Abstract
Activated protein C (APC) resistance is a major risk factor for venous thrombosis. Factor V (FV) gene mutations like FVLeiden (R506Q) and FVR2 (H1299R) may cause APC resistance either by reducing the susceptibility of FVa to APC-mediated inactivation or by interfering with the cofactor activity of FV in APC-catalyzed FVIIIa inactivation. We quantified the APC cofactor activity expressed by FVLeiden and FVR2 and determined the relative contributions of reduced susceptibility and impaired APC cofactor activity to the APC resistance associated with these mutations. Plasmas containing varying concentrations of normal FV, FVLeiden, or FVR2 were assayed with an APC resistance assay that specifically measures the APC cofactor activity of FV in FVIIIa inactivation, and with the activated partial thromboplastin time (aPTT)-based assay, which probes both the susceptibility and APC cofactor components. FVR2 expressed 73% of the APC cofactor activity of normal FV, whereas FVLeiden exhibited no cofactor activity in FVIIIa inactivation. Poor susceptibility to APC and impaired APC cofactor activity contributed equally to FVLeiden-associated APC resistance, whereas FVR2-associated APC resistance was entirely due to the reduced APC cofactor activity of FVR2. Thrombin generation assays confirmed the importance of the anticoagulant activity of FV and indicated that FVLeiden homozygotes are exposed to a higher thrombotic risk than heterozygotes because their plasma lacks normal FV acting as an anticoagulant protein.
Introduction
The protein C pathway is a major anticoagulant mechanism that down-regulates the prothrombin- and intrinsic factor X (FX)-activating complexes via inactivation of their respective cofactors, activated factors V (FVa) and VIII (FVIIIa).1 Cofactor inactivation occurs by limited proteolysis at amino acid positions 306, 506, and 679 in FVa 2 and 336, 562, and 740 in FVIIIa.3 These reactions are catalyzed by the serine protease activated protein C (APC) and stimulated by the APC cofactor protein S. In vitro experiments using purified proteins have shown that FV also stimulates the APC-mediated inactivation of FVIIIa.4-6 To express full APC cofactor activity, FV must retain (part of) the B domain4,6,7 and be cleaved by APC at Arg506.8 Although this anticoagulant function of FV is still poorly characterized, a recent report suggests that it plays a critical antithrombotic role in vivo.9
Functional defects of the protein C pathway, due to inherited or acquired conditions, determine a plasma phenotype known as APC resistance,10 which is a prevalent and important risk factor for venous thrombosis.11-13 A plasma is termed “APC resistant” when the addition of exogenous APC fails to prolong its clotting time in an activated partial thromboplastin time (aPTT) assay.10 Since the discovery of APC resistance, several other methods have been developed to detect this condition and to study the underlying molecular mechanism.
To date, a few FV gene mutations have been identified in association with APC resistance. In principle, these mutations may cause APC resistance either by reducing the susceptibility of FVa to APC-mediated inactivation (susceptibility component) or by interfering with the APC cofactor activity of FV in FVIIIa inactivation (APC cofactor activity component). The FV R506Q mutation (FVLeiden14 ) is the major cause of hereditary APC resistance in the white population. Due to the loss of the APC cleavage site at Arg506, FV(a)Leiden is less susceptible to inactivation by APC15,16 and expresses reduced APC cofactor activity in FVIIIa inactivation.8,17 The common FV gene haplotype marked by the H1299R (R2) polymorphism18,19 has also been reported to cause mild APC resistance, particularly in the homozygous condition.19,20 Although the underlying molecular mechanism remains unknown, it has been shown that FV(a)R2 is inactivated by APC at the same rate as normal FVa, whereas its cofactor activity in APC-mediated FVIIIa inactivation is impaired.20 The FV R306T (FVCambridge21 ) and FV R306G (FVHong Kong22 ) mutations, which affect the APC cleavage site at Arg306, are rather rare and confer only mild APC resistance.23,24 Among all these mutations, only FVLeiden has been conclusively associated with an increased risk of venous thrombosis,25 whereas FVR2 has been reported to increase FVLeiden-related thrombosis risk in doubly heterozygous individuals.26,27
Although the relative weight of the susceptibility and APC cofactor activity components in FVLeiden-associated APC resistance has never been quantified,28 it is generally believed that resistance of FVaLeiden to APC-mediated inactivation is the predominant underlying defect.29 In the present study we have dissected the relative contributions of the susceptibility and APC cofactor activity components to the APC resistance associated with the FVLeiden and FVR2 mutations. Our findings not only indicate that poor APC cofactor activity of FV is a major component of APC resistance, but they also provide evidence for an important role of the anticoagulant function of FV in the in vivo regulation of thrombin formation.
Materials and methods
Plasma samples
Venous blood was drawn in 129 mM sodium citrate (9:1 vol/vol). Platelet-poor plasma was obtained by centrifugation at 3000g (25 minutes at room temperature) followed by a second centrifugation at 20 000g (30 minutes at 4°C), after which samples were aliquoted, snap-frozen in liquid nitrogen, and stored at -80°C until use.
A normal plasma pool was prepared by pooling the plasma of 74 healthy blood donors. Moreover, plasma was obtained from 2 FVR2 homozygotes and 2 FVLeiden homozygotes. All blood samples were collected from consenting individuals in accordance with the Helsinki protocol. FV genotypes were ascertained by polymerase chain reaction (PCR) amplification of genomic DNA followed by restriction analysis (R506Q and H1299R mutations). The FVR2-homozygous individuals were homozygous for the whole HR2 haplotype, as determined by direct sequencing of FV exons 8, 13, 16, and 25. The demographic characteristics of the subjects who donated blood, as well as the FV, FVIII, and protein S levels of all plasmas, are shown in Table 1. Congenitally FV-deficient plasma was purchased from George King Bio-Medical (Overland Park, KS).
Definition of sample plasmas
Plasma . | Source . | Age, y . | Sex . | % FV . | % FVIII . | % protein S . |
|---|---|---|---|---|---|---|
| Normal pool | Healthy blood donors (n = 74) | 40.0 (± 8.9 SD) | M/F (45/29) | 100 | 100 | 100 |
| FVR2 homozygote | Healthy blood donor | 55 | M | 77 | 150 | 132 |
| FVR2 homozygote | Coronaropathic patient | 49 | M | 80 | 82 | 104 |
| FVLeiden homozygote | Healthy blood donor | 28 | F | 91 | 76 | 74 |
| FVLeiden homozygote | Thrombotic patient | 75 | M | 121 | 155 | 116 |
| FV-deficient plasma | Commercial (George King) | — | — | < 1 | 118 | 113 |
Plasma . | Source . | Age, y . | Sex . | % FV . | % FVIII . | % protein S . |
|---|---|---|---|---|---|---|
| Normal pool | Healthy blood donors (n = 74) | 40.0 (± 8.9 SD) | M/F (45/29) | 100 | 100 | 100 |
| FVR2 homozygote | Healthy blood donor | 55 | M | 77 | 150 | 132 |
| FVR2 homozygote | Coronaropathic patient | 49 | M | 80 | 82 | 104 |
| FVLeiden homozygote | Healthy blood donor | 28 | F | 91 | 76 | 74 |
| FVLeiden homozygote | Thrombotic patient | 75 | M | 121 | 155 | 116 |
| FV-deficient plasma | Commercial (George King) | — | — | < 1 | 118 | 113 |
— indicates information not provided by the firm.
APC resistance assays
Measurement of APC resistance with the Immunochrom APC Response assay. Normal pooled plasma and individual plasmas from subjects with different FV genotypes (2 FVR2 homozygotes and 2 FVLeiden homozygotes; Table 1), respectively, were mixed with FV-deficient plasma in various proportions, ranging from 2.5% to 100% sample plasma, and assayed with the Immunochrom APC Response kit (Progen Biotechnik, Heidelberg, Germany). This test30 specifically quantifies the ability of APC and its cofactors protein S and FV to inhibit FXa generation by the intrinsic FX-activating complex via inactivation of FVIIIa. Phospholipids, CaCl2, and all relevant proteins (FIXa, FX, and APC) are exogenously added, except for FVIII, protein S, and FV, which are contributed by the plasma. The assay was performed as previously described,31 and the APC-sensitivity ratio (APCsr) was expressed as the ratio of the amounts of FXa generated in the absence and in the presence of APC. A low APCsr indicates a defect in the inactivation of FVIIIa and consequent APC resistance. APCsr's were measured 4 times in duplicate for normal plasma, 2 times in duplicate for FVR2-homozygous plasma, and 3 times in duplicate for FVLeiden-homozygous plasma.
Measurement of APC resistance with the Coatest APC Resistance assay. Dilutions of sample plasmas in FV-deficient plasma were also assayed with the Coatest APC Resistance kit (Chromogenix, Mölndal, Sweden)—that is, the classical aPTT-based APC resistance test that reflects the effect of APC on both FVa and FVIIIa inactivation. The test was performed according to the manufacturer's instructions in an ACL 300 Research coagulometer (Instrumentation Laboratory, Milan, Italy). The APCsr was expressed as the ratio of the clotting times determined in the presence and absence of APC. Also in this assay, a low APCsr indicates APC resistance. APCsr's were measured 6 times in duplicate for normal plasma, 4 times in duplicate for FVR2-homozygous plasma, and 3 times in duplicate for FVLeiden-homozygous plasma.
Statistics. Regression lines in Figures 1 and 2 were calculated according to the least squares method. After performing an analysis of variance (ANOVA) on the regression, the slopes of the normal FV and FVR2 lines were compared using the t test for parallelism of regression lines.
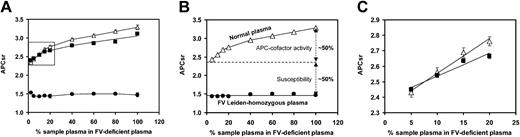
Effect of FV level on the APCsr determined with the Immunochrom APC Response test. The FV level was varied by mixing sample plasma with FV-deficient plasma in different proportions, and the APCsr was determined as described in “Materials and methods.” Each point is the average of 2 or more duplicate experiments, with error bars representing 1 standard error above and below the mean. Δ indicates normal plasma; ▪, FVR2-homozygous plasma; and •, FVLeiden-homozygous plasma.
Effect of FV level on the APCsr determined with the Immunochrom APC Response test. The FV level was varied by mixing sample plasma with FV-deficient plasma in different proportions, and the APCsr was determined as described in “Materials and methods.” Each point is the average of 2 or more duplicate experiments, with error bars representing 1 standard error above and below the mean. Δ indicates normal plasma; ▪, FVR2-homozygous plasma; and •, FVLeiden-homozygous plasma.
Effect of FV level on the APCsr determined with the Coatest APC Resistance assay. (A) Plot of the APCsr as a function of FV concentration. The FV level was varied by mixing sample plasma with FV-deficient plasma in different proportions, and the APCsr was determined as described in “Materials and methods.” (B) The difference in APCsr between normal plasma and FVLeiden-homozygous plasma consists of 2 components: (1) poor susceptibility of FVaLeiden to APC, quantified by the difference between the y-axis intercepts of the APCsr plots of normal and FVLeiden-homozygous plasma; and (2) impaired APC cofactor activity of FVLeiden in FVIIIa inactivation, whose contribution to the APCsr is deduced by subtracting the susceptibility component from the total APCsr difference between normal and FVLeiden-homozygous plasma measured in full plasma. (C) Magnification of the inset in panel A. Each point is the average of 3 or more duplicate experiments, with error bars representing 1 standard error above and below the mean. Δ indicates normal plasma; ▪, FVR2-homozygous plasma; and •, FVLeiden-homozygous plasma.
Effect of FV level on the APCsr determined with the Coatest APC Resistance assay. (A) Plot of the APCsr as a function of FV concentration. The FV level was varied by mixing sample plasma with FV-deficient plasma in different proportions, and the APCsr was determined as described in “Materials and methods.” (B) The difference in APCsr between normal plasma and FVLeiden-homozygous plasma consists of 2 components: (1) poor susceptibility of FVaLeiden to APC, quantified by the difference between the y-axis intercepts of the APCsr plots of normal and FVLeiden-homozygous plasma; and (2) impaired APC cofactor activity of FVLeiden in FVIIIa inactivation, whose contribution to the APCsr is deduced by subtracting the susceptibility component from the total APCsr difference between normal and FVLeiden-homozygous plasma measured in full plasma. (C) Magnification of the inset in panel A. Each point is the average of 3 or more duplicate experiments, with error bars representing 1 standard error above and below the mean. Δ indicates normal plasma; ▪, FVR2-homozygous plasma; and •, FVLeiden-homozygous plasma.
Measurement of FV, FVIII, and protein S levels in plasma
The FV concentration in plasma was measured via a prothrombinase-based chromogenic assay.32 Plasma was diluted 1:1000 in a buffer containing 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (pH 7.7 at room temperature), 175 mM NaCl, 0.5% bovine serum albumin (BSA), 3 mM CaCl2, and 10 μM phospholipid vesicles (dioleoylphosphatidylserine [DOPS]/dioleoylphosphatidylcholine [DOPC] 20:80 mol/mol) and activated with 1 nM thrombin at 37°C for 10 minutes. Prothrombin activation was started by the addition of 5 nM FXa and 1 μM prothrombin (final concentrations) and stopped after 2 minutes by the addition of (ice-cold) buffer containing EDTA (ethylenediaminetetraacetic acid) (19.6 mM final). The amount of thrombin formed, which is a measure of the FVa present in the reaction mixture, was quantitated using the chromogenic substrate S2238. The FV concentration was calculated from a calibration curve constructed with known amounts of purified plasma FV.
FVIII levels were calculated from the data obtained with the Immunochrom APC Response assay. Because FVIII is the limiting factor under the assay conditions, the FXa generation rate is directly proportional to the FVIII concentration in plasma. Therefore, the measurement obtained in the absence of APC reflects the amount of FVIII present in a given plasma, which was expressed as percentage of the FVIII present in pooled normal plasma determined in the same experiment.
Protein S levels were measured with an enzyme-linked immunosorbent assay (ELISA)33 in plasma samples diluted 1:800 or 1:1600 in a buffer containing 25 mM HEPES (pH 7.7 at room temperature), 175 mM NaCl, and 0.5% BSA. Serial dilutions of normal plasma were used as a reference.
Reconstitution experiments
Human FV was purified from pooled normal plasma and from the plasma of an FVLeiden-homozygous individual, as described.34 Purified FV was desalted on a PD-10 column (Pharmacia Biotech, Uppsala, Sweden), and the FV concentration was measured as described above. Congenitally FV-deficient plasma (George King Bio-Medical) was supplemented with purified FV to various final concentrations, ranging from 0.6 nM (2.5%) to 23 nM (100%), and the APCsr of the reconstituted plasmas was determined with the Coatest APC Resistance assay kit (Chromogenix).
Measurement of thrombin generation
The plasma concentration of FV was varied between 10% and 120% of the normal level by adding a variable amount of purified normal FV or FVLeiden to congenitally FV-deficient plasma (George King Bio-Medical). In a similar way, FV-deficient plasma was reconstituted with normal FV and/or FVLeiden to simulate normal plasma (100% normal FV), FVLeiden-heterozygous plasma (50% normal FV and 50% FVLeiden), FVLeiden-homozygous plasma (100% FVLeiden), and FVLeiden-pseudohomozygous35 plasma (50% FVLeiden only). Plasma doubly heterozygous for FVLeiden and FVR2 was obtained by mixing FVLeiden-homozygous and FVR2-homozygous plasmas in equal proportions.
Thrombin generation was measured essentially as described.36 Plasma was mixed with a low-affinity fluorogenic substrate for thrombin (Z-Gly-Gly-Arg-AMC; BACHEM, Bubendorf, Switzerland; 300 μM final), and coagulation was initiated with a mixture containing recombinant tissue factor (TF; Dade Innovin; Behring, Marburg, Germany), synthetic phospholipid vesicles (DOPS/DOPC/dioleoylphosphatidylethanolamine [DOPE] 20:60:20 mol/mol/mol), and CaCl2 (final concentrations in plasma, about 320 pg/mL TF [about 6.8 pM], 15 μM phospholipids, and 16 mM added CaCl2), in the absence or presence of APC (5, 10, or 20 nM, as specified below). The formation of thrombin was followed continuously in a SPECTRAmax GEMINI XS fluorometer (Molecular Devices, Sunnyvale, CA) using 368-nm (excitation) and 460-nm (emission) filters. A thrombin standard (Synapse b.v., Maastricht, The Netherlands) was used to correct the raw fluorescence data for innerfilter effect and substrate consumption and to convert relative fluorescence units (RFUs) into nanomolars of thrombin. After subtraction of the signal attributable to the α2-macroglobulin (α2M)-thrombin complex, the first derivative of the data was calculated to obtain the fully corrected thrombin generation curve, and the underlying area (endogenous thrombin potential [ETP]) was determined according to Hemker et al.37 Each time course of thrombin generation was measured in duplicate.
Results
APC cofactor activity of FVLeiden and FVR2
The concentration of normal FV, FVLeiden, and FVR2 in plasma was varied between 2.5% and 100% by mixing normal plasma, FVLeiden-homozygous plasma, and FVR2-homozygous plasma, respectively, with FV-deficient plasma. The APCsr of the plasma mixtures was determined with the Immunochrom APC Response test, which specifically probes the APC resistance resulting from poor APC cofactor activity of FV in FVIIIa inactivation.30 As shown in Figure 1, the APCsr increased linearly with increasing concentrations of normal FV and FVR2, while it remained virtually constant and independent of the FV concentration in the case of FVLeiden. This indicates that normal FV and, to a lesser extent, FVR2 act as cofactors in APC-catalyzed FVIIIa inactivation, whereas FVLeiden lacks APC cofactor activity in FVIIIa inactivation.
Taking into account the actual amount of FV present in the different plasmas (Table 1), we calculated the slopes of the lines (ΔAPCsr/IU FV), which are a measure of the APC cofactor activity of the FV contained in the respective plasma samples. The slopes obtained with FVR2 (ΔAPCsr = 0.45/IU FV, 95% confidence interval [CI], 0.35 to 0.56) and normal FV (ΔAPCsr = 0.62/IU FV, 95% CI, 0.55 to 0.69) were significantly different (P < .005) and indicated that FVR2 expresses 73% of the APC cofactor activity of normal FV. FVLeiden (ΔAPCsr = -0.14/IU FV, 95% CI, -0.23 to -0.06) expresses no APC cofactor activity and might even slightly inhibit APC-catalyzed FVIIIa inactivation.
Effect of FV concentration on the APCsr determined with the Coatest APC Resistance assay
We also determined the effect of FV concentration on the APCsr measured with the Coatest APC Resistance assay (Figure 2A). Because in this test clotting is initiated via the intrinsic pathway, both FVIIIa and FVa inactivation by APC contribute to the test result (APCsr). Consequently, variation of the FV concentration in plasma can affect the APCsr by modifying the rates of both FVIIIa inactivation, in which FV acts as a cofactor of APC, and FVa inactivation, in which FVa is the substrate of APC.
As shown in Figure 2A, the dependence of the APCsr on the FV concentration in plasma was different for FVLeiden (closed circles) and normal FV (open triangles). Variation of the concentration of FVLeiden between 2.5% and 100% did not significantly affect the APCsr, which remained constant at about 1.5. This not only confirms that FVLeiden does not express APC cofactor activity but also shows that, under the assay conditions, the susceptibility of FVa to APC is independent of the FV concentration. In contrast, variation of the amount of normal FV from 2.5% to 100% caused the APCsr to increase from 2.3 to 3.3. Because the susceptibility of FVa is independent of the FV concentration, this increase in APCsr is attributable to an increase in the APC cofactor activity at increasing FV concentration. At very low FV levels, where the APC cofactor activity is negligible, the difference between the APCsr of plasmas containing normal FV and FVLeiden is entirely due to the difference in the susceptibility of normal and mutant FVa for APC, which does not change when the FV concentration is increased. Thus, the difference in APCsr between plasmas containing normal FV and FVLeiden at any given FV concentration can be resolved in a susceptibility component, which is constant and independent of the FV concentration, and an APC cofactor activity component, which progressively increases at increasing FV (Figure 2B). At 100% FV (ie, in full plasma), the difference between the APCsr of normal plasma and FVLeiden-homozygous plasma (ΔAPCsr = 1.81; Figure 2A) is due for 49% to the fact that FVaLeiden is less susceptible to APC-mediated inactivation than normal FVa and for 51% to the fact that FVLeiden expresses no APC cofactor activity in the inactivation of FVIIIa (Figure 2B).
It should be emphasized that the FV-deficient plasma used to dilute sample plasmas, in contrast to many other commercially available FV-deficient plasmas,38 had a normal protein S level (Table 1). This congenitally FV-deficient plasma was chosen to avoid varying the protein S levels (and thereby affect the APCsr) when mixing sample plasma and FV-deficient plasma in different proportions.
The variation of the APCsr with the concentration of FVR2 (Figure 2A, closed squares) was similar to that observed with normal FV (Figure 2A, open triangles). This is in line with the report that FVR2 has a minor effect on the APCsr determined with the aPTT-based APC resistance test.20
Because the increase in APCsr with the FV concentration is due to APC cofactor activity, the initial slopes of the lines in Figure 2A can be taken as a measure of the APC cofactor activity expressed by the FV contained in the respective plasma. Figure 2C shows that the initial slope of the APCsr plot as a function of the FV concentration was actually lower for FVR2 (ΔAPCsr = 1.86/IU FV; 95% CI, 0.65-3.08) than for normal FV (ΔAPCsr = 2.24/IU FV; 95% CI, 1.36-3.13), again suggesting that FVR2 expresses reduced APC cofactor activity, but confidence intervals largely overlapped and the difference did not reach significance.
Because the plasma from the 2 FVLeiden-homozygous donors might not have been representative for all FVLeiden homozygotes, we repeated the Coatest experiment using FV-deficient plasma reconstituted with varying amounts of purified normal FV or FVLeiden. Plasma reconstituted with normal FV exhibited a similar dependence of the APCsr on the FV concentration as normal pooled plasma, while plasma reconstituted with FVLeiden behaved the same as the plasma from the FVLeiden-homozygous individual, showing a constant APCsr of 1.5 (data not shown). Therefore, our results can be generalized to any homozygous carrier of FVLeiden.
Effect of the APC cofactor activity of FV on TF-initiated thrombin generation
The experiments described above indicate that impaired APC cofactor activity of FV is an important component of FVLeiden-associated APC resistance as measured with the Coatest assay. To evaluate the role of the anticoagulant function of FV within a more physiologic setting, we used a thrombin generation assay in which coagulation was initiated via the extrinsic pathway at a low TF concentration (6.8 pM). Control experiments performed in FVIII-deficient plasma with and without added FVIII demonstrated that the intrinsic FX-activating complex significantly contributes to FXa (and thus thrombin) generation under these experimental conditions, both in the absence and presence of APC (data not shown).
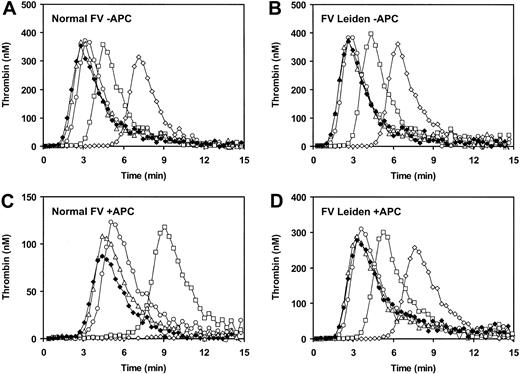
First of all, we performed an FV titration of thrombin generation in FV-deficient plasma reconstituted with purified normal FV or FVLeiden. In the absence of APC (Figure 3A-B), increasing the concentration of normal FV or FVLeiden up to about 60% of the normal plasma concentration resulted in a gradual increase of the amount of thrombin formed and in a decrease of the time of onset of thrombin generation. At higher FV concentrations both the time of onset of thrombin generation and the total amount of thrombin formed did not further change. In the presence of APC (10 nM), increasing the concentration of normal FV caused an initial increase of thrombin generation (up to 60% FV), which was followed by a progressive decrease of thrombin generation (Figure 3C). This decrease is presumably attributable to the increasing anticoagulant activity expressed by increasing amounts of normal FV. Conversely, when the same experiment was performed with FVLeiden, which expresses no APC cofactor activity, thrombin generation in the presence of APC steadily increased toward a maximum, and hardly any decrease at the highest FV concentrations was observed (Figure 3D).
Effect of FV concentration on thrombin generation. Congenitally FV-deficient plasma was reconstituted with varying amounts of purified normal FV (A,C) or FVLeiden (B,D). Thrombin generation after extrinsic activation of plasma in the absence (A-B) or presence (C-D) of 10 nM APC was measured as described in “Materials and methods.” ⋄ indicates 10% FV; □, 20% FV; ○, 60% FV; Δ, 100% FV; and ⋄, 120% FV.
Effect of FV concentration on thrombin generation. Congenitally FV-deficient plasma was reconstituted with varying amounts of purified normal FV (A,C) or FVLeiden (B,D). Thrombin generation after extrinsic activation of plasma in the absence (A-B) or presence (C-D) of 10 nM APC was measured as described in “Materials and methods.” ⋄ indicates 10% FV; □, 20% FV; ○, 60% FV; Δ, 100% FV; and ⋄, 120% FV.
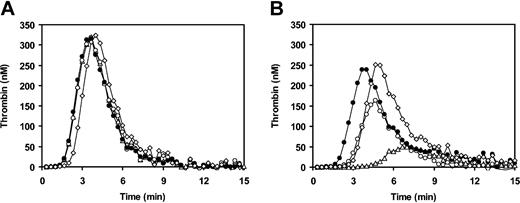
We also simulated normal, FVLeiden-heterozygous, FVLeiden-homozygous, and FVLeiden-pseudohomozygous plasmas by reconstituting FV-deficient plasma with the appropriate amount of purified normal FV and/or FVLeiden. Following initiation of coagulation with TF in the absence of APC (Figure 4A), all plasmas yielded superimposable thrombin generation curves, except for FVLeiden-pseudohomozygous plasma, which showed a slightly longer lag phase due to its lower (50%) FV concentration. In the presence of 20 nM APC (Figure 4B), strong inhibition of thrombin generation was observed in FV-deficient plasma reconstituted with normal FV (open triangles). In contrast, simulated FVLeiden-homozygous (100% FVLeiden, closed circles) and pseudohomozygous (50% FVLeiden, open diamonds) plasmas were fully resistant to APC, with identical ETPs in the presence and absence of APC. In FVLeiden-heterozygous plasma (open circles), which differs from pseudohomozygous plasma by the additional presence of 50% normal FV, about half as much thrombin was formed as in pseudohomozygous plasma. This reduction in thrombin generation is likely due to the anticoagulant activity expressed by normal FV in the presence of APC.
Thrombin generation in FV-deficient plasma reconstituted with normal FV and/or FVLeiden. Congenitally FV-deficient plasma was reconstituted with purified normal FV and/or FVLeiden to simulate normal plasma (Δ), FVLeiden-heterozygous plasma (○), FVLeiden-homozygous plasma (•), and FVLeiden-pseudohomozygous plasma (⋄). Thrombin generation after extrinsic activation of plasma in the absence (A) and presence (B) of 20 nM APC was measured as described in “Materials and methods.”
Thrombin generation in FV-deficient plasma reconstituted with normal FV and/or FVLeiden. Congenitally FV-deficient plasma was reconstituted with purified normal FV and/or FVLeiden to simulate normal plasma (Δ), FVLeiden-heterozygous plasma (○), FVLeiden-homozygous plasma (•), and FVLeiden-pseudohomozygous plasma (⋄). Thrombin generation after extrinsic activation of plasma in the absence (A) and presence (B) of 20 nM APC was measured as described in “Materials and methods.”
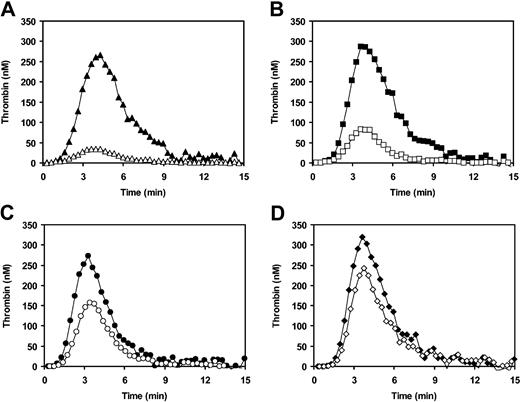
Because FVR2 expresses reduced APC cofactor activity in FVIII(a) inactivation (Figures 1 and 2C), we expected that more thrombin would be generated in FVR2-homozygous plasma than in normal plasma following activation in the presence of APC. As a matter of fact, the amount of thrombin formed in the plasma from an FVR2-homozygous individual in the presence of APC (5 nM) was 23% of that in the absence of APC (Figure 5B), as compared with a residual thrombin generation of 13% in normal pooled plasma (Figure 5A). In other words, while virtually equal amounts of thrombin were formed in the absence of APC, in the presence of APC the plasma from the FVR2 homozygote generated 1.8 times more thrombin than normal pooled plasma. To exclude the possibility that increased thrombin generation in FVR2-homozygous plasma in the presence of APC was due to plasma components other than FV, we repeated the experiment after a 5-fold dilution of both plasmas in FV-deficient plasma and verified that the difference persisted (data not shown).
Thrombin generation in plasmas containing FVR2 and/or FVLeiden. Thrombin generation after extrinsic activation of plasma in the absence (filled symbols) and presence (open symbols) of 5 nM APC was measured as described in “Materials and methods.” (A) Normal pooled plasma. (B) Plasma of an FVR2-homozygous individual. (C) FVLeiden-heterozygous plasma obtained by pooling plasma from 2 unrelated FVLeiden-heterozygous individuals. (D) Simulated FVLeiden/FVR2-doubly heterozygous plasma obtained by mixing plasma from an FVLeiden-homozygous individual and from an FVR2-homozygous individual in equal proportions.
Thrombin generation in plasmas containing FVR2 and/or FVLeiden. Thrombin generation after extrinsic activation of plasma in the absence (filled symbols) and presence (open symbols) of 5 nM APC was measured as described in “Materials and methods.” (A) Normal pooled plasma. (B) Plasma of an FVR2-homozygous individual. (C) FVLeiden-heterozygous plasma obtained by pooling plasma from 2 unrelated FVLeiden-heterozygous individuals. (D) Simulated FVLeiden/FVR2-doubly heterozygous plasma obtained by mixing plasma from an FVLeiden-homozygous individual and from an FVR2-homozygous individual in equal proportions.
Because it has been reported that coinheritance of FVR2 enhances the APC resistance19,39 and the thrombotic risk26,27 associated with FVLeiden, we also investigated the combined effect of FVR2 and FVLeiden on thrombin generation in the presence and absence of APC. To this end, a simulated FVLeiden/FVR2-doubly heterozygous plasma, prepared by mixing FVLeiden-homozygous and FVR2-homozygous plasmas in equal proportions, was compared with pooled plasma from 2 FVLeiden-heterozygous individuals. In the presence of 5 nM APC, residual thrombin generation (relative to thrombin generation in the same plasma triggered in the absence of APC) was 55% in FVLeiden-heterozygous plasma (Figure 5C) and 79% in FVLeiden/FVR2-doubly heterozygous plasma (Figure 5D).
Discussion
In the original report on APC resistance, Dahlbäck and coworkers observed that the addition of normal plasma to APC-resistant plasma corrected the abnormal phenotype, and they concluded that APC resistance was due to the defect of a new APC cofactor,10 which later turned out to be FV.40 Subsequently, the ability of FV to stimulate APC-mediated inactivation of FVIIIa was proven directly in model systems containing purified proteins.4-6 However, after the FVLeiden mutation was discovered as the main cause of APC resistance,14 little attention was paid to the role of FV as an APC cofactor. The abolition of the APC cleavage site at Arg506, which impaired the inactivation of FVaLeiden in in vitro studies,15,16,29,41 appeared a satisfactory explanation for the APC resistance associated with the mutation. Only when it was reported that FVLeiden is a poor cofactor in APC-catalyzed FVIIIa inactivation8,17 was it realized that reduced APC cofactor activity may also contribute to FVLeiden-associated APC resistance.
Here we demonstrate that FV plays a crucial role in the APC-mediated inactivation of FVIIIa in plasma and that this anticoagulant activity of FV is lost in FVLeiden (Figure 1). This result extends to the plasma environment previous reports on the APC cofactor activities of purified and recombinant FV and FVLeiden determined in model systems of FVIIIa inactivation.4-6,8 Furthermore, we show that, although reduced susceptibility of FVaLeiden to APC-catalyzed inactivation remains an important determinant of FVLeiden-associated APC resistance, impaired APC cofactor activity of FVLeiden in FVIIIa inactivation accounts for about half of the difference between the APCsr of healthy individuals and FVLeiden homozygotes, as measured with the Coatest APC Resistance assay (Figure 2B).
To gain an insight into the physiologic role of the anticoagulant activity of FV, we measured thrombin generation in plasma after initiation of coagulation via the extrinsic pathway. This assay allows the evaluation of the overall efficiency of the coagulation system under conditions that are close to the in vivo situation.42 To ensure that FXa formed via the intrinsic FX-activating complex contributes to thrombin generation, coagulation was initiated with a low TF concentration. As illustrated in Figure 3C, increasing the concentration of normal FV in plasma triggered in the presence of APC led to an initial increase of thrombin generation, followed by a gradual decrease at FV concentrations higher than 60%. This remarkable pattern is likely explained by the dual function of FV in coagulation. At low FV concentrations the procoagulant activity of FV prevails over the anticoagulant activity, leading to progressively higher thrombin generation at increasing FV concentrations. However, at an FV level of 60%, the procoagulant activity of FV is saturated (Figure 3A), and a further increase of the FV concentration results in increased anticoagulant activity and consequently decreased thrombin generation. This interpretation is supported by the parallel experiment conducted with FVLeiden (Figure 3D), which showed hardly any inhibition of thrombin generation in the presence of APC at high FV concentrations, thus confirming that FVLeiden expresses no anticoagulant activity.
The physiopathologic relevance of the APC cofactor activity of FV is well illustrated by the comparison of heterozygosity, homozygosity, and pseudohomozygosity35 for the FVLeiden mutation as shown in Figure 4. FV-deficient plasma reconstituted with 50% purified FVLeiden (which corresponds to the plasma of an FVLeiden-pseudohomozygous individual) was fully resistant to APC in the thrombin generation test. However, when 50% normal FV was added to this plasma, thrombin generation in the presence of APC was reduced by half (Figure 4B) due to the anticoagulant activity expressed by the normal FV. Conversely, the addition to FVLeiden-pseudohomozygous plasma of 50% FVLeiden (which does not express APC cofactor activity) was ineffective in decreasing thrombin generation in the presence of APC (Figure 4B). These findings may explain the respective thrombotic risks associated with heterozygosity, homozygosity, and pseudohomozygosity for the FVLeiden mutation. Moreover, they suggest that the reason why FVLeiden homozygotes are exposed to a higher thrombotic risk than heterozygotes is not the presence of twice as much FVLeiden in their plasma but the absence of normal FV capable of expressing anticoagulant activity. This is in line with the recent in vivo observation that the thrombotic lethality of FVLeiden-pseudohomozygous mice with heterozygous tissue factor pathway inhibitor (TFPI) deficiency (FVQ/-TFPI+/- genotype) can be corrected by transgene expression of wild-type FV.9
In contrast to FVLeiden, the APC cofactor activity of FVR2 is reduced to 73% of that of normal FV (Figures 1 and 2C). However, because FVR2 is expressed at a lower level than normal FV,18,20,39 the overall APC cofactor activity contained in the plasma of a typical FVR2 homozygote with an FV level of about 75% is actually about half of that present in normal plasma. Because it has been reported that FVaR2 and normal FVa are inactivated at equal rates by APC, both in the presence and in the absence of protein S,20 reduced APC cofactor activity in FVIIIa inactivation appears to be the only determinant of the mild APC resistance observed in carriers of the FVR2 haplotype. The pathophysiologic consequences of the decreased anticoagulant activity of FVR2 are apparent in the thrombin generation assay, which shows that FVR2-homozygous plasma forms almost twice as much thrombin as normal plasma in the presence of 5 nM APC (Figure 5A-B). Although the plasma of only a single FVR2-homozygous individual was used in the thrombin generation experiments, increased thrombin formation in the presence of APC was still observed after 1:5 dilution in FV-deficient plasma, which strongly reduces the interference by plasma components other than FV. Therefore, in spite of the inconclusive results of epidemiologic studies (see Castaman et al43 and references therein), our experiments provide evidence for the mild prothrombotic character of FVR2, both per se (Figure 5A-B) and in combination with FVLeiden (Figure 5C-D).
In conclusion, our study shows (1) that the impaired APC cofactor activity of FVLeiden and FVR2 in FVIIIa inactivation contributes prominently to the APC resistance associated with carriership of these mutations and (2) that the anticoagulant activity of FV plays a pivotal role in the regulation of thrombin formation.
Prepublished online as Blood First Edition Paper, February 19, 2004; DOI 10.1182/blood-2003-10-3578.
Supported by a research grant (2000.021) from the Dutch Heart Foundation. E.C. was supported by a Long-Term Fellowship of the European Molecular Biology Organization (EMBO).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr K. Hamulyák and R. van Oerle from the Haematology Department of Maastricht Academic Hospital for providing the plasmas from FVR2- and FVLeiden-homozygous individuals as well as the normal plasma pool. We also thank M. C. L. G. D. Thomassen and E. J. P. Magdeleyns for technical assistance. Prof. C. Scapoli from the Department of Biology of the University of Ferrara (Italy) is gratefully acknowledged for assistance with the statistical analysis.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal