Abstract
Enforced expression of Hoxb4 dramatically increases the regeneration of murine hematopoietic stem cells (HSCs) after transplantation and enhances the repopulation ability of human severe combined immunodeficiency (SCID) repopulating cells. Therefore, we asked what physiologic role Hoxb4 has in hematopoiesis. A novel mouse model lacking the entire Hoxb4 gene exhibits significantly reduced cellularity in spleen and bone marrow (BM) and a subtle reduction in red blood cell counts and hemoglobin values. A mild reduction was observed in the numbers of primitive progenitors and stem cells in adult BM and fetal liver, whereas lineage distribution was normal. Although the cell cycle kinetics of primitive progenitors was normal during endogenous hematopoiesis, defects in proliferative responses of BM Lin- Sca1+ c-kit+ stem and progenitor cells were observed in culture and in vivo after the transplantation of BM and fetal liver HSCs. Quantitative analysis of mRNA from fetal liver revealed that a deficiency of Hoxb4 alone changed the expression levels of several other Hox genes and of genes involved in cell cycle regulation. In summary, the deficiency of Hoxb4 leads to hypocellularity in hematopoietic organs and impaired proliferative capacity. However, Hoxb4 is not required for the generation of HSCs or the maintenance of steady state hematopoiesis.
Introduction
Hematopoiesis relies on the unique abilities of relatively few hematopoietic stem cells to self-renew and generate progenitors that will differentiate into the mature cells forming the blood system. This dynamic process is tightly regulated by a complex of internal and external signals, such as transcription factors, growth factors, and cell cycle regulators (for reviews, see Orkin1 and Verfaillie2 ). Many transcription factors, including homeobox (Hox) transcription factors, have been shown to be key players in the proliferation and differentiation of early progenitor cells.3,4-6 Specific expression patterns of multiple Hox genes have been detected in normal and leukemic hematopoiesis.7,8 Enforced expression of Hox genes has been shown to affect the ability of progenitors and stem cells to proliferate and differentiate.9-17 One of these genes, Hoxb4, has been implicated in the regulation of hematopoietic stem cell regeneration,8 and retrovirally engineered overexpression in murine bone marrow cells dramatically increases the stem cell pool ex vivo and in vivo, resulting in faster, more complete recovery of the stem cells in transplantation studies with no adverse effect on differentiation or lineage distribution.14,18-21 This is in contrast to the overexpression of other Hox genes, which can perturb the proliferation and lineage commitment of primitive progenitors and can give rise to hematopoietic malignancies.10,11,13,15,16,22-24 However, recent studies have suggested that the effect of Hoxb4 is concentration dependent and is not necessarily restricted to proliferation.25-27 Thus, the level of Hoxb4 expression has to be within a specific range for Hoxb4 to increase stem cell proliferation without adverse effects on differentiation. Although enforced expression of Hoxb4 in hematopoietic cells has been studied in detail, its physiologic role in hematopoiesis is poorly understood. Recently, we described a mouse model deficient in Hoxb3 and Hoxb4, showing reduced proliferative capacity of the stem cell pool without otherwise perturbing hematopoiesis.28 Here we report a novel mouse model in which the Hoxb4 gene alone has been completely removed through the Cre/loxP technique. Hoxb4-deficient mice have a phenotype similar to that of double Hoxb3/Hoxb4 knockout (KO) mice, although the effects are milder in the Hoxb4-/- mice. The phenotype observed seems mainly confined to the stem cell pool, resulting in reduced proliferative capacity of bone marrow and fetal liver hematopoietic stem cells (HSCs) without affecting differentiation or lineage choice. Deficiency of Hoxb4 or Hoxb3 and Hoxb4 affects the expression of other Hox genes and the expression of cell cycle regulators, indicating a complex regulatory role of these Hox genes. Collectively, these findings indicate that Hoxb4 improves proliferative recruitment of HSCs in settings demanding high proliferation, such as transplantation, but that it has a less prominent role in normal endogenous hematopoiesis.
Materials and methods
Cloning Hoxb4 and generating a targeting construct
A genomic bacteria artificial chromosome (BAC; Stratagene, La Jolla, CA) clone containing more than 100 kb of the Hoxb gene cluster28 was used for generating the Hoxb4 targeting construct. Two overlapping clones, a 6-kb EagI5′ clone (pBS-EagI) and a 9-kb EcoRI 3′ clone (pBS-ERI), were used. The 6-kb EagI fragment was digested with HindIII, and the loxP flanked (floxed) neomycin expression cassette28 was blunted and inserted into the HindIII site. pBS-ERI was opened with EagI, the 3′EagI fragment was isolated and blunted, and a single loxP site, isolated from pGZM (gift from H. Gu, National Institutes of Health, Bethesda, MD), was inserted as a blunt fragment. The 5′ EagI/Neo fragment and the 3′ EagI/loxP fragment were then joined together, creating pBS-KO1. A 12-kb PshAI/ClaI fragment was then isolated from pBS-KO1 and ligated into SmaI/ClaI pBS, creating pBS-KO2. The thymidine kinase (tk) gene controlled by the PGK promoter was isolated from pPNT (gift from Dr R. Jaenish, Massachusetts Institute of Technology, Boston, MA) by EcoRI and HindIII digests. This fragment was blunt ligated into the ClaI site in the 3′ polylinker of pBSKO-2, generating pBS-KO2-tk with approximately 1.7-kb 5′ and 5.5-kb 3′ homologous arms.
Gene targeting in ES cells
Embryonic stem (ES) cells were targeted using standard protocols as detailed in Bjornsson et al.28 To verify homologous recombination (HR), the surviving colonies were screened by polymerase chain reaction (PCR) using the B4U5′ext primer 1 (P1) (GTTGACATAAACACTCCGCTCATA) and P2 (internal neospecific primer) (CGAAGTTATTAGGTCTGAAGAAGGAG). Positive clones were further analyzed by Southern blot using external probes (data not shown). Ten micrograms DNA was digested with either PstIor KpnI for the detection of the 3′ loxP site or a single integration of the neomycin cassette, respectively (Figure 1). Correctly targeted clones were expanded, and approximately 2 × 106 cells were electroporated with 15 μg plasmid pIC-Cre, resulting in the excision of the neomycin gene either alone or together with a total excision of the Hoxb4 gene (null mutation). Neomycin-sensitive (neos) clones were identified and analyzed by PCR using primers P3 (GGAAGCAAGAAAAGGAGGAAGAAAGGA), P4 (CAAAGTGGGTACAGACAGGGAGGAAAG), and P5 (GTTGACATAAACACTCCGCTCATA). For generating chimeras, independently targeted clones bearing either the Hoxb4-floxed or the Hoxb4-deleted version were used according to published techniques.29 Chimeric males were mated to C57BL/6J females, resulting in offspring with a 129Sv/C57BL/6J genetic background. For screening of germline offspring, DNA was isolated from tail biopsy specimens and was analyzed using PCR (Figure 1). Total RNA was isolated from peripheral blood and bone marrow (RNeasy; Qiagen, Hilden, Germany) of Hoxb4 mice and used for reverse transcription–PCR (RT-PCR) to verify the presence or absence of the RNA transcripts.
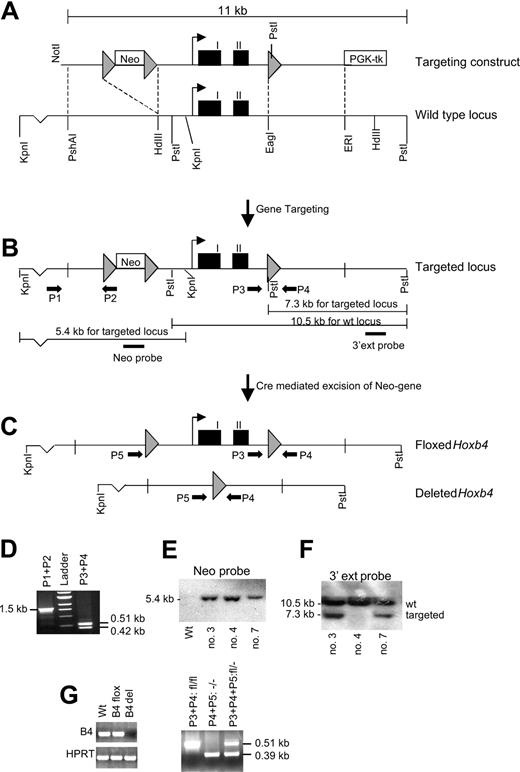
Generation of Hoxb4-deficient mice. (A) The targeting construct compared with the WT locus. Gray arrowheads indicate loxP sites; black boxes, Hoxb4 exons. (B) Targeted locus showing the strategy for detection of homologous recombination (HR) events. Black arrows indicate PCR primers (P1-P5), and resultant bands are shown in panel D. Two Southern blot strategies are shown. The PstI digest using the 3′ external probe confirms the homologous integration of the 3′ loxP site, and the KpnI digest using the neo probe verifies a single homologous integration site for the construct (see panels E and F). (C) Targeted locus after Cre-mediated recombination events, resulting in either a floxed or a deleted Hoxb4 gene. (D) Findings from the PCR analysis verifying homologous recombination at the 5′ end of the construct (P1 and P2) and the presence of the 3′ loxP site (P3 and P4). (E-F) Southern blot analysis using KpnIor Pst I digests, respectively. All the clones (3, 4, and 7) have a single integration site of the neomycin cassette, but clone 4 does not have the single 3′ loxP site. (G) RT-PCR results showing normal expression of Hoxb4 in floxed mice, but no expression can be detected in mice carrying the deleted version. (H) PCR analysis for genotyping mice that carry the deleted Hoxb4 version. Neo indicates neomycin-resistance gene driven by the thymidine kinase promoter; PGK-tk, thymidine kinase driven by the PGK promoter; HdIII, HindIII; ERI, EcoRI; ext probe, external probe; and kb, kilobase pairs.
Generation of Hoxb4-deficient mice. (A) The targeting construct compared with the WT locus. Gray arrowheads indicate loxP sites; black boxes, Hoxb4 exons. (B) Targeted locus showing the strategy for detection of homologous recombination (HR) events. Black arrows indicate PCR primers (P1-P5), and resultant bands are shown in panel D. Two Southern blot strategies are shown. The PstI digest using the 3′ external probe confirms the homologous integration of the 3′ loxP site, and the KpnI digest using the neo probe verifies a single homologous integration site for the construct (see panels E and F). (C) Targeted locus after Cre-mediated recombination events, resulting in either a floxed or a deleted Hoxb4 gene. (D) Findings from the PCR analysis verifying homologous recombination at the 5′ end of the construct (P1 and P2) and the presence of the 3′ loxP site (P3 and P4). (E-F) Southern blot analysis using KpnIor Pst I digests, respectively. All the clones (3, 4, and 7) have a single integration site of the neomycin cassette, but clone 4 does not have the single 3′ loxP site. (G) RT-PCR results showing normal expression of Hoxb4 in floxed mice, but no expression can be detected in mice carrying the deleted version. (H) PCR analysis for genotyping mice that carry the deleted Hoxb4 version. Neo indicates neomycin-resistance gene driven by the thymidine kinase promoter; PGK-tk, thymidine kinase driven by the PGK promoter; HdIII, HindIII; ERI, EcoRI; ext probe, external probe; and kb, kilobase pairs.
Mice were bred, kept in ventilated racks, and fed autoclaved food and water in the animal facility of The Biomedical Center, Lund University. The Ethical Committee for Animal Research approved all animal experiments.
Clonogenic assays
Hematopoietic cells were harvested as described.28 For myeloid clonogenic progenitor assays, bone marrow (BM) cells were cultured in 35-mm Petri dishes. For culture colony-forming unit (CFU-C) assay, rich methylcellulose (M3534 containing stem cell factor [SCF; 50 ng/mL], interleukin-3 [IL-3; 10 ng/mL], and IL-6 [10 ng/mL]; Stem Cell Technologies, Vancouver, BC, Canada) with the addition of 5 U/mL human erythropoietin (hEPO; Janssen-Cilag, Sollentuna, Sweden) was used. For erythroid blast-forming unit (BFU-E) assay, serum-free methylcellulose (M3236; Stem Cell Technologies) supplemented with 50 ng/mL SCF (Amgen, Thousand Oaks, CA), 50 ng/mL thrombopoietin (TPO; Kirin Brewery, Tokyo, Japan), and 5 U/mL hEPO was used. Colonies were scored on days 7 to 12.
Proliferation recruitment
For single-cell cultures, Lin- Sca1+ c-kit+ (LSK) cells were used as described.28 The following cytokines were used in various combinations (see “Results”): SCF (Amgen), TPO (Kirin), Flt-3 ligand (FL; Immunex, Seattle, WA), granulocyte colony-stimulating factor (G-CSF; Amgen), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-3 (GM-CSF and IL-3 were gifts from Novartis, East Hanover, NJ).
Cell cycle analysis
Lineage-negative BM cells (CD4, CD8, CD5, Gr1, Mac1, B220, and Ter119-) were stained with c-kit–allophycocyanin, (c-kit–APC) and Sca1-phycoerythrin (Sca1-PE) and were preserved in 0.4% formaldehyde (LabKemi, Stockholm, Sweden) and 0.2% Triton-X (Sigma) overnight. The next day, the cells were labeled with Ki67-fluorescein isothiocyanate (Ki67-FITC) and 7AAD (Sigma) and were analyzed on a FACSCalibur (Becton Dickinson, Erembodegen, Belgium).
Bone marrow transplantation experiments
For standard competitive transplantation experiments, 2 × 105 fresh BM cells from Hoxb4-/- or control littermates (Ly5.2) were transplanted, together with 8 × 105 BM cells from B6.SJL (Ly5.1). When including 5-fluorouracil (5-FU; Nycomed, Stockholm, Sweden) treatment in the transplantation study, mutant and normal littermates were injected with 5-FU (150 mg/kg) on day 1 and day 5. On day 6, BM cells were harvested, and one tenth of a femur equivalent was transplanted together with 2 × 105 fresh B6.SJL BM cells. Lethally irradiated B6.SJL cells were used as recipients.
Fluorescence-activated cell sorter analysis
Hematopoietic cell suspensions for lineage analysis (peripheral blood [PB], BM, spleen, thymus, and lymph nodes) were stained with an FITC-conjugated lineage cocktail containing anti-Mac1, anti-Gr1, anti-B220, and anti-CD4 antibodies and with PE-conjugated anti-CD3, anti-CD8, and anti-Ter119 antibodies. For analysis of reconstitution in mice that underwent transplantation, PE-conjugated anti-CD45.1 (Ly5.1) and APC–(biotin-)anti-CD45.2 (Ly5.2) antibodies were used. For estimating LSK CD34lo/- cells, the cells were incubated in a lineage cocktail (described above) of purified antibodies, then labeled with Tricolor-conjugated goat F(ab')2 antirat immunoglobulin G(H+L) (IgG[H+L]) (Caltag Laboratories, Burlingame, CA) and with Sca1-FITC, c-kit-PE, and CD34-(biotin)–APC.
Fetal liver studies
Breeder pairs were set up late in the afternoon, and mice with vaginal plugs observed the following morning were designated 0.5 days postcoitum (dpc). Fetal livers were dissected from 14.5 dpc mice, and single-cell suspensions were prepared by drawing liver cells through 23-gauge needle and then filtering them through a 50-μm cell strainer.
Fetal liver transplantation
For fetal liver transplantation experiments, 2 × 105 Hoxb4-/- or Hoxb4+/+ cells (Ly5.2) derived from 14.5-day-old embryos were used in competition with 6 × 105 B6.SJL cells (Ly5.1) and were transplanted into lethally irradiated (B6.SJL × C57BL/6)F1 recipients (expressing both Ly5.1 and Ly5.2).
Analysis of primitive hematopoietic fetal liver cells (Lin-/loSca+AA4.1+)
Cells were stained as previously described for LSK cells; however, Mac1 was excluded from the lineage cocktail, the cells were labeled with PE-conjugated Sca1 antibody, and an FITC-conjugated AA4.1 antibody was used instead of the c-kit antibody. Day-14.5 Ly5.2 fetal liver cells from 3 to 4 individual fetuses of each genotype were injected into lethally irradiated Ly5.1/Ly5.2 mice at a concentration of 10 000, 20 000, 50 000, or 100 000 cells, mixed with 300 000 unfractionated Ly5.1 bone marrow cells. Each cohort was composed of 6 mice. Contributions of fetal liver–derived blood cells were analyzed after 1 and 3 months. Mice positive for more than 1% Ly5.2 cells of all nucleated peripheral blood cells of myeloid (Mac1, Gr1) and lymphoid (B220, CD3) cells by fluorescence-activated cell sorter (FACS) analysis were considered positive. Poisson statistics30 were then applied to estimate the frequency of competitive repopulating units (CRUs) based on an analysis of the number of negative recipients as a function of the number of test cells injected, as previously described.31,32
Quantitative RT-PCR
Day-14.5 fetal liver cells were depleted of Ter119 cells by use of magnetic bead–conjugated antibodies, and total RNA was extracted using TriZol (Gibco BRL, Grand Island, NY). cDNA was transcribed (Superscript II; Gibco BRL), and quantitative PCR (Table 1) was performed on a LightCycler (Roche Diagnostics, Mannheim, Germany) and analyzed with LightCycler Software version 5.32. Values for each PCR product were normalized against HPRT giving a relative intensity (RI) for comparison of knockout and normal fetal liver. For a few genes (Hoxa4, c4, d4), the level of expression was too low for detection using SybrGreen I. For detection of these mRNAs, primers and TaqMan probes from Applied Biosystems (Foster City, CA) were used and analyzed in an ABI Prism 7700 Sequence Detection System (Applied Biosystems).
Primers used in quantitative RT-PCR assays
Target gene . | 5′ sequence . | 3′ sequence . |
|---|---|---|
| HPRT | cacaggactagaacacctgc | gctggtgaaaaggacctc |
| Hoxa9 | ggtgactgtcccacgcttga | gagtggagcgagcatgtag |
| Hoxb2 | ccagctggagtgcctgtt | gagggtgttgtgtacctt |
| Hoxb3 | gcagaaagccacctactacg | ccattgagctccttgctctt |
| Hoxb5 | ccggactatcagttgctaa | ggacgtcgcctgcctgaa |
| c-myc | cctgtacctcgtccgatt | acgttgtgtgtccgcctctt |
| c-jun | ggtgcctacggcatcagtaa | gtagtggtgatgtgcccatt |
| jun-B | gtggggvaggcagctactt | gcgcccagggacacgtt |
| CXCR4 | ggaccggtacctcgccatt | ccacaggctatcggggtaa |
| cyclinD1 | ccagctcctgtgctgcgaa | catccaggtggccacgatt |
Target gene . | 5′ sequence . | 3′ sequence . |
|---|---|---|
| HPRT | cacaggactagaacacctgc | gctggtgaaaaggacctc |
| Hoxa9 | ggtgactgtcccacgcttga | gagtggagcgagcatgtag |
| Hoxb2 | ccagctggagtgcctgtt | gagggtgttgtgtacctt |
| Hoxb3 | gcagaaagccacctactacg | ccattgagctccttgctctt |
| Hoxb5 | ccggactatcagttgctaa | ggacgtcgcctgcctgaa |
| c-myc | cctgtacctcgtccgatt | acgttgtgtgtccgcctctt |
| c-jun | ggtgcctacggcatcagtaa | gtagtggtgatgtgcccatt |
| jun-B | gtggggvaggcagctactt | gcgcccagggacacgtt |
| CXCR4 | ggaccggtacctcgccatt | ccacaggctatcggggtaa |
| cyclinD1 | ccagctcctgtgctgcgaa | catccaggtggccacgatt |
Statistical analysis
Statistical analysis (except for CRU determination) was performed using the Mann-Whitney rank sum test or the Student t test. A P value of less than .05 was considered significant.
Results
Generation of a targeted Hoxb4 mouse model
To study the physiologic role of Hoxb4 in hematopoiesis, we generated a mouse model deficient of Hoxb4. We used the Cre/loxP system to generate conditional Hoxb4 KO mice to avoid possible developmental abnormalities that could affect the analysis of hematopoiesis. To remove both exons and the intron of Hoxb4 as well as closely flanking sequences, loxP sites were introduced 1.4 kb upstream and 0.5 kb downstream of the start and stop codon, respectively (Figure 1). Correctly targeted 129Sv ES clones were identified by Southern blot analysis and PCR (Figure 1D-F) and were used to generate chimeric mice. In addition to generating mice carrying the floxed (flanked by loxP sites) Hoxb4 allele, we also generated null mutant mice from ES clones in which the Hoxb4 gene had been excised (Figure 1C). Both mouse lines were born at normal Mendelian ratios and appeared healthy. Expression of Hoxb4 seemed not to be affected by the presence of the loxP sites in homozygous Hoxb4 flox/flox mice (Hoxb4fl/fl), and no expression of Hoxb4 was detectable in the null mutants (Hoxb4-/-) (Figure 1G). Because the null mutant mice reproduced normally, they were the focus of our studies.
Reduced cellularity in hematopoietic organs of Hoxb4-/- mice
No abnormality in hematopoietic organs (bone marrow, spleen, thymus, and lymph nodes) was observed. However, a mild reduction in cellularity was detected in various hematopoietic organs of Hoxb4-/- mice. Analysis of PB showed a small but significant reduction in red blood cell count (P = .04) and in hemoglobin values (P = .04), whereas the white blood cell count was normal (Table 2). A significant reduction in cellularity was also observed in BM (P = .04) and spleen (P = .03) (Table 2). The reduction in cellularity was not caused by alterations in specific mature hematopoietic compartments because flow cytometry (FACS) analysis on cells derived from PB, BM, spleen, and thymus (antibodies against Gr1, Mac1, B220, CD3, CD4, CD8, and Ter119) showed no significant difference between null mutants and normal littermates (data not shown). These findings indicate that the cause for the reduced cellularity lies within the primitive hematopoietic cell compartment, as was observed for the Hoxb3/b4 mutants.28
Cellularity in hematopoietic organs of WT and Hoxb4-deficient mice
Hematopoietic parameter . | Hoxb4+/+ . | Hoxb4-/- . | P . |
|---|---|---|---|
| Spleen weight, mg | 105 ± 13 | 89 ± 9 | .05 |
| Spleen cellularity, × 106 | 154 ± 20 | 128 ± 25 | .03 |
| Red blood cell count, × 1012/L | 8 ± 0.5 | 7.6 ± 0.4 | .04 |
| Hemoglobin, g/L | 142 ± 6 | 136 ± 5 | .04 |
| White blood cell count, × 109/L | 4.3 ± 0.6 | 4.0 ± 0.6 | .5 |
| Bone marrow cellularity, × 106/2 femurs | 56 ± 8 | 46 ± 6 | .04 |
Hematopoietic parameter . | Hoxb4+/+ . | Hoxb4-/- . | P . |
|---|---|---|---|
| Spleen weight, mg | 105 ± 13 | 89 ± 9 | .05 |
| Spleen cellularity, × 106 | 154 ± 20 | 128 ± 25 | .03 |
| Red blood cell count, × 1012/L | 8 ± 0.5 | 7.6 ± 0.4 | .04 |
| Hemoglobin, g/L | 142 ± 6 | 136 ± 5 | .04 |
| White blood cell count, × 109/L | 4.3 ± 0.6 | 4.0 ± 0.6 | .5 |
| Bone marrow cellularity, × 106/2 femurs | 56 ± 8 | 46 ± 6 | .04 |
n = 10 mice/genotype, 12 to 16 weeks of age. Values are mean ± SD.
Hoxb4 deficiency does not affect colony-forming ability in vitro
Colony-forming assays were performed by plating out fresh BM cells in methylcellulose designed for the general enrichment of CFU-C (SCF, IL-3, IL-6, EPO) or, more specifically, for BFU-E (SCF, TPO, EPO). Colonies were enumerated on day 8 and days 10 to 12 for BFU-E and CFU-C, respectively. Hoxb4-/--derived cells showed lower colony-forming ability than control cells, but this difference was not significant (BFU-E colonies: KO 11 ± 3 vs wild-type [WT] 15 ± 3 per 105 BM cells; P = .2) (CFU-C colonies: KO 19 ± 5 vs WT 23 ± 4 per 104 BM cells; P = .3). Correlation of these data with the reduced BM cellularity observed in the Hoxb4-deficient mice showed a mild, nonsignificant reduction in the absolute numbers of CFU-C and BFU-E progenitors in the KO mice (P = .1; data not shown).
Hoxb4 deficiency causes a mild reduction in absolute numbers of primitive hematopoietic progenitors without affecting cell cycle distribution during endogenous hematopoiesis
Given that ectopic expression of HOXB4 enhances the regeneration of HSCs, we wanted to analyze whether Hoxb4 deficiency would result in a reduced stem cell pool with possible alterations in cell cycle distribution. We estimated the proportion of Lin- Sca1+ c-kit+CD34lo/- (LSKCD34) cells in BM by FACS analysis and found no significant difference between Hoxb4-deficient mice and control littermates. When correlated with the observed reduction seen in BM cellularity, a mild, nonsignificant reduction in absolute numbers of LSKCD34 cells was observed (P = .2). For analysis of cell cycle distribution, LSK progenitors were analyzed by 7AAD and Ki67 staining. The lack of Hoxb4 does not alter the ratio of LSK progenitors in active cycle compared with G0/G1 at steady state (data not shown), indicating that the cause for reduced cellularity and size of hematopoietic organs may have its roots during development.
Proliferative response of primitive hematopoietic progenitors is reduced in Hoxb4-/- mice
To determine whether the observed reductions in total cellularity and primitive hematopoietic populations size was caused by proliferation defects in Hoxb4-/- progenitors, we analyzed the proliferation recruitment of LSK cells. LSK progenitors were sorted from BM and grown in a single-cell assay measuring recruitment into proliferation and survival. LSK cells were plated out for 10-day culture in serum-free medium supplemented with various cytokines (SCF; SCF and TPO; SCF, TPO, and FL; or the multicytokine mix, SCF, FL, TPO, G-CSF, IL-3, and GM-CSF) on day 1 (for proliferation recruitment) or on day 5 (multicytokine mix, for viability analysis). No significant difference was noted in total number of clones (P = .2) between Hoxb4-/- and controls nor in numbers of clones in the survival analysis (P = .4; data not shown). However, the proliferative response of primitive progenitors was significantly different because lower numbers of high proliferative clones were observed for the Hoxb4-/- cells compared with cells derived from normal littermates (P = .02; Figure 2). This phenomenon was only seen when maximum cytokine stimulation was used, indicating an active role for Hoxb4 in situations demanding high proliferation of primitive LSK progenitors.
Reduced proliferative response in Hoxb4-/- LSK cells. Enumeration of positive wells after 10-day single-cell assay (Lin-Sca1+c-kit+) with SCF, FL, TPO, G-CSF, GM-CSF, and IL-3 in Terasaki plates. No significant difference was seen in the total number of positive wells (□). However, a significant reduction in wells positive for more than 50% of the well covered by cells (high proliferative clones, ▦) was seen in wells with Hoxb4-/- cells (mean ± SD). Data are from 4 donors of each phenotype.
Reduced proliferative response in Hoxb4-/- LSK cells. Enumeration of positive wells after 10-day single-cell assay (Lin-Sca1+c-kit+) with SCF, FL, TPO, G-CSF, GM-CSF, and IL-3 in Terasaki plates. No significant difference was seen in the total number of positive wells (□). However, a significant reduction in wells positive for more than 50% of the well covered by cells (high proliferative clones, ▦) was seen in wells with Hoxb4-/- cells (mean ± SD). Data are from 4 donors of each phenotype.
Reduced repopulating ability of bone marrow–derived HSCs deficient in Hoxb4
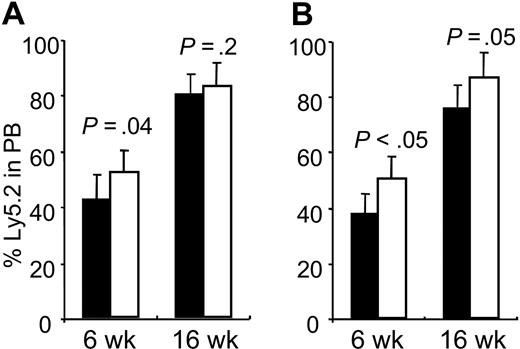
To further investigate the physiologic role of Hoxb4 in the proliferation of primitive hematopoietic progenitors and stem cells, we performed competitive transplantation using fresh BM cells from Hoxb4-/- and Hoxb4+/+ mice. Repopulating ability and lineage distribution of mutant cells was compared with those of normal littermates in competition with B6.SJL competitor cells (Ly5.1) in lethally irradiated recipients (Ly5.1). Our results were in line with previously reported data using Hoxb3/b4-/- BM cells, with significantly lower repopulating ability of the knockoutderived cells after 4 to 6 weeks but less pronounced differences at 12 to 16 weeks (Figure 3). The lineage commitment of engrafted Hoxb4-deficient cells was not affected (data not shown). For additional proliferative stress, we performed a secondary transplantation using cells from the primary Hoxb4 BM recipients. Secondary recipient reconstitution of mutant cells was significantly lower than for normal cells at week 6 but less prominent at week 16 (P < .05 and P = .05, respectively). Collectively, these findings suggest that the regenerative capacity of HSCs is reduced in Hoxb4-/- mice.
Reduced competitive repopulation of Hoxb4-/- BM cells. Contribution of wt (□) and Hoxb4-/- (▪) cells in peripheral blood of primary (A) and secondary (B) recipients after 6 and 12 weeks, in competition with B6.SJL BM cells. Primary recipients showed significantly reduced reconstitution at 6 weeks after BMT, whereas the difference at 12 weeks was only marginal. In secondary recipients, reduced reconstitution by Hoxb4-/- BM was even more pronounced at 6 weeks after transplantation (P < .05), but at 12 weeks the difference was no longer significant (P = .05) (mean ± SD; n = 4).
Reduced competitive repopulation of Hoxb4-/- BM cells. Contribution of wt (□) and Hoxb4-/- (▪) cells in peripheral blood of primary (A) and secondary (B) recipients after 6 and 12 weeks, in competition with B6.SJL BM cells. Primary recipients showed significantly reduced reconstitution at 6 weeks after BMT, whereas the difference at 12 weeks was only marginal. In secondary recipients, reduced reconstitution by Hoxb4-/- BM was even more pronounced at 6 weeks after transplantation (P < .05), but at 12 weeks the difference was no longer significant (P = .05) (mean ± SD; n = 4).
Hoxb4-/- stem cells display increased tolerance to 5-FU treatment
To further analyze the effect of Hoxb4 on the proliferation of primitive hematopoietic progenitors and stem cells in vivo, we set up a competitive transplantation study in combination with the antimitotic drug 5-FU. 5-FU treatment is selectively cytotoxic for cycling cells, forcing the quiescent HSCs to go into cycle. A second 5-FU injection depletes the activated stem cells. Hoxb4-/- mice and healthy littermates were injected with 5-FU (150 mg/kg) on days 1 and 5, and BM was harvested on the following day. These Hoxb4-/- or Hoxb4+/+ BM cells were transplanted together with B6.SJL cells into lethally irradiated B6.SJL recipients. At 6 and 17 weeks after transplantation, the Hoxb4-/-cells showed significantly higher engraftment than the Hoxb4+/+ cells (P = .01 [data not shown] and P = .04, respectively; Figure 4), demonstrating that Hoxb4-deficient stem cells have higher tolerance to 5-FU treatment.
Hoxb4-/-BM cells have increased tolerance to 5-FU treatment. (A) In vivo repopulation of Hoxb4-/--derived and Hoxb4+/+-derived donor cells after double treatment with 5-FU (day 1 [d1] and day 5 [d5]). The 5-FU–treated cells were competed against fresh B6.SJL cells in lethally irradiated B6.SJL recipients. (B) Level of reconstitution was measured as peripheral blood cells at 17 weeks after transplantation (mean ± SD) (each bar = 16 recipient mice from 4 donors).
Hoxb4-/-BM cells have increased tolerance to 5-FU treatment. (A) In vivo repopulation of Hoxb4-/--derived and Hoxb4+/+-derived donor cells after double treatment with 5-FU (day 1 [d1] and day 5 [d5]). The 5-FU–treated cells were competed against fresh B6.SJL cells in lethally irradiated B6.SJL recipients. (B) Level of reconstitution was measured as peripheral blood cells at 17 weeks after transplantation (mean ± SD) (each bar = 16 recipient mice from 4 donors).
HSC pool in day-14.5 fetal liver is marginally reduced in embryos deficient in Hoxb4
Because the data from our proliferation recruitment assay of adult LSK cells in vitro and stem cell transplantation in vivo suggested a defect in proliferative capacity, we wanted to study this further in embryonic hematopoiesis. Expansion of the stem cell population has been reported to occur in the fetal liver on days 11 to 15, after which the hematopoietic cells seed to the bone marrow.33,34 Therefore, we analyzed the repopulating Lin-Sca1+AA4.1+ (LSA) cells35 in day-14.5 fetal liver from Hoxb4 mutants and littermate control fetuses. However, as observed previously, the loss of Hoxb4 alone results in a weak phenotype, this time only marginally affecting the size of the LSA population (Table 3).
Cellularity of fetal liver day 14.5 in Hoxb4-deficient mice
. | Hoxb4+/+* . | Hoxb4-/-† . | P . |
|---|---|---|---|
| Total cellularity, ×106 | 20.3 | 19.0 | NS |
| Lin-Scal+ AA4.1+, ×104 | 5.5 | 4.8 | NS |
. | Hoxb4+/+* . | Hoxb4-/-† . | P . |
|---|---|---|---|
| Total cellularity, ×106 | 20.3 | 19.0 | NS |
| Lin-Scal+ AA4.1+, ×104 | 5.5 | 4.8 | NS |
NS indicates not significant.
n = 14
n = 8
Transplantation of fetal liver cells indicates delayed proliferative response of HSC
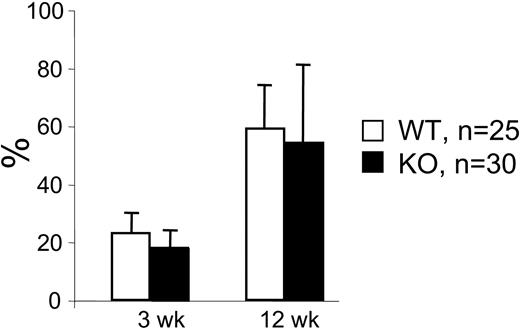
To further analyze whether the fetal liver stem cell pool of mutant mice harbored functional defects, we performed competitive repopulation studies. Fetal liver cells from Hoxb4-deficient or normal mice underwent transplantation with fresh B6.SJL BM cells into lethally irradiated (B6.SJL × C57BL/6) fetal liver recipients. The results shown in Figure 5 are reminiscent of the data from the transplantation of adult BM cells, suggesting a delayed proliferative response of mutant HSCs as they lag behind their normal counterparts during the initial repopulation period, a difference that fades with time.
Competitive repopulation of 14.5 dpc fetal liver cells. Fetal liver cells from wt and Hoxb4-/- 14.5 dpc fetuses were transplanted in competition with B6.SJL bone marrow cells. Blood samples were analyzed at 3, 6, and 12 weeks after BMT for the contribution of fetal liver–derived blood cells. No significant difference in contributions of cells from wt compared with Hoxb4-/--derived fetal liver cells was seen at any time point. Data from 25 wt and 30 Hoxb4-deficient fetal livers (mean ± SD).
Competitive repopulation of 14.5 dpc fetal liver cells. Fetal liver cells from wt and Hoxb4-/- 14.5 dpc fetuses were transplanted in competition with B6.SJL bone marrow cells. Blood samples were analyzed at 3, 6, and 12 weeks after BMT for the contribution of fetal liver–derived blood cells. No significant difference in contributions of cells from wt compared with Hoxb4-/--derived fetal liver cells was seen at any time point. Data from 25 wt and 30 Hoxb4-deficient fetal livers (mean ± SD).
Lower CRU numbers in fetal livers of Hoxb4 KO mice
Assays measuring CRU are used to enumerate HSCs in murine models.31,32 In light of our data suggesting a proliferative defect in mutant HSCs, we transplanted fetal liver cells in limiting dilution with B6.SJL support cells into lethally irradiated mice. Blood samples were assayed at 1 month and 3 months to determine the contribution of fetal liver–derived cells in the blood. Mild reductions in the numbers of CRU were seen at both time points in the recipients of Hoxb4-/- FL cells compared with WT (352 ± 91 vs 416 ± 83 at 1 month and 356 ± 46 vs 429 ± 112 at 3 months, respectively). These data support the findings of a reduced capacity of mutant HSCs from BM and fetal liver to repopulate lethally irradiated recipients in a competitive repopulation transplantation assay (see the previous paragraph).
Altered gene expression in mice lacking Hoxb4
How the Hoxb4 gene and its target genes are regulated is largely unknown. Recent data suggest that Hoxb4 binds to the promoter region of c-myc36 ; the AP-1 complex is another suggested target.37 Other data suggest that the different Hox genes are regulated by neighboring Hox genes in a cascade of expression in a 3′ to 5′ order.38,39 Therefore, we asked whether the deficiency of Hoxb3/b4 or Hoxb4 alone affected the regulation of other Hox genes in hematopoietic cells. We performed quantitative RT-PCR on fetal livers 14.5 dpc of KO and WT mice. Ter119-depleted fetal liver cells were tested for the expression of Hoxa4, Hoxa9, Hoxb2, Hoxb3, Hoxb5, Hoxc4, Hoxd4, c-myc, Jun-B, p21, cyclinD1, CXCR4, and c-Jun. A cut-off level at a 2-fold increase or decrease was chosen, giving a significant reduction of mRNA for Hoxb2, b3, b5, jun-B and cyclinD1 in Hoxb4-deficient fetal liver cells (n = 14; P < .05), whereas the level of Hoxa4 was slightly but not significantly increased (Table 4). mRNA levels from the paralogous genes Hoxc4 and Hoxd4 were too low to be reliably detected in fetal liver samples from WT and KO mice. Although they did not reach the 2-fold change (1.6 and 1.9, respectively) and, therefore, were not scored as positive, p21 and c-myc levels were significantly increased. For the other genes tested, no significant reduction or increase could be seen (Table 4). For comparison, Table 4 also includes gene expression levels from the Hoxb3/b4-/- mice.28 These findings support the notion that Hoxb4 is involved in the regulation of c-myc and that there is a complex interregulation in the expression of clustered Hox genes.
Quantitative RT-PCR
. | Hoxb4 Ter119-depleted fetal liver . | . | . | Hoxb3/b4 Ter119-depleted fetal liver . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene . | wt; n = 9 . | b4-/-; n = 10-14 . | FD . | wt; n = 8 . | b3/b4-/-; n = 5-9 . | FD . | ||||
| HoxA4 | 0.29 ± 0.12 | 0.38 ± 0.10 | +1.3 | 0.29 ± 0.12 | 0.51 ± 0.17 | +1.7 | ||||
| HoxA9 | 3.28 ± 1.67 | 2.13 ± 2.10 | -1.5 | 2.76 ± 1.09 | 0.46 ± 0.83 | -6.0† | ||||
| HoxB2 | 2.80 ± 1.72 | 0.72 ± 0.44 | -3.9† | 2.85 ± 0.74 | 0.76 ± 0.23 | -3.8† | ||||
| HoxB3 | 1.68 ± 0.65 | 0.19 ± 0.39 | -8.8† | — | — | — | ||||
| HoxB5 | 2.33 ± 0.68 | 0.15 ± 0.13 | -16‡ | 2.25 ± 0.64 | 0.48 ± 0.25 | -4.7† | ||||
| HoxC4 | VL | VL | — | VL | VL | — | ||||
| HoxD4 | VL | VL | — | VL | VL | — | ||||
| p21 | 1.31 ± 0.80 | 2.13 ± 0.75 | +1.6* | 1.24 ± 0.74 | 2.97 ± 1.65 | +2.4* | ||||
| c-myc | 2.11 ± 2.10 | 3.92 ± 1.33 | +1.9* | 2.11 ± 1.11 | 5.67 ± 3.29 | +2.7 | ||||
| Jun-B | 3.56 ± 0.94 | 0.75 ± 0.69 | -4.7* | 3.55 ± 0.93 | 1.16 ± 0.91 | -3.1* | ||||
| CyclinD1 | 1.57 ± 0.55 | 0.43 ± 0.28 | -3.6‡ | 1.57 ± 0.54 | 0.81 ± 0.53 | -1.9* | ||||
| c-Jun | 0.41 ± 0.27 | 0.21 ± 0.14 | -2.0 | ND | ND | — | ||||
| CXCR4 | 1.75 ± 0.99 | 0.94 ± 0.77 | -1.9 | 1.69 ± 0.89 | 1.50 ± 0.97 | -1.1 | ||||
. | Hoxb4 Ter119-depleted fetal liver . | . | . | Hoxb3/b4 Ter119-depleted fetal liver . | . | . | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene . | wt; n = 9 . | b4-/-; n = 10-14 . | FD . | wt; n = 8 . | b3/b4-/-; n = 5-9 . | FD . | ||||
| HoxA4 | 0.29 ± 0.12 | 0.38 ± 0.10 | +1.3 | 0.29 ± 0.12 | 0.51 ± 0.17 | +1.7 | ||||
| HoxA9 | 3.28 ± 1.67 | 2.13 ± 2.10 | -1.5 | 2.76 ± 1.09 | 0.46 ± 0.83 | -6.0† | ||||
| HoxB2 | 2.80 ± 1.72 | 0.72 ± 0.44 | -3.9† | 2.85 ± 0.74 | 0.76 ± 0.23 | -3.8† | ||||
| HoxB3 | 1.68 ± 0.65 | 0.19 ± 0.39 | -8.8† | — | — | — | ||||
| HoxB5 | 2.33 ± 0.68 | 0.15 ± 0.13 | -16‡ | 2.25 ± 0.64 | 0.48 ± 0.25 | -4.7† | ||||
| HoxC4 | VL | VL | — | VL | VL | — | ||||
| HoxD4 | VL | VL | — | VL | VL | — | ||||
| p21 | 1.31 ± 0.80 | 2.13 ± 0.75 | +1.6* | 1.24 ± 0.74 | 2.97 ± 1.65 | +2.4* | ||||
| c-myc | 2.11 ± 2.10 | 3.92 ± 1.33 | +1.9* | 2.11 ± 1.11 | 5.67 ± 3.29 | +2.7 | ||||
| Jun-B | 3.56 ± 0.94 | 0.75 ± 0.69 | -4.7* | 3.55 ± 0.93 | 1.16 ± 0.91 | -3.1* | ||||
| CyclinD1 | 1.57 ± 0.55 | 0.43 ± 0.28 | -3.6‡ | 1.57 ± 0.54 | 0.81 ± 0.53 | -1.9* | ||||
| c-Jun | 0.41 ± 0.27 | 0.21 ± 0.14 | -2.0 | ND | ND | — | ||||
| CXCR4 | 1.75 ± 0.99 | 0.94 ± 0.77 | -1.9 | 1.69 ± 0.89 | 1.50 ± 0.97 | -1.1 | ||||
cDNA was from Ter119-depleted day-14.5 fetal liver cells from wt and Hoxb4-/- and Hoxb3/b4-/- mice. HPRT was used to normalize cDNA concentration for each PCR product.
Values are presented as mean ± SD for the relative intensity of cDNA from the indicated gene compared with HPRT. FD indicates fold difference; — indicates not applicable; VL, very low levels, not or barely detectable; and ND, not done.
P < .05
P ≤ .01
P ≤ .001
Discussion
Understanding the complex regulation of HSCs is crucial for future therapeutic applications. Several studies show that the enforced expression of HOXB4 can be used to expand HSCs ex vivo and in vivo.14,18-20,27,40 Here we have addressed the question of the physiologic role for Hoxb4 in hematopoiesis in a novel gene-targeting mouse model with complete deletion of the Hoxb4 gene. The deficiency of Hoxb4 leads to a 20% reduction in cellularity of spleen and bone marrow and causes a mild reduction in peripheral blood cellularity, particularly red cells, without major alterations in lineage distribution. A mild quantitative reduction was observed in the absolute numbers of myeloid progenitors, seemingly without any qualitative changes. A subtle reduction was observed in the number of cells enriched for long-term repopulating ability (LSK CD34lo/-), and Hoxb4-/- LSK progenitors displayed reduced recruitment into proliferation in vitro when stimulated by strong cytokine signals. Furthermore, Hoxb4-/- BM and fetal liver stem cells showed reduced regenerative capacity in competitive transplantation studies, and BM stem cells displayed increased tolerance to treatment with 5-FU. Expansion of primitive hematopoietic cells during development is significantly reduced; however, collectively the studies suggest a redundant role for Hoxb4 in normal adult hematopoiesis. The phenotype of this model is strikingly similar to the one observed in the double Hoxb3/b4 KO model,28 but with a less pronounced phenotype, suggesting a cooperation between Hoxb3 and Hoxb4 or possibly a dosage effect as has been observed in compound Hox KO models.41-44 Interestingly, we detected more pronounced down-regulation of HoxA9 in Hoxb3/4-/- fetal liver cells than in Hoxb4-/- mice, offering a possible explanation for why the hematopoietic phenotype in Hoxb3/4-/- mice is more pronounced given the report of a severe stem cell defect in HoxA9-deficient mice.45
The strong proliferative effect caused by enforced HOXB4 expression in HSCs contrasts with the relatively mild proliferation defects in Hoxb4-deficient HSCs. The deficiency of Hoxb4 can possibly be compensated for by neighboring or paralogous Hox genes, explaining the relative redundancy. However, expression levels of neighboring Hox genes (Hoxb2, Hoxb3, and Hoxb5) was significantly reduced, ruling out a positive compensatory effect from Hox proteins encoded by these genes. RNA expression levels generated by the paralogous Hox genes Hoxa4, Hoxc4, and Hoxd4 were analyzed and demonstrated a mild (1.3-fold) up-regulation of Hoxa4 (not statistically significant) and very low or undetectable levels of Hoxc4 and Hoxd4. A positive compensatory effect from the Hoxa4 up-regulation is theoretically possible, and the low levels of Hoxc4 and Hoxd4 mRNAs indicate a minor role for these genes in regulating the function of hematopoietic progenitors. An elegant study by Greer et al46 demonstrates a redundancy for the paralogous genes Hoxa3 and Hoxd3, where the proteins encoded by these genes are shown to carry out identical biologic functions. Additionally, the HxRE1 and HxRE2 sequences in the Hoxb4 promoter, shown to be the binding sites for NF-Y and USF1/2,47 have also been identified in the 5′ noncoding sequences of HoxA4, HoxC4, and HoxD4, indicating similar regulatory mechanisms of these paralogous genes. However, we did not find a compensatory increase, but rather a decrease, in the expression of several Hox genes in the mutant fetal liver cells, suggesting that the structure of the cluster is essential for proper regulation.48
Analysis of the thorax structure in the Hoxb4-/- and Hoxb3/b4-/- mice showed that 40% of the mice were missing the lowest rib pair as the only major skeletal defect (A.C.M.B. et al, unpublished studies, January 2003), contrasting an earlier report in which Ramirez-Solis et al49 describe a split sternum in a differently targeted Hoxb4 mouse model. They describe two targeted models: one with an inserted double-selection cassette plus stop codon in the first exon that resulted in a split sternum and one with a stop codon inserted in the second exon with milder axial/atlas transformations that did not affect the sternum.49 It is, therefore, clear that different targeting procedures have significant impacts on the observed phenotype. Inserting a selection cassette driven by strong promoters clearly affects the phenotype and the complex regulation within the Hox cluster.50 However, complete removal of the Hoxb4 gene brings Hoxb3 and Hoxb5 together, which might give rise to alternative possibilities for neighboring genes to affect or rescue the phenotype.
The mechanism for HOX protein–mediated regulation is poorly understood, and definitive data on downstream targets for Hox proteins are lacking. It has been suggested that thrombopoietin mediates its positive effects on the proliferation of HSCs through p38 mitogen-activated protein kinase (MAPK) and the upstream stimulating factor (USF-1), which binds to the promoter of Hoxb4, inducing its expression.51,52 Other studies indicate an alternative function for HOX genes as modulators of chromatin by affecting the acetylation of histones, functioning either as repressors or activators.53-55 Studies in cell lines have indicated that HOXB4 is involved in the down-regulation of c-myc expression, thereby promoting differentiation.36 The level of c-myc in our Ter119-depleted, Hoxb4-deficient fetal liver cells was increased, although only modestly, compared with that in WT fetal liver cells, but with the reduced proliferative capacity of the KO cells seen here, these findings support the data of Pan et al.36 The HOXB cluster also seems to be involved in the proliferative response of activated human T and NK cells because HOXB gene expression is sequentially induced in a 3′ → 5′ cluster direction (ie, from HOXB1 through HOXB9) after activation,38,39 and our data from quantitative RT-PCR confirm that the removal of one or both genes in this study affects the expression levels of genes in the same cluster. Studies in Rat-1 fibroblasts give further insight into the proliferative response caused by HOXB4 overexpression, showing activation of the AP-1 complex genes Fra-1 and Jun-B followed by up-regulation of the cell cycle regulator, cyclinD1.37 In conjunction, we show that both Jun-B and cyclinD1 were significantly decreased in KO fetal liver cells from both our KO models. A function for HOXB4 in embryonal hematopoiesis has been suggested in a recent report by Kyba et al56 in promoting the switch between primitive and definitive hematopoiesis, though our data show that Hoxb4 is not the key player in that process.
Safe expansion of HSCs ex vivo is essential for therapeutic applications, and much effort has been put into the search for growth factor combinations that stimulate stem cell regeneration with only modest success.57,58 Enforced HOXB4 expression causes a 40-fold net increase in murine stem cells ex vivo, and a recent study on human cord blood cells generated a 3- to 4-fold increase in CRU numbers in a NOD/SCID transplantation model.19,27 To avoid permanent overexpression of HOXB4 and to avoid the risk for insertional mutagenesis, new approaches have recently been developed by which the HOXB4 protein is added to the cells, either by coculture of human cord blood CD34+ cells with a murine HOXB4-producing cell line59 or by the addition of the purified protein to murine bone marrow cells.60 These exciting findings provide proof-of-principle for the potential use of the Hoxb4 protein in ex vivo stem cell expansion.
We conclude that the physiologic role of Hoxb4 in hematopoiesis is to enhance the proliferative response of long-term repopulating HSCs. Hoxb4 deficiency has subtle effects on steady state hematopoiesis, resulting in reduced cellularity of various hematopoietic organs without perturbing lineage distribution. However, in conditions that call for rapid proliferation, such as bone marrow transplantation, the regenerative capacity of the stem cell pool is significantly reduced, resulting in lower engraftment levels in irradiated recipients. The deficiency of Hoxb4 affects hematopoiesis at the fetal stage, resulting in slightly reduced expansion of the stem cell pool in fetal liver, as shown here by competitive repopulation and CRU studies. Although this effect is reflected in the adult hematopoietic system, Hoxb4 does not seem to be a prominent regulator of HSC proliferation in endogenous hematopoiesis after birth.
Prepublished online as Blood First Edition Paper, February 12, 2004; DOI 10.1182/blood-2003-10-3557.
Supported by grants from Cancerfonden, Sweden; Barncancerfonden, Sweden; Astra Draco AB (now AstraZeneca); and the Donation Funds Lund University Hospital (S.K.), and by a clinical research award (ALF) from Lund University Hospital. J.M.B. was supported by a Graduate Student Award from the Medical Faculty, Lund University. Lund Strategic Center for Stem Cell Biology and Cell Therapy is supported by a center of excellence grant from The Foundation for Strategic Research.
A.C.M.B., J.M.B., and M.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr C. Largman for providing the Hoxb4 probe and Dr R. Fässler for providing the RI ES cells. We also thank Mary-Ann Sällström for help with blastocyst injections, Kristina Sundgren and Eva Gynnstam for expert animal care, Lilian Wittman for help with animal experiments, and Jonas Larsson, Keith Humphries, Sten Eirik Jacobsen, and members of the Department of Stem Cell Biology, Lund University, for helpful advice and discussions.


![Figure 4. Hoxb4-/- BM cells have increased tolerance to 5-FU treatment. (A) In vivo repopulation of Hoxb4-/--derived and Hoxb4+/+-derived donor cells after double treatment with 5-FU (day 1 [d1] and day 5 [d5]). The 5-FU–treated cells were competed against fresh B6.SJL cells in lethally irradiated B6.SJL recipients. (B) Level of reconstitution was measured as peripheral blood cells at 17 weeks after transplantation (mean ± SD) (each bar = 16 recipient mice from 4 donors).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/11/10.1182_blood-2003-10-3557/6/m_zh80110461880004.jpeg?Expires=1767704344&Signature=LltFEN9Sy9S~BcWRLMkFONGobkyNJVqAc7CJqbqYR3kxgcby8SEnR8R5NTFi~wiNHjYzOKosUvtiwu2QRgNBJ4~lord9umwYlEObHKDB-bdFcu2RpaWrRf2cffuFaPCrxtLIDGuHBbalYFbTcLe6eMRaR9y--Eudm8VSIxMmvIjrEIpYNIcfIhAs4CFC4WSwYwjNxalnO9HGhVP6v80Jr5BeMIcR8YSlV96Exvvs2EjVecpN1nplfxt0HQQwVPIihQgC~Gm7t29m1P0e-J8pKWL4t4Jc3~ienrxsVVs8cC7XtbVef81acb5-3k07U~~SaoHbYMksraYm~92Hi7BLGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal