Abstract
Thymus-derived regulatory T lymphocytes of CD4+CD25+ phenotype regulate a large variety of beneficial and deleterious immune responses and can inhibit lethal graft-versus-host disease in rodents. In vitro, CD4+CD25+ T cells require specific major histocompatibility complex (MHC)/peptide ligands for their activation, but once activated they act in an antigen-nonspecific manner. In vivo, regulatory T cells are also activated in an antigen-specific fashion, but nothing is known about antigen specificity of their suppressor-effector function. Here we show that CD4+CD25+ regulatory T lymphocytes isolated from naive mice and activated in vitro with allogeneic antigen-presenting cells (APCs) induced specific long-term tolerance to bone marrow grafts disparate for major and minor histocompatibility antigens; whereas “target” bone marrow was protected, third-party bone marrow was rejected. Importantly, in mice injected with a mix of target and third-party bone marrows, protection and rejection processes took place simultaneously. These results indicate that CD4+CD25+ regulatory T cells can act in an antigen-specific manner in vivo. Our results suggest that CD4+CD25+ regulatory T cells could in the future be used in clinical settings to induce specific immunosuppression. (Blood. 2004;103:4216-4221)
Introduction
Due to the random rearrangements of genes encoding T- and B-lymphocyte antigen receptors, a significant number of autospecific and potentially autoreactive lymphocytes develop in primary lymphoid organs.1-3 Central tolerance (ie, induced in primary lymphoid organs) eliminates (by deletion) or functionally inactivates (by induction of anergy) such dangerous lymphocytes. In absence of central tolerance induction, a strongly self-reactive T-cell repertoire develops.4,5 However, when central tolerance is partially defective, self-tolerance can be maintained by peripheral mechanisms.6-8 Several types of peripheral tolerance mechanisms control lymphocytes having escaped central tolerance and are known to play a crucial role in preventing autoimmunity (for reviews see Sprent et al6 and Stockinger9 ).
One of these peripheral tolerance mechanisms was discovered using the day-3 thymectomy model of multiorgan autoimmunity in mice.10 The pathology can be prevented by injection of CD4+CD25+ lymphocytes, which appear after day 3 of life in the peripheral lymphoid organs of normal mice. CD4+CD25+ regulatory T cells do not only inhibit autoimmunity, they can also inhibit experimental inflammatory bowel disease induced by injection of CD4+CD45RBhigh cells into severe combined immunodeficiency (SCID) mice or recombination-activating gene (RAG)-deficient animals.11 Moreover, they contribute to the fine control of immunity to infectious agents such as parasites and viruses.12,13 An undesired side effect of the activity of CD4+CD25+ regulatory T lymphocytes is the occasional incapacity of the immune system to eliminate tumor cells.14,15 Therefore, regulatory T lymphocytes play a crucial role in the pathophysiologic maintenance of immunologic tolerance (reviewed in Singh et al,11 Sakaguchi,16 Shevach,17 and Bach18 ).
Natural (ie, thymus-derived) CD4+CD25+ regulatory T cells can induce transplantation tolerance in mice. Lethal graft-versus-host disease (GVHD) can be significantly reduced by injection of freshly isolated or ex vivo-cultured regulatory T cells.19-22 In one report, freshly isolated CD4+CD25+ regulatory T cells from naive animals were shown to induce limited tolerance to minor histocompatibility antigen-disparate skin grafts.23 Intrathymic injection of donor-strain antigen results in a tolerant state, which was shown to be due to development of CD4+CD25+ regulatory thymocytes.24
Regulatory T cells with a CD4+CD25+ phenotype are also known to be involved in experimental systems in which tolerance to alloantigens is induced in vivo with antibodies to T-cell surface antigens (eg, CD4, CD8, or CD15423,25-29 ) or with the active form of vitamin D3 and mycophenolate mofetil.30 In these systems, however, it is not clear if thymus-derived regulatory T cells (that can be found in naive animals) are involved or if these cells are induced in the periphery.31,32
Like all other T-cell-receptor αβ (TCRαβ)-expressing T lymphocytes, thymus-derived CD4+CD25+ regulatory T lymphocytes are antigen specific, at least during their activation phase. These cells have been shown to proliferate in an antigen-specific manner in vivo.33-35 Myelin basic protein-specific CD4+CD25+ regulatory T cells protect better against experimental autoimmune encephalomyelitis than regulatory T cells with a restricted (but non-myelin basic protein-specific) TCR repertoire.36 Regulatory T cells activated in vitro with host-type antigen-presenting cells (APCs) inhibit GVHD more potently than cells activated with third-party APCs.21,22 Also, experimentally induced CD4+CD25+ regulatory T cells act in a specific manner and protect target but not third-party allografts.27,29 In vitro, however, CD4+CD25+ regulatory T cells only require interaction with specific major histocompatibility complex (MHC)/peptide complexes during their activation phase. Once activated, their suppressor-effector function is completely non-antigen specific.37 The antigen specificity observed in vivo could therefore potentially be explained by the hypothesis that regulatory T cells are activated only in hosts presenting antigens for which they are specific. In mice in which these antigens are not expressed, the regulatory T cells are not activated and immunosuppression does not take place.
It remains therefore entirely unclear if CD4+CD25+ regulatory T cells are antigen specific during their effector phase in vivo. Such antigen specificity is required for a potential future use of these cells in induction of specific immunosuppression in patients. Here we present an experimental model in which transplanted bone marrow, disparate for major and minor histocompatibility antigens, is protected from rejection by host T cells through injection of CD4+CD25+ regulatory T cells cultured ex vivo. Using this model we show that CD4+CD25+ regulatory T cells can act in an alloantigen-specific manner during their effector phase. The model described here should prove useful in deciphering the in vivo mechanisms of specific immunosuppression mediated by CD4+CD25+ regulatory T lymphocytes. Moreover, our data suggest a clinical potential of these cells in protocols aimed at induction of specific tolerance to allogeneic grafts.
Materials and methods
Mice
All mice were used at 6 to 10 weeks of age. C57BL/6 (B6, H-2b), (C57BL/6xDBA/2)F1 (B6D2F1, H-2bd), and (C57BL/6xCBA/J)F1 (B6CBAF1, H-2bk) mice were purchased from Janvier (Le Genest St Isle, France). All experiments involving animals were performed in compliance with the relevant laws and institutional guidelines (INSERM; approval no. 31-13) and have been approved by the local ethics committee (Midi-Pyrénees, France; ref MP/01/31/10/03).
Purification of T-cell subsets
Erythrocyte-depleted (Lympholite-M; Cedarlane Laboratories, Hornby, ON, Canada) splenocytes were enriched in CD4+ T cells by magnetic depletion with sheep antirat antibody-coated Dynabeads (Dynal Biotech, Oslo, Norway) after incubation with a cocktail of the following rat monoclonal antibodies (mAb): anti-NK1.1 (PK136), anti-CD8 (53.6.7), anti-FcγRIII (2.4G2), and anti-MHC class II molecules (M5). The resulting population was then labeled with phycoerythrin (PE)-labeled anti-CD25 mAb PC61 (BD PharMingen, Heidelberg, Germany; eBioscience, San Diego, CA) and CD4+CD25+ cells enriched with anti-PE microbeads using AutoMACS (Miltenyi Biotec, Paris, France). Cell purity was checked by flow cytometry on a FACSCalibur (BD Biosciences, San Jose, CA) using PE-labeled anti-CD25 mAb PC61 and fluorescein isothiocyanate (FITC)-labeled anti-CD4 (GK1.5). Positively sorted CD4+CD25+ T cells were routinely more than 95% pure.
CD4+ or CD8+ T cells were similarly isolated using negative selection with Dynabeads (PK136, 2.4G2, M5, and 53.6.7 or GK1.5) followed by positive selection on AutoMACS (with PE-labeled anti-CD4 mAb GK1.5 or anti-CD8 mAb 53.6.7; BD Pharmingen; eBioscience). Purity of populations routinely exceeded 98%.
Ex vivo culture of CD4+CD25+ T cells
CD4+CD25+ T cells (2 × 103/well) from B6 mice were cocultured with 5 × 105 γ-irradiated (17 Gy [1700 rad]) total splenocytes from B6D2F1 or B6CBAF1 mice in 96-well round-bottom plates for 14 days. Cells were cultured in RPMI 1640 (Eurobio, Les Ulis, France) supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, penicillin, streptomycin, 10 mM Hepes (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 50 μM 2-mercaptoethanol (2-ME), 1 mM nonessential amino acids, 1 mM sodium pyruvate, and 100 U/mL interleukin-2 (IL-2; supernatant of phorbol myristate acetate [PMA]-stimulated EL4.IL-2 cells; American Type Culture Collection [ATCC], Manassas, VA). At day 7, 100 μL fresh medium was added and cells were cultured for another 7 days.
Bone marrow chimeras
Bone marrow from femurs and tibias was collected in Dulbecco modified Eagle medium (DMEM; Eurobio) supplemented with 10% FCS, 2 mM l-glutamine, penicillin, and streptomycin. Single-cell suspensions were washed in complete medium. Thy1+ and NK1.1+ cells were eliminated using AT83 and PK136 Abs, respectively, and rabbit complement (Saxon Europe, Suffolk, United Kingdom). Cells (5 × 106) from each donor were then injected intravenously into γ-irradiated hosts (8.5 Gy [850 rad]; 137Cs source, 6.3 Gy/min [630 rad/min]) that were kept on antibiotic-containing water (0.28% pediatric suspension of bactrim; Roche, Basel, Switzerland) for the complete duration of the experiment. Effector and/or regulatory T cells were coinjected with bone marrow preparations.
Fluorescence-activated cell sorter (FACS) analysis
For analysis of bone marrow from femurs and tibias, mice were killed 15 days after bone marrow and T-cell transfer. For peripheral blood mononuclear cell (PBMC) analysis, blood samples were taken at indicated time points. Cells were labeled with anti-H-2Kb-FITC and anti-H-2Kd-PE or with anti-H-2Kk-FITC and anti-H-2Kb-PE (BD Pharmingen; eBioscience). The relative percentages of semiallogeneic cells shown in the figures are calculated as follows: (% of semiallogeneic cells in experimental mouse/mean % of semiallogeneic cells in mice injected with bone marrow only) × 100.
Results
Ex vivo-expanded CD4+CD25+ regulatory T lymphocytes inhibit CD4+ and CD8+ T-cell-mediated rejection of allogeneic bone marrow
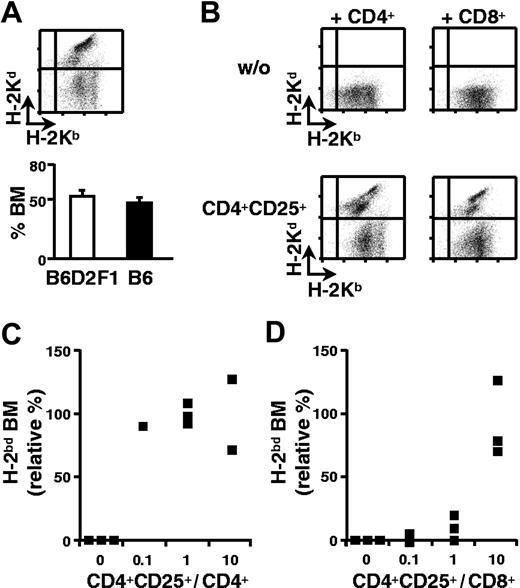
We have adapted our previously described mouse model for bone marrow transplantation in which B6 (H-2b) hosts are lethally irradiated and reconstituted with a 1:1 mixture of syngeneic (B6) and semiallogeneic B6D2F1 (H-2bd) bone marrow.4,8 Equivalent percentages of syngeneic and allogeneic cells were found in the bone marrow 2 weeks after transfer (Figure 1A). When host-type CD4+ T cells were coinjected, the semiallogeneic bone marrow was eliminated and the retained syngeneic bone marrow allowed for survival of the host (Figure 1B). Since we have been unable to induce tolerance to semiallogeneic bone marrow with freshly isolated regulatory T cells (data not shown), we evaluated the regulatory potential of CD4+CD25+ T cells activated and expanded in vitro. Purified B6 CD4+CD25+ splenic T cells were cultured in vitro with B6D2F1 APCs in the presence of IL-2. In 2-week cultures, a 10- to 20-fold expansion was reproducibly obtained (data not shown). When such in vitro-cultured regulatory T cells were coinjected with B6 CD4+ T lymphocytes, rejection of semiallogeneic bone marrow was inhibited very efficiently, even at a ratio of 1 regulatory to 10 CD4+ effector T cells (Figure 1B-C).
CD4+CD25+ regulatory T cells preactivated in vitro induce CD4+ and CD8+ T-cell tolerance to semiallogeneic bone marrow. B6 mice were lethally irradiated and grafted with a 1:1 mixture of B6 (H-2b) and B6D2F1 (H-2bd) bone marrow (5 × 106 cells each). Two weeks later equivalent percentages of syngeneic and semiallogeneic bone marrow were observed in bone marrow (A). Irradiated mice were coinjected with the 2 types of bone marrow and 3 × 105 CD4+ (panel B, left column; panel C) or CD8+ T cells (panel B, right column; panel D) with (panel B, bottom row) or without (panel B, top row) CD4+CD25+ regulatory T cells from B6 mice in vitro preactivated with B6D2F1 APCs (panel B; regulatory-to-effector T-cell ratio = 10; panels C-D as indicated). Mice were analyzed 2 weeks later. (Panel A, top; panel B) FACS analysis of bone marrow, (panels C-D) relative percentage (see “Materials and methods”) of semiallogeneic bone marrow. ▪ indicates individual mice from a representative experiment of 4 performed. Error bars in panel A indicate SEM.
CD4+CD25+ regulatory T cells preactivated in vitro induce CD4+ and CD8+ T-cell tolerance to semiallogeneic bone marrow. B6 mice were lethally irradiated and grafted with a 1:1 mixture of B6 (H-2b) and B6D2F1 (H-2bd) bone marrow (5 × 106 cells each). Two weeks later equivalent percentages of syngeneic and semiallogeneic bone marrow were observed in bone marrow (A). Irradiated mice were coinjected with the 2 types of bone marrow and 3 × 105 CD4+ (panel B, left column; panel C) or CD8+ T cells (panel B, right column; panel D) with (panel B, bottom row) or without (panel B, top row) CD4+CD25+ regulatory T cells from B6 mice in vitro preactivated with B6D2F1 APCs (panel B; regulatory-to-effector T-cell ratio = 10; panels C-D as indicated). Mice were analyzed 2 weeks later. (Panel A, top; panel B) FACS analysis of bone marrow, (panels C-D) relative percentage (see “Materials and methods”) of semiallogeneic bone marrow. ▪ indicates individual mice from a representative experiment of 4 performed. Error bars in panel A indicate SEM.
Rejection of allogeneic grafts is also mediated by CD8+ T lymphocytes. It was therefore important to assess if regulatory T cells could inhibit CD8+ T cells in vivo. Lethally irradiated B6 mice were reconstituted with a mixture of syngeneic and semiallogeneic bone marrow cells. They were simultaneously injected with purified CD8+ T lymphocytes with or without preactivated regulatory T cells. The data shown in Figures 1B and 1D indicate that CD8+ T lymphocytes fully rejected semiallogeneic bone marrow and that coinjection of in vitro-expanded regulatory T cells (at a ratio of 10 CD4+CD25+ to 1 CD8+ T cell) could inhibit this rejection. The higher ratios of regulatory T lymphocytes required to inhibit graft rejection by CD8+ (compared with CD4+) T cells are probably due to the much higher efficiency of CD8+ cells in graft rejection in our system (data not shown). In conclusion, CD4+CD25+ regulatory T lymphocytes are capable of inhibiting in vivo alloreactivity of CD4+ as well as of CD8+ T cells.
Regulatory T cells induce durable tolerance of total splenocytes to allogeneic bone marrow
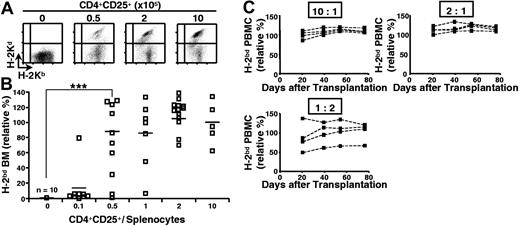
We then assessed if regulatory T lymphocytes could also inhibit semiallogeneic bone marrow rejection by total splenocytes. Lethally irradiated B6 hosts were reconstituted with a mixture of syngeneic and semiallogeneic bone marrow cells, injected with B6 splenocytes, and coinjected (or not) with titrated numbers of B6 CD4+CD25+ T lymphocytes that had been preactivated with B6D2F1 APCs. As shown in Figures 2A-B, coinjected regulatory T cells inhibited semiallogeneic bone marrow rejection in a dose-dependent fashion. Already at a CD4+CD25+ regulatory T-cell-tosplenocyte ratio of 1:2, significant protection of semiallogeneic bone marrow was observed.
Preactivated CD4+CD25+ regulatory T cells durably prevent semiallogeneic bone marrow rejection by total splenocytes. B6 mice were lethally irradiated and grafted with a 1:1 mixture of B6 (H-2b) and B6D2F1 (H-2bd) bone marrow (5 × 106 cells each) and coinjected with 105 B6 splenocytes and regulatory T cells preactivated with B6D2F1 APCs in vitro (regulatory-to-effector T-cell ratio as indicated). Bone marrow was analyzed 2 weeks later (A-B; combined data from 4 independent experiments), and PBMCs were analyzed at indicated time points (C; representative individual mice). (A) FACS analysis of bone marrow. (B-C) Relative percentage of semiallogeneic cells (see “Materials and methods”). ***P < .001 (Student t test). Horizontal bars in panel B indicate the mean.
Preactivated CD4+CD25+ regulatory T cells durably prevent semiallogeneic bone marrow rejection by total splenocytes. B6 mice were lethally irradiated and grafted with a 1:1 mixture of B6 (H-2b) and B6D2F1 (H-2bd) bone marrow (5 × 106 cells each) and coinjected with 105 B6 splenocytes and regulatory T cells preactivated with B6D2F1 APCs in vitro (regulatory-to-effector T-cell ratio as indicated). Bone marrow was analyzed 2 weeks later (A-B; combined data from 4 independent experiments), and PBMCs were analyzed at indicated time points (C; representative individual mice). (A) FACS analysis of bone marrow. (B-C) Relative percentage of semiallogeneic cells (see “Materials and methods”). ***P < .001 (Student t test). Horizontal bars in panel B indicate the mean.
In these experiments, bone marrow engraftment was analyzed after 2 weeks. To assess if the tolerance was durable, we reconstituted lethally irradiated B6 mice with syngeneic and semiallogeneic bone marrow, coinjected them with B6 splenocytes and preactivated B6 regulatory T cells, and analyzed peripheral blood mononuclear cells from 1 to 11 weeks after reconstitution. Figure 2C shows that the tolerance was durable: up to 11 weeks after transfer no signs of rejection were observed at regulatory T-cell-splenocyte ratios of 2:1 and 10:1. At a regulatory T-cell-splenocyte ratio of 1:2, levels of protection were quite variable among the 4 animals analyzed. Interestingly, once established, the percentage of allogeneic cells in PBMCs did not significantly change during the period in which the animals were analyzed. Therefore, even partial protection appears to be stable in time. We are currently investigating the underlying mechanisms.
Preactivated regulatory T cells protect allogeneic bone marrow in an MHC-haplotype-specific manner
We next evaluated if the protection of allogeneic grafts by activated regulatory T cells was specific. B6 CD4+CD25+ T cells were preactivated in vitro with B6D2F1 (H-2bd) APCs in the presence of IL-2. These cells were then injected into lethally irradiated B6 hosts together with B6 splenocytes, B6 bone marrow, and B6D2F1 (H-2bd) or B6CBAF1 (H-2bk) bone marrow. The results in Figure 3A show that B6D2F1 bone marrow was more efficiently protected than B6CBAF1 cells, most notably at lower regulatory T-cell-to-splenocyte ratios.
Prevention of semiallogeneic bone-marrow rejection mediated by CD4+CD25+ regulatory T cells is MHC-haplotype specific. B6 mice were lethally irradiated, grafted with a 1:1 mixture of B6 (H-2b) plus B6D2F1 (H-2bd) or B6CBAF1 (H-2bk) bone marrow (5 × 106 cells each), and coinjected with 105 B6 splenocytes and with regulatory T cells from B6 mice preactivated with B6D2F1 (A) or B6CBAF1 (B) APCs in vitro (regulatory-to-effector T-cell ratios as indicated). Bone marrow was analyzed 2 weeks later. Relative percentage of semiallogeneic H-2bd (□) or H-2bk (▪) cells in the individual mice is indicated (see “Materials and methods”). *P < .05 (Student t test). Combined data from 3 independent experiments are shown. Horizontal bars indicate the mean.
Prevention of semiallogeneic bone-marrow rejection mediated by CD4+CD25+ regulatory T cells is MHC-haplotype specific. B6 mice were lethally irradiated, grafted with a 1:1 mixture of B6 (H-2b) plus B6D2F1 (H-2bd) or B6CBAF1 (H-2bk) bone marrow (5 × 106 cells each), and coinjected with 105 B6 splenocytes and with regulatory T cells from B6 mice preactivated with B6D2F1 (A) or B6CBAF1 (B) APCs in vitro (regulatory-to-effector T-cell ratios as indicated). Bone marrow was analyzed 2 weeks later. Relative percentage of semiallogeneic H-2bd (□) or H-2bk (▪) cells in the individual mice is indicated (see “Materials and methods”). *P < .05 (Student t test). Combined data from 3 independent experiments are shown. Horizontal bars indicate the mean.
Since this specific protection might hypothetically be due to differences in the kinetics and/or potency of rejection of the 2 types of bone marrow, we also performed the reciprocal experiment and tested if B6 regulatory T cells activated in vitro with B6CBAF1 APCs acted in a specific manner (Figure 3B). At a regulatory T-cell-to-splenocyte ratio of 0.5, B6CBAF1 bone marrow was more efficiently protected than B6D2F1 cells. When using higher regulatory T-cell-to-splenocyte ratios the specificity was lost.
Regulatory T cells protect target bone marrow while allowing third-party alloreactivity to develop simultaneously
Since the observed specificity in the protection of allogeneic bone marrow might be due to several factors other than specificity in the effector function of regulatory T cells (eg, differential survival, homeostatic expansion, or activation in the distinct experimental mice), we then tested if the specificity was maintained when both types of semiallogeneic bone marrow (ie, B6D2F1 and B6CBAF1) were transferred (together with syngeneic bone marrow) into the same host. When the 3 types of bone marrow were injected without effector T cells, practically equivalent percentages of syngeneic (B6) and the 2 types of allogeneic (B6D2F1 and B6CBAF1) cells were found in bone marrow 2 weeks later (Figure 4A). As shown in Figures 4B (top) and 4C, addition of syngeneic effector splenocytes resulted in the clearance of both allogeneic populations, and regulatory T cells preactivated with B6D2F1 APCs preferentially inhibited rejection of B6D2F1 (compared with B6CBAF1) bone marrow, especially at lower regulatory-to-effector T-cell ratios. At higher regulatory-to-effector T-cell ratios, specificity was gradually lost and cells of both semiallogeneic origins were efficiently preserved.
Simultaneous protection of target bone marrow and rejection of third-party cells. B6 mice were lethally irradiated; grafted with a 1:1:1 mixture of B6 (H-2b), B6D2F1 (H-2bd), and B6CBAF1 (H-2bk) bone marrow (5 × 106 cells each); and coinjected with 105 B6 splenocytes and regulatory T cells preactivated with B6D2F1 or B6CBAF1 APCs in vitro (as indicated, regulatory T-cell-to-splenocyte ratios as indicated). Bone marrow was analyzed 2 weeks later. (A) Percentages of the 3 types of bone marrow recovered from control mice (ie, mice that had not received splenocytes). Error bars indicate SD. (B) H-2Kd and H-2Kk expression by bone marrow cells recovered from individual mice that had received bone marrow, splenocytes, and titrated numbers of preactivated regulatory T cells, as indicated. Numbers above the bars indicate the percentage of cells within the gate. (C) Summary of all data depicted as ratios of relative percentages of H-2bk to H-2bd bone marrow cells. □ indicates mice injected with regulatory T cells preactivated with H-2bd APCs; ▪, mice injected with regulatory T cells activated with H-2bk APCs. **P < .01 (Wilcoxon test). Combined data from 3 independent experiments are shown. Horizontal bars indicate the mean.
Simultaneous protection of target bone marrow and rejection of third-party cells. B6 mice were lethally irradiated; grafted with a 1:1:1 mixture of B6 (H-2b), B6D2F1 (H-2bd), and B6CBAF1 (H-2bk) bone marrow (5 × 106 cells each); and coinjected with 105 B6 splenocytes and regulatory T cells preactivated with B6D2F1 or B6CBAF1 APCs in vitro (as indicated, regulatory T-cell-to-splenocyte ratios as indicated). Bone marrow was analyzed 2 weeks later. (A) Percentages of the 3 types of bone marrow recovered from control mice (ie, mice that had not received splenocytes). Error bars indicate SD. (B) H-2Kd and H-2Kk expression by bone marrow cells recovered from individual mice that had received bone marrow, splenocytes, and titrated numbers of preactivated regulatory T cells, as indicated. Numbers above the bars indicate the percentage of cells within the gate. (C) Summary of all data depicted as ratios of relative percentages of H-2bk to H-2bd bone marrow cells. □ indicates mice injected with regulatory T cells preactivated with H-2bd APCs; ▪, mice injected with regulatory T cells activated with H-2bk APCs. **P < .01 (Wilcoxon test). Combined data from 3 independent experiments are shown. Horizontal bars indicate the mean.
Again, to exclude the hypothetical possibility that the observed specificity was due to differences in the kinetics and/or potency of rejection of the 2 types of bone marrow, we then performed the reciprocal experiment. Regulatory T cells preactivated with B6CBAF1 cells preferentially protected B6CBAF1 (compared with B6D2F1) bone marrow, again most notably at lower regulatory-to-effector T-cell ratios (Figures 4B bottom and 4C). These results definitively show that preactivated regulatory T cells can act in an antigen-specific manner during their effector function in vivo.
Discussion
Like all other T lymphocytes, CD4+CD25+ regulatory T cells are antigen specific and are thought to act in an antigen-specific manner in vivo. In vitro, however, antigen specificity of these cells is limited to the activation phase, while the effector phase is completely antigen nonspecific. While in several in vivo models regulatory T cells have been shown to act in an antigen-specific manner, in none of these systems has a distinction been made between antigen-specific activation and antigen-specific suppressor-effector function.
Using a novel experimental system in which allogeneic bone marrow is durably protected from rejection by in vitro-activated regulatory T cells, we show here that regulatory T cells can act in an antigen-specific manner during their effector phase in vivo. Our results suggest that CD4+CD25+ regulatory T cells could become a powerful tool to induce specific tolerance to allogeneic grafts in clinical settings.
CD4+CD25+ regulatory T cells have been used in a variety of systems to inhibit immunopathology. All these models have in common that injection of T lymphocytes must be followed by homeostatic proliferation. In the colitis model, disease is induced in lymphopenic mice.11 GVHD models rely on lethally irradiated recipients, which are therefore lymphopenic.19-21,38 The induction of transplantation tolerance (GVHD or graft rejection) with experimentally induced CD4+CD25+ regulatory T cells has also been studied in lymphopenic mice.23,25-29 In the experimental models of autoimmunity induced by day-3 thymectomy or in nude mice, homeostatic expansion of injected T lymphocytes also certainly occurs.10 Since regulatory T cells are known to regulate homeostatic expansion and to expand themselves in immunodeficient mice,39,40 it has been suggested that regulatory T cells could outcompete pathogenic T cells during the expansion phase and thus inhibit immunopathology.41 Such a scenario could also explain the regulatory T-cell-induced tolerance we observed when mice were injected with only one type of allogeneic bone marrow (target or third-party). In experiments in which the mice were coinjected with 2 types of allogeneic bone marrow, however, target bone marrow was preferentially protected (notably at low regulatory-to-effector T-cell ratios). Therefore, in contrast to the above-mentioned reports, in our system tolerance cannot be explained by differential homeostatic expansion of alloreactive versus innocuous regulatory T cells.
T-cell tolerance induced by CD4+CD25+ regulatory T lymphocytes has never before been shown to be antigen specific in the effector phase. In GVHD and transplantation models, tolerance was studied in separate hosts.21,22,27,29 Since homeostatic proliferation of regulatory T cells depends upon MHC class II expression40 and potentially even upon interaction with specific antigen,33 antigen specificity in vivo may be due to lack of homeostatic proliferation in animals lacking the “target” tissue. In these models, antigen specificity is probably also due to the absence of specific ligands capable of activating the suppressor-effector function of regulatory T lymphocytes. These hypothetical explanations may also apply to the specific tolerance we observed when target and third-party bone marrows were injected (together with splenocytes and regulatory T cells) into separate hosts. However, we also observed preferential protection of target bone marrow in mice in which third-party cells were simultaneously rejected. One could argue that the specific protection was due to differences in the kinetics and/or potency of rejection of the 2 types of bone marrow, but such objections can be ruled out since identical results were obtained in reciprocal experiments. Since target bone marrow was protected in these mice, regulatory T cells had clearly been activated but protected third-party bone marrow much less efficiently. Therefore, our data directly show that CD4+CD25+ regulatory T cells can act in an antigen-specific manner during their effector phase.
Several reason(s) could explain why protection is not entirely antigen specific, especially at higher regulatory-to-effector T-cell ratios. Since this lack of specificity was also observed in mice in which target bone marrow was absent, regulatory T cells are sufficiently cross-reactive with third-party antigens to cause partial protection. Such a cross-reactivity may in part be due to “indirect antigen presentation” by host-type MHC molecules that are expressed by both types of semiallogeneic bone marrows. Direct cross-reactivity toward allogeneic MHC molecules most likely also plays an important role.3 In contrast to the C57BL/6 hosts we used, both donor strains (B6D2F1 and B6CBAF1) present several mouse mammary tumor virus-encoded endogenous superantigens.42 Therefore, superantigen reactivity of injected regulatory T cells may cause very significant cross-reactivity. In the mice in which we injected both types of allogeneic bone marrow, a contribution of non-antigen-specific “bystander” mechanisms formally cannot be excluded. In any case, our data directly indicate that immunosuppression mediated by CD4+CD25+ regulatory T cells can act in an antigen-specific manner, not only in the activation but also very importantly during the in vivo effector phase. It will be important to assess if the antigen-specific suppressor-effector function of CD4+CD25+ T cells will allow for clearance of infection of donor-type cells (eg, by viruses).
Allogeneic bone marrow or hematopoietic stem cell transplantation is extensively used to correct hereditary defects such as primary immunodeficiencies, metabolic diseases, β-thalassemia major, and sickle cell anemia (for review see Sullivan et al43 ). These protocols require very frequent transfusions and/or immuno-suppression to obtain and maintain hematopoietic chimerism. Since immunosuppressive drugs do not act in an antigen-specific manner, their use is associated with opportunistic infections and increased tumor incidence. Patients suffering from the abovementioned congenital diseases would benefit from the availability of specific immunosuppressive protocols. The results we have obtained with our experimental model indicate that specific immunosuppression can be achieved with CD4+CD25+ regulatory T lymphocytes. They should open the way to development of clinical approaches aimed at induction of specific and durable tolerance to bone marrow and hematopoietic stem cell allografts. Moreover, regulatory T cells could potentially also be used to induce specific and durable immunologic tolerance to organ allografts, and we are currently investigating this intriguing possibility.
In conclusion, the data presented here indicate that regulatory T cells can induce antigen-specific immunosuppression. It will be interesting to assess how antigen-specific in vivo tolerance is achieved. The model we describe here may prove particularly useful in addressing this and other questions concerning the in vivo functioning of CD4+CD25+ regulatory T cells. Moreover, our results suggest the feasibility of development of clinical protocols in which CD4+CD25+ regulatory T lymphocytes would be used to induce specific tolerance to allogeneic hematopoietic stem cell or organ grafts.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2004-01-0005.
Supported by grants from the “Etablissement Français des Greffes” and the “Région Midi-Pyrénées.”
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to acknowledge the staff of the animal facility for excellent animal care; Carine Chanut for expert technical assistance; Drs Jean-Charles Guéry, Etienne Joly, and Abdelhadi Saoudi for critical reading of the manuscript; Drs Ronald N. Germain and Guy Laurent for helpful discussions; and Dr Clemens Utzny for advice on statistical analysis.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal