse signal joint T-cell receptor excision circles (sjTRECs)
Molecules of mouse sjTRECs were quantitated by real-time polymerase chain reaction (PCR) as previously described.22,23 Genomic DNA from 100 000 thymocytes was prepared by homogenization in 1 mL TRIZOL Reagent (Life Technologies, Gaithersburg, MD) using an Omni International homogenizer (Warrenton, VA). Thymus DNA (1 μg) was amplified by real-time PCR to determine the number of mouse sjTRECs per microgram of DNA. The total number of sjTRECs/thymus was determined by multiplying by total DNA recovered per 100 000 thymocytes and total thymocytes per thymus.
Intracellular cytokine staining
This assay was described in detail previously.24 Briefly, prior to intracellular cytokine staining, spleen cells were first activated with 10 nM phorbol 12-myristate 13-acetate (PMA; Sigma) and 0.5 μM ionomycin (Sigma) for 4 hours in the presence of brefeldin A (10 μg/mL; Sigma). Brefeldin A disaggregates the Golgi complex and enables intracellular proteins to accumulate.25,26 Cells were washed once and stained with surface markers before fixation and permeabilization. Cells were fixed in 100 μL reagent A (Cell permeabilization kit; Caltag) for 15 minutes at room temperature. After fixation, cells were washed and resuspended in 100 μL reagent B (Cell permeabilization kit; Caltag) containing anticytokine antibodies. Cells were then incubated for 20 minutes at 4°C and washed once before analysis. Irrelevant isotype-matched control antibody produced less than 1% fluorescent cells. The stained cells were analyzed using EPICS XL equipped with System II software (Coulter).
Cardiac transplantation
The method has been described in detail before.22 Briefly, the dorsum of the pinna of an adult mouse was cleaned with 70% ethanol, and a pouch 3 to 4 mm in diameter was prepared. The heart from a neonatal mouse (< 48 hours old) was placed in the pouch. Residual fluid was cleared from the pouch with a cotton swab after transplantation. Cardiac grafts were examined under a stereomicroscope at 10-fold magnification daily after day +6. Grafts resumed visual cardiac contraction between 6 to 7 days after transplantation. Grafts that were never observed to be contracting were considered surgical failure, and those data were excluded (none in this study). Rejection was defined as the point of cessation of the visible allograft cardiac activity. All grafts were monitored daily until day +60 after cardiac transplantation.
Statistical analysis
Comparisons between different groups were done by analysis of variance. All statistical analyses were performed using Statview software (SAS Institute, Cary, NC).
Results
T-cell-depleted bone marrow cell dose correlates with the speed of phenotypic recovery of lymphocyte and lymphocyte subsets after allogeneic T-cell-depleted bone marrow transplantation
To test our hypothesis, we first performed the experiments using T-cell-depleted bone marrow as a stem cell source in an allogeneic transplantation model. Lethally irradiated BALB/c mice were injected with either 1 × 107 or 5 × 107 T-cell-depleted bone marrow cells from CD45.2 mice. The absolute numbers of total lymphocyte and lymphocyte subsets in spleen were monitored at various time points after transplantation. The results are shown in Table 1. Significant differences in the absolute counts of T cells (CD3+, CD4+, CD8+, CD4+CD62L+, and CD8+CD62L+) and B cells (B220+) in spleen between low- and high-dose groups were observed as early as 14 days after transplantation. These differences persisted on day +28. By 42 days, the absolute counts of T cells and B cells in the 1 × 107 group became equivalent to those in the 5 × 107 group. On day +42, the levels of T cells and T-cell subsets in both groups were only half of the pretransplantation level. Similar results were also obtained in peripheral blood (data not shown).
Immune recovery in spleen after allogeneic T-cell-depleted bone marrow transplantation
. | Day + 14 . | . | . | Day + 28 . | . | . | Day + 42 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotypes . | 1 × 107 . | 5 × 107 . | P . | 1 × 107 . | 5 × 107 . | P . | 1 × 107 . | 5 × 107 . | P . | ||||||
| n | 5 | 5 | — | 5 | 5 | — | 5 | 5 | — | ||||||
| Total spleen cells | 140 ± 38 | 296 ± 78 | .0083 | 207 ± 22 | 261 ± 50 | .0591 | 215 ± 38 | 250 ± 74 | .3676 | ||||||
| CD3+ | 9 ± 2 | 18 ± 5 | .0133 | 39 ± 10 | 65 ± 22 | .0660 | 60 ± 8 | 68 ± 17 | .4176 | ||||||
| CD4+ | 7 ± 2 | 15 ± 5 | .0159 | 33 ± 8 | 55 ± 20 | .0764 | 49 ± 6 | 55 ± 13 | .4305 | ||||||
| CD8+ | 2 ± 0.8 | 2 ± 0.6 | .3444 | 6 ± 2 | 10 ± 3 | .0448 | 9 ± 5 | 13 ± 4 | .1774 | ||||||
| CD4+CD62L+ | 2 ± 0.4 | 3 ± 0.9 | .0627 | 15 ± 5 | 24 ± 6 | .0373 | 34 ± 8 | 32 ± 17 | .8334 | ||||||
| CD8+CD62L+ | 0.2 ± 0.0 | 0.4 ± 0.1 | .0349 | 3 ± 1 | 5 ± 0.3 | .0559 | 9 ± 2 | 12 ± 3 | .2335 | ||||||
| B220+ | 77 ± 31 | 222 ± 69 | .0062 | 137 ± 15 | 178 ± 37 | .0750 | 110 ± 17 | 159 ± 55 | .1358 | ||||||
. | Day + 14 . | . | . | Day + 28 . | . | . | Day + 42 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phenotypes . | 1 × 107 . | 5 × 107 . | P . | 1 × 107 . | 5 × 107 . | P . | 1 × 107 . | 5 × 107 . | P . | ||||||
| n | 5 | 5 | — | 5 | 5 | — | 5 | 5 | — | ||||||
| Total spleen cells | 140 ± 38 | 296 ± 78 | .0083 | 207 ± 22 | 261 ± 50 | .0591 | 215 ± 38 | 250 ± 74 | .3676 | ||||||
| CD3+ | 9 ± 2 | 18 ± 5 | .0133 | 39 ± 10 | 65 ± 22 | .0660 | 60 ± 8 | 68 ± 17 | .4176 | ||||||
| CD4+ | 7 ± 2 | 15 ± 5 | .0159 | 33 ± 8 | 55 ± 20 | .0764 | 49 ± 6 | 55 ± 13 | .4305 | ||||||
| CD8+ | 2 ± 0.8 | 2 ± 0.6 | .3444 | 6 ± 2 | 10 ± 3 | .0448 | 9 ± 5 | 13 ± 4 | .1774 | ||||||
| CD4+CD62L+ | 2 ± 0.4 | 3 ± 0.9 | .0627 | 15 ± 5 | 24 ± 6 | .0373 | 34 ± 8 | 32 ± 17 | .8334 | ||||||
| CD8+CD62L+ | 0.2 ± 0.0 | 0.4 ± 0.1 | .0349 | 3 ± 1 | 5 ± 0.3 | .0559 | 9 ± 2 | 12 ± 3 | .2335 | ||||||
| B220+ | 77 ± 31 | 222 ± 69 | .0062 | 137 ± 15 | 178 ± 37 | .0750 | 110 ± 17 | 159 ± 55 | .1358 | ||||||
Lethally irradiated BALB/c mice (H2d) received transplants of 2 different doses (1 × 107 and 5 × 107) of T-cell-depleted bone marrow cells from the CD45.2 mice (H2b). Mice were killed at different time points after transplantation. Spleen cells were prepared and analyzed by flow cytometry. This is a representative of 3 similar experiments. Similar results were also obtained in peripheral blood (data not shown). All values represent total cell counts (× 106) per spleen (mean ± SD). The values of normal BALB/c mice (n = 20) are as follows: total spleen cells, 252 ± 55; CD3+, 133 ± 26; CD4+, 113 ± 24; CD8+, 20 ± 2; and B220+, 122 ± 25. CD45.2 mice indicate CD57BL/6, CD45.2, Thy1.2 mice.—indicates not applicable.
T-cell-depleted bone marrow cell dose correlates with the speed of phenotypic recovery of lymphocyte and lymphocyte subsets after congenic T-cell-depleted bone marrow transplantation
We then tested the same hypothesis in a congenic transplantation model. Lethally irradiated CD45.2 mice were injected with graded numbers (8 × 105, 4 × 106, and 1 × 107) of T-cell-depleted bone marrow cells from the CD45.1 mice. The absolute numbers of total, donor-type, and host-type lymphocyte and lymphocyte subsets in peripheral blood were monitored at different time points starting on day 28 after transplantation, and the results were plotted in Table 2. Although the differences were not always statistically significant among different dose groups, there was always a trend toward quicker total CD4+ T-cell recovery after infusing more T-cell-depleted bone marrow cells up to 70 days after transplantation. The dose-dependent recovery of total CD8+ T cells was less apparent. However, the number of both donor-type CD4+ and donor-type CD8+ T cells was directly correlated with the number of T-cell-depleted bone marrow cells infused starting on day 28 and up to 70 days after transplantation. Interestingly, there was a reversed correlation between the number of host-type CD4+ and CD8+ T cells and the number of T-cell-depleted bone marrow cells infused at all time points, suggesting that T-cell-depleted bone marrow cells can promote engraftment. The number of total B cells, all of which were donor origin (Figure 2), correlated directly with the T-cell-depleted bone marrow cell dose up to 70 days after transplantation. Some of the values exceeded the pretransplantation level up to 70 days after transplantation.
Immune recovery in peripheral blood after congenic T-cell-depleted bone marrow transplantation
. | Total . | . | . | Donor-type . | . | Host-type . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | CD4+ . | CD8+ . | B220+ . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | ||||
| Before | |||||||||||
| transplantation | 773 ± 147 | 379 ± 69 | 2912 ± 714 | — | — | — | — | ||||
| Day + 28 | |||||||||||
| 8 × 105 | 476 ± 165 | 216 ± 63 | 190 ± 192‡ | 29 ± 36† | 12 ± 16† | 447 ± 182 | 204 ± 76 | ||||
| 4 × 106 | 526 ± 75 | 190 ± 47 | 702 ± 151 | 48 ± 59 | 13 ± 14 | 478 ± 96 | 177 ± 53 | ||||
| 1 × 107 | 565 ± 116 | 212 ± 54 | 1641 ± 476 | 201 ± 96 | 59 ± 30 | 364 ± 54 | 153 ± 34 | ||||
| Day + 35 | |||||||||||
| 8 × 105 | 781 ± 220 | 371 ± 116* | 1112 ± 878† | 158 ± 99† | 40 ± 32† | 623 ± 240† | 331 ± 137† | ||||
| 4 × 106 | 824 ± 171 | 278 ± 42 | 2093 ± 683 | 223 ± 122 | 57 ± 37 | 601 ± 136 | 221 ± 44 | ||||
| 1 × 107 | 973 ± 250 | 340 ± 67 | 2787 ± 885 | 568 ± 188 | 167 ± 51 | 405 ± 95 | 173 ± 23 | ||||
| Day + 42 | |||||||||||
| 8 × 105 | 1037 ± 296† | 526 ± 256 | 1544 ± 1220‡ | 446 ± 140† | 132 ± 39† | 592 ± 224* | 395 ± 240† | ||||
| 4 × 106 | 1039 ± 334 | 387 ± 102 | 2752 ± 974 | 532 ± 267 | 156 ± 80 | 507 ± 120 | 230 ± 57 | ||||
| 1 × 107 | 1408 ± 289 | 505 ± 112 | 4627 ± 1554 | 1010 ± 255 | 306 ± 82 | 398 ± 70 | 199 ± 41 | ||||
| Day + 56 | |||||||||||
| 8 × 105 | 1619 ± 557 | 727 ± 264 | 3246 ± 2993* | 1109 ± 446* | 389 ± 186* | 510 ± 195† | 339 ± 148* | ||||
| 4 × 106 | 1744 ± 411 | 697 ± 126 | 4604 ± 1598 | 1236 ± 400 | 428 ± 128 | 508 ± 124 | 269 ± 55 | ||||
| 1 × 107 | 1944 ± 403 | 769 ± 170 | 6273 ± 2401 | 1600 ± 375 | 564 ± 131 | 344 ± 47 | 204 ± 47 | ||||
| Day + 70 | |||||||||||
| 8 × 105 | 1571 ± 687* | 778 ± 350 | 3742 ± 3436* | 1214 ± 565† | 475 ± 246† | 357 ± 184 | 303 ± 166 | ||||
| 4 × 106 | 1606 ± 237 | 721 ± 76 | 5403 ± 1564 | 1249 ± 253 | 477 ± 108 | 358 ± 96 | 245 ± 67 | ||||
| 1 × 107 | 2059 ± 361 | 936 ± 180 | 7765 ± 2145 | 1811 ± 357 | 730 ± 139 | 249 ± 35 | 207 ± 53 | ||||
. | Total . | . | . | Donor-type . | . | Host-type . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | CD4+ . | CD8+ . | B220+ . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | ||||
| Before | |||||||||||
| transplantation | 773 ± 147 | 379 ± 69 | 2912 ± 714 | — | — | — | — | ||||
| Day + 28 | |||||||||||
| 8 × 105 | 476 ± 165 | 216 ± 63 | 190 ± 192‡ | 29 ± 36† | 12 ± 16† | 447 ± 182 | 204 ± 76 | ||||
| 4 × 106 | 526 ± 75 | 190 ± 47 | 702 ± 151 | 48 ± 59 | 13 ± 14 | 478 ± 96 | 177 ± 53 | ||||
| 1 × 107 | 565 ± 116 | 212 ± 54 | 1641 ± 476 | 201 ± 96 | 59 ± 30 | 364 ± 54 | 153 ± 34 | ||||
| Day + 35 | |||||||||||
| 8 × 105 | 781 ± 220 | 371 ± 116* | 1112 ± 878† | 158 ± 99† | 40 ± 32† | 623 ± 240† | 331 ± 137† | ||||
| 4 × 106 | 824 ± 171 | 278 ± 42 | 2093 ± 683 | 223 ± 122 | 57 ± 37 | 601 ± 136 | 221 ± 44 | ||||
| 1 × 107 | 973 ± 250 | 340 ± 67 | 2787 ± 885 | 568 ± 188 | 167 ± 51 | 405 ± 95 | 173 ± 23 | ||||
| Day + 42 | |||||||||||
| 8 × 105 | 1037 ± 296† | 526 ± 256 | 1544 ± 1220‡ | 446 ± 140† | 132 ± 39† | 592 ± 224* | 395 ± 240† | ||||
| 4 × 106 | 1039 ± 334 | 387 ± 102 | 2752 ± 974 | 532 ± 267 | 156 ± 80 | 507 ± 120 | 230 ± 57 | ||||
| 1 × 107 | 1408 ± 289 | 505 ± 112 | 4627 ± 1554 | 1010 ± 255 | 306 ± 82 | 398 ± 70 | 199 ± 41 | ||||
| Day + 56 | |||||||||||
| 8 × 105 | 1619 ± 557 | 727 ± 264 | 3246 ± 2993* | 1109 ± 446* | 389 ± 186* | 510 ± 195† | 339 ± 148* | ||||
| 4 × 106 | 1744 ± 411 | 697 ± 126 | 4604 ± 1598 | 1236 ± 400 | 428 ± 128 | 508 ± 124 | 269 ± 55 | ||||
| 1 × 107 | 1944 ± 403 | 769 ± 170 | 6273 ± 2401 | 1600 ± 375 | 564 ± 131 | 344 ± 47 | 204 ± 47 | ||||
| Day + 70 | |||||||||||
| 8 × 105 | 1571 ± 687* | 778 ± 350 | 3742 ± 3436* | 1214 ± 565† | 475 ± 246† | 357 ± 184 | 303 ± 166 | ||||
| 4 × 106 | 1606 ± 237 | 721 ± 76 | 5403 ± 1564 | 1249 ± 253 | 477 ± 108 | 358 ± 96 | 245 ± 67 | ||||
| 1 × 107 | 2059 ± 361 | 936 ± 180 | 7765 ± 2145 | 1811 ± 357 | 730 ± 139 | 249 ± 35 | 207 ± 53 | ||||
Lethally irradiated (10.5 Gy) CD45.2 mice received transplants of 3 different doses (8 × 105, 4 × 106, and 1 × 107) of T-cell-depleted bone marrow cells from the congenic CD45.1 mice. Peripheral blood was harvested at various time points after transplantation. The blood samples were then stained and analyzed by flow cytometry. The absolute counts were determined using Flow-Count fluorospheres. Data represent absolute count (mean ± SD/μL blood) in peripheral blood from 8 to 10 animals per group per time point. All B cells were donor origin (Figure 2). CD45.2 mice indicate C57BL/Ka, CD45.2, Thy1.2 mice. CD45.1 mice indicate C57BL/Ka, CD45.1, Thy1.1 mice.—indicates not applicable.
P < .05, in any one of the following comparisons: 8 × 105 versus 4 × 106, 8 × 105 versus 1 × 107, and 4 × 106 versus 1 × 107.
P < .05, any 2 of the following comparisons: 8 × 105 versus 4 × 106, 8 × 105 versus 1 × 107, and 4 × 106 versus 1 × 107.
P < .05, all of the following comparisons: 8 × 105 versus 4 × 106, 8 × 105 versus 1 × 107, and 4 × 106 versus 1 × 107.
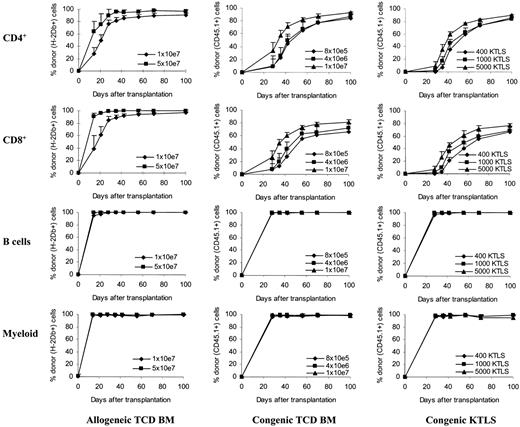
Kinetics of donor T-cell chimerism. Peripheral blood was obtained at various time points after stem cell transplantation. Peripheral blood was then stained with monoclonal antibodies and the red blood cells were lysed. The stained whole-blood samples were then analyzed by flow cytometry. T-cell subsets and B cells were gated based on their expression of CD4, CD8, and B220 molecules. Myeloid cells were gated based on forward and side scatters (the gate was confirmed using anti-CD45, anti-CD11b, and anti-Gr-1 in the preliminary studies). The donor cells were determined by anti-H2Db antibody in allogeneic T-cell-depleted bone marrow recipients or by anti-CD45.1 antibody in congenic stem cell recipients. The values (mean + SD) represent the results from 4 to 10 animals per group per time point. Allogeneic TCD BM recipients: P < .05 between groups at all time points (CD4+); for CD8+, P < .05 except on day +28 (P = .09), day +42 (P = .08), and day +56 (P = .08). Congenic TCD BM recipients: except between 8 × 105 and 4 × 106, P < .05 in both CD4+ and CD8+ cells. Congenic KTLS recipients: for CD4+, except on day +100 (P < .05, 1000 KTLS vs 5000 KTLS), P < .05 in at least 2 of the following comparisons: 400 KTLS versus 1000 KTLS, 400 KTLS versus 5000 KTLS, and 1000 KTLS versus 5000 KTLS; for CD8+, P < .05 in at least 2 of the following comparisons: 400 KTLS versus 1000 KTLS, 400 KTLS versus 5000 KTLS, and 1000 KTLS versus 5000 KTLS. The percentages of donor cells in B cells and myeloid cells were more than 95% in all groups at all time points tested. TCD BM indicates T-cell-depleted bone marrow; KTLS, c-Kit+Thy1.1lowLin-/lowSca-1+ hematopoietic stem cells.
Kinetics of donor T-cell chimerism. Peripheral blood was obtained at various time points after stem cell transplantation. Peripheral blood was then stained with monoclonal antibodies and the red blood cells were lysed. The stained whole-blood samples were then analyzed by flow cytometry. T-cell subsets and B cells were gated based on their expression of CD4, CD8, and B220 molecules. Myeloid cells were gated based on forward and side scatters (the gate was confirmed using anti-CD45, anti-CD11b, and anti-Gr-1 in the preliminary studies). The donor cells were determined by anti-H2Db antibody in allogeneic T-cell-depleted bone marrow recipients or by anti-CD45.1 antibody in congenic stem cell recipients. The values (mean + SD) represent the results from 4 to 10 animals per group per time point. Allogeneic TCD BM recipients: P < .05 between groups at all time points (CD4+); for CD8+, P < .05 except on day +28 (P = .09), day +42 (P = .08), and day +56 (P = .08). Congenic TCD BM recipients: except between 8 × 105 and 4 × 106, P < .05 in both CD4+ and CD8+ cells. Congenic KTLS recipients: for CD4+, except on day +100 (P < .05, 1000 KTLS vs 5000 KTLS), P < .05 in at least 2 of the following comparisons: 400 KTLS versus 1000 KTLS, 400 KTLS versus 5000 KTLS, and 1000 KTLS versus 5000 KTLS; for CD8+, P < .05 in at least 2 of the following comparisons: 400 KTLS versus 1000 KTLS, 400 KTLS versus 5000 KTLS, and 1000 KTLS versus 5000 KTLS. The percentages of donor cells in B cells and myeloid cells were more than 95% in all groups at all time points tested. TCD BM indicates T-cell-depleted bone marrow; KTLS, c-Kit+Thy1.1lowLin-/lowSca-1+ hematopoietic stem cells.
Hematopoietic stem cell dose correlates with the speed of phenotypic recovery of lymphocyte and lymphocyte subsets after congenic purified stem cell transplantation
Although we have demonstrated that more T-cell-depleted bone marrow cells infused resulted in more rapid immune recovery in both congenic (Table 1) and allogeneic (Table 2) settings, we were unsure whether this was a result of the increase in infused stem cells—as the higher-dose T-cell-depleted bone marrow groups received not only more stem cells but also more non-stem cells (such as progenitors, residual mature T cells, and B cells). To test the hypothesis directly, we then performed transplantation using FACS-purified stem cells. The congenic transplantation model was used because 10 times more stem cells are needed to rescue lethally irradiated recipients in allogeneic compared with congenic settings,12 and it was technically difficult to sort the large number of purified stem cells needed for this experiment. Lethally irradiated CD45.2 mice were injected with graded numbers (400, 1000, and 5000) of KTLS cells, which corresponded with T-cell-depleted bone marrow cell doses used in Table 2 (eg, 400 KTLS = 8 × 105 T-cell-depleted bone marrow cells) from the congenic CD45.1 mice. Identical to the transplantation using T-cell-depleted bone marrow, the absolute numbers of total, donor-type, and host-type lymphocyte and lymphocyte subsets in peripheral blood were monitored at different time points starting on day 28, and those in the spleen were monitored on day +42 after transplantation. The results for peripheral blood and spleen are summarized in Tables 3 and 4, respectively.
Immune recovery in peripheral blood after congenic purified hematopoietic stem cell transplantation
. | Total . | . | . | Donor-type . | . | Host-type . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | CD4+ . | CD8+ . | B220+ . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | ||||
| Before transplantation | 773 ± 147 | 379 ± 69 | 2912 ± 714 | — | — | — | — | ||||
| Day + 28 | |||||||||||
| 400 KTLS | 429 ± 98* | 265 ± 79 | 77 ± 60‡ | 2 ± 1† | 1 ± 1† | 427 ± 98* | 264 ± 78 | ||||
| 1000 KTLS | 548 ± 100 | 292 ± 101 | 465 ± 267 | 10 ± 6 | 5 ± 3 | 538 ± 97 | 287 ± 99 | ||||
| 5000 KTLS | 653 ± 213 | 316 ± 134 | 1481 ± 464 | 65 ± 71 | 23 ± 20 | 588 ± 176 | 292 ± 125 | ||||
| Day + 35 | |||||||||||
| 400 KTLS | 633 ± 202† | 383 ± 122 | 438 ± 364‡ | 48 ± 31‡ | 13 ± 8‡ | 431 ± 98† | 278 ± 83 | ||||
| 1000 KTLS | 866 ± 143 | 412 ± 135 | 1201 ± 482 | 152 ± 80 | 45 ± 26 | 714 ± 114 | 367 ± 112 | ||||
| 5000 KTLS | 1028 ± 214 | 458 ± 137 | 2897 ± 540 | 366 ± 127 | 115 ± 38 | 662 ± 149 | 342 ± 135 | ||||
| Day + 42 | |||||||||||
| 400 KTLS | 1130 ± 198† | 575 ± 135 | 1556 ± 1408‡ | 401 ± 150‡ | 113 ± 44‡ | 729 ± 185 | 462 ± 155 | ||||
| 1000 KTLS | 1345 ± 211 | 628 ± 118 | 3056 ± 839 | 645 ± 151 | 208 ± 56 | 700 ± 123 | 420 ± 111 | ||||
| 5000 KTLS | 1563 ± 349 | 700 ± 184 | 4651 ± 845 | 937 ± 263 | 320 ± 101 | 626 ± 102 | 379 ± 89 | ||||
| Day + 56 | |||||||||||
| 400 KTLS | 1486 ± 640 | 724 ± 328 | 2571 ± 2575* | 929 ± 464† | 304 ± 169† | 558 ± 219 | 420 ± 191 | ||||
| 1000 KTLS | 1371 ± 282 | 641 ± 83 | 2543 ± 1317 | 857 ± 187 | 317 ± 71 | 514 ± 159 | 324 ± 57 | ||||
| 5000 KTLS | 1816 ± 353 | 819 ± 158 | 4973 ± 1015 | 1387 ± 272 | 503 ± 103 | 429 ± 90 | 315 ± 97 | ||||
| Day + 70 | |||||||||||
| 400 KTLS | 2109 ± 619* | 983 ± 302† | 5721 ± 4035* | 1561 ± 486† | 547 ± 185† | 548 ± 174 | 436 ± 153† | ||||
| 1000 KTLS | 1597 ± 507 | 680 ± 154 | 4316 ± 2137 | 1189 ± 434 | 413 ± 139 | 408 ± 113 | 267 ± 28 | ||||
| 5000 KTLS | 2476 ± 521 | 1111 ± 234 | 8148 ± 1873 | 2048 ± 441 | 802 ± 172 | 428 ± 84 | 309 ± 87 | ||||
. | Total . | . | . | Donor-type . | . | Host-type . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
. | CD4+ . | CD8+ . | B220+ . | CD4+ . | CD8+ . | CD4+ . | CD8+ . | ||||
| Before transplantation | 773 ± 147 | 379 ± 69 | 2912 ± 714 | — | — | — | — | ||||
| Day + 28 | |||||||||||
| 400 KTLS | 429 ± 98* | 265 ± 79 | 77 ± 60‡ | 2 ± 1† | 1 ± 1† | 427 ± 98* | 264 ± 78 | ||||
| 1000 KTLS | 548 ± 100 | 292 ± 101 | 465 ± 267 | 10 ± 6 | 5 ± 3 | 538 ± 97 | 287 ± 99 | ||||
| 5000 KTLS | 653 ± 213 | 316 ± 134 | 1481 ± 464 | 65 ± 71 | 23 ± 20 | 588 ± 176 | 292 ± 125 | ||||
| Day + 35 | |||||||||||
| 400 KTLS | 633 ± 202† | 383 ± 122 | 438 ± 364‡ | 48 ± 31‡ | 13 ± 8‡ | 431 ± 98† | 278 ± 83 | ||||
| 1000 KTLS | 866 ± 143 | 412 ± 135 | 1201 ± 482 | 152 ± 80 | 45 ± 26 | 714 ± 114 | 367 ± 112 | ||||
| 5000 KTLS | 1028 ± 214 | 458 ± 137 | 2897 ± 540 | 366 ± 127 | 115 ± 38 | 662 ± 149 | 342 ± 135 | ||||
| Day + 42 | |||||||||||
| 400 KTLS | 1130 ± 198† | 575 ± 135 | 1556 ± 1408‡ | 401 ± 150‡ | 113 ± 44‡ | 729 ± 185 | 462 ± 155 | ||||
| 1000 KTLS | 1345 ± 211 | 628 ± 118 | 3056 ± 839 | 645 ± 151 | 208 ± 56 | 700 ± 123 | 420 ± 111 | ||||
| 5000 KTLS | 1563 ± 349 | 700 ± 184 | 4651 ± 845 | 937 ± 263 | 320 ± 101 | 626 ± 102 | 379 ± 89 | ||||
| Day + 56 | |||||||||||
| 400 KTLS | 1486 ± 640 | 724 ± 328 | 2571 ± 2575* | 929 ± 464† | 304 ± 169† | 558 ± 219 | 420 ± 191 | ||||
| 1000 KTLS | 1371 ± 282 | 641 ± 83 | 2543 ± 1317 | 857 ± 187 | 317 ± 71 | 514 ± 159 | 324 ± 57 | ||||
| 5000 KTLS | 1816 ± 353 | 819 ± 158 | 4973 ± 1015 | 1387 ± 272 | 503 ± 103 | 429 ± 90 | 315 ± 97 | ||||
| Day + 70 | |||||||||||
| 400 KTLS | 2109 ± 619* | 983 ± 302† | 5721 ± 4035* | 1561 ± 486† | 547 ± 185† | 548 ± 174 | 436 ± 153† | ||||
| 1000 KTLS | 1597 ± 507 | 680 ± 154 | 4316 ± 2137 | 1189 ± 434 | 413 ± 139 | 408 ± 113 | 267 ± 28 | ||||
| 5000 KTLS | 2476 ± 521 | 1111 ± 234 | 8148 ± 1873 | 2048 ± 441 | 802 ± 172 | 428 ± 84 | 309 ± 87 | ||||
Lethally irradiated (10.5 Gy) CD45.2 mice received transplants of 3 different doses (400, 1000, and 5000) of KTLS cells that corresponded with T-cell-depleted bone marrow cell doses used in Table 2 (eg, 400 KTLS = 8 × 105 T-cell-depleted bone marrow cells) from the congenic CD45.1 mice. Peripheral blood was harvested at various time points after stem cell transplantation. The blood samples were then stained and analyzed by flow cytometry. The absolute counts were determined using Flow-Count fluorospheres. Data represent absolute count (mean ± SD/μL blood) in peripheral blood from 4 to 15 animals per group per time point. All B cells were donor origin (Figure 2). KTLS indicates c-Kit+Thy1.1lowLin−/lowSca-1+ hematopoietic stem cells; —, not done. CD45.2 mice indicates C57BL/6, CD45.2, Thy1.2 mice. CD45.1 mice indicates C57BL/Ka, CD45.1, Thy1.1 mice.
P < .05, in any one of the following comparisons: 400 KTLS versus 1000 KTLS, 400 KTLS versus 5000 KTLS, and 1000 KTLS versus 5000 KTLS.
P < .05, any 2 of the following comparisons: 400 KTLS versus 1000 KTLS, 400 KTLS versus 5000 KTLS, and 1000 KTLS versus 5000 KTLS.
P < .05, all of the following comparisons: 400 KTLS versus 1000 KTLS, 400 KTLS versus 5000 KTLS, and 1000 KTLS versus 5000 KTLS.
T-lymphocyte recovery in spleen after congenic purified hematopoietic stem cell transplantation (day + 42)
. | Groups . | . | . | P . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 400 KTLS . | 1000 KTLS . | 5000 KTLS . | 400 vs 1000 . | 400 vs 5000 . | 1000 vs 5000 . | ||||
| n | 4 | 7 | 4 | — | — | — | ||||
| Total | ||||||||||
| CD3+ | 19.6 ± 4.3 | 28.5 ± 5.6 | 30.4 ± 2.9 | .0115 | .0076 | .5369 | ||||
| CD4+ | 11.3 ± 3.0 | 18.0 ± 3.4 | 18.9 ± 1.2 | .0032 | .0029 | .6087 | ||||
| CD8+ | 6.3 ± 2.3 | 8.3 ± 2.2 | 7.3 ± 2.2 | .1814 | .5309 | .5028 | ||||
| Naive CD4+ | 8.5 ± 3.5 | 14.4 ± 2.9 | 15.3 ± 1.3 | .0054 | .0049 | .6346 | ||||
| Naive CD8+ | 0.9 ± 0.5 | 2.7 ± 1.0 | 3.4 ± 0.8 | .0088 | .0018 | .1941 | ||||
| Donor-type | ||||||||||
| CD3+ | 5.3 ± 2.3 | 11.8 ± 3.6 | 13.0 ± 0.6 | .0032 | .0023 | .5197 | ||||
| CD4+ | 3.2 ± 1.6 | 7.5 ± 2.3 | 7.3 ± 0.4 | .0026 | .0079 | .8462 | ||||
| CD8+ | 0.9 ± 0.5 | 2.6 ± 1.1 | 2.5 ± 0.4 | .0101 | .0253 | .8679 | ||||
| Naive CD4+ | 3.3 ± 1.8 | 7.5 ± 2.9 | 7.8 ± 0.7 | .0135 | .0172 | .8248 | ||||
| Naive CD8+ | 0.4 ± 0.2 | 1.5 ± 0.7 | 1.8 ± 0.4 | .0111 | .0039 | .3274 | ||||
| Host-type | ||||||||||
| CD3+ | 14.3 ± 2.1 | 16.7 ± 3.5 | 17.4 ± 2.6 | .2372 | .1757 | .7111 | ||||
| CD4+ | 8.1 ± 1.6 | 10.4 ± 2.0 | 11.6 ± 1.6 | .0631 | .0175 | .3117 | ||||
| CD8+ | 5.4 ± 1.8 | 5.7 ± 1.7 | 4.8 ± 1.9 | .7743 | .6792 | .4554 | ||||
| Naive CD4+ | 5.2 ± 1.9 | 6.9 ± 1.9 | 7.5 ± 1.3 | .1330 | .0881 | .6381 | ||||
| Naive CD8+ | 0.6 ± 0.3 | 1.2 ± 0.4 | 1.6 ± 0.4 | .0333 | .0050 | .1677 | ||||
. | Groups . | . | . | P . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
. | 400 KTLS . | 1000 KTLS . | 5000 KTLS . | 400 vs 1000 . | 400 vs 5000 . | 1000 vs 5000 . | ||||
| n | 4 | 7 | 4 | — | — | — | ||||
| Total | ||||||||||
| CD3+ | 19.6 ± 4.3 | 28.5 ± 5.6 | 30.4 ± 2.9 | .0115 | .0076 | .5369 | ||||
| CD4+ | 11.3 ± 3.0 | 18.0 ± 3.4 | 18.9 ± 1.2 | .0032 | .0029 | .6087 | ||||
| CD8+ | 6.3 ± 2.3 | 8.3 ± 2.2 | 7.3 ± 2.2 | .1814 | .5309 | .5028 | ||||
| Naive CD4+ | 8.5 ± 3.5 | 14.4 ± 2.9 | 15.3 ± 1.3 | .0054 | .0049 | .6346 | ||||
| Naive CD8+ | 0.9 ± 0.5 | 2.7 ± 1.0 | 3.4 ± 0.8 | .0088 | .0018 | .1941 | ||||
| Donor-type | ||||||||||
| CD3+ | 5.3 ± 2.3 | 11.8 ± 3.6 | 13.0 ± 0.6 | .0032 | .0023 | .5197 | ||||
| CD4+ | 3.2 ± 1.6 | 7.5 ± 2.3 | 7.3 ± 0.4 | .0026 | .0079 | .8462 | ||||
| CD8+ | 0.9 ± 0.5 | 2.6 ± 1.1 | 2.5 ± 0.4 | .0101 | .0253 | .8679 | ||||
| Naive CD4+ | 3.3 ± 1.8 | 7.5 ± 2.9 | 7.8 ± 0.7 | .0135 | .0172 | .8248 | ||||
| Naive CD8+ | 0.4 ± 0.2 | 1.5 ± 0.7 | 1.8 ± 0.4 | .0111 | .0039 | .3274 | ||||
| Host-type | ||||||||||
| CD3+ | 14.3 ± 2.1 | 16.7 ± 3.5 | 17.4 ± 2.6 | .2372 | .1757 | .7111 | ||||
| CD4+ | 8.1 ± 1.6 | 10.4 ± 2.0 | 11.6 ± 1.6 | .0631 | .0175 | .3117 | ||||
| CD8+ | 5.4 ± 1.8 | 5.7 ± 1.7 | 4.8 ± 1.9 | .7743 | .6792 | .4554 | ||||
| Naive CD4+ | 5.2 ± 1.9 | 6.9 ± 1.9 | 7.5 ± 1.3 | .1330 | .0881 | .6381 | ||||
| Naive CD8+ | 0.6 ± 0.3 | 1.2 ± 0.4 | 1.6 ± 0.4 | .0333 | .0050 | .1677 | ||||
Lethally irradiated (10.5 Gy) CD45.2 mice received transplants of 3 different doses (400, 1000, and 5000) of KTLS cells that corresponded with T-cell-depleted bone marrow cell doses used in Table 2 (eg, 400 KTLS = 8 × 105 T-cell-depleted bone marrow cells) from the congenic CD45.1 mice. Mice were killed 42 days after transplantation. Spleen cells were prepared and analyzed by flow cytometry. The absolute counts were determined using Flow-Count fluorospheres. Naive T cells refer to T cells with CD62L +CD44− phenotype. All values represent total cell counts (× 106) per spleen (mean ± SD). The values of normal recipient-type mice (n = 5) are as follows: CD3+, 59 ± 7; CD4+, 28 ± 3; CD8+, 22 ± 4; naive CD4+, 26 ± 3; and naive CD8+, 15 ± 3. The values of normal donor-type mice (n = 5) are as follows: CD3+, 61 ± 10; CD4+, 31 ± 6; CD8+, 24 ± 4; naive CD4+, 28 ± 6; and naive CD8+, 17 ± 4. KTLS indicates c-Kit+Thy1.1lowLin−/lowSca-1+ hematopoietic stem cells; —indicates not applicable. CD45.2 mice indicates C57BL/6, CD45.2, Thy1.2 mice. CD45.1 mice indicates C57BL/Ka, CD45.1, Thy1.1 mice.
In peripheral blood, total CD4+ and CD8+ T cells recovered quicker in a KTLS cell dose-dependent manner on days 28, 35, and 42. After day 56, the dose-dependent differences between dose groups became less apparent. The differences in total T-cell recovery resulted mainly from regeneration of donor-type T cells from KTLS because the absolute numbers of donor-type CD4+ and CD8+ T cells were different among 3 different dose groups up to 70 days after transplantation. By contrast, the numbers of host-type CD4+ and CD8+ T cells were mostly similar between different dose groups and remained very consistent at different time points. Total B cells, all of which were donor derived (Figure 2), were significantly different between different dose groups up to 70 days after transplantation. All differences between different dose groups became statistically insignificant by day 98 (data not shown). Similar to the recipients of congenic T-cell-depleted bone marrow recipients, some of the values exceeded the pretransplantation level up to 98 days after transplantation.
In the day +42 spleen, there were significant differences in most of the total and donor-type CD3+, CD4+, CD8+, naive CD4+ (CD62L+CD44-), and naive CD8+ (CD62L+CD44-) T-cell numbers (P < .05) between the recipients of 400 KTLS cells and other dose groups except in total CD8+. Most numbers of host-type T cells were similar between different dose groups except in CD4+ between 400 and 5000 KTLS and in naive CD8+ between 400 and 1000 or 5000 KTLS.
These data suggested that more rapid peripheral T-cell and B-cell recovery is associated with more stem cells in the graft after purified stem cell transplantation. This might be resulting from quicker regeneration of donor-type T cells because the numbers of those cells were increased in parallel (Tables 3, 4).
Kinetics of chimerism
To further determine whether the accelerated recovery of T-cell numbers was a result of accelerated donor cell engraftment and differentiation with more T-cell-depleted bone marrow and KTLS cells, the kinetics of donor chimerism on T cells as well as B cells and myeloid cells was followed over time and the results were plotted in Figure 2. The percentages of donor cells in B cells and myeloid cells were more than 95% in all groups at all time points tested (Figure 2).
In the recipients of allogeneic T-cell-depleted bone marrow, donor chimerism was detected using anti-H-2Db antibody starting on day 14 after transplantation. Donor T cells comprised 28% of CD4+ and 39% of CD8+ T cells in the 1 × 107 group, and 64% of CD4+ and 91% of CD8+ T cells in the 5 × 107 group on day +14 (Figure 2, P < .05). The proportion of donor-type cells increased over time in both groups. By day +42, donor T cells comprised 85% of CD4+ and 92% of CD8+ T cells in the 1 × 107 group, and 95% of CD4+ and 97% of CD8+ T cells in the 5 × 107 group (Figure 2, P < .05 in CD4+).
In the recipients of congenic T-cell-depleted bone marrow, donor chimerism was examined using anti-CD45.1 antibody starting on day 28 after transplantation. On day +28, the donor-type CD4+ T cells comprised 9.8%, 9.2%, and 40% of total CD4+ T cells and the donor-type CD8+ T cells comprised 8.4%, 7.7%, and 26.6% of total CD8+ T cells in the recipients of 8 × 105, 4 × 106, and 1 × 107 T-cell-depleted bone marrow cells, respectively (P < .05, 1 × 107 vs other groups). The percentages of donor-type T cells increased continuously over time in all groups. By day +42, the donor-type CD4+ T cells comprised 44.3%, 49.3%, and 71.1% of total CD4+ T cells and the donor-type CD8+ T cells comprised 28.1%, 39.2%, and 60% of total CD8+ T cells in the recipients of 8 × 105, 4 × 106, and 1 × 107 T-cell-depleted bone marrow cells, respectively (P < .05, 1 × 107 vs other groups). By day +70, there were still differences between different dose groups (P < .05, 1 × 107 vs other groups).
The development of donor chimerism in both CD4+ and CD8+ T cells was delayed in the recipients of KTLS cells compared with the recipients of T-cell-depleted bone marrow especially in the allogeneic setting (Figure 2). The donor-type T cells could not be detected until day +28 after transplantation. On day +28, donor-type CD4+ T cells comprised 0.5%, 1.7%, and 9% of total CD4+ T cells and donor-type CD8+ T cells comprised 0.4%, 1.7%, and 7.3% of total CD8+ T cells in the recipients of 400, 1000, and 5000 KTLS cells, respectively (P < .05, 5000 KTLS vs others). Subsequently, the donor-type T cells were generated rapidly. By day +42, the donor-type T cells comprised 35.4%, 45.4%, and 59.5% in CD4+ T cells (P < .05, 5000 KTLS vs others) and 21.5%, 35.2%, and 45.5% in CD8+ T cells (P < .05, 400 KTLS vs others) in the recipients of 400, 1000, and 5000 KTLS cells, respectively. By day +100, the donor-type T cells comprised 85.5%, 83.7%, and 89.8% in CD4+ T cells and 67%, 68.4%, and 77.2% in CD8+ T cells in the recipients of 400, 1000, and 5000 KTLS cells, respectively.
Hematopoietic stem cell dose correlates with thymic output after congenic stem cell transplantation
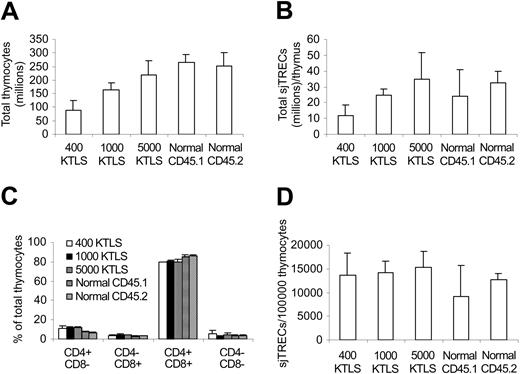
Increase in the numbers of naive T cells (Table 4) suggested that thymopoiesis was enhanced after infusing higher numbers of stem cells. To determine whether this was the case directly, we next measured the total thymocyte numbers and sjTRECs in the KTLS cell recipients 42 days after transplantation, at the time when the differences in peripheral donor-type T-cell counts were significant between different dose groups (Tables 3, 4). As shown in Figure 3A, more thymocytes were detected in higher KTLS cell dose groups (P < .05). Similarly, more sjTRECs per thymus were also detected in higher KTLS cell dose groups (P < .05, 400 KTLS vs 5000 KTLS; P = .1, 1000 KTLS vs others). However, there was no difference in the frequencies of thymocyte subsets and sjTRECs per 100 000 CD4+ or CD8+ T cells between different dose groups except in percent CD4-8- (400 KTLS vs 1000 KTLS, Figure 3C-D). It is important to point out that all thymocytes were donor derived (data not shown).
Thymocyte and signal joint T-cell excision circle (sjTREC) reconstitution. Thymocytes were harvested and counted 42 days after congenic stem cell transplantation. sjTRECs were determined by quantitative real-time PCR. The values represent mean ± SD from 3 to 6 animals per group. (A) Total thymocytes. P < .05 except 5000 KTLS versus normals and normal CD45.1 versus normal CD45.2. (B) Total sjTRECs per thymus. P < .05, 400 KTLS versus 5000 KTLS; P = .1, 1000 KTLS versus 400 KTLS and 5000 KTLS. (C) Percentage of thymocyte subsets. P = not significant (NS) between transplantation recipients except 400 KTLS versus 1000 KTLS in percent CD4-8-; P < .05, transplantation recipients versus normals. (D) Frequency of sjTRECs in thymocytes. P < .05, normal CD45.1 versus 1000 KTLS and 5000 KTLS. KTLS indicates c-Kit+Thy1.1lowLin-lowSca-1+ hematopoietic stem cells; CD45.1 mice, C57BL/Ka, CD45.1, Thy1.1 mice; and CD45.2 mice, C57BL/6, CD45.2, Thy1.2 mice.
Thymocyte and signal joint T-cell excision circle (sjTREC) reconstitution. Thymocytes were harvested and counted 42 days after congenic stem cell transplantation. sjTRECs were determined by quantitative real-time PCR. The values represent mean ± SD from 3 to 6 animals per group. (A) Total thymocytes. P < .05 except 5000 KTLS versus normals and normal CD45.1 versus normal CD45.2. (B) Total sjTRECs per thymus. P < .05, 400 KTLS versus 5000 KTLS; P = .1, 1000 KTLS versus 400 KTLS and 5000 KTLS. (C) Percentage of thymocyte subsets. P = not significant (NS) between transplantation recipients except 400 KTLS versus 1000 KTLS in percent CD4-8-; P < .05, transplantation recipients versus normals. (D) Frequency of sjTRECs in thymocytes. P < .05, normal CD45.1 versus 1000 KTLS and 5000 KTLS. KTLS indicates c-Kit+Thy1.1lowLin-lowSca-1+ hematopoietic stem cells; CD45.1 mice, C57BL/Ka, CD45.1, Thy1.1 mice; and CD45.2 mice, C57BL/6, CD45.2, Thy1.2 mice.
Cytokine-producing potential at a per-cell level
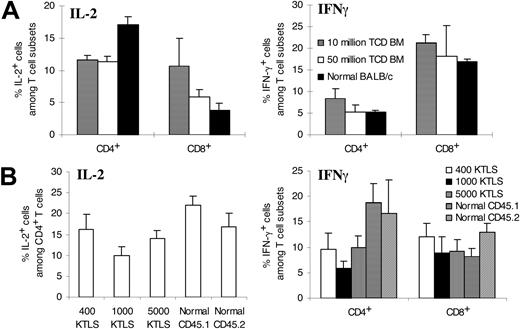
To determine the functional recovery of T cells, we next obtained the spleens on day +28 (allogeneic T-cell-depleted bone marrow recipients) or on day +42 (congenic KTLS recipients) and measured the T-cell production of IL-2 and IFN-γ, which are 2 critical cytokines in T-cell responses.27 Cytokine-producing potential of individual T cells was tested at a per-cell level by intracellular cytokine staining following stimulation with PMA plus ionomycin. As shown in Figure 4A, CD4+ and CD8+ T cells from the recipients of 1 × 107 or 5 × 107 allogeneic T-cell-depleted bone marrow cells produced comparable amounts of IL-2 and interferon-γ (IFN-γ). When compared with those from normal BALB/c mice, CD4+ T cells from both groups produced less IL-2 (Figure 4A).
Ability of individual T cells to produce cytokines. Spleen cells were harvested 28 days after allogeneic T-cell-depleted bone marrow transplantation or 42 days after congenic stem cell transplantation. Spleen cells were then stimulated by phorbol 12-myristate 13-acetate and ionomycin in the presence of brefeldin A. Cells were stained with surface markers before fixation and permeabilization. After permeabilization, cells were stained with anticytokine antibody. The stained samples were analyzed by flow cytometry. Irrelevant isotype-matched control antibody produced less than 1% fluorescent cells. The values represent mean ± SD of 3 to 7 animals per group. (A) Allogeneic T-cell-depleted bone marrow transplantation. For IL-2, P < .05 except 10 million TCD BM versus 50 million TCD BM (CD4+); P = NS except 10 million TCD BM versus normal BALB/c (CD8+). For IFN-γ, P = NS except 10 million TCD BM versus other groups in CD4+ (P < .05). (B) Congenic KTLS cell transplantation. IL-2 production by CD8+ T cells was not studied due to technical failure. P = NS except 1000 KTLS versus others and normal CD45.1 versus others (IL-2); P = NS except KTLS recipients versus normal (IFN-γ among CD4+); P = NS except normal CD45.2 versus others (except 400 KTLS) and 400 KTLS versus normal CD45.1 (IFN-γ among CD8+). KTLS indicates c-Kit+Thy1.1lowLin-/lowSca-1+ hematopoietic stem cells; CD45.1 mice, C57BL/Ka, CD45.1, Thy1.1 mice; and CD45.2 mice, C57BL/6, CD45.2, Thy1.2 mice.
Ability of individual T cells to produce cytokines. Spleen cells were harvested 28 days after allogeneic T-cell-depleted bone marrow transplantation or 42 days after congenic stem cell transplantation. Spleen cells were then stimulated by phorbol 12-myristate 13-acetate and ionomycin in the presence of brefeldin A. Cells were stained with surface markers before fixation and permeabilization. After permeabilization, cells were stained with anticytokine antibody. The stained samples were analyzed by flow cytometry. Irrelevant isotype-matched control antibody produced less than 1% fluorescent cells. The values represent mean ± SD of 3 to 7 animals per group. (A) Allogeneic T-cell-depleted bone marrow transplantation. For IL-2, P < .05 except 10 million TCD BM versus 50 million TCD BM (CD4+); P = NS except 10 million TCD BM versus normal BALB/c (CD8+). For IFN-γ, P = NS except 10 million TCD BM versus other groups in CD4+ (P < .05). (B) Congenic KTLS cell transplantation. IL-2 production by CD8+ T cells was not studied due to technical failure. P = NS except 1000 KTLS versus others and normal CD45.1 versus others (IL-2); P = NS except KTLS recipients versus normal (IFN-γ among CD4+); P = NS except normal CD45.2 versus others (except 400 KTLS) and 400 KTLS versus normal CD45.1 (IFN-γ among CD8+). KTLS indicates c-Kit+Thy1.1lowLin-/lowSca-1+ hematopoietic stem cells; CD45.1 mice, C57BL/Ka, CD45.1, Thy1.1 mice; and CD45.2 mice, C57BL/6, CD45.2, Thy1.2 mice.
In the recipients of congenic KTLS cells (Figure 4B), no consistent difference was observed in cytokine production by T cells between different dose groups. T cells from KTLS cell recipients produced or had a trend to produce less IL-2 and IFN-γ when compared with normal donor- or recipient-type mice.
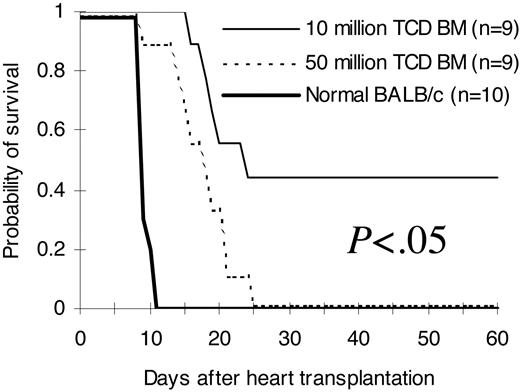
T-cell-depleted bone marrow cell dose correlates with the ability to reject third-party hearts in allogeneic T-cell-depleted bone marrow recipients
To investigate whether improved phenotypic immune reconstitution (Tables 1, 2, 3, 4; Figures 2, 3) after infusion of higher stem cell doses has functional relevance in vivo, we transplanted hearts from third-party C3H/HeJ newborn mice into pouches created in the pinnae of lethally irradiated BALB/c recipients of C57BL/6 T-cell-depleted bone marrow on day 14 after bone marrow transplantation. As demonstrated in Figure 5, normal recipient-type BALB/c mice rejected third-party hearts between day 9 and day 11 (median, 9 days) after heart transplantation. Mice from the 5 × 107 T-cell-depleted bone marrow cell group rejected third-party hearts between day +9 to day +25 (median, 17 days). Third-party hearts were rejected following a significant delay in the 1 × 107 T-cell-depleted bone marrow cell group. The first third-party heart was rejected on day +16 in this group. Close to half of them were not rejected up to 60 days after heart transplantation (median, 23 days; P < .05 compared with the 5 × 107 group).
Ability to reject third-party hearts. Hearts from third-party C3H/HeJ newborn mice (H2k) were transplanted into pouches created in the pinnae of BALB/c (H2d) recipients of C57BL/6 (H2b) T-cell-depleted bone marrow 14 days after bone marrow transplantation. Graft survival was assessed daily by evaluation of the presence or absence of heart contractions. The hearts were followed for 60 days after heart transplantation. The comparisons were among all different groups. The results were pooled from 2 independent experiments. TCD BM indicates T-cell-depleted bone marrow.
Ability to reject third-party hearts. Hearts from third-party C3H/HeJ newborn mice (H2k) were transplanted into pouches created in the pinnae of BALB/c (H2d) recipients of C57BL/6 (H2b) T-cell-depleted bone marrow 14 days after bone marrow transplantation. Graft survival was assessed daily by evaluation of the presence or absence of heart contractions. The hearts were followed for 60 days after heart transplantation. The comparisons were among all different groups. The results were pooled from 2 independent experiments. TCD BM indicates T-cell-depleted bone marrow.
Discussion
In the current study, we demonstrated that hematopoietic stem cell dose correlates directly with the speed of phenotypic (Tables 1, 2, 3, 4; Figures 2, 3) and functional (Figures 4, 5) immune reconstitution after transplantation. Higher numbers of stem cells resulted in more rapid T-cell (Tables 1, 2, 3, 4; Figures 2, 3) and B-cell (Tables 1, 2, 3) recovery. Since the delay in T-cell recovery is a major problem after hematopoietic cell transplantation,5 this study was focused on whether and how higher stem cell dose could result in more rapid T-cell recovery.
Immune function relies not only on the number (quantity) but also on the function (quality) of immunocompetent cells. Therefore, T cells were followed quantitatively and qualitatively following transplantation of different doses of bone marrow stem cells in this study. Phenotypic analyses in both congenic (Table 2; Figure 2) and allogeneic (Table 1; Figure 2) settings demonstrated that more T-cell-depleted bone marrow cells infused are associated with more rapid regeneration of T cells. Further experiments using purified stem cells (Tables 3, 4) directly demonstrated that hematopoietic stem cell dose correlates with the speed of phenotypic T- and B-cell recovery. To further determine whether there is an effect of stem cell dose on functional T-cell recovery, the ability of T cells to secrete cytokines was measured at a per-cell level. As demonstrated in Figure 4, both CD4+ and CD8+ T cells from different T-cell-depleted bone marrow or stem cell dose groups produced comparable amounts of IL-2 and IFN-γ, suggesting that the quality of regenerated T cells is not affected by the stem dose. Increased quantity with equal quality at a per-cell level suggests that higher numbers of stem cells may result in more potent immune responses as a whole. Indeed, we directly demonstrated in vivo (Figure 5) that higher doses of T-cell-depleted bone marrow cells dramatically shorten the time required for rejection of third-party heart grafts in an allogeneic transplantation model. These data suggest that higher doses of stem cells may hasten the immune recovery and therefore may shorten the time of exposure to life-threatening opportunistic infections in stem cell transplant recipients. However, it is important to point out that there may be an upper limit for this effect. This effect is not a result of enhanced myeloid engraftment because by the time we first detected donor-derived T cells in the peripheral blood (day +28) in the KTLS recipients, the peripheral blood counts including white cells, hematocrit, and platelets have recovered to the pretransplantation level even in the recipients of as few as 100 KTLS cells.12 Moreover, both myeloid and B cells were fully donor chimeric at least on day +14 in allogeneic recipients and on day +28 in congenic recipients (Figure 2).
The most efficient way to reconstitute the T-cell repertoire after hematopoietic cell transplantation is via the thymus.28 Using this pathway, new T cells are generated from donor hematopoietic stem/progenitor cells via thymopoiesis. This recapitulation of T-cell ontogeny involves rearrangement of T-cell receptor genes and positive and negative selection in the thymus.5,28 T cells generated by thymopoiesis maintain repertoire diversity and immunocompetence to a variety of antigens. Increase in naive T-cell counts (Table 4) suggests that this pathway is used in hematopoietic stem cell recipients. Increased numbers of donor-derived total thymocyte numbers (Figure 3A) and total sjTRECs per thymus (Figure 3B) directly demonstrate that transplantation of higher numbers of hematopoietic stem cells enhances thymopoiesis. This conclusion is further supported by the observation that only donor- but not host-type T cells were increased over time after purified stem cell transplantation (Tables 3, 4; Figure 2). Unchanged proportion of thymocyte subsets (Figure 3C) and similar numbers of sjTRECs per 100 000 thymocytes (Figure 3D) between different stem cell dose groups suggest that the machinery of T-cell receptor gene rearrangement is not altered with more stem cells infused. Taken together, we conclude that the accelerated recovery of T cells is simply a result of more input of lymphoid stem cells and/or T-cell precursors into the thymus.
The accelerated recovery of T cells after hematopoietic cell transplantation can also be a result of expansion of host radioprotective T cells and/or generation of new T cells in the host thymus from residual host stem cells.28 However, these pathways are not important in the acceleration of T-cell recovery with higher numbers of stem cells since only the donor- and not host-type T cells increased over time (Tables 1, 2, 3, 4; Figure 2). The thymic-independent pathway cannot be completely excluded. However, this pathway is generally considered to contribute minimally to the regeneration of classical peripheral T cells,29 especially as thymopoiesis was robust in all stem cell recipients (Figure 3).
One of the biggest concerns of such a strategy is whether it would also increase the incidence of GVHD in the allogeneic setting. However, neither clinical nor histologic GVHD was observed in any hematopoietic cell recipients (data not shown). The lack of GVHD is a consequence of a lack of or limited numbers of mature T cells in the stem cell graft and because host-reactive CD4+ T cells derived from stem cells are presumably deleted in the host thymus (negative selection) regardless of stem cell dose.30 As long as no mature T cell is present in the graft, the risk of GVHD will not increase with more stem cells infused.
In summary, we have demonstrated that more stem cells are associated with more rapid immune reconstitution after both allogeneic and congenic hematopoietic stem cell transplantation. The accelerated T-cell recovery is a result of enhanced thymopoiesis and regeneration of donor-type T cells in the thymus. A higher number of stem cells through either ex vivo expansion31 or a combination of stem cell units from different donors32 may speed up the immune reconstitution after hematopoietic cell transplantation, especially in adult umbilical cord blood transplantation, in which marked delay in T-cell recovery was observed probably due to insufficient numbers of available stem cells.9 Indeed, it has already been shown that a higher dose of CD34+ peripheral blood cells results in significantly faster T-cell recovery.33
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-07-2534.
Supported by National Institutes of Health grant P01-HL67314.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Dr John F. Whitesides and Patrice McDermott from the Duke Human Vaccine Institute Flow Cytometry Core Facility (supported by National Institutes of Health grant AI-51445) for the cell sorting.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal