Abstract
Crystallographic structures indicate that γ-chain residue Asn308 participates in D:D interactions and indeed substitutions of γAsn308 with lysine or isoleucine have been identified in dysfibrinogens with impaired polymerization. To probe the role of Asn308 in polymerization, we synthesized 3 variant fibrinogens: γAsn308 changed to lysine (γN308K), isoleucine (γN308I), and alanine (γN308A). We measured thrombin-catalyzed polymerization by turbidity, fibrinopeptide release by high-performance liquid chromatography, and factor XIIIa–catalyzed cross-linking by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. In the absence of added calcium, polymerization was clearly impaired with all 3 variants. In contrast, at 0.1 mM calcium, only polymerization of γN308K remained markedly abnormal. The release of thrombin-catalyzed fibrinopeptide B (FpB) was delayed in the absence of calcium, whereas at 1 mM calcium FpB release was delayed only with γN308K. Factor XIIIa–catalyzed γ-γ dimer formation was delayed with fibrinogen (in absence of thrombin), whereas with fibrin (in presence of thrombin) γ-γ dimer formation of only γN308K was delayed. These data corroborate the recognized link between FpB release and polymerization. They show fibrin cross-link formation likely depends on the structure of protofibrils. Together, our results show substitution of Asn308 with a hydrophobic residue altered neither polymer formation nor polymer structure at physiologic calcium concentrations, whereas substitution with lysine altered both.
Introduction
Fibrinogen is a 340-kDa glycoprotein that circulates in the blood at 5.88 to 11.76 μM (2-4 g/L). It is composed of a pair of 3 polypeptide chains, Aα,Bβ, and γ, connected by 29 intrachain and interchain disulfide bonds.1 The 6 chains are arranged into 3 globular nodules. The central E nodule contains the N-termini of all chains and the distal D nodules contain the C-termini of the β, γ, and a short segment of the α chains. Coiled coils composed of all 3 chains link the E and D nodules.2 The C-terminal residues of Aα chains form globular nodules that are associated with one another, as well as with the E nodule.3 Recent crystallographic studies have provided high-resolution structures of the C-terminal region of the γ chain, which contains functional sites for fibrin polymerization, calcium binding, platelet aggregation, and factor XIII (FXIII) cross-linking.4-6
During coagulation, thrombin cleaves fibrinogen, releasing fibrinopeptides A (FpA) and B (FpB) from the N-termini of the Aα and Bβ chains, respectively, and converting fibrinogen to fibrin monomers (for reviews, see Blomback1 and Doolittle2 ). Fibrin monomers polymerize spontaneously through a 2-step process, with a second step being calcium-dependent.7,8 The first step begins with the release of FpA. This exposes the “A” site, which interacts with the “a” site in the D nodule of another molecule, leading to the formation of half-staggered, double-stranded protofibrils.9,10 Within protofibrils, individual fibrin molecules also interact through the D nodule sites called the D:D interface.11-13 Subsequently, during the second step, the release of FpB exposes the “B” site, which likely interacts with the “b” site in the D nodule of another molecule to promote lateral aggregation of the protofibrils.5,6,8,10 During fibrin assembly FXIIIa catalyzes the formation of intermolecular isopeptide bonds, thereby cross-linking the individual fibrin molecules within the fibrin matrix.14 The final product is a complex, stable, insoluble, branching network of fibrin fibers and bundles of fibers.
High-resolution crystal structures have identified the D nodule residues that likely compose the “a” and “b” sites, as well as several calcium-binding sites.6,15,16 These and subsequent crystal structures also identified the residues that likely form the D:D interface.16,17 This interface is unusual in 2 ways: it is not symmetric as might be expected for interactions between identical nodules and it is not supported by a significant network of hydrogen bonds or salt links. Nevertheless, specific residues within the γ chain appear to be critical to this interface.
The analysis of dysfibrinogens has provided useful information for understanding the molecular details that promote thrombincatalyzed polymerization.18-21 Analysis of 2 dysfibrinogens with substitutions at γ-chain residue 308, Asn308 to lysine and Asn308 to isoleucine, indicated that this residue has an important role in polymerization.22-25 As is the case with most dysfibrinogens, these substitutions occurred in individuals who are heterozygous for the mutation. This complicates functional analysis because fibrinogen in these individuals is a complex mixture of normal and abnormal molecules.26,27 We have circumvented this complication by synthesizing recombinant variant fibrinogens and have examined the changes in function associated with changes in primary structure.28 Here we describe analysis of 3 variant fibrinogens with substitutions at Asn308 in the γ chain: γN308K and γN308I, analogous to dysfibrinogens, and γN308A. Our data indicate that this residue has an important role in the D:D interactions that are critical for normal polymerization.
Materials and methods
Materials
All chemicals were reagent grade from Sigma (St Louis, MO), unless otherwise noted. Human γ-thrombin and FXIII were purchased from Enzyme Research Laboratories (South Bend, IN).
Preparation of variant fibrinogens
The construction of mutant expression vectors and the synthesis and purification of the recombinant fibrinogens have been described.29-31 Briefly, recombinant fibrinogens were expressed in Chinese hamster ovary (CHO) cells cultured in serum-free medium. Fibrinogen was purified from harvested culture medium by immunoaffinity chromatography using a calcium-dependent monoclonal antibody (IF-1; Iatron Laboratories, Tokyo, Japan). Fibrinogen was eluted with 5 mM EDTA (ethylenediaminetetraacetic acid), and fractions were pooled and dialyzed against 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4; 0.12 M NaCl (HBS), first with 1 mM CaCl2, and subsequently against HBS without added calcium. Dialyzed samples were aliquoted and stored at -80° C. The fibrinogen concentration was determined from the A280, assuming a 1 mg/mL solution has an absorbance of 1.51.32 As described previously, normal recombinant fibrinogen purified by this procedure is 96% clottable.30
Thrombin-catalyzed fibrin polymerization
Polymerization was followed by turbidity at 350 nm using a UV-110-02 spectrophotometer (Shimadzu, Tokyo, Japan). Reactions were performed in a final volume of 100 μL as described elsewhere.30 Briefly, fibrinogen (90 μL at 0.3 mg/mL) in HBS, with or without added CaCl2, was mixed with human α-thrombin (10 μL at 0.5 U/mL) and changes in turbidity were monitored at ambient temperature. Two parameters, lag period and the maximum slope, were obtained from the turbidity curves, as described elsewhere.33 The reactions were performed in triplicate.
Thrombin-catalyzed fibrinopeptide release
Thrombin-catalyzed fibrinopeptide release was monitored by reversedphase high-performance liquid chromatography (HPLC), essentially as described.34,35 Briefly, fibrinogen (0.1 mg/mL) in HBS, with or without 1 mM CaCl2, was incubated with human α-thrombin (0.005 U/mL) at ambient temperature and the reactions stopped by incubation at 100° C. Fibrinopeptides were separated by HPLC and their concentrations determined from the peak areas measured with the ISCO Chemresearch 2.4 software. The data were fit to first-order kinetics (DeltaGraph; Deltapoint, Monterey, CA), assuming the release of FpB followed the release of FpA. Specificity constants, kcat/Km, were determined as described.34,35 Reactions were performed in triplicate.
FXIIIa-catalyzed cross-linking of fibrin or fibrinogen
FXIII (50 U/mL) was activated with human α-thrombin (1 U/mL) for 60 minutes at 37° C in HBS with 5 mM CaCl2. To examine cross-linking of fibrin, fibrinogen (final concentration, 0.47 mg/mL) was incubated at 37° C with FXIIIa (final concentration, 3.3 U/mL) and human α-thrombin (final concentration, 0.07 U/mL) in HBS and 0.67 mM calcium. To examine cross-linking of fibrinogen, hirudin (10 U/mL) was added to thrombin- -activated FXIIIa (final concentration, 3.3 U/mL) prior to incubation with fibrinogen (final concentration, 0.47 mg/mL) in HBS and 0.67 mM calcium. The reactions were stopped at various times by addition of an equal volume of sodium dodecyl sulfate (SDS) sample buffer with 2-mercaptoethanol and incubation (5 minutes) at 100° C. Samples equivalent to 4.7 μg fibrinogen were separated on 8% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie brilliant blue R-250.31
Results
Synthesis and characterization of recombinant fibrinogens
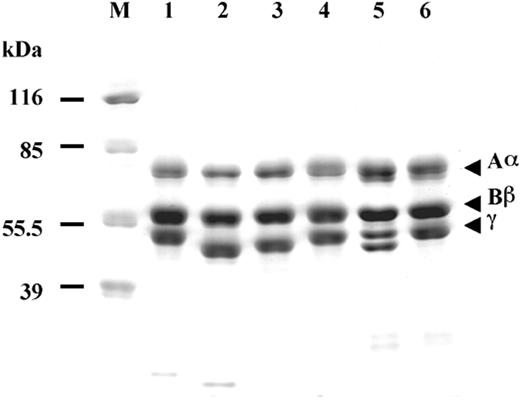
We synthesized 3 variant fibrinogens with single amino acid substitutions in the C-terminal region of the γ chain: γN308K, γN308I, and γN308A, where Asn308 is changed to lysine, isoleucine, and alanine, respectively. The variant fibrinogens were synthesized in cultured CHO cells and purified from the culture medium, as described.27,35 SDS-PAGE run under reducing conditions (Figure 1) showed the usual pattern of 3 bands corresponding to the Aα, Bβ and γ chains, except, as previously reported,24,31 the migration of the γ-chain band in γN308K and γN308I was faster than normal. The unusual γ-chain migration was most obvious in fibrinogen Matsumoto II (γN308K) purified from plasma, which showed 2 distinct bands corresponding to the normal and variant γ chains in this heterozygous individual (compare lane 5 to lane 1 or 6 in Figure 1). Similar shifts in band mobility have been reported for several dysfibrinogens with substitutions or small deletions in the γ chain.36-41
Characterization of recombinant fibrinogens. Coomassie-stained 10% SDS-PAGE run under reducing conditions; normal recombinant fibrinogen (lane 1), γN308K fibrinogen (lane 2), γN308I fibrinogen (lane 3), γN308A fibrinogen (lane 4), plasma fibrinogen from Matsumoto II patient (lane 5), and plasma fibrinogen from normal individual (lane 6). Positions of the molecular markers are indicated on the left (M).
Characterization of recombinant fibrinogens. Coomassie-stained 10% SDS-PAGE run under reducing conditions; normal recombinant fibrinogen (lane 1), γN308K fibrinogen (lane 2), γN308I fibrinogen (lane 3), γN308A fibrinogen (lane 4), plasma fibrinogen from Matsumoto II patient (lane 5), and plasma fibrinogen from normal individual (lane 6). Positions of the molecular markers are indicated on the left (M).
Thrombin-catalyzed fibrin polymerization
Thrombin-catalyzed fibrin polymerization was monitored as the change in turbidity at 350 nm, as described in “Materials and methods.” Representative curves are shown in Figure 2. We measured 2 parameters from these curves: the lag time, which reflects the formation of protofibrils, and the maximum slope (Vmax), which reflects the rate at which protofibrils laterally aggregate with each other to form a fibrin fiber. When the experiments were performed in 20 mM HEPES, pH 7.4, 0.12 M NaCl and no added calcium (Figure 2A), polymerization was abnormal for all 3 variants. Polymerization of γN308I fibrinogen was the most impaired, with the longest lag time and the slowest rise in turbidity. Compared to normal fibrinogen, the lag times of γN308A and γN308K fibrinogens are longer by 2- and 3.5-fold, respectively. The slopes of each of the polymerization curves were approximately the same as normal. Thus, under these conditions, the substitution of the bulky, hydrophobic isoleucine side chain resulted in the greatest delay in polymerization. As seen in Figure 2B, the results were markedly different in the presence of 1 mM CaCl2. The increase in calcium concentration led to a substantial improvement such that the polymerizations of γN308A and γN308I fibrinogens became similar to the polymerization of normal fibrinogen. The turbidity curves of γN308A and γN308I were similar to one another and to the curve of normal fibrinogen. In contrast, the curve with γN308K fibrinogen remained markedly different from normal because the increase in calcium concentration only modestly improved the turbidity profile. In this respect, the addition of calcium improved polymerization of γN308K fibrinogen to the same extent as the addition of calcium improved polymerization of normal fibrinogen.
Thrombin-catalyzed fibrin polymerization. Polymerization of fibrinogen (0.27 mg/mL) was initiated with thrombin (0.05 U/mL) and the change in turbidity at 350 nm was followed with time. Representative polymerization curves of normal (▪), γN308K (•), γN308I (▴), and γN308A (⋄) fibrinogens in the absence of added CaCl2 (A) and in the presence of 1 mM CaCl2 (B) are shown.
Thrombin-catalyzed fibrin polymerization. Polymerization of fibrinogen (0.27 mg/mL) was initiated with thrombin (0.05 U/mL) and the change in turbidity at 350 nm was followed with time. Representative polymerization curves of normal (▪), γN308K (•), γN308I (▴), and γN308A (⋄) fibrinogens in the absence of added CaCl2 (A) and in the presence of 1 mM CaCl2 (B) are shown.
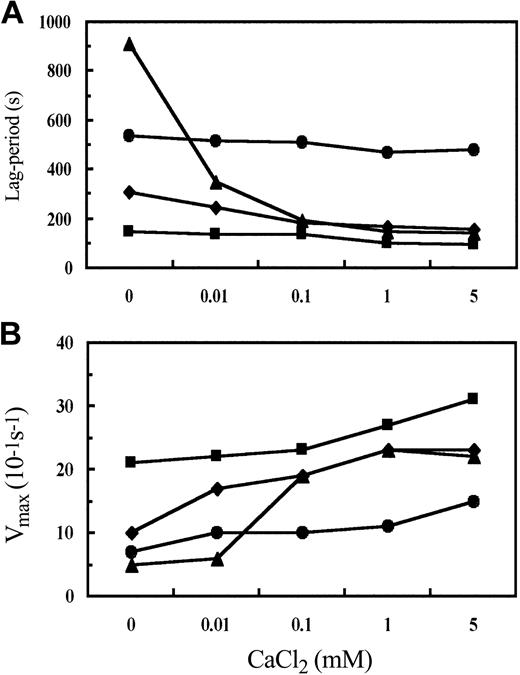
We further characterized the influence of calcium by performing polymerization experiments at several calcium concentrations. The average values for lag time and Vmax from 3 experiments are plotted in Figure 3 as a function of calcium concentration. The concentration of calcium had a marked influence on the polymerization of both hydrophobic substitutions and was greatest for the isoleucine substitution. For example, with γN308I fibrinogen, the lag period was 6-fold longer and the Vmax 5-fold slower in the absence of added calcium relative to 1 mM CaCl2. With γN308A fibrinogen the lag period was 1.9-fold longer and the Vmax 2.3-fold slower in the absence of added calcium relative to 1 mM CaCl2. The comparable values for normal fibrinogen were a 1.5-fold longer lag period and a 1.3-fold slower Vmax. As a result, at 1 mM CaCl2 polymerization of γN308I fibrinogen and γN308A fibrinogen was essentially normal. Overall with the variants γN308I and γN308A the most substantive influence of calcium occurred at less than 0.1 mM CaCl2. In contrast, with γN308K fibrinogen, the changes with calcium concentration were analogous to those seen with normal fibrinogen; the lag period was 1.1-fold longer and the Vmax 1.6-fold slower in the absence of added calcium relative to 1 mM CaCl2. As a result, the difference between normal fibrinogen and γN308K was relatively constant at all calcium concentrations, such that the respective curves in Figure 3 parallel one another. These experiments also showed that raising the calcium concentration to 5 mM had a minimal impact on polymerization of all 4 fibrinogens (Figure 3).
Influence of CaCl2 concentration on polymerization parameters. Thrombin-catalyzed fibrin polymerization was performed as indicated for Figure 2. For each calcium concentration, polymerization was performed 3 times. Average values of lag time (A) and Vmax (B) for normal (▪), γN308K (•), γN308I (▴), and γN308A (⋄) fibrinogens were determined as described in “Materials and methods.”
Influence of CaCl2 concentration on polymerization parameters. Thrombin-catalyzed fibrin polymerization was performed as indicated for Figure 2. For each calcium concentration, polymerization was performed 3 times. Average values of lag time (A) and Vmax (B) for normal (▪), γN308K (•), γN308I (▴), and γN308A (⋄) fibrinogens were determined as described in “Materials and methods.”
Calcium dependence of fibrinopeptide release
Thrombin-catalyzed fibrinopeptide release was monitored by HPLC and the data plotted as percent of peptide released as a function of time, as described in “Materials and methods.” Because polymerization showed an unusual dependence on calcium concentration, we examined fibrinopeptide release with no added calcium and 1 mM CaCl2. Average specificity constants, kcat/Km, from 3 experiments are given in Table 1. As expected, the kinetics of FpA release was the same for all 4 fibrinogens and the rate of FpA release was not influenced by calcium.
Specificity constants of thrombin-catalyzed fibrinopeptide release in the presence and the absence of added CaCl2
. | No added CaCl2, kcat/Km ± SD . | . | 1 mM CaCl2, kcat/Km ± SD . | . | ||
|---|---|---|---|---|---|---|
| Fibrinogen . | FpA . | FpB (P) . | FpA (P) . | FpB (P) . | ||
| Normal | 13.6 ± 3.8 | 5.9 ± 1.4 | 10.9 ± 1.4 (.3768*) | 6.8 ± 0.3 (.3091*) | ||
| γN308K | 11.9 ± 2.1 | 2.2 ± 0.3 (.0027†) | 11.4 ± 0.7 (.1231*) | 2.9 ± 0.2 (.0015†; .0634*) | ||
| γN308A | 11.6 ± 2.0 | 4.9 ± 1.8 (.0474†) | 11.2 ± 1.1 (.1732*) | 8.6 ± 0.4 (.0159†; .0530*) | ||
| γN308I | 11.6 ± 0.4 | 3.0 ± 1.0 (.0097†) | 11.3 ± 0.5 (.1276*) | 7.2 ± 0.3 (.1091†; .0189*) | ||
. | No added CaCl2, kcat/Km ± SD . | . | 1 mM CaCl2, kcat/Km ± SD . | . | ||
|---|---|---|---|---|---|---|
| Fibrinogen . | FpA . | FpB (P) . | FpA (P) . | FpB (P) . | ||
| Normal | 13.6 ± 3.8 | 5.9 ± 1.4 | 10.9 ± 1.4 (.3768*) | 6.8 ± 0.3 (.3091*) | ||
| γN308K | 11.9 ± 2.1 | 2.2 ± 0.3 (.0027†) | 11.4 ± 0.7 (.1231*) | 2.9 ± 0.2 (.0015†; .0634*) | ||
| γN308A | 11.6 ± 2.0 | 4.9 ± 1.8 (.0474†) | 11.2 ± 1.1 (.1732*) | 8.6 ± 0.4 (.0159†; .0530*) | ||
| γN308I | 11.6 ± 0.4 | 3.0 ± 1.0 (.0097†) | 11.3 ± 0.5 (.1276*) | 7.2 ± 0.3 (.1091†; .0189*) | ||
In contrast, the kinetics of FpB release from the variant fibrinogens was different from normal fibrinogen, and a strong calcium dependence of FpB release was apparent with γN308A and γN308I fibrinogens. In the absence of added calcium, FpB release from all variants was slower than normal with γN308A being closest to normal and γN308K being the most different. At 1 mM calcium, FpB release from γN308I was indistinguishable from normal, whereas FpB release from γN308A was slightly faster than normal. In contrast, FpB release from γN308K at 1 mM calcium was not different from FpB release in the absence of added calcium, such that FpB release remained slower than normal. Thus, for all the variants the kinetics of FpB release correlated directly with polymerization. Under conditions where polymerization was impaired, FpB release was also slower, and when polymerization was normal, then FpB release was also normal. This correlation has been reported previously and was interpreted as indicating that FpB is released from small fibrin polymers.9,42-44
FXIIIa-catalyzed cross-linking of fibrin or fibrinogen
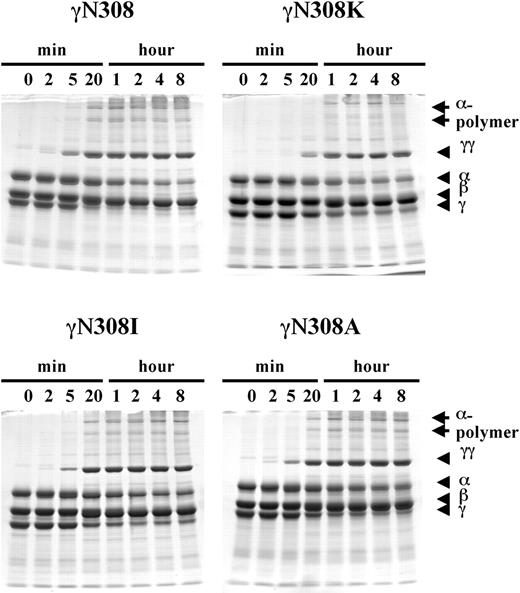
Cross-linking of fibrin α and γ chains was performed in the presence of FXIIIa and thrombin, and the reaction products analyzed by SDS-PAGE as described in “Materials and methods.” Because FXIIIa is a calcium-dependent enzyme,45,46 we measured cross-linking only in the presence of calcium. The data are presented in Figure 4. With normal fibrin (Figure 4; γN308), the expected γ-γ dimer and α-polymer bands were readily apparent. As previously reported, the γ-γ dimer appeared first, weakly evident at the earliest time point, 2 minutes, and the 2 α-polymer bands appeared later, evident at 5 minutes.47 With longer incubation, the intensity of γ-γ dimer and α-polymer bands increased, whereas the intensity of α- and γ-chain bands decreased. With γN308I and γN308A fibrins, the γ-γ dimer and 2 α-polymer bands were evident after 2 and 20 minutes, respectively. As with normal fibrinogen, the intensities of these bands increased with time, whereas the intensities of the α- and γ-chain bands decreased. In contrast, with γN308K fibrin cross-link formation was delayed, the γ-γ dimer and α-polymer bands being evident only after 20 and 60 minutes, respectively (Figure 4; γN308K). The FXIIIa-catalyzed cross-linking of fibrin correlated with polymerization similarly to the correlation of FpB release with polymerization. When polymerization was impaired (γN308K fibrin) the cross-linking was delayed. When polymerization was normal (γN308A and γN308I fibrins) the cross-linking was normal.
FXIIIa-catalyzed cross-linking of fibrin. Cross-linking of fibrin by FXIIIa was examined with 8% SDS-PAGE, under reducing conditions as described in “Materials and methods.” Fibrinogen (0.47 mg/mL) was mixed with FXIIIa (3.3 U/mL) and thrombin (0.07 U/mL), and the reaction was incubated for the specified time at 37° C in 20 mM HEPES, pH 7.4; 0.12 M NaCl, and 0.67 mM CaCl2 buffer. The reduced fibrin chains (α, β, γ, cross-linked γ-γ dimer, and cross-linked α-chain polymers) are indicated on the right side of the gels.
FXIIIa-catalyzed cross-linking of fibrin. Cross-linking of fibrin by FXIIIa was examined with 8% SDS-PAGE, under reducing conditions as described in “Materials and methods.” Fibrinogen (0.47 mg/mL) was mixed with FXIIIa (3.3 U/mL) and thrombin (0.07 U/mL), and the reaction was incubated for the specified time at 37° C in 20 mM HEPES, pH 7.4; 0.12 M NaCl, and 0.67 mM CaCl2 buffer. The reduced fibrin chains (α, β, γ, cross-linked γ-γ dimer, and cross-linked α-chain polymers) are indicated on the right side of the gels.
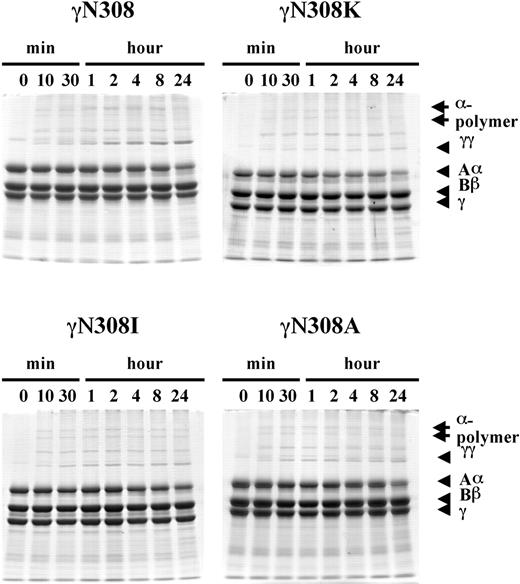
To circumvent the role of polymerization and examine the influence of the substitution per se, we examined FXIIIa-catalyzed cross-linking of fibrinogen.48 FXIII was first activated with thrombin, hirudin was added to inhibit the thrombin, the enzyme mixture was added to the fibrinogen solutions, and reaction products analyzed by SDS-PAGE; the data are presented in Figure 5. With all 4 fibrinogens, γ-γ dimer and α polymers were visible in aliquots removed at the earliest time point, 10 minutes. With normal fibrinogen (Figure 5; γN308), the intensity of the γ-γ dimer band had increased noticeably through the observed period. In contrast, with all 3 γN308 variant fibrinogens the intensity of the γ-γ dimer band remained essentially constant throughout the incubation. These results indicated that all the substitutions influenced crosslinking of soluble fibrinogen. This differs from the cross-linking of polymerized fibrin. We conclude that polymerization, rather than the substitution per se, influenced the fibrin cross-linking data shown in Figure 4. Specifically, the data show that γ-γ dimer formation was normal for the γN308A fibrin and γN308I fibrin variants because polymerization of these variants was normal at this calcium concentration. In contrast, γ-γ dimer formation with γN308K fibrin was delayed because polymerization of this fibrin was delayed.
FXIIIa-catalyzed cross-linking of fibrinogen. Cross-linking of fibrinogen by FXIIIa was examined with 8% SDS-PAGE, under reducing conditions as indicated for Figure 4, except that hirudin was added to FXIIIa prior to addition to fibrinogen and no thrombin was added to fibrinogen. The reduced fibrinogen chains (Aα, Bβ, γ, cross-linked γ-γ dimer, and cross-linked α-chain polymers) are indicated on the right side of the gels.
FXIIIa-catalyzed cross-linking of fibrinogen. Cross-linking of fibrinogen by FXIIIa was examined with 8% SDS-PAGE, under reducing conditions as indicated for Figure 4, except that hirudin was added to FXIIIa prior to addition to fibrinogen and no thrombin was added to fibrinogen. The reduced fibrinogen chains (Aα, Bβ, γ, cross-linked γ-γ dimer, and cross-linked α-chain polymers) are indicated on the right side of the gels.
Discussion
Our results support the conclusion that the C-terminal γ-chain residue Asn308 is important for normal D:D interactions and thus for normal polymerization. We examined 3 variants at this position, and in the absence of added calcium, each showed impaired polymerization. In contrast, at 0.1 mM calcium, polymerization of 2 of the variants, γN308A and γN308I, was only slightly impaired, whereas polymerization of γN308K differed markedly from normal. These data suggest that the lysine substitution directly disrupts a critical aspect of polymerization, whereas the isoleucine or alanine substitutions do so only indirectly, in conjunction with calcium binding. Thus, the hydrophobic variants may disrupt calcium binding and thereby a critical aspect of polymerization, or they may disrupt a critical aspect of polymerization that is evident only in the absence of calcium. This remarkable calcium dependence was also apparent in the kinetics of thrombin-catalyzed FpB release, such that impaired FpB release always correlated with impaired polymerization. These results lend strong support to the previous conclusion that FpB is cleaved from small fibrin polymers, not fibrin monomers.9,42-44 Similarly, FXIIIa-catalyzed γ-γ dimer formation in fibrin was impaired only whenever polymerization was impaired. Because FXIIIa is a calcium-dependent enzyme, we did not examine the influence of calcium on γ-γ dimer formation. Nevertheless, because FXIIIa-catalyzed cross-linking of fibrinogen (Figure 5) was equally abnormal for all variants, we conclude that the data showed impaired fibrin cross-link formation (γN308K; Figure 4) correlated with impaired polymerization (γN308K; Figure 2B). Furthermore, these data suggest that the D:D conformation in the fibrin polymer is a more effective substrate for FXIIIa-catalyzed γ-γ dimer formation than the D:D conformation in fibrinogen, presumably because the D:D/E complex is a more effective substrate.
Because the mutations at Asn308 in γ chain have been identified in several heterozygous dysfibrinogens, we anticipated the impaired polymerization of the recombinant variant proteins. We were, however, surprised that polymerization of these homogeneous recombinant variants was relatively normal. In our previous studies with recombinant fibrinogens analogous to the heterozygous dysfibrinogens Vlissingen/Frankfurt IV and Matsumoto I, polymerization of the recombinant proteins was substantially more impaired than the polymerization of fibrinogen isolated from these heterozygous patients.26,39,49 Indeed, neither recombinant protein polymerized at all. Data from the recombinant proteins and their dysfibrinogen counterparts are summarized in Table 2. The nearnormal polymerization of the γN308I variant at physiologic calcium concentration might have been anticipated from the preliminary report on fibrinogen Baltimore III, where it was noted that addition of more than 7 mM calcium to patient plasma normalized the clotting time.50 However, subsequent analysis of fibrinogen purified from the heterozygous Baltimore III patient showed no disparity between calcium binding to the normal and abnormal γ chains.22 Analysis of the patient's fibrinogen in the absence of added calcium also showed that the kinetics of FpB release did not correlate with fibrin monomer polymerization, because the former was normal whereas the latter was delayed (Table 2).50 In contrast, with homozygous recombinant γN308I fibrinogen, the Baltimore III substitution, both polymerization and FpB release were impaired in the absence of added calcium, and both were essentially normal as calcium concentration was increased to 0.1 mM. This difference likely reflects the experimental conditions because FpB release from the patient's fibrinogen was measured at higher fibrinogen and thrombin concentrations where fibrinopeptide release is rapid such that the decreased rate of FpB release might not be apparent (Table 2).
Summary of variant fibrinogens and their functions
Fibrinogen . | Substitution . | Allele* . | Fibrin polymerization† . | FpA release . | FpB release‡ . | FXIIIa-catalyzed cross-linking . | References . |
|---|---|---|---|---|---|---|---|
| Kyoto I | γN308K | Hetero | AMn | N | N (5.0/1.0) | ND | Yoshida et al24 |
| Bicetre II | γN308K | Hetero | AMn | ND | ND | A | Grailhe et al25 |
| Marchi et al51 | |||||||
| Matsumoto II | γN308K | Hetero | ATh | ND | ND | ND | Okumura et al23,27 |
| γK308 | γN308K | Recomb | ATh | N | A (0.1/0.005) | A | Okumura et al27 |
| Baltimore III | γN308I | Hetero | AMn | N | N (4.0/0.33) | ND | Bantia et al22 |
| Ebert and Bell50 | |||||||
| γI308 | γN308I | Recomb | ATh/NTh§ | N | A/N (0.1/0.005)§ | N | This paper |
| Matsumoto I | γD364H | Hetero | ATh | N | N (1.0/0.2) | ND | Hogan et al26 |
| Okumura et al29 | |||||||
| γH364 | γD364H | Recomb | ATh | N | N (0.09/0.01)∥ | ND | Hogan et al26 |
| Okumura et al49 | |||||||
| Vlissingen/Frankfurt IV | γΔ19N,320D | Hetero | AMn | N | N (4.0/2.0) | ND | Hogan et al26 |
| Koopman et al40 | |||||||
| γΔ319,320 | γD319N,320D | Recomb | ATh | N | A (0.1/0.01) | A | Hogan et al26,39 |
Fibrinogen . | Substitution . | Allele* . | Fibrin polymerization† . | FpA release . | FpB release‡ . | FXIIIa-catalyzed cross-linking . | References . |
|---|---|---|---|---|---|---|---|
| Kyoto I | γN308K | Hetero | AMn | N | N (5.0/1.0) | ND | Yoshida et al24 |
| Bicetre II | γN308K | Hetero | AMn | ND | ND | A | Grailhe et al25 |
| Marchi et al51 | |||||||
| Matsumoto II | γN308K | Hetero | ATh | ND | ND | ND | Okumura et al23,27 |
| γK308 | γN308K | Recomb | ATh | N | A (0.1/0.005) | A | Okumura et al27 |
| Baltimore III | γN308I | Hetero | AMn | N | N (4.0/0.33) | ND | Bantia et al22 |
| Ebert and Bell50 | |||||||
| γI308 | γN308I | Recomb | ATh/NTh§ | N | A/N (0.1/0.005)§ | N | This paper |
| Matsumoto I | γD364H | Hetero | ATh | N | N (1.0/0.2) | ND | Hogan et al26 |
| Okumura et al29 | |||||||
| γH364 | γD364H | Recomb | ATh | N | N (0.09/0.01)∥ | ND | Hogan et al26 |
| Okumura et al49 | |||||||
| Vlissingen/Frankfurt IV | γΔ19N,320D | Hetero | AMn | N | N (4.0/2.0) | ND | Hogan et al26 |
| Koopman et al40 | |||||||
| γΔ319,320 | γD319N,320D | Recomb | ATh | N | A (0.1/0.01) | A | Hogan et al26,39 |
A indicates abnormal; N, normal; and ND, not done.
Hetero or recomb indicate fibrinogen from a heterozygous individual or recombinant expression, respectively
Polymerization was measured for fibrin monomers (Mn) or catalyzed by thrombin (Th)
Numbers in parentheses indicate fibrinogen (mg/mL)/thrombin (U/mL)
Experiments with no CaCl2 (A) or with added CaCl2 (N)
Fibrinopeptide release was performed in the presence of 10 mM CaCl2
As reported previously, at 1 mM CaCl2 polymerization of the recombinant γN308K was delayed relative to polymerization of fibrinogen isolated from the heterozygous Matsumoto II patient (Table 2).27 Further, the parallel calcium dependence seen when comparing normal fibrinogen and the γN308K variant could have been anticipated from previous calcium-binding studies that showed normal calcium binding to fibrinogen isolated from the heterozygous Kyoto I patient (Table 2).24 In these studies, Yoshida et al24 measured calcium binding to D1 fragments isolated from the patient's fibrinogen (γN308K substitution) and found essentially identical binding to normal and variant fragments, with Kd = 3.2 μM for the variant and 2.8 μM for the normal fragment. Thus, polymerization of the homogenous recombinant lysine variant did mimic polymerization of fibrinogen isolated from the analogous heterozygous patients, although the former was more impaired than the latter.24,27 As reported here, the delayed polymerization of γN308K was associated with the delayed FpB release and FXIIIa-catalyzed fibrin cross-linking, which cannot be corrected with calcium.
The role of residue γAsn308 in polymerization has been considered in 2 reviews that correlated dysfibrinogens with crystallographic structures of fibrinogen fragments.19,21 The side chain of γAsn308 forms hydrogen bonds with the backbone atoms of γTyr278 and of γGly309. These hydrogen bonds would be absent in any of the variants we examined. Introduction of a positively charged lysine at γ308 would further destabilize the complex interactions, as the positively charged side chain of γArg275 also forms hydrogen bonds with the backbone of γGly309. Positive charge repulsion is also likely with γLys321. In the FXIIIa cross-linked D-dimer structure, a cluster of residues including γAsn308, γGly309, γGly268, γArg275, and γMet310 is found at or near the D:D interface between adjacent molecules.11 These residues are likely to be buried during formation of protofibrils, such that the altered polymerizations seen here reflect impaired protofibril formation. Therefore, it would be expected that substitutions at γ308 would disrupt the initial alignment of fibrin monomers into protofibrils, and thereby impair FpB release. Further, the disruption of the D:D interface would be expected to influence FXIIIa-catalyzed γ-γ dimer formation in fibrin. The analyses of γN308K fibrinogen were consistent with these expectations. Furthermore, our analyses with γN308A and γN308I fibrinogens suggest a hydrophobic side chain can be accommodated within the normal D:D interface, implying the positive charge on lysine mediated the impaired polymerization of γN308K fibrinogen.
In conclusion, our results show substitution of Asn308 in γ chain of fibrinogen with a hydrophobic residue isoleucine and alanine altered neither polymer formation nor polymer structure at physiologic calcium concentrations, whereas substitution with lysine, had altered both.
Prepublished online as Blood First Edition Paper, February 5, 2004; DOI 10.1182/blood-2003-12-4296.
Supported by a grant from the Charitable Trust Clinical Pathology Research Foundation of Japan (N.O.) and National Institutes of Health grant HL-31048 (S.T.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors acknowledge S. Kani for helping in the preparation of the figures.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal